Abstract
Angiogenesis is essential for cyclic endometrial growth, implantation, and pregnancy maintenance. Vasculogenesis, the formation of new blood vessels by bone marrow (BM)-derived endothelial progenitor cells (EPCs), has been shown to contribute to endometrial vasculature. However, it is unknown whether vasculogenesis occurs in neovascularization of the decidua during pregnancy. To investigate the contribution of BM-derived EPCs to vascularization of the pregnant uterus, we induced non-gonadotoxic submyeloablation by 5-fluorouracil administration to wild-type FVB/N female mice recipients followed by BM transplantation from transgenic mice expressing green fluorescent protein (GFP) under regulation of Tie2 endothelial-specific promoter. Following 1 month, Tie2-GFP BM-transplanted mice were bred and sacrificed at various gestational days (ED6.5, ED10.5, ED13.5, ED18.5, and postpartum). Bone-marrow-transplanted non-pregnant and saline-injected pregnant mice served as controls (n = 5–6/group). Implantation sites were analyzed by flow cytometry, immunohistochemistry, and immunofluorescence. While no GFP-positive EPCs were found in non-pregnant or early pregnant uteri of BM-transplanted mice, GFP-positive EPCs were first detected in pregnant uterus on ED10.5 (0.12%) and increased as the pregnancy progressed (1.14% on ED13.5), peaking on ED18.5 (1.42%) followed by decrease in the postpartum (0.9%). The percentage of endothelial cells that were BM-derived out of the total endothelial cell population in the implantation sites (GFP+CD31+/CD31+) were 9.3%, 15.8%, and 6.1% on ED13.5, ED18.5, and postpartum, respectively. Immunohistochemistry demonstrated that EPCs incorporated into decidual vasculature, and immunofluorescence showed that GFP-positive EPCs colocalized with CD31 in vascular endothelium of uterine implantation sites, confirming their endothelial lineage. Our findings indicate that BM-derived EPCs contribute to vasculogenesis of the pregnant mouse decidua.
Keywords: endothelial progenitor cells (EPCs), bone marrow, pregnancy, vasculogenesis, mouse
Bone marrow-derived endothelial progenitor cells contribute to decidual neovascularization of the pregnant uterus via vasculogenesis
Introduction
Angiogenesis is the formation of new blood vessels from pre-existing ones, and is an essential process for tissue growth and development. Angiogenesis is limited to embryonic development and pathological conditions, with the exception of the female reproductive tract (i.e., ovary, uterus, and placenta), which, in the adult, normally undergoes physiological angiogenesis [1]. The uterus undergoes cyclic changes in angiogenesis, which plays an important role in implantation, decidualization, placentation, fetal growth, and maintenance of pregnancy [2–4]. It is widely believed that extensive new blood vessel formation and vascular remodeling are essential to deliver sufficient oxygen and nutrients to the uterus as well as to the developing embryo [5, 6]. Accordingly, vascular insufficiencies during pregnancy have been associated with a number of pregnancy complications ranging from spontaneous miscarriage to fetal growth restriction and preeclampsia [7–10]. Therefore, formation of vascular network both in placenta and uterus is crucial for successful gestation.
Neovascularization can occur via either angiogenesis or vasculogenesis [11]. In contrast to angiogenesis, which is based on pre-existing blood vessels, vasculogenesis involves de novo formation of blood vessels from bone marrow (BM)-derived endothelial progenitor cells (EPCs). Although vasculogenesis was initially thought to be restricted to the embryogenesis period, it has been shown to contribute to neovascularization in a variety of organs in the adult (postnatal vasculogenesis) [12, 13]. BM-derived EPCs have been shown to contribute to vasculogenesis during wound healing [14], postmyocardial infarction [15], fracture healing [16], limb ischemia [17], and tumor growth [18, 19]. We have previously shown that BM-derived progenitors can give rise to non-hematopoietic cells in the endometrium of adult humans and mice [20, 21]. Furthermore, BM-derived EPCs contribute to new blood vessel formation in adult reproductive organs, where they have been shown to integrate as endothelial cells in the human and mouse endometrium [22, 23] and ovary corpus luteum (CL) [24]. Moreover, studies have demonstrated that new blood vessel formation in endometriosis lesions also involves vasculogenesis from circulating BM-derived EPCs, which home to the endometriotic lesions and are incorporated into the microvascular endothelium [25, 26]. Interestingly, Sugawara et al. investigated circulating EPCs in the peripheral blood of pregnant women and found that the number of circulating EPCs increased gradually throughout gestation [27]. However, it is still unknown whether vasculogenesis plays a role in new blood vessel formation in the pregnant uterus.
We hypothesized that BM-derived EPCs may be recruited to the uterus and contribute to its vascularization during pregnancy. To investigate our hypothesis and characterize the contribution of EPCs to the pregnant mouse uterus, we utilized our previously described 5-fluorouracil (5-FU)-based non-gonadotoxic submyeloablation bone marrow transplant (BMT) protocol [28], performing BMT from mice expressing green fluorescent protein (GFP) under the regulation of endothelial-specific Tie2 promoter into wild-type mice.
Materials and Methods
Animals
FVB/N wild-type mice and Tie2-GFP-expressing mice (Tg(Tie2-GFP)287Sato/J) were purchased from the Jackson Laboratory. The mice were maintained at 24°C under controlled environmental conditions (lights on from 7 am to 7 pm) and had free access to tap water and standard pellet food. Male Tie2-GFP mice were used as syngeneic BM donors for BMT to FVB/N wild-type female mice. All animals were treated under an approved Yale University institutional animal care and use committee protocol.
Submyeloablation mouse model
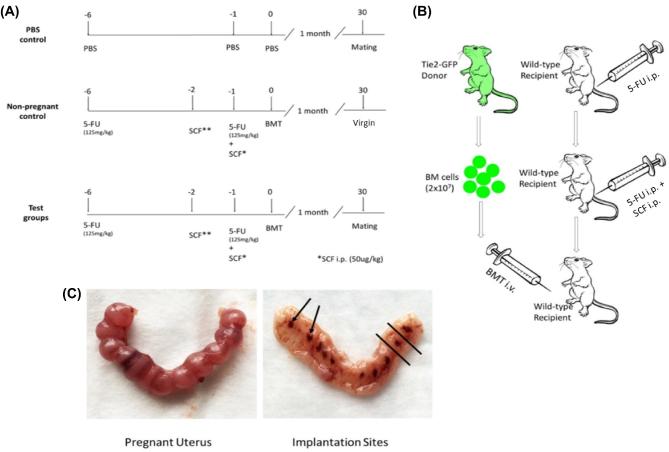
Six-week-old FVB/N wild-type female mice underwent 5-FU-based non-gonadotoxic submyeloablation as previously described [28]. Experimental BMT-recipient mice received 125 mg/kg 5-fluoruracil (Sigma-Aldrich) by ip injections on days 6 and 1 before BMT. In addition, mice were injected ip with 50 μg/kg (150 μg/kg total) stem cell factor (SCF, R&D Systems) at 21 h and 9 h before and 3 h after the second 5-FU dose (Figure 1A and B). The control group received iv phosphate-buffered saline (PBS) injection instead of BM cells, and PBS ip injections in lieu of 5-FU and SCF (Figure 1A and B). Mice were monitored for general toxicity daily after drug treatment by measuring weights and assessing their wellbeing. Acute 5-FU-induced toxicities began to be manifested within 3–4 days after treatment and were resolved by 2 weeks in these studies, consistent with our prior experience [28].
Figure 1.
Non-gonadotoxic submyeloablation mouse model and harvest of implantation sites. (A) Mice received either phosphate-buffered saline (PBS) or 5-fluorouracil (5-FU) on days 6 and 1 before bone marrow transplant (BMT). In addition, stem cell factor (SCF) was administered by three separate 50 μg/kg doses at 21 and 9 h before and 3 h after the second 5-FU dose. (B) 5-FU or PBS was administered ip, while bone marrow (BM) was given iv via retro-orbital injection. (C) ED10.5 pregnant uterus is shown prior to dissection (left), and following longitudinal dissection and removal of the placenta and embryo (right). The implantation sites are shown (arrows). The region between the black lines depicts the implantation area removed for subsequent flow cytometry analysis.
Bone marrow transplantation
Mice underwent BMT 24 h following the second dose of 5-FU. Transplantation of fresh BM cells was performed as described previously [28]. Bone marrow cells were obtained by flushing the femurs and tibias of donor FVB/N-TgN (Tie2/GFP) 287 Sato mice (8-week-old) with cold sterile DMEM/F12 (Sigma-Aldrich). Recipient mice underwent transplantation via retro-orbital vein injection with 2 × 107 unfractionated BM cells resuspended in 100 μL of PBS. Subsequently, the mice were monitored and allowed 4 weeks for complete recovery. In addition, 4 weeks after BMT, peripheral blood was obtained by venipuncture for analysis of donor chimerism using flow cytometry and confirmation of successful BM engraftment.
Breeding experiments
For breeding experiments, BMT-recipient female mice were divided into pregnant and non-pregnant groups. Mice in the pregnant groups were mated after recovery on day 30 post-BMT with FVB/N male mice of proven fertility and were checked for vaginal plugs at 7 am daily. The morning of vaginal plug detection was considered embryonic day (ED) 0.5. Successfully bred female mice were sacrificed at various gestational time points including ED6.5, ED10.5, ED13.5, ED18.5, or postpartum day 1. In addition, virgin mice that underwent BMT from GFP donors served as the non-pregnant group. For each time point, mice of corresponding gestational age, which did not undergo BMT and were injected with PBS instead of BM cells, served as controls. The engraftment of GFP-positive EPCs and their characterization in the uterus and uterine implantation sites was performed by flow cytometry and immunohistochemistry/fluorescence.
Flow cytometry
Mice were anesthetized by inhalation of Forane (isoflurane; Abbott Laboratories) and sacrificed by cervical dislocation, and then transcardially perfused with 10 mL PBS. The uteri were extracted, and fetal/placental parts were removed from the uterus. Uterine implantation sites were dissected as shown in Figure 1C. Three implantation sites were finely minced, and subsequently digested with a solution of Hanks’ balanced salt solution (Life Technologies, Inc.) containing HEPES (25mM), collagenase B (1 mg/mL; Roche Diagnostics), and deoxyribonuclease I (0.1 mg/mL; Sigma-Aldrich) for 60 min at 37°C. Other uterine tissues were fixed in 4% paraformaldehyde. After digestion, the samples were filtered using 70 μm filters and centrifuged at 2000 rpm for 8 min at 4°C. Cell pellets were suspended in PBS, followed by incubation for 30 min at room temperature with PerCp/Cy5.5 conjugated anti-mouse CD31 antibody (#102419, Biolegend). Propidium iodide was added to exclude nonviable cells. Isotype-identical antibodies served as controls (PerCP/Cy5.5 rat IgG2a; #400532, Biolegend). Flow cytometry was performed on a fluorescence-activated cell sorting Beckman Coulter MoFlo machine (Beckman Coulter). Gates were applied to forward-scatter/side-scatter dot plots to exclude nonviable cells and cell debris. Appropriate unstained and antibody IgG isotype controls were used for setting compensation and determining gates. Data were analyzed using the software FlowJo V10 (FlowJo).
Histology and immunohistochemistry
Uterine tissues were fixed in 4% paraformaldehyde and embedded in paraffin. Tissue sections (5 μm) were mounted on slides followed by deparaffinization and rehydration. Slides were then boiled in sodium citrate (pH 6.0) for antigen retrieval. Sections were blocked with 5% rabbit serum (Vector Laboratories) followed by incubating with a goat polyclonal anti-GFP primary antibody (1:1000; ab6673, Abcam) overnight at 4°C. Sections were then incubated with a biotinylated rabbit anti-goat antibody (1:200; Vector Laboratories) for 1 h. Detection was performed using ABC Vectorstain Elite reagents with DAB plus H2O2 (Vector Laboratories). Tissue sections were counterstained with hematoxylin (Sigma-Aldrich). Images of stained sections were captured using an Olympus BX-51 microscope (Olympus).
Immunofluorescence
For colocalization of GFP with CD31, immunofluorescence was performed following antigen retrieval as above. Sections were blocked with 5% donkey serum for 1 h followed by overnight incubation with primary antibody at 4°C. The primary antibodies used in this study were polyclonal goat anti-GFP antibody (ab6673, 1:300), rabbit anti-CD31 (ab28634, 1:100), an endothelial cell marker (all antibodies from Abcam). Alexa Fluor 564-conjugated donkey anti-goat (1:200; Life Technologies) and Alexa Fluor 488-conjugated donkey anti-rabbit (1:200; Life Technologies) were used as secondary antibodies. After counterstaining with Vectashield fluorescent mounting media with 4′6-diamidino-2-phenylindole (DAPI) (Vector Laboratories), the sections were mounted under coverslips. For negative controls, sections were incubated without primary and/or secondary antibodies. Images were captured using a confocal laser microscope (LSM710; Carl Zeiss) and analyzed using the ZEN software (Carl Zeiss).
Statistical analysis
Data were assessed for normal distribution with a Shapiro–Wilk normality test using GraphPad Prism 6 software (GraphPad Software). Normally distributed data were analyzed using the Student's unpaired two-tailed t test for comparison of two groups, and one-way ANOVA with Tukey's multiple comparison test for multiple group comparison. If data were not normally distributed, or if distribution could not be determined due to small sample size, data were analyzed using a Mann–Whitney U test. P < 0.05 was considered statistically significant.
Results
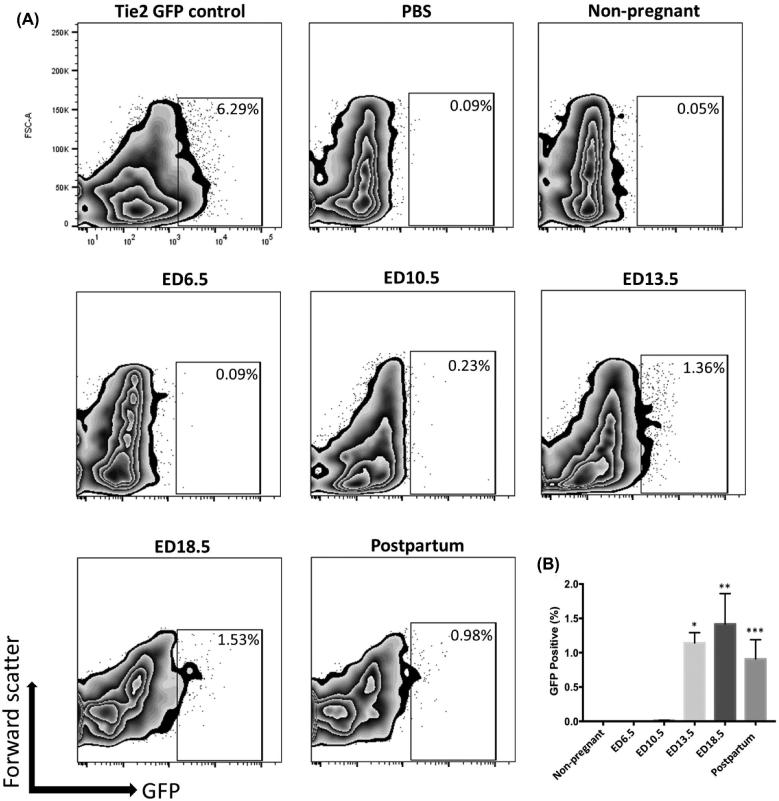
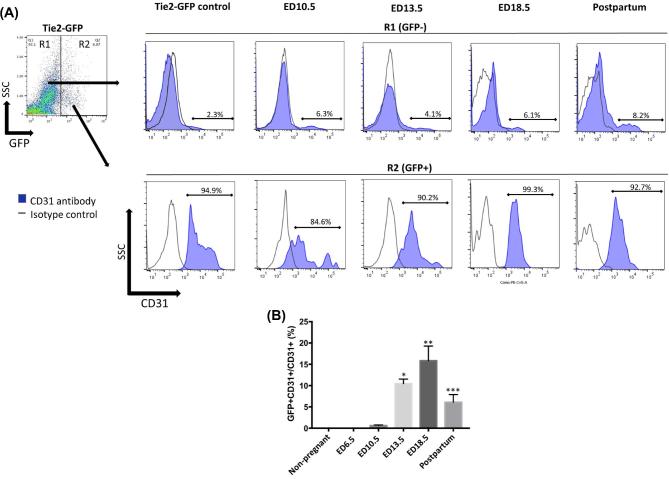
To investigate the temporal contribution of BM-derived EPCs to the pregnant uterus, BMT was performed from mice expressing GFP under regulation of Tie2 promoter into wild-type female mice following our previously described 5-FU-based non-gonadotoxic submyeloablation protocol [28]. The pregnant mice in this 5FU submyeloablation model do not show any difference in placental weights, embryo weights or placental/embryo ratios as compared to controls (Figure S1). Recipient female mice were sacrificed at various gestational time points and the percentage of GFP-positive cells in implantation sites of uteri following removal of the embryo/placenta was determined by flow cytometry. CD31 is a specific marker of endothelial cells, and CD31 antibody staining was performed to identify the endothelial populations. The mean percentage of GFP+ EPCs out of the total cells in the uterus of Tie2-GFP transgenic control mice was 6.62% (Figure 2A), indicating that overall endothelial cells comprise about 6% of cells in the normal uterus. No GFP+ EPCs were found in PBS control (Figure 2A). After transplant of BM from Tie2-GFP transgenic to wild-type mice, no GFP-positive cells were detected in non-pregnant or early pregnant uteri (ED6.5). In BM-transplanted mice, GFP-positive EPCs were first detected in the uterus on ED10.5, accounting for 0.12% ± 0.01% of total uterine cells (Figure 2A and B). This contribution increased to 1.14% ± 0.18% of total uterine cells on ED13.5, further increase towards late gestation peaking on ED18.5 (1.42% ± 0.40%), followed by a decrease in the postpartum period (day 1) (0.90% ± 0.28%) (Figure 2A and B). Accordingly, significantly increased EPCs engraftment was observed in ED 13.5, ED18.5, and postpartum day 1 groups as compared with non-pregnant group (P < 0.01, P < 0.001, P < 0.05, respectively) (Figure 2B). The GFP+ uterine cell populations at the various gestational time points were mostly positive for CD31 surface marker, confirming their endothelial lineage (Figure 3A). The percentage of endothelial cells that were BM-derived out of the total endothelial cell population of the implantation site (GFP+CD31+/total CD31+) were 9.3%, 15.8%, and 6.1% on ED13.5, ED18.5, and postpartum, respectively. The proportion of BM-derived endothelial cells in ED13.5, ED18.5, and postpartum groups was also significantly increased as compared with non-pregnant control group (P < 0.0001, P < 0.0001, P < 0.05, respectively) (Figure 3B).
Figure 2.
Flow cytometry analysis of GFP-positive bone marrow (BM)-derived EPCs in the pregnant uterus. (A) Representative zebra plots of flow cytometry analysis of uterine tissue cells showing GFP-positive endothelial progenitor cells (EPCs) found in the uteri of Tie2-GFP positive control, PBS negative control, and non-pregnant group, and in the implantation sites on pregnant days ED 6.5, 10.5, 13.5, 18.5, and postpartum day 1; (B) Mean percentage of GFP-positive EPCs in the uteri of non-pregnant, ED6.5, ED10.5, ED13.5, ED18.5, and postpartum groups; n = 6 mice per group. Data in graphs are presented as mean ± SEM; *P<0.005 vs. non-pregnant group; **P<0.001 vs. non-pregnant group; ***P<0.05 vs. non-pregnant group.
Figure 3.
Flow cytometry analysis of endothelial cells in the pregnant uterus. (A) Cells gated in R2 are bone marrow (BM)-derived (GFP+) uterine cells while cells gated in R1 are non-BM-derived resident (GFP-) uterine cells. Histograms represent GFP+ cells (R2) or GFP- cells (R1) from ED10.5, 13.5, 18.5, and postpartum day 1 uterine tissues that are stained with the CD31 antibody (filled blue) and isotype control (black line). Tie2-GFP mice served as positive controls. (B) Mean percentage of BM-derived EPCs (GFP+CD31+) out of the total endothelial cell population (CD31+) in the uterine implantation sites in non-pregnant, ED6.5, ED10.5, ED13.5, ED18.5, and postpartum groups; n = 6 mice per group. Data in graphs are presented as mean ± SEM; *P<0.005 vs. non-pregnant group; **P<0.001 vs. non-pregnant group; ***P<0.05 vs. non-pregnant group.
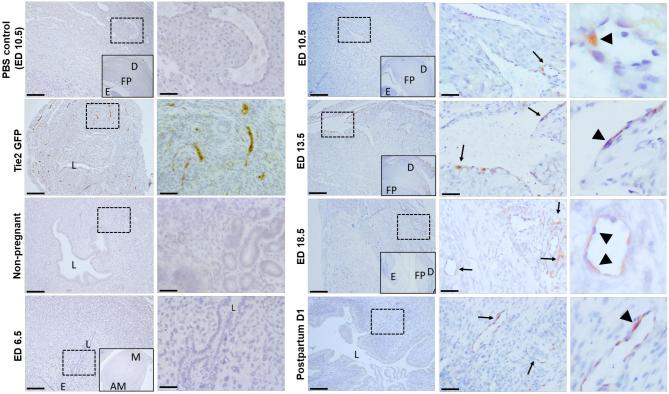
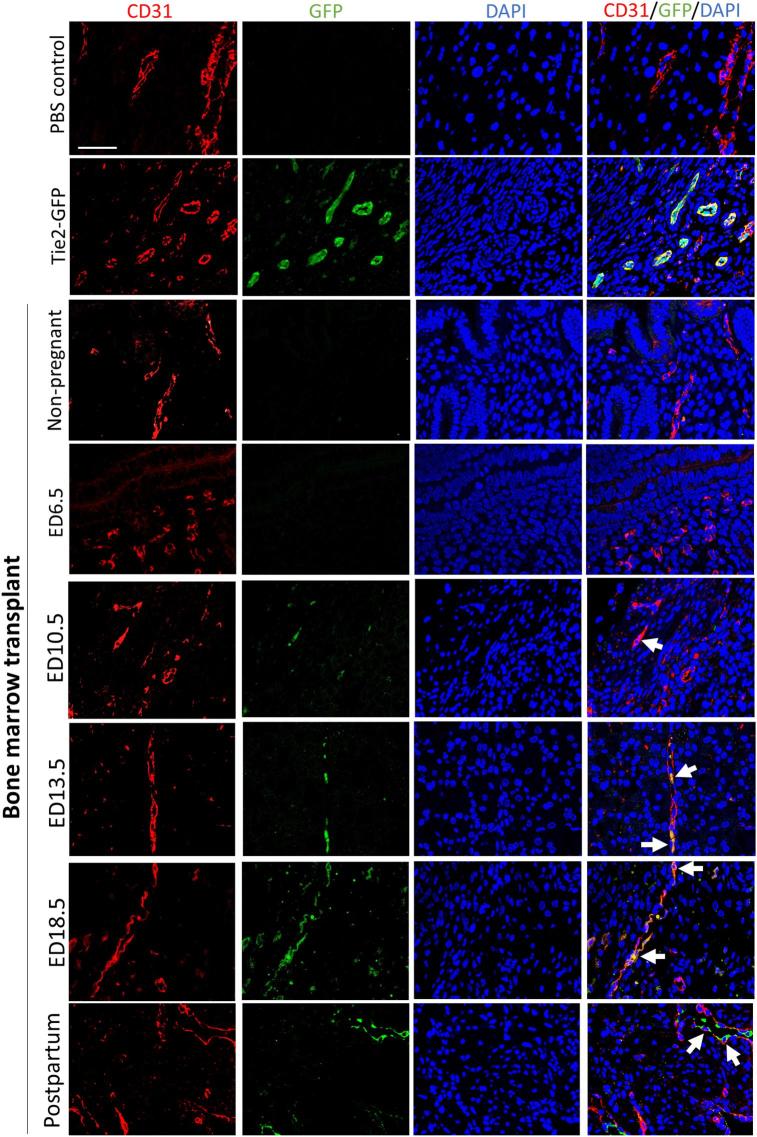
Immunohistochemical staining using GFP antibody further revealed the localization of EPCs in the pregnant uterus. As the positive control, GFP-positive EPCs were found to be incorporated into the endothelial lining of the microvessels in the uteri of Tie2-GFP mice (Figure 4), no GFP signal was detected in cells from uteri of PBS control, non-pregnant, and ED6.5 groups (Figure 4). GFP-positive cells were first found to localize in the microvessels of the decidua on ED10.5. However, more GFP-positive cells were detected showing greater EPCs recruitment to pregnant uteri of ED13.5, ED18.5, and postpartum day 1 groups compared with the non-pregnant group (Figure 4). In addition, the analysis of the localization of these GFP-positive EPCs in the uterus revealed that most of them were incorporated into the endothelial lining of the microvessels, whereas a few were sporadically distributed in the surrounding endometrial stroma and myometrium.
Figure 4.
Immunohistochemical detection of GFP-positive endothelial cells in uterine sections. Immunostaining of uterine sections using anti-GFP antibody. The inset within left panels of pregnant sections (ED6.5, ED10.5, ED13.5, ED18.5, and PBS ED10.5 control) is a lower magnification image of the panel for orientation, showing the mesometrial (M) and anti-mesometrial (AM) areas, the decidua (D), fetal side of the placenta (FP) and embryo (E). The right panels of each time point are higher magnification images of the dashed respective rectangle area in the left panel. GFP-positive endothelial cells (brown) are seen in uterine blood vessels of Tie2-GFP positive control mice while no GFP positive staining is seen in PBS, non-pregnant and ED6.5 groups. Arrows point to decidual blood vessels with incorporated GFP-positive endothelial cells (arrowheads) in ED10.5, ED13.5, ED18.5, and postpartum day 1 groups. Representative of 3–4 mice for each group. Scale bars in the lower magnification images = 200 um. Scale bars in the higher magnification images = 50 um.
To confirm the integration of BM-derived (GFP+) EPCs into uterine blood vessels, we performed immunofluorescence analysis using CD31 (endothelial marker) and GFP antibodies. On ED10.5, ED13.5, ED18.5, and postpartum day 1, we observed that GFP+ cells colocalized with CD31, indicating that these BM-derived EPCs integrated into blood vessels as endothelial cells, contributing to the microvasculature of pregnant uteri (Figure 5). No GFP-positive cells were detected in the uteri of non-pregnant or ED6.5 groups (Figure 5) consistent with the flow cytometry data. Immunofluorescence-based analysis also demonstrated that almost all BM-derived GFP cells in the uterus colocalized with CD31, and were localized in the decidual stroma, whereas no GFP positive cells were detected in either the decidual glandular or luminal epithelium.
Figure 5.
Immunofluorescence colocalization of GFP-positive endothelial cells in uterine sections. Uterine tissues from Tie2-GFP positive control, PBS control, non-pregnant, ED6.5, ED10.5, ED13.5, ED18.5, and postpartum groups were co-stained with anti-CD31 (endothelial cell marker, red) antibody and anti-GFP antibody (green). White arrows indicate the BM-derived EPCs (GFP+ cells) showing colocalization of CD31 and GFP. Representative of 3–4 mice for each group. Scale bar = 50 um.
Discussion
In the present study, we used a non-gonadotoxic submyeloablation BMT mouse model to characterize the spatial and temporal dynamics of BM-derived EPCs in the uterus during pregnancy. We show that vasculogenesis contributes to uterine vessel neovascularization during pregnancy, accounting for up to 15–20% of total uterine endothelial cells at late-gestation. These findings suggest that BM-derived EPCs play a potentially important role in the regulation and maintenance of uterine vascular development and integrity during pregnancy.
During the course of gestation, uterine volume increases approximately 1000-fold and uterine blood flow increases between 20- and 50-fold [29]. Accordingly, neovascularization is crucial to support the blood supply demands of a rapidly growing pregnant uterus. Neovascularization has been proposed to occur in the placenta via classic angiogenesis mechanisms as well as vasculogenesis [3, 30–32]. However, only angiogenesis has been known to contribute to the decidual vascular formation and remodeling in the pregnant uterus [33, 34]. In our current study, we demonstrate for the first time another mechanism for uterine neovascularization in pregnancy, the incorporation of BM-derived EPCs into the pregnant decidua by vasculogenesis. BM-derived GFP-positive EPCs were first detected in decidual vasculature at mid-gestation. They continue to increase, reaching a peak at the end of gestation (ED18.5), accounts for up to 15–20% of total uterine endothelial cells. The rest of the endothelial cells in the pregnant uterus are most likely derived from local proliferation of pre-existing blood vessels.
In prior studies, some researchers have shown that the extent of BM-derived EPCs incorporation into the endothelium of newly forming blood vessels by the mechanism of vasculogenesis was low, only a rare number of EPCs could be detected within the developing microvascular networks. Kizuka et al. [24] studied the change of BM-derived GFP-positive cells in the theca cell layer of the preovulatory follicle and in the CL during CL formation in an irradiated BMT model and found the proportion of GFP positive cells out of CD34/CD31-positive cells estimated to be less than 5%. Ahn et al. [35] also reported that only rare numbers of EPCs can be detected within the developing microvascular networks of growing tumors. However, in our study, we found that up to 15–20% of all endothelial cells within the pregnant uterus originate from circulating BM-derived EPCs. This degree of donor chimerism is relatively high, which is consistent with previous study by Laschke et al. [26]. The authors examined vasculogenesis in a mouse endometriosis model using Tie2-GFP BM-transplanted mice, demonstrating that approximately 18% of all endothelial cells in the endometriotic lesions were BM-derived EPCs [26]. They suggested that the endometrial tissue is the target tissue for engraftment of BM-derived stem cells and is also well adapted to the process of stem cell homing. BM-derived progenitor cells are known to give rise to various endometrial cells in the uterus and their recruitment to the non-pregnant endometrium was shown to be stimulated by tissue injury and ischemia [36]. Our data indicate that pregnancy is a physiological stimulus for BM-derived EPCs recruitment to the uterus in the second and third trimester. This is consistent with a human study showing mobilization of circulating EPCs in pregnancy, with the number of EPCs in peripheral blood increasing gradually throughout gestation [27].
It is well established that estrogen levels keep rising as pregnancy progresses. Interestingly, the number of EPCs in maternal circulation has been found to gradually increase with gestational age, correlating with serum estradiol levels [27]. In addition, estrogens can mobilize EPCs from the bone marrow in vivo and can inhibit the senescence of EPCs and stimulate VEGF production in vitro [37]. Ovariectomy has been shown to decrease the number of circulating EPCs, whereas treatment with E2 or selective estrogen receptor alpha agonist restores circulating EPCs in rats [38]. Moreover, estrogens were shown to increase the number of BM-derived EPCs incorporated into endometriotic lesions in a mouse model of endometriosis [39]. In the present study, we found that the number of BM-derived EPCs incorporated into the pregnant uterus kept increasing as pregnancy progressed and decreased in the postpartum period. Taken together, these data suggest that estrogens may play an important physiological role in mediating mobilization of EPCs from bone marrow to maternal circulation during pregnancy, contributing to the increased incorporation of these BM-derived EPCs into the decidual vasculature of the pregnant uterus. It would be interesting to explore the mechanism(s) underlying the increased homing of EPCs to the pregnant uterus, and whether the CXCL12-CXCR4 axis plays a role.
In our study, no BM-derived EPCs were found in early pregnant uteri of BM-transplanted mice, and were first detected on ED10.5. Christofferson et al. reported that embryo implantation induced a vascular shut-down in the primary decidual zone and the area remained avascular until day 10 of pregnancy in the rat uterus. Subsequently, maternal blood volume at implantation sites starts to increase on days 10–12 of pregnancy [40]. Since the recruitment of EPCs into ischemic tissues is critically dependent on blood perfusion and associated remodeling processes in the microvasculature [41], it is not surprising that GFP-positive EPCs were detected only on ED10.5, coinciding with the establishment of a new microvasculature with maternal uterine blood vessels ingrowth.
Preeclampsia is characterized by impaired, shallow trophoblast invasion of spiral arteries. The resulting reduced uteroplacental perfusion is thought to induce a series of hypoxia/ischemia events, accompanied by an imbalance between vasodilative/vasopressive factors, angiogenic factors, a dysfunction of the renin–angiotensin system as well as endothelium activation [42]. Interestingly, several studies reported that circulating EPCs levels are decreased in preeclampsia. Sugawara et al. [43] reported a decrease in circulating EPCs number and increased EPCs cellular senescence. Others have also found decreased number of EPCs in preeclampsia [44] as well as in intrauterine growth restriction [45]. Gammill et al. have reported that EPCs number increases in healthy pregnancy but similar increase was not observed in women with preeclampsia [46]. Moreover, there is evidence in humans that synthesis of estrogens is altered in preeclamptic pregnancies, with a decrease in 17β-estradiol, estriol, and other estrogen metabolites, such as 2-methoxyestradiol, as well as its enzyme catechol-o-methyltransferase (COMT) [47, 48]. Given the role of estrogens in stimulating EPCs mobilization and function, such aberrations in estrogen metabolism may explain the observations of decreased EPCs number and/or function in preeclampsia. Our findings that EPCs contribute and integrate into endothelium of uterine neovessels at mid/late-gestation, a period when preeclampsia manifestations tend to appear, suggests that EPCs may play an important physiological role in maintaining uterine vascular endothelial health, and that dysfunction in this process may contribute to the pathogenesis of preeclampsia.
In summary, we demonstrate that BM-derived EPCs contribute to vascularization of the mouse uterus during pregnancy. These findings indicate that the process of vasculogenesis is an integral part of the mechanism of pregnant uterine neovascularization, which is crucial for maintenance of pregnancy and fetal growth. Further studies of vasculogenesis in the pregnant uterus may provide potential new preventive and therapeutic strategies to some pregnancy pathologies, which are characterized by maternal vasculature dysfunction, including preeclampsia and fetal growth restriction. EPCs may be a good biomarker to diagnose and evaluate therapeutic efficacy for these diseases.
Supplementary Material
Notes
Conference Presentation: This work was presented in part as an oral presentation at the Society for Reproductive Investigation (SRI) 64th annual scientific meeting, 2017, Orlando, Florida, USA.
Edited by Dr. Peter J. Hansen
Footnotes
Grant Support: This work was supported by the funds from NICHD grant 5K12HD04701813 (to R.T.), Ferring/New England Fertility Society grant (to R.T.), American Society for Reproductive Medicine (ASRM) grant (to R.T.), and the Robert E. Leet and Clara Guthrie Patterson Fellowship award (to R.T.).
Supplementary data
Supplementary Figure S1. Comparison of placental and fetal weights between 5FU-myeloablated mice and controls. Placental weights (A), embryo weights (B), and placenta to embryo ratio (C) were measured on ED13.5 and ED16.5 in pregnant mice, which underwent bone marrow transplant following 5-FU submyeloablation vs. control PBS-injected mice; n = 6 mice per group. Data in graphs are presented as mean ± SEM.
References
- 1. Torry RJ, Rongish BJ. Angiogenesis in the uterus: potential regulation and relation to tumor angiogenesis. Am J Reprod Immunol 1992; 27:171–179. [DOI] [PubMed] [Google Scholar]
- 2. Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med 2012; 18:1754–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zygmunt M, Herr F, Münstedt K, Lang U, Liang OD. Angiogenesis and vasculogenesis in pregnancy. Eur J Obstet Gynecol Reprod Biol 2003; 110:S10–S18. [DOI] [PubMed] [Google Scholar]
- 4. Fraser HM, Lunn SF. Angiogenesis and its control in the female reproductive system. Br Med Bull 2000; 56:787–797. [DOI] [PubMed] [Google Scholar]
- 5. Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, Wang H. Molecular cues to implantation. Endocr Rev 2004; 25:341–373. [DOI] [PubMed] [Google Scholar]
- 6. Reynolds LP, Grazul-Bilska AT, Redmer DA. Angiogenesis in the female reproductive organs: pathological implications. Int J Exp Pathol 2002; 83:151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hiby SE, Walker JJ, O'shaughnessy KM, Redman CW, Carrington M, Trowsdale J, Moffett A. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med 2004; 200:957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gourvas V, Dalpa E, Konstantinidou A, Vrachnis N, Spandidos DA, Sifakis S. Angiogenic factors in placentas from pregnancies complicated by fetal growth restriction (review). Mol Med Rep 2012; 6: 23–27. [DOI] [PubMed] [Google Scholar]
- 9. Banerjee P, Ghosh S, Dutta M, Subramani E, Khalpada J, Chakravarty B, Chaudhury K. Identification of key contributory factors responsible for vascular dysfunction in idiopathic recurrent spontaneous miscarriage. PLoS One 2013; 8:e80940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Torry DS, Hinrichs M, Torry RJ. Determinants of placental vascularity. Am J Reprod Immunol 2004; 51:257–268. [DOI] [PubMed] [Google Scholar]
- 11. Tal R, Segars JH. The role of angiogenic factors in fibroid pathogenesis: potential implications for future therapy. Hum Reprod Update 2014; 20:194–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997; 275:964–966. [DOI] [PubMed] [Google Scholar]
- 13. Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 1999; 85:221–228. [DOI] [PubMed] [Google Scholar]
- 14. Gill M, Dias S, Hattori K, Rivera ML, Hicklin D, Witte L, Girardi L, Yurt R, Himel H, Rafii S. Vascular trauma induces rapid but transient mobilization of VEGFR2(+)AC133(+) endothelial precursor cells. Circ Res 2001; 88:167–174. [DOI] [PubMed] [Google Scholar]
- 15. Edelberg JM, Tang L, Hattori K, Lyden D, Rafii S. Young adult bone marrow-derived endothelial precursor cells restore aging-impaired cardiac angiogenic function. Circ Res 2002; 90:E89–E93. [DOI] [PubMed] [Google Scholar]
- 16. Matsumoto T, Kuroda R, Mifune Y, Kawamoto A, Shoji T, Miwa M, Asahara T, Kurosaka M. Circulating endothelial/skeletal progenitor cells for bone regeneration and healing. Bone 2008; 43:434–439. [DOI] [PubMed] [Google Scholar]
- 17. Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med 1999; 5:434–438. [DOI] [PubMed] [Google Scholar]
- 18. Peters BA, Diaz LA, Polyak K, Meszler L, Romans K, Guinan EC, Antin JH, Myerson D, Hamilton SR, Vogelstein B, Kinzler KW, Lengauer C. Contribution of bone marrow-derived endothelial cells to human tumor vasculature. Nat Med 2005; 11:261–262. [DOI] [PubMed] [Google Scholar]
- 19. Kopp HG, Ramos CA, Rafii S. Contribution of endothelial progenitors and proangiogenic hematopoietic cells to vascularization of tumor and ischemic tissue. Curr Opin Hematol 2006; 13:175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Du H, Taylor HS. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells 2007; 25:2082–2086. [DOI] [PubMed] [Google Scholar]
- 21. Taylor HS. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA 2004; 292:81–85. [DOI] [PubMed] [Google Scholar]
- 22. Gil-Sanchis C, Cervelló I, Khurana S, Faus A, Verfaillie C, Simón C. Contribution of different bone marrow-derived cell types in endometrial regeneration using an irradiated murine model. Fertil Steril 2015; 103:1596–1605.e1. [DOI] [PubMed] [Google Scholar]
- 23. Mints M, Jansson M, Sadeghi B, Westgren M, Uzunel M, Hassan M, Palmblad J. Endometrial endothelial cells are derived from donor stem cells in a bone marrow transplant recipient. HumReprod 2008; 23:139–143. [DOI] [PubMed] [Google Scholar]
- 24. Kizuka F, Tokuda N, Takagi K, Adachi Y, Lee L, Tamura I, Maekawa R, Taketani T, Tamura H, Suzuki T, Owada Y, Sugino N. Involvement of bone marrow-derived vascular progenitor cells in neovascularization during formation of the corpus luteum in mice. Biol Reprod 2012; 87:55, 1–7. [DOI] [PubMed] [Google Scholar]
- 25. Becker CM, Beaudry P, Funakoshi T, Benny O, Zaslavsky A, Zurakowski D, Folkman J, D’Amato RJ, Ryeom S. Circulating endothelial progenitor cells are up-regulated in a mouse model of endometriosis. Am J Pathol 2011; 178:1782–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Laschke MW, Nickels RM, Scheuer C, Menger MD. Endothelial progenitor cells contribute to the vascularization of endometriotic lesions. Am J Pathol 2011; 178:442–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sugawara J, Mitsui-Saito M, Hoshiai T, Hayashi C, Kimura Y, Okamura K. Circulating endothelial progenitor cells during human pregnancy. J Clin Endocrinol Metab 2005; 90:1845–1848. [DOI] [PubMed] [Google Scholar]
- 28. Tal R, Liu Y, Pluchino N, Shaikh S, Mamillapalli R, Taylor HS. A murine 5-Fluorouracil-based submyeloablation model for the study of bone marrow-derived cell trafficking in reproduction. Endocrinology 2016; 157:3749–3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Magness R, Ford SP. Maternal cardiovascular adaptation and uterine circulation-physiology and pathophysiology. In: Stress and Developmental Programming of Health and Disease: Beyond Phenomenology, Zhang L, Longo LD (eds). Hauppauge, NY: Nova Science; 2014; 341–374. [Google Scholar]
- 30. Demir R, Kayisli UA, Cayli S, Huppertz B. Sequential steps during vasculogenesis and angiogenesis in the very early human placenta. Placenta 2006; 27:535–539. [DOI] [PubMed] [Google Scholar]
- 31. Herr F, Baal N, Widmer-Teske R, McKinnon T, Zygmunt M. How to study placental vascular development? Theriogenology 2010; 73:817–827. [DOI] [PubMed] [Google Scholar]
- 32. Grazul-Bilska AT, Johnson ML, Borowicz PP, Bilski JJ, Cymbaluk T, Norberg S, Redmer DA, Reynolds LP. Placental development during early pregnancy in sheep: effects of embryo origin on vascularization. Reproduction 2014; 147:639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Douglas NC, Tang H, Gomez R, Pytowski B, Hicklin DJ, Sauer CM, Kitajewski J, Sauer MV, Zimmermann RC. Vascular endothelial growth factor receptor 2 (VEGFR-2) functions to promote uterine decidual angiogenesis during early pregnancy in the mouse. Endocrinology 2009; 150:3845–3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim M, Park HJ, Seol JW, Jang JY, Cho YS, Kim KR, Choi Y, Lydon JP, Demayo FJ, Shibuya M, Ferrara N, Sung HK et al.. VEGF-A regulated by progesterone governs uterine angiogenesis and vascular remodelling during pregnancy. EMBO Mol Med 2013; 5:1415–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ahn GO, Brown JM. Role of endothelial progenitors and other bone marrow-derived cells in the development of the tumor vasculature. Angiogenesis 2009; 12:159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Du H, Naqvi H, Taylor HS. Ischemia/reperfusion injury promotes and granulocyte-colony stimulating factor inhibits migration of bone marrow-derived stem cells to endometrium. Stem Cells Dev 2012; 21:3324–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Robb AO, Mills NL, Newby DE, Denison FC. Endothelial progenitor cells in pregnancy. Reproduction 2007; 133:1–9. [DOI] [PubMed] [Google Scholar]
- 38. Bolego C, Rossoni G, Fadini GP, Vegeto E, Pinna C, Albiero M, Boscaro, E, Agostini C, Avogaro A, Gaion RM, Cignarella A. Selective estrogen receptor-alpha agonist provides widespread heart and vascular protection with enhanced endothelial progenitor cell mobilization in the absence of uterotrophic action. FASEB J 2010; 24:2262–2272. [DOI] [PubMed] [Google Scholar]
- 39. Rudzitis-Auth J, Nenicu A, Nickels RM, Menger MD, Laschke MW. Estrogen stimulates homing of endothelial progenitor cells to endometriotic lesions. Am J Pathol 2016; 186:2129–2142. [DOI] [PubMed] [Google Scholar]
- 40. Christofferson RHB, Wassberg BE, Nilsson BO. Angiogenesis in the rat uterus during pregnancy. In: Glasser SR, Mulholland J, Psychoyos A (eds), Endocrinology of Embryo-Endometrium Interactions: Reproductive Biology. Boston, MA: Springer; 1994:77–92. [Google Scholar]
- 41. Capla JM, Ceradini DJ, Tepper OM, Callaghan MJ, Bhatt KA, Galiano RD, Levine JP, Gurtner GC. Skin graft vascularization involves precisely regulated regression and replacement of endothelial cells through both angiogenesis and vasculogenesis. Plast Reconstr Surg 2006; 117:836–844. [DOI] [PubMed] [Google Scholar]
- 42. Tal R. The role of hypoxia and hypoxia-inducible factor-1alpha in preeclampsia pathogenesis. Biol Reprod 2012; 87:1–8. [DOI] [PubMed] [Google Scholar]
- 43. Sugawara J, Mitsui-Saito M, Hayashi C, Hoshiai T, Senoo M, Chisaka H, Yaegashi N, Okamura K. Decrease and senescence of endothelial progenitor cells in patients with preeclampsia. J Clin Endocrinol Metab 2005; 90:5329–5332. [DOI] [PubMed] [Google Scholar]
- 44. Kwon JY, Maeng YS, Kwon YG, Kim YH, Kang MH, Park YW. Decreased endothelial progenitor cells in umbilical cord blood in severe preeclampsia. Gynecol Obstet Invest 2007; 64:103–108. [DOI] [PubMed] [Google Scholar]
- 45. Calcaterra F, Taddeo A, Colombo E, Cappelletti M, Martinelli A, Calabrese S, Mavilio D, Cetin I, Della Bella S. Reduction of maternal circulating endothelial progenitor cells in human pregnancies with intrauterine growth restriction. Placenta 2014; 35:431–436. [DOI] [PubMed] [Google Scholar]
- 46. Gammill HS, Lin C, Hubel CA. Endothelial progenitor cells and preeclampsia. Front Biosci 2007; 12:2383–2394. [DOI] [PubMed] [Google Scholar]
- 47. Jobe SO, Tyler CT, Magness RR. Aberrant synthesis, metabolism, and plasma accumulation of circulating estrogens and estrogen metabolites in preeclampsia implications for vascular dysfunction. Hypertension 2013; 61:480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kanasaki K, Palmsten K, Sugimoto H, Ahmad S, Hamano Y, Xie L, Parry S, Augustin HG, Gattone VH, Folkman J, Strauss JF, Kalluri R. Deficiency in catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature 2008; 453:1117–1121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.