Abstract
The change from the state of pregnancy to the state of parturition, which we call uterine transitioning, requires the actions of inflammatory mediators and results in an activated uterus capable of performing the physiology of labor. Interleukin (IL)-1β and prostaglandin (PG)F2α are two key mediators implicated in preparing the uterus for labor by regulating the expression of uterine activation proteins (UAPs) and proinflammatory cytokines and chemokines. To investigate this process, primary human myometrial smooth muscle cells (HMSMC) isolated from the lower segment of women undergoing elective cesarean sections at term (not in labor) were used to test the inflammatory cytokine and UAP outputs induced by PGF2α and IL-1β alone or in sequential combinations. PGF2α and IL-1β regulate mRNA abundance of the PGF2α receptor FP, the IL-1 receptor system, interleukin 6, and other UAPs (OXTR, COX2), driving positive feedback interactions to further amplify their own proinflammatory effects. Sequential stimulation of HMSMC by PGF2α and IL-1β in either order results in amplified upregulation of IL-6 and COX-2 mRNA and protein, compared to their effects individually. These profound increases were unique to myometrium and not observed with stimulation of human fetal membrane explants. These results suggest that PGF2α and IL-1β act cooperatively upstream in the birth cascade to maximize amplification of IL-6 and COX-2, to build inflammatory load and thereby promote uterine transition. Targeting PGF2α or IL-1β, their actions, or intermediates (e.g. IL-6) would be an effective therapeutic intervention for preterm birth prevention or delay.
Keywords: myometrium, cytokines, prostaglandins, amplification, parturition, inflammation
PGF2α and IL-1β act cooperatively upstream in the birth cascade to maximize amplification of IL-6 and COX-2, contributing to increased inflammatory burden and promoting uterine transition.
Introduction
The core tenet of human physiology is centered on maintaining homeostatic balance or equilibrium of the body's internal environment. However, pregnancy and parturition force uterine physiology to deviate from normal homeostasis to enable extensive transitioning over a short time period [1–3]. Positive feedback mechanisms are involved in the transition from uterine quiescence to labor because maintenance of pregnancy beyond term gestation (>40 weeks) compromises health of the mother and her fetus(es) [4, 5].
Birth is a complex physiological event; it has recently been shown that 471 [6] and 796 [7] genes change in expression (increase or decrease) in preparation for labor in the human myometrium and choriodecidua. We refer to the physiological change from the state of pregnancy to the state of parturition as uterine transitioning [8]. As a result, the uterus becomes activated to perform the physiology of labor [9]. During pregnancy, a high progesterone to estrogen ratio supports a state of growth and myometrial relaxation [10]. Near the end of gestation, there is a functional withdrawal of progesterone signaling in humans and an upregulation of uterine activation proteins (UAPs) regulated by an increase in estrogen and proinflammatory mediators [9, 11, 12]. There are many different UAPs that contribute to the activation of the uterus for labor by increasing or decreasing at term [9, 13, 14]. Of these, our group routinely tracks the mRNA expression of three genes and their proteins that increase at term as markers of activation [8]: cyclooxygenase (COX)-2, an inducible enzyme catalyzing a key intermediate step in the synthesis of prostaglandins (previously known as PGHS-2) [15], receptors for significant uterotonic contractile stimulators prostaglandin (PG)F2α receptor (FP) [9] and the oxytocin receptor (OXTR) [16].
Parturition is an inflammatory event; without the presence of intrauterine infection [17, 18] proinflammatory cytokines, chemokines, prostaglandins, and their receptors increase in expression near to parturition [9, 15, 19]. Stimulated by damage associated molecular patterns (DAMPs) released by the maturing fetus, aging placenta, and increasingly physiologically stressed uterus [20], their consequent “inflammatory burden” steadily increases throughout the gestational period [21]. Parturition occurs when proinflammatory mediators are upregulated and amplified until their signals exceed a threshold level whereby they stimulate functional progesterone withdrawal and complete uterine transition to its activated state for labor [21, 22].
Two very powerful mediators exert considerable control over expression of UAPs in human myometrium: interleukin (IL)-1β and PGF2α [2, 23]. IL-1β is a proinflammatory cytokine that promotes the expression of numerous prolabor genes, regulates UAP expression, and amplifies the proinflammatory response [23–25]. The modulation of IL-1β activity by rytvela (101.10), an allosteric IL-1 receptor antagonist, prolongs gestation in mouse models of preterm birth induced by IL-1β, lipopolysaccharide, and lipoteichoic acid [26] and improves neonatal and fetal developmental outcomes [27]. IL-1β induces many proinflammatory cytokines and chemokines, including IL-6 [28, 29], and upregulates COX-2 expression resulting in increased prostaglandin synthesis [30, 31].
Prostaglandins have been described as the “triggers” of labor [32] as they increase in abundance in gestational tissues and fluids when approaching parturition [33–39], the inhibition of their synthesis delays birth [32, 40], and exogenous prostaglandin treatment initiates contraction of the myometrium [41–43]. PGF2α is a key signaling mediator in parturition as it is involved not only in stimulating uterine contraction but also in mediating uterine transition through the regulation of UAP expression and amplification of proinflammatory cytokine and chemokine production [2, 3]. Moreover, allosteric modulation of the FP receptor delays preterm birth in both mice and sheep [44, 45].
Nearly all we know about the involvement of IL-1β and PGF2α in parturition has derived from in vitro studies examining each mediator in isolation or ex vivo and in vivo studies inhibiting an individual mediator [2, 3, 24, 26, 42, 44, 46–49]. Yet the human PGF2α receptor gene promoter encoding PTGFR contains four NFκB transcription factor binding sites and two NF-IL-6 binding sites, suggesting transcriptional regulation of FP by both IL-1β and IL-6 [25, 50]. Such data suggest that neither IL-1β nor PGF2α act in isolation, but rather, in concert to affect UAP and possibly cytokine expression thereby amplifying the proinflammatory process that terminates pregnancy. The intent of the present study was to examine closely, and for the first time, the sequential roles of PGF2α and IL-1β in regulating the transition of the uterus for parturition using primary human myometrium smooth muscle cells (HMSMC) and human fetal membrane (hFM) explants. We hypothesized that PGF2α and IL-1β act cooperatively in the birth cascade to sequentially promote proinflammatory amplification of UAPs and cytokines in the uterus.
Materials and methods
Cell culture of primary human myometrium smooth muscle cells
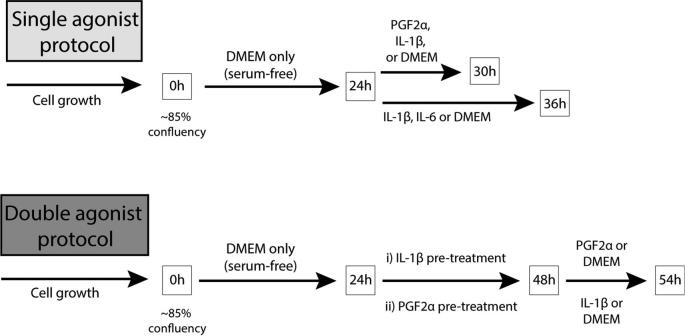
HMSMCs were isolated from lower uterine segment myometrial biopsies collected from nonlaboring pregnant women undergoing elective cesarean sections at term (>37 weeks’ gestational age) at the Royal Alexandra Hospital in Edmonton, AB, using a validated and published protocol [2, 3, 51, 52]. Ethics approval was received from the University of Alberta Research Ethics Board, Study ID Pro00069209. Myometrial tissue was washed, dissected into small pieces, and dissociated using Hank balanced salt solution (HBSS, Gibco, Thermo Fisher Scientific, Waltham, MA) containing 2.0 mg/mL collagenase (Sigma-Aldrich, St. Louis, MO), 200 ng/mL DNAse I (Roche Diagnostics, Basel, Switzerland), and 1x antibiotic/antimycotic (100 U/mL penicillin G sodium, 100 μg/mL streptomycin sulfate, and 0.25 μg/mL amphotericin B, HyClone, GE Healthcare Life Sciences, Mississauga, ON, Canada). Following 20 min of digestion at 37°C with agitation, supernatant was discarded and replaced with 10 mL of fresh dissociation medium. Incubation then continued for 2.5 h before the remaining solution was filtered through a 100-μm filter and centrifuged at 1250 × g for 5 min. The resulting cell pellet was washed twice with Dulbecco modified Eagle medium (DMEM) (HyClone, GE Healthcare Life Sciences) before resuspension. The cell solution was then plated in a 25 cm2 flask maintained at 37°C and 5% CO2 in DMEM containing 10% fetal bovine serum (Gibco, Thermo Fisher Scientific) and 1x antibiotic/antimycotic as described above. After 15-min incubation at 37°C, the HMSMC-containing solution was moved to a new flask. Upon reaching confluence, cells were passaged using 0.05% trypsin-EDTA according to the manufacturer-supplied protocol (Gibco, Thermo Fisher Scientific). At the seventh passage, cells were plated into six-well plates at a density of 2 × 105 cells/mL; once reaching ∼80–90% confluency they were starved in serum-free DMEM for 24 h before undergoing cell treatments (Figure 1).
Figure 1.
Cell culture treatment protocol. (A) Single agonist protocol: after 24 h in serum-free media, HMSMC were treated with PGF2α for 6 h (0.1, 1, or 10 μM), IL-1β for 6 or 12 h (1 or 5 ng/mL), or IL-6 for 12 h (5 or 15 ng/mL), in order to demonstrate their effects alone and determine optimal concentrations for subsequent treatments. (B) Double agonist protocol: after 24 h in serum-free media, HMSMC were treated with (i) IL-1β for 24 h (5 ng/mL), HBSS wash, then PGF2α for 6 h (10 μM) or (ii) PGF2α for 24 h (10 μM), HBSS wash, then IL-1β for 6 h (5 ng/mL).
We first treated cells for 6, 12, or 24 h with single agonists, PGF2α (0.1, 1, or 10 μM), IL-1β (1 or 5 ng/mL), or IL-6 (5 or 15 ng/mL), in order to demonstrate their effects alone and determine optimal concentrations for subsequent treatments (PGF2α: Cayman Chemical Company, Ann Arbor, MI; IL-1β, IL-6: Millipore Sigma, Etobicoke, ON, Canada). All treatment solutions were diluted into serum-free DMEM containing 0.1% albumin (Sigma-Aldrich). Then we treated HMSMC with two agonists to explore their effects in tandem. Six hours is sufficient to measure an abundant upregulation in proinflammatory outputs by HMSMC as well as the upregulation of many UAPs. However, the preliminary data showed a peak upregulation (or downregulation) in receptors such as FP and OXTR after 24 h of PGF2α or IL-1β stimulation. We hypothesized that pretreatment with IL-1β for 24 h would result in a maximal upregulation in the PGF2α receptor FP, resulting in an amplified subsequent response to PGF2α. The double agonist protocol group was treated according to one of the following schedules: (1) prestimulation with IL-1β for 24 h (5 ng/mL), a brief wash with HBSS, then subsequent 6-h treatment with PGF2α (10 μM); or (2) prestimulation for 24 h with PGF2α (10 μM), a brief wash with HBSS, then a second 6-h treatment of IL-1β (5 ng/mL). All treatment concentrations were selected after testing a range of concentrations and consulting the literature, and represent physiological concentrations in the cell milieu [2, 47, 53, 54].
Extraction of human fetal membrane explants
To demonstrate the exclusivity of responsiveness to two agonists in HMSMC, we tested our two agonist protocols in hFM. Intact placentas were obtained with consent from pregnant women undergoing elective cesarean sections at term while not in labor (>37 weeks’ gestational age) at the Royal Alexandra Hospital, Edmonton, AB, with ethics approval received from the University of Alberta Research Ethics board. Following a protocol outlined by Yin et al. [55, 56], whole fetal membranes were extracted from the placenta. Human fetal membrane tissue explants were then excised with a 6-mm tissue punch and washed in HBSS. Human fetal membranes were then plated with chorion facing down in 12-well Netwell transwells (Corning Life Sciences, Tewksbury, MA) in DMEM Nutrient Mixture F12 (HyClone, GE Healthcare Life Sciences) containing 15% FBS and 1x antibiotic/antimycotic. Following a 48-h acclimation period at 37°C and 5% CO2 (with fresh medium every 24 h), hFM explants were treated according to the double agonist protocol described in the previous section.
Quantitative RT-PCR
Trizol reagent (Ambion, Thermo Fisher Scientific) was used for RNA extraction following HMSMC treatments using the manufacturer-supplied protocol. Total RNA (500 ng) was reverse transcribed using qScript cDNA SuperMix (Quanta Biosciences, Beverly, MA) also using the manufacturer-supplied protocol, resulting in a total reaction volume of 20 μL. The resulting cDNA was used in quantitative PCR reactions (25 ng/μL). Human IL6, COX2, FP, OXTR, IL1R1, IL1R2, IL1RAcP, IL1RAcPb, and GAPDH primer sequences, product sizes, and accession numbers are provided in Table 1. The annealing temperature of all primers was 60°C, except for IL-6, which was 58°C. To ensure amplification of template cDNA and not genomic DNA, all 3′ and 5′ primers were designed to span exon–exon boundaries, therefore impeding the primer binding to genomic DNA due to the intron presence.
Table 1.
Primer sequences used in quantitative polymerase chain reaction (qPCR).
| Target gene | Forward primer (5′ → 3′) | Reverse primer (5′ → 3′) | Size of PCR product (bp) | Accession number |
|---|---|---|---|---|
| GAPDH | GAAGGTGAAGGTCGGAGTC | GAAGATGGTGATGGGATTTC | 226 | BC025925 |
| IL6 | CAAAGATGGCTGAAAAAGATGGA | CTGTTCTGGAGGTACTCTAGGT | 118 | NM_000600 |
| COX2 (PGHS2) | GCTGGAACATGGAATTACCCA | CTTTCTGTACTGCGGGTGGAA | 98 | NM_000963 |
| FP | TCCTGTATTTGTTGGAGCCCATTTCTGGTTAC | TCCATGTTGCCATTCGGAGAGCAAAAAG | 115 | BC112965 |
| OXTR | ATGGACAAGAACGAGTGTCGGTGAG | GAGTGGCATTCCTGGGTCATATGG | 155 | X64878 |
| IL1R1 | AGAGGAAAACAAACCCACAAGG | CTGGCCGGTGACATTACAGAT | 106 | KJ891450 |
| IL1R2 | TGGCACCTACGTCTGCACTACT | TTGCGGGTATGAGATGAACG | 112 | KJ892439 |
| IL1RAcP | GGGCAGGTTCTGGAAGCA | GCTAGACCGCCTGGGACTTT | 64 | AH009309 |
| IL1RAcPb | TCCAAGCACCGAGGGAAGT | AGGTGATTCTCTCCTTCACAGTAGGT | 71 | FJ998418 |
Each 20 μL reaction was run in duplicates and included 1 μL of cDNA, 10 μL of 2x PerfeCTa SYBR Green FastMix for iQ (Quanta Biosciences), 0.5 μL of 10 μM forward primer, 0.5 μL of 10 μM reverse primer, and 8 μL water. With the use of iCycler IQ technology and software (Bio-Rad Laboratories, Hercules, CA), two-step quantitative PCR was completed under the following conditions: 10 min at 95°C, 45 cycles of 15 s at 95°C, and 1 min at the annealing temperature. Following amplification, melt curve analysis was performed for each plate to ensure that amplification of nonspecific products did not occur. PCR products from the primers used in this study have been confirmed previously in our lab by gel electrophoresis followed by sequencing to verify amplification of the correct products. Standard curves for target genes and GAPDH were generated by serial dilutions of cDNA samples and analyzed with iCycler IQ software (Bio-Rad Laboratories). The amplification efficiency for each primer set was determined manually by converting the slope of the standard curve using the algorithm E = 10 –1/slope in a Microsoft Excel spreadsheet (Microsoft Corporation, Redmond, WA). The mean threshold cycle for each gene was calculated from duplicate reactions, then corrected for the efficiency of the reaction and expressed relative to a vehicle-treated control sample for each experiment. Target gene levels were then expressed relative to GAPDH levels using the following formula [57]: 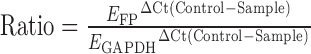 . GAPDH is very consistently expressed in HMSMC, remains unchanged with treatment, and has been selected as the housekeeping gene for analysis of myometrial tissue and HMSMC in previous publications [2, 14, 58].
. GAPDH is very consistently expressed in HMSMC, remains unchanged with treatment, and has been selected as the housekeeping gene for analysis of myometrial tissue and HMSMC in previous publications [2, 14, 58].
Multiplex assay
Supernatants were collected from HMSMC and immediately stored at –80°C. The Bio-Rad custom human cytokine multiplex kits were used as per the manufacturer's instructions with a Bio-Plex 200 suspension array system and corresponding Bio-Plex 200 software, version 6.1 (Bio-Rad Laboratories). Briefly, magnetic beads coated with antibodies targeting the cytokines of interest were added to each well of the 96-well plate and washed twice. Supernatant samples and cytokine standards were then added to the plate in duplicate and incubated with the magnetic beads at room temperature on a shaker for 1 h. The beads were then incubated with biotinylated detection antibodies, and then streptavidin tagged with a phycoerythrin fluorescent reporter which strongly binds to the biotinylated detection antibody. Beads were washed, and then resuspended in assay buffer for quantification of analytes using the Luminex-based reader in the Bio-Plex system. Each analyte's concentration was calculated by measuring the median fluorescence intensity (MFI) signal of the phycoerythrin fluorescent reporter per bead (at least 50 beads per analyte). MFI signals were compared to a standard curve generated by the manufacturer-supplied cytokine standards. Concentration outputs were normalized to a ratio of cell density (2 × 105 cells/mL).
Western blot
Following treatment, HMSMC were washed twice with PBS and placed on ice on a shaker for 15 min with RIPA buffer containing 0.05% Tris, 0.01% EDTA, 0.001% Triton-X-100, 0.005% PMSF, and 1x Halt protease inhibitor cocktail (Thermo Fisher Scientific). Total cell lysates were then collected using a cell scraper and centrifuged at 4°C for 10 min at 15 000 × g to remove cell debris. Total protein concentrations were calculated using Precision Red Advanced Protein Assay Reagent (Cytoskeleton Inc, Denver, CO) using a Nanodrop 1000 spectrophotometer system (Thermo Fisher Scientific).
Total protein (50 μg) from each sample was combined with 1x loading buffer (250 mM Tris-HCl containing 4% sodium dodecyl sulfate, 10% glycerol, 2% β-mercaptoethanol, and 0.002% bromophenol blue) and heated for 5 min at 95°C. Protein lysates were separated by SDS-PAGE using polyacrylamide gels containing acrylamide and bisacrylamide, and transferred to nitrocellulose membranes by electroblotting. Membranes were incubated at room temperature for 2 h with Bløk fluorescent blocking buffer (EMD Millipore, Etobicoke, ON, Canada) before incubation with specific primary antibodies: anti-COX-2 at 1:1000 (sc-1745, Santa Cruz Biotechnology, Dallas, TX) and anti-GAPDH at 1:5000 (PA1–987, Pierce Protein Biology, Thermo Fisher Scientific). Membranes were washed three times with filtered PBS containing 0.1% Tween 20 (Sigma-Aldrich) and incubated for 45 min with 1:2500 secondary IRDye 680LT or 800CW antibody (LI-COR Biosciences, Lincoln, NE) at room temperature. COX-2 and GAPDH were then detected and quantified using the Odyssey LI-COR Biosciences Infrared Imaging System and application software V3.0 (LI-COR Biosciences). Relative protein expression levels were calculated by obtaining a ratio of COX-2 to GAPDH band intensities via densitometry and expressed relative to a vehicle-treated control sample for each patient group.
Statistical analysis
Results are expressed as mean ± SEM. Data were log10-transformed and analyzed by repeated measures one-way analysis of variance (ANOVA) followed by Tukey post hoc test when significance was achieved (P < 0.05) to discriminate between treatments (GraphPad Prism, La Jolla, CA). Data sets comparing only two treatment groups (Figure 3B) were analyzed using the paired samples t-test on log10-transformed data. Significance is indicated with letters designating outcomes; different letters denote significant differences at P < 0.05. If there is overlap between letter groups (such as AB vs A), post hoc differences between those two groups are not significantly different. If no overlap is depicted between letters (such as A vs B and C), then the group is significantly different from all other groups.
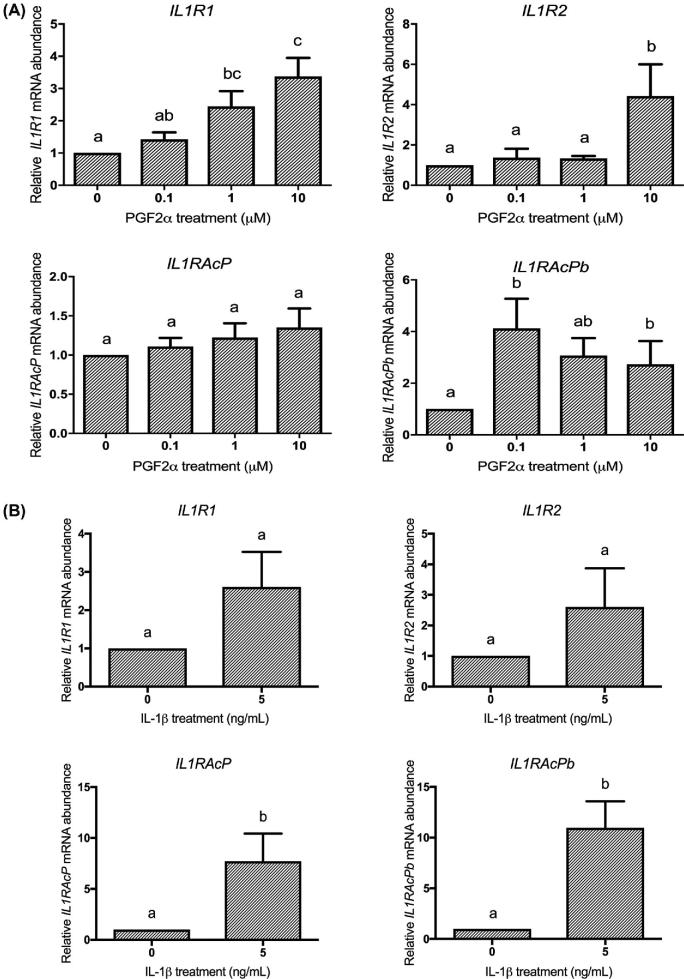
Figure 3.
PGF2α and IL-1β regulate HMSMC mRNA expression of the IL-1 receptor system. HMSMC were stimulated with (A) PGF2α (0.1, 1, or 10 μM) for 6 h or (B) IL-1β (5 ng/mL) for 12 h. Data are presented as relative change (x-fold) from control values, mean ± SEM. N = 7–9 patients. Statistical analysis: (A) repeated measures one-way ANOVA on log10-transformed data followed by Tukey post hoc analysis, (B) paired t-test on log10-transformed data. Groups with statistically significant post hoc differences are represented with different letter designations, P < 0.05.
Results
PGF2α and IL-1β regulate mRNA expression of IL6 and UAPs
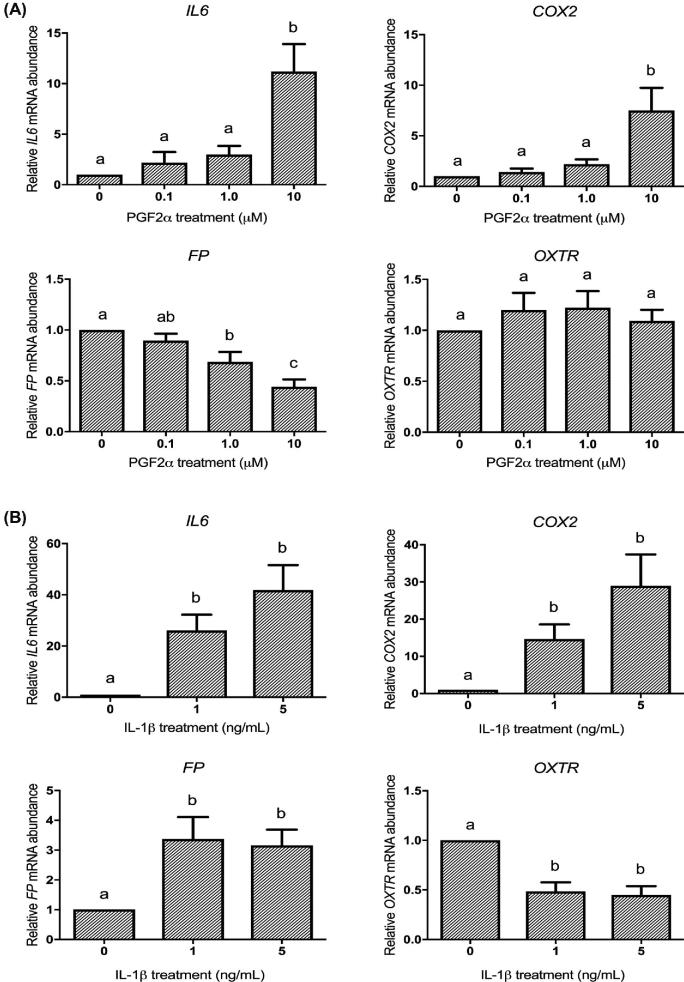
We tested a series of agonist concentrations for PGF2α and IL-1β on UAPs in HMSMC to determine the concentration to be used in subsequent tests of both agonists together. PGF2α stimulated a concentration-dependent increase in IL6 and COX2 abundance after 6 h, resulting in an 8.8- and 5.9-fold upregulation, respectively (Figure 2A). From this, we selected 10 μM as our PGF2α concentration for subsequent tests. IL-1β treatment also induced a dose-dependent increase in IL6 and COX2 mRNA by 41.9- and 28.9-fold, respectively (Figure 2B). We selected 5 ng/mL as the IL-1β concentration to use in the subsequent dual treatment studies, as this concentration demonstrated the maximal effect. PGF2α and IL-1β had opposite effects on FP mRNA expression: 10 μM PGF2α downregulated FP from 1 to 0.44, whereas 5 ng/mL IL-1β significantly increased FP mRNA expression by 3-fold. OXTR mRNA expression did not respond to PGF2α at any concentration but decreased from 1 to 0.44 in response to 5 ng/mL IL-1β (Figure 2A and B).
Figure 2.
PGF2α and IL-1β regulate HMSMC mRNA expression of proinflammatory cytokine IL6 and UAPs COX2, FP, and OXTR. HMSMC were stimulated for 6 h with (A) PGF2α (0.1, 1, or 10 μM) or (B) IL-1β (1 or 5 ng/mL). Data are presented as relative change (x-fold) from control values, mean ± SEM. N = 5–7 patients. Repeated measures one-way ANOVA on log10-transformed data followed by Tukey post hoc analysis. Groups with statistically significant post hoc differences are represented with different letter designations, P < 0.05.
IL-1β and PGF2α effects on the IL-1 receptor system
In another study, we reported that IL-1β administration to pregnant rats upregulated the IL-1 receptor and accessory protein system in the uterus near term [19]. We therefore tested the effects of PGF2α and IL-1β on the IL-1 receptor and accessory protein in HMSMC. After 6 h of PGF2α treatment at 10 μM, mRNA abundance of IL1R1 and IL1R2 increased by 3.4-fold and 4.4-fold, respectively (Figure 3A). IL1RAcP mRNA increased by 1.35-fold (not significant (NS)), while IL1RAcPb reached a maximal increase of 4.3-fold at 0.1 μM PGF2α. IL-1β also regulated mRNA abundance of the IL-1 receptor system. While IL-1β increased both IL1R1 and IL1R2 by 2.6-fold (NS), a significant 7.7- and 11-fold increase in mRNA abundance of IL1RAcP and IL1RAcPb were elicited (Figure 3B). Unlike PGF2α and IL-1β, stimulation of HMSMC with IL-6 (at 5 or 15 ng/mL) did not substantially alter mRNA expression of UAPs COX2, FP, or OXTR, or the IL-1 receptors and accessory proteins (Supplementary Figure S1) thereby demonstrating the specificity of the effects with PGF2α and IL-1β.
Sequential treatments of HMSMC with IL-1β and PGF2α induce sizable increases in IL-6
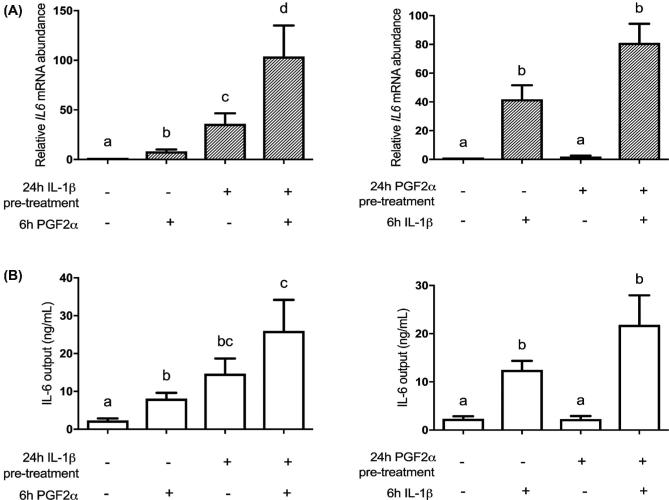
Since it is likely that in vivo PGF2α and IL-1β act in concert and each is a powerful stimulant of IL-6 mRNA and protein expression, we systematically explored the priming of myometrium by careful sequential treatment of HMSMC with the two agonists and appropriate controls. HMSMC were stimulated for 24 h with 5 ng/mL IL-1β followed by DMEM or 10 μM PGF2α for 6 h (Figure 4A). IL-1β treatment alone upregulated IL6 mRNA abundance by 36-fold. PGF2α treatment (6 h) increased IL6 abundance by only 8-fold when administered on its own. However, IL-1β treatment for 24 h followed by PGF2α treatment for 6 h produced a 104-fold increase in IL6 mRNA abundance (Figure 4A). Reversing the order of treatments with PGF2α for 24 h then IL-1β for 6 h, we observed a similarly large response. PGF2α for 24 h alone induced a 1.9-fold increase in IL6 abundance whereas just IL-1β for 6 h produced a 42-fold increase in IL6. PGF2α followed by IL-1β however stimulated an 81-fold increase in IL6 (Figure 4A).
Figure 4.
Sequential stimulation of HMSMC with PGF2α and IL-1β amplifies upregulation of IL6. HMSMC were either (1) prestimulated for 24 h with IL-1β (5 ng/mL), washed, and subsequently stimulated with PGF2α (10 μM) for 6 h, or (2) prestimulated for 24 h with PGF2α, washed, and stimulated with IL-1β. (A) IL6 mRNA data are presented as relative change (x-fold) from control values, mean ± SEM, n = 5–7. (B) IL-6 protein levels in cell culture supernatant are presented as concentration output (ng/mL), mean ± SEM, n = 6. Repeated measures one-way ANOVA statistical testing was performed on log10-transformed data followed by Tukey post hoc analysis. Groups with statistically significant post hoc differences are represented with different letter designations, P < 0.05.
We observed a less pronounced effect of IL-1β and PGF2α treatments on IL-6 protein outputs from HMSMC compared to IL6 mRNA. The 24-h IL-1β treatment increased IL-6 output by HMSMC from 2.3 ± 0.5 to 14.7 ± 4.0 ng/mL. PGF2α stimulation for 6 h produced an output of 8.1 ± 1.5 ng/mL, but the combined treatment sequence together produced 26 ± 8.2 ng/mL IL-6 (Figure 4B). Again, reversing the treatment order, the 24-h PGF2α treatment alone led to an IL-6 output of 2.3 ± 0.5 ng/mL and 6 h IL-1β stimulated 12.5 ± 1.9 ng/mL. However, PGF2α then IL-1β produced an IL-6 protein output of 21.8 ± 6.1 ng/mL from HMSMC (Figure 4B).
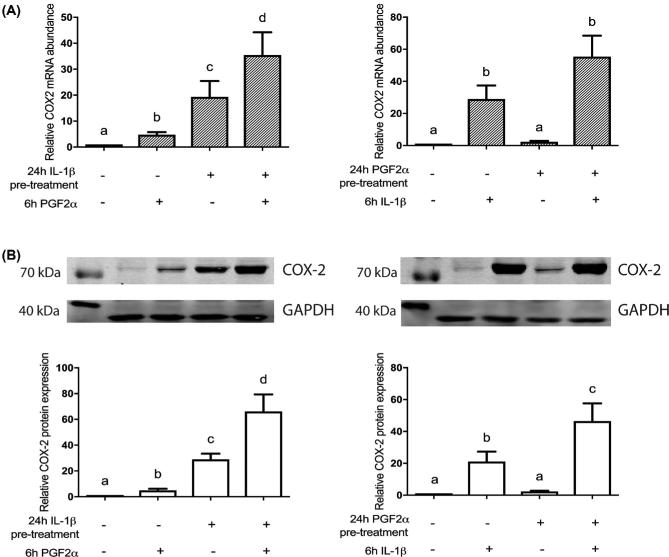
IL-1β and PGF2α in tandem induce large increases in COX-2 mRNA and protein expression in HMSMC
Given the substantial responses in IL-6 expression, we tested IL-1β then PGF2α on COX-2 expression. IL-1β alone for 24 h stimulated COX2 mRNA abundance by 19.4-fold from control levels; 6 h of PGF2α on its own increased COX2 abundance by 4.8-fold, whereas IL-1β then PGF2α stimulated a 35.4-fold increase in COX2 abundance (Figure 5A). Reversing the order of administration, we observed a similar response (Figure 5A). HMSMC stimulated by PGF2α for 24 h experienced a 2.35-fold increase in COX2 abundance, and 6 h IL-1β induced a 28.9-fold increase in COX2 expression. Exposure to both sequentially however induced a large 55.4-fold increase in COX2 mRNA abundance.
Figure 5.
Sequential stimulation of HMSMC with PGF2α and IL-1β amplifies upregulation of COX-2 mRNA and protein expression. HMSMC were either (1) prestimulated for 24 h with IL-1β, washed, and subsequently stimulated with PGF2α for 6 h, or (2) prestimulated for 24 h with PGF2α, washed, and stimulated with IL-1β. (A) COX2 mRNA; (B) COX-2 protein expression quantified using densitometry (representative blots included). Data are presented as relative change (x-fold) from 6 h vehicle control values (first lane), mean ± SEM. N = 5–7 patients. Repeated measures one-way ANOVA statistical testing was performed on log10-transformed data followed by Tukey post hoc analysis. Groups with statistically significant post hoc differences are represented with different letter designations, P < 0.05.
Additionally, we observed the same response for COX-2 protein from cell lysates collected in response to IL-1β and PGF2α. IL-1β increased COX-2 protein by 29-fold, while 6-h PGF2α treatment alone increased relative COX-2 protein abundance by 5-fold. IL-1β followed by PGF2α treatment stimulated a large 66.2-fold increase in COX-2 protein. When the treatment order was reversed, similar protein expression changes were measured. PGF2α alone increased COX-2 protein mass by 2.4-fold, while 6 h IL-1β on its own increased COX-2 protein expression by 21.1-fold from control levels. Together, PGF2α then IL-1β increased COX-2 protein abundance by 46-fold (Figure 5B).
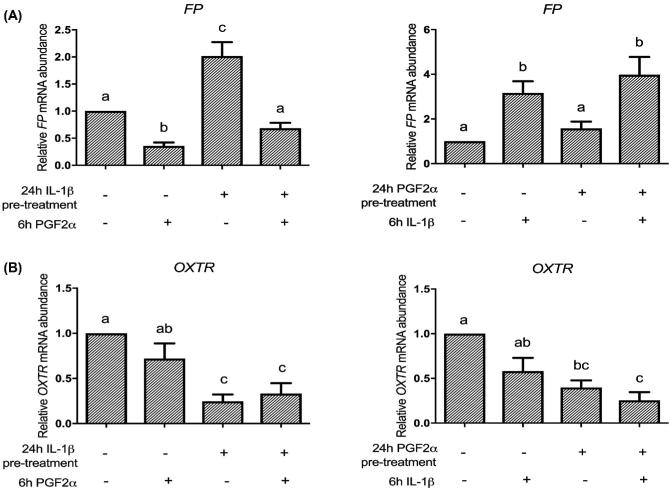
Sequential treatments of IL-1β and PGF2α do not have an amplification effect on FP or OXTR mRNA in HMSMC
Unlike their cumulative stimulatory effects on IL-6 and COX-2 expression, sequential IL-1β and PGF2α treatment, regardless of order of treatment, did not result in high levels of FP and OXTR induction (Figure 6). IL-1β treatment alone upregulated FP by 2-fold, whereas 6-h PGF2α treatment decreased FP expression from 1 to 0.36. Together, they decreased FP mRNA expression to 0.68 of control (Figure 6A). When the treatment order was reversed, 24 h of PGF2α stimulated a 1.6-fold increase in FP abundance whereas IL-1β treatment alone increased FP abundance by 3.2-fold. Consecutively, they exhibited a 4-fold increase in FP mRNA expression (Figure 6A). Sequential stimulation of HMSMC by IL-1β and PGF2α led to a downregulation of OXTR abundance regardless of order of administration (Figure 6B).
Figure 6.
Sequential stimulation of HMSMC with PGF2α and IL-1β does not cooperatively increase upregulation of FP and OXTR mRNA expression. HMSMC were either (1) prestimulated for 24 h with IL-1β, washed, and stimulated with PGF2α for 6 h, or (2) prestimulated for 24 h with PGF2α, washed, and stimulated with IL-1β. Data are presented as relative change (x-fold) from control values, mean ± SEM. N = 5 patients. Repeated measures one-way ANOVA statistical testing was performed on log10-transformed data followed by Tukey post hoc analysis. Groups with statistically significant post hoc differences are represented with different letter designations, P < 0.05.
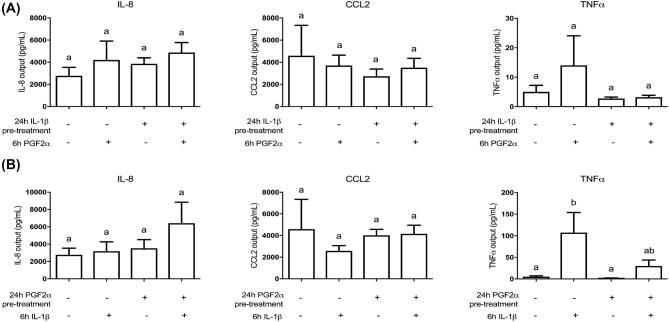
Unlike IL-6, sequential IL-1β and PGF2α do not induce high levels of IL-8, CCL2, or TNFα
Since IL-6 was highly responsive to the treatment sequence of PGF2α then IL-1β, we investigated whether other proinflammatory cytokines and chemokines were regulated in a similar way. IL-8 and CCL2 protein synthesis, as measured by multiplex assay, did not significantly change when treated with PGF2α, IL-1β, or either sequential treatment of the two (Figure 7). PGF2α stimulation alone for 24 h had no effect on TNFα output, although 6 h of IL-1β treatment on its own increased TNFα from 5.0 ± 2.2 to 107.3 ± 46.7 pg/mL. Interestingly, PGF2α stimulation before IL-1β suppressed the IL-1β-induced effect (Figure 7).
Figure 7.
Unlike IL-6, sequential stimulation of HMSMC with PGF2α and IL-1β does not cooperatively increase upregulation of IL-8, CCL2, or TNFα. HMSMC were either (A) prestimulated for 24 h with IL-1β (5 ng/mL), washed, and stimulated with PGF2α (10 μM) for 6 h, or (B) prestimulated for 24 h with PGF2α, washed, and stimulated with IL-1β. Data are presented as concentration output (pg/mL) into cell culture supernatant, mean ± SEM, n = 3. Repeated measures one-way ANOVA statistical testing was performed on log10-transformed data followed by Tukey post hoc analysis. Groups with statistically significant post hoc differences are represented with different letter designations, P < 0.05.
IL-1β and PGF2α do not drive IL6 and COX2 mRNA in hFM explants
A logical question that some may ask is, are these extremely large responses of COX-2 and IL-6 to sequential IL-1β/PGF2α treatments unique to the myometrium or do other intrauterine tissues respond similarly? We addressed this question using hFM explants because this excellent model contains the other intrauterine tissues, amnion, chorion, and some decidua vera, all together. The result indicates, however, that the very large response relationship involving IL-1β and PGF2α stimulation in tandem is apparently unique to the myometrium, since hFM explants treated according to the same protocol did not demonstrate significant upregulation of COX2 or IL6 (Figure 8). COX2 mRNA decreased from 1 to 0.8 with PGF2α treatment. IL-1β treatment, whether alone or followed by a second treatment of PGF2α, upregulated COX2 by only 2.3-fold (Figure 8A). PGF2α (6 h) increased IL6 abundance by only 1.3-fold, and IL-1β treatment alone upregulated IL6 mRNA abundance by 12-fold. IL-1β treatment followed by PGF2α yielded a 6.7-fold increase in IL6 mRNA abundance (Figure 8A). Reversing the order of treatments with PGF2α then IL-1β, we observed that IL6 and COX2 increased with 6 h IL-1β stimulation but not 24 h PGF2α treatment alone (Figure 8B). The 24-h IL-1β treatment alone increased FP mRNA expression in hFM, but no other treatments resulted in significant changes in FP or OXTR (Figure 8B).
Figure 8.
Unlike HMSMC, sequential stimulation of hFM explants with PGF2α and IL-1β does not cooperatively increase upregulation of IL6 and COX2 mRNA expression. 6 mm hFM explants were either (A) prestimulated for 24 h with IL-1β (5 ng/mL), washed, and stimulated with PGF2α (10 μM) for 6 h, or (B) prestimulated for 24 h with PGF2α, washed, and stimulated with IL-1β. Data are presented as relative change (x-fold) from control values, mean ± SEM, n = 6. Repeated measures one-way ANOVA statistical testing was performed on log10-transformed data followed by Tukey post hoc analysis. Groups with statistically significant differences are represented with different letter designations, P < 0.05.
Discussion
In this study, we confirmed our earlier observation that PGF2α and IL-1β are individually important proinflammatory mediators in myometrial cells [2, 3, 26]. But here we considerably expand upon that observation to propose that they are acting in tandem to drive positive feedback interactions that amplify the proinflammatory cascade involved in labor induction. Sequential stimulation of HMSMC by PGF2α then IL-1β (in either order) results in a tremendous upregulation of IL-6 and COX-2, and this appears to be exclusive to the myometrium. In addition, we made the intriguing discovery that IL-1β and PGF2α each stimulate increases in members of the broad IL-1 family in HMSMC including the IL1R1 receptor and its accessory proteins that confirm our in vivo observations [19]. Together, these salient observations illustrate the involvement of positive feedback or feed-forward relationships in amplification of the inflammatory load to the uterus, which is critical for expressing the UAPs that convert the uterus from a physiological state of pregnancy to a state of parturition [21]. A limitation to this study is the lack of in vivo functional assays confirming the observed in vitro interactions in primary term nonlaboring HMSMCs. In the future, the pairing of these in vitro outcomes with functional evidence (such as myometrial contraction experiments) would provide additional contextual value and further understanding of the physiological phenomenon of uterine transition for labor.
IL-1β has an influential role in orchestrating the gene regulation of proinflammatory and prolabor mediators in gestational tissues. One hour of IL-1β stimulation upregulates 98 genes in PHM1–41 uterine myocytes by at least 3-fold [24]. Microarray data of decidual cells stimulated with IL-1β demonstrated a significant upregulation of 350 transcripts, a downregulation of 78 transcripts, as well as the predicted activation of 57 transcription factors and inhibition of 22 [48]. Ishiguro et al. demonstrated that changes in uterine sensitivity to IL-1β occur at term in the rat through an upregulation of IL1R1 and accessory proteins IL1RAcP and IL1RAcPb and a downregulation in IL1R2 [19]. An IL-1β-induced preterm birth mouse model exhibited increased IL1R1 and IL1RAcP in the myometrium, and a highly specific antagonist to IL-1R1, rytvela, blocked IL-1β- and infection-induced preterm delivery in mice [26]. In the rhesus macaque, IL1R1, IL1R2, and IL1RAcP were upregulated in amniochorion and decidua after intra-amniotic IL-1β injection [59]. Our model corroborates these findings in the human, as HMSMC treated with IL-1β upregulated mRNA expression of IL1R accessory proteins, and showed increasing trends in IL1R1 and IL1R2.
Similar to IL-1β, PGF2α also stimulated an increase in mRNA abundance of IL1R1, IL1R2, and IL1RAcPb in HMSMC. IL-1RAcPb is an isoform of the IL-1 accessory protein that when complexed with IL-1β and IL-1R1 does not activate NFκB as does IL-1RAcP, but instead acts through p38 MAPK and Src phosophorylation [60]. IL-1RAcPb had only been identified in the central nervous system [61] until we described an increase in its expression in the pregnant rat uterus at delivery [19]. Here we show that IL1RAcPb is expressed in the pregnant human uterus (HMSMC) and is regulated by both IL-1β and PGF2α.
This intriguing effect is an excellent example that PGF2α has many more roles than its stimulation of myometrial contractility [2, 3]. For instance, we confirmed that PGF2α stimulates COX-2 expression in HMSMC as it does in amnion, myometrium, and endometrial adenocarcinoma tissue [2, 62, 63]. In the decidua, PGF2α increases the concentration of matrix metalloproteinases (MMP)-2 and -9 while decreasing the concentration of their tissue inhibitor, TIMP-1 [46]. MMP-2, -3, and -9, in turn, catalyze the inactive pro-IL-1β into biologically active IL-1β independent of caspase-1 thereby further amplifying the proinflammatory response through positive feedback [64]. In HMSMC, IL-6 is upregulated by both PGF2α and IL-1β, corroborating previous findings that proinflammatory cytokine release is increased by PGF2α [3]. Completing the feed-forward cycle, we showed that IL-1β stimulates upregulation of the PGF2α receptor FP [2, 25]. Hence, there is ample evidence that both PGF2α and IL-1β are mutually stimulatory in HMSMC and thereby may contribute considerably to the expression of UAPs that transition the pregnant uterus to the parturient uterus.
But without question the most significant observation of this study is that when the two agonists stimulate HMSMC sequentially, in either order, a profound increase in the output of IL-6 and COX-2 occurs. The process of synergy is defined generally by the Concise Oxford English Dictionary as the “interaction or cooperation of two or more organizations, substances, or other agents to produce a combined effect greater than the sum of their separate effects” [65]. Our data clearly demonstrate that sequential treatments of PGF2α and IL-1β produce a synergistic effect in HMSMC using this definition. For example, in Figure 5B COX-2 protein levels increased 29-fold after 24-h IL-1β, and 5-fold with 6-h PGF2α stimulation. Instead of a 34-fold additive response in response to IL-1β then PGF2α stimulation, COX-2 increased 66-fold. Reversing the order of treatments led to a PGF2α-stimulated increase of COX-2 protein by 2.4-fold and an IL-1β-stimulated increase of 21-fold, but together they stimulated an increase of COX-2 protein levels of 46-fold—not the additive 23-fold. Unlike IL-1β, PGF2α pretreatment of HMSMC alone did not result in a significant increase in IL-6 and COX-2, only increasing expression levels to a maximum level of 2.5×. However, a PGF2α priming effect still exists in HMSMC, as IL-6 and COX-2 outputs from PGF2α-primed HMSMC were 2× higher then outputs from HMSMC stimulated with 6 h of IL-1β without the PGF2α prestimulation. Synergistic outputs were characterized for IL-6 but not for other cytokines, and this effect was not observed in fetal membrane explants suggesting it is unique for myometrium.
The concept of immune synergy is well established in the literature. In a study of gastrointestinal tumor growth, chemokines CXCL6 and CCL2 together stimulated a significantly higher mobilization of leukocyte migration than the sum of each alone [66]. IL-8 synergizes with a series of chemokines including CCL2 to induce amplified neutrophil chemotaxis [67]. In chondrocytes, inflammatory synergy results in cartilage destruction; high-mobility group box-1 (HMGB-1), a DAMP, synergizes with IL-1β to produce high levels of MMPs [68], and IL-1α works in concert with glucocorticoids and mineralocorticoids to synergistically increase lipocalin-2 and MMP-13 [69]. The challenges and complications of experimental demonstration of pharmacological synergy in a system are thoroughly described in a review by Foucquier and Guedj [70]. To clearly affirm pharmacological synergy in a system, large data sets are required including extended concentration response curves with many more than the three concentration points included in this study, as well as the completion of multiple mathematical frameworks. We are not arguing that the results presented in this paper constitute this definition of pharmacological synergy. Additionally, our agonists were not administered in combination but sequentially, instead modeling a priming effect. Combined stimulation of HMSMC with a mixture of PGF2α and IL-1β for 30 h instead of sequential stimulation of IL-1β followed by the PGF2α (and vice versa) does not result in amplification of COX2 or IL6 mRNA expression (Leimert, unpublished). We contend that the amplification of IL-6 and COX-2 presented in this paper in response to sequential PGF2α/IL-1β stimulation is not modeling classical “pharmacological synergy” of drug combination but cooperative inflammatory amplification. It is possible that there are multiple individual steps or net increments that are additive, and only more investigation will be able to tell. We demonstrate clearly, however, that in many situations described in our study, the overall output effect is higher than the additive effects of PGF2α and IL-1β individually. Prestimulation of HMSMC with IL-1β (or PGF2α) seems to have a priming effect on the cells, resulting in a heightened subsequent response to the second agonist.
We do not know the full range of mechanisms involved in our observed synergistic responses, but it is conceivable that one element involved is that both PGF2α and IL-1β stimulate expression increases in the others’ receptor as we measured here. Other studies suggest additional mechanistic detail. Amnion-derived WISH cells displayed synergistic upregulation of COX-2 induced by co-treatment of IL-1β with epidermal growth factor (EGF). Inhibition of NFκB decreased IL-1β/EGF-induced COX-2 by only 44% suggesting that at least one signaling mechanism other than NFκB is involved in the synergistic relationship [71]. IL-6 and COX-2 were both synergistically upregulated in aortic smooth muscle cells by combined treatments of cytokines Oncostatin M and IL-1β due to both transcriptional and post-transcriptional regulation [72]. IL-6 transcription may be cooperatively regulated, as multiple regulatory elements can synergize to induce greater levels of IL-6 induction. NFIL-6 and NFκB individually both induced a 2-fold increase in IL-6 (as measured by luciferase activity), but together the transcription factors interacted with both promoter binding sites to induce over 40-fold increases in IL-6 [73]. Additionally, all three transcription factors can interact and induce an even greater effect. AP-1, NFκB, and NFIL-6 can form a complex and interact with the IL-6 promoter, resulting in nearly 300 times the level of IL-6 induction, much greater than the three regulatory elements separately, or even paired combinations [74]. IL-1β and PGF2α stimulate several intracellular pathways in uterine tissues, and it is likely that synergistic responses could occur through intricate regulation of several of these [3, 75–80]. Interestingly, the COX-2 gene promoter also contains NFκB and NFIL-6 regulatory elements [81].
While IL-1β was a potent stimulator of proinflammatory mediator output from HMSMC, IL-6 did not significantly alter mRNA expression of COX2, FP, OXTR, IL1R1, IL1RAcP, or IL1RAcPb. We established that the IL-6R mRNA was expressed in these cells (data not shown). In contrast, we found that IL-6 stimulation of the hFM explants we used to compare against the synergistic effects of HMSMC produced several cytokines (TNFα, IFNγ, CCL21, and IL-1β protein) (Olson, unpublished), demonstrating specificity of tissue responsiveness. It is possible that the high levels of IL-6 produced by the myometrium are not acting on the uterine musculature itself, but on local leukocytes or other cell types in the surrounding gestational tissues.
Unlike their combined effects on IL-6 or COX-2, sequential IL-1β and PGF2α treatment did not result in amplified FP and OXTR. This is not surprising as PGF2α and IL-1β have opposing effects on FP expression in HMSMC, and both downregulate (or have no effect) on OXTR. Increases in receptors of contractile mediators (uterotonic receptors) are upregulated in the final step of the birth cascade; in rats, OXTR does not increase until just a few hours before delivery [82]. Culmination of the inflammatory load may result in the triggering of functional progesterone withdrawal [11] and uterine activation, including FP and OXTR increase. In the myometrium, PGF2α upregulates the PR-A/PR-B ratio [83] and IL-1β increases the abundance and stability of the PR-A protein [84, 85], contributing to functional progesterone withdrawal.
In conclusion, our work suggests that a process we term inflammatory amplification contributes to the facilitation of uterine transition from pregnancy to parturition. In the in vitro HMSMC model, we established PGF2α and IL-1β as key triggers or upstream drivers of this process, and IL-6 and COX-2 as key targets. Existing preterm birth therapies target mechanisms occurring at the final stages of the birth cascade, when excessive amplification has already taken place. IL-1β and IL-6 are upstream mediators in the birth cascade [17, 86–89] and now we advocate that PGF2α is also a key mediator, interacting to induce amplification of other prolabor mechanisms. Alteration of the activity of these key mediators through targeting their receptors, for example by novel allosteric modulators [26, 44, 90], may suppress inflammatory amplification and uterine transition. Targeting not only uterine contraction but also inflammatory amplification and uterine transition presents a promising path for therapeutic development for preterm birth prevention or delay.
Supplementary data
Supplemental Figure S1. IL-6 treatment does not induce HMSMC mRNA expression of UAPs, IL-1β, or the IL-1 receptor system. HMSMC were stimulated for 12 h with IL-6 (5 or 15 ng/mL). (A) Target mRNA data are presented as relative change (x-fold) from control values, mean ± SEM. (B) IL-1β protein levels were measured in supernatants via multiplex, presented as mean ± SEM concentration output (pg/mL). (A) N = 6–9 patients, (B) n = 4. Repeated measures one-way ANOVA was performed on log10-transformed data followed by Tukey post hoc analysis. Groups with statistically significant differences are represented with different letter designations, P < 0.05.
Acknowledgments
The authors would like to thank Donna Dawson, BScN, for her assistance in the recruitment and collection of myometrial biopsies at the Royal Alexandra Hospital in Edmonton, AB, as well as the pregnant women who volunteered to participate in our study.
Notes
Conference Presentation: Presented in part at the 62nd Annual Scientific Meeting of the Society for Reproductive Investigation (SRI), March 25–28, 2015, San Francisco, California, and the 63rd Annual Scientific Meeting of the Society for Reproductive Investigation (SRI), March 16–19, 2016, Montréal, Quebec.
Edited by Dr. Romana Nowak
Funding
Grant support: This research was funded by: Canadian Institutes of Health Research (CIHR) #119513, Global Alliance for the Prevention of Prematurity and Stillbirth (GAPPS), an initiative of Seattle Children's #12005, and the March of Dimes (MOD) #21FY12-161. KB Leimert received a graduate studentship from the Women and Children's Health Research Institute, through the generosity of the Stollery Children's Hospital Foundation and supporters of the Lois Hole Hospital for Women. BSE Verstraeten received a PhD fellowship from the Research Foundation – Flanders (FWO).
References
- 1. Christiaens I, Zaragoza DB, Guilbert L, Robertson SA, Mitchell BF, Olson DM. Inflammatory processes in preterm and term parturition. J Reprod Immunol 2008; 79:50–57. [DOI] [PubMed] [Google Scholar]
- 2. Xu C, Long A, Fang X, Wood SL, Slater DM, Ni X, Olson DM. Effects of PGF2α on the expression of uterine activation proteins in pregnant human myometrial cells from upper and lower segment. J Clin Endocrinol Metab 2013; 98:2975–2983. [DOI] [PubMed] [Google Scholar]
- 3. Xu C, Liu W, You X, Leimert K, Popowycz K, Fang X, Wood SL, Slater DM, Sun Q, Gu H, Olson DM, Ni X. PGF2α modulates the output of chemokines and pro-inflammatory cytokines in myometrial cells from term pregnant women through divergent signaling pathways. Mol Hum Reprod 2015; 21:603–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Galal M, Symonds I, Murray H, Petraglia F, Smith R. Postterm pregnancy. Facts Views Vis Obgyn 2012; 4:175–187. [PMC free article] [PubMed] [Google Scholar]
- 5. Linder N, Hiersch L, Fridman E, Klinger G, Lubin D, Kouadio F, Melamed N. Post-term pregnancy is an independent risk factor for neonatal morbidity even in low-risk singleton pregnancies. Arch Dis Child Fetal Neonatal Ed 2017; 102:F286–F290. [DOI] [PubMed] [Google Scholar]
- 6. Mittal P, Romero R, Tarca AL, Gonzalez J, Draghici S, Xu Y, Dong Z, Nhan-Chang CL, Chaiworapongsa T, Lye S, Kusanovic JP, Lipovich L et al.. Characterization of the myometrial transcriptome and biological pathways of spontaneous human labor at term. J Perinat Med 2010; 38:617–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stephen GL, Lui S, Hamilton SA, Tower CL, Harris LK, Stevens A, Jones RL. Transcriptomic profiling of human choriodecidua during term labor: Inflammation as a key driver of labor. Am J Reprod Immunol 2015; 73:36–55. [DOI] [PubMed] [Google Scholar]
- 8. Leimert KB, Messer A, Gray T, Fang X, Chemtob S, Olson DM. Maternal and fetal intrauterine tissue crosstalk promotes proinflammatory amplification and uterine transition. Biol Reprod 2018doi:10.1093/biolre/ioy232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cook JL, Zaragoza DB, Sung DH, Olson DM. Expression of myometrial activation and stimulation genes in a mouse model of preterm labor: myometrial activation, stimulation, and preterm labor. Endocrinology 2000; 141:1718–1728. [DOI] [PubMed] [Google Scholar]
- 10. Csapo A. Progesterone block. Am J Anat 1956; 98:273–291. [DOI] [PubMed] [Google Scholar]
- 11. Mesiano S, Chan EC, Fitter JT, Kwek K, Yeo G, Smith R. Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor A expression in the myometrium. J Clin Endocrinol Metab 2002; 87:2924–2930. [DOI] [PubMed] [Google Scholar]
- 12. Di WL, Lachelin GC, McGarrigle HH, Thomas NS, Becker DL. Oestriol and oestradiol increase cell to cell communication and connexin43 protein expression in human myometrium. Mol Hum Reprod 2001; 7:671–679. [DOI] [PubMed] [Google Scholar]
- 13. Arthur P, Taggart MJ, Zielnik B, Wong S, Mitchell BF. Relationship between gene expression and function of uterotonic systems in the rat during gestation, uterine activation and both term and preterm labour. J Physiol 2008; 586:6063–6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nadeau-Vallée M, Boudreault A, Leimert K, Hou X, Obari D, Madaan A, Rouget R, Zhu T, Belarbi L, Brien M, Beaudry-Richard A, Olson DM et al.. Uterotonic neuromedin U receptor 2 and its ligands are upregulated by inflammation in mice and humans, and elicit preterm birth. Biol Reprod 2016; 95:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cook JL, Shallow MC, Zaragoza DB, Anderson KI, Olson DM. Mouse placental prostaglandins are associated with uterine activation and the timing of birth. Biol Reprod 2003; 68:579–587. [DOI] [PubMed] [Google Scholar]
- 16. Fuchs AR, Fields MJ, Freidman S, Shemesh M, Ivell R. Oxytocin and the timing of parturition. Influence of oxytocin receptor gene expression, oxytocin secretion, and oxytocin-induced prostaglandin F2 alpha and E2 release. Adv Exp Med Biol 1995; 395:405–420. [PubMed] [Google Scholar]
- 17. Elliott CL, Loudon JA, Brown N, Slater DM, Bennett PR, Sullivan MH. IL-1beta and IL-8 in human fetal membranes: changes with gestational age, labor, and culture conditions. Am J Reprod Immunol 2001; 46:260–267. [DOI] [PubMed] [Google Scholar]
- 18. Romero R, Miranda J, Chaiworapongsa T, Korzeniewski SJ, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, Kim CJ, Yeo L. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol 2014; 72:458–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ishiguro T, Takeda J, Fang X, Bronson H, Olson DM. Interleukin (IL)-1 in rat parturition: IL-1 receptors 1 and 2 and accessory proteins abundance in pregnant rat uterus at term - regulation by progesterone. Physiol Rep 2016; 4: pii:e12866 (doi:10.14814/phy2.12866). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nadeau-Vallée M, Obari D, Palacios J, Brien M, Duval C, Chemtob S, Girard S. Sterile inflammation and pregnancy complications: a review. Reproduction 2016; 152:R277–R292. [DOI] [PubMed] [Google Scholar]
- 21. Keelan JA. Intrauterine inflammatory activation, functional progesterone withdrawal, and the timing of term and preterm birth. J Reprod Immunol 2018; 125:89–99. [DOI] [PubMed] [Google Scholar]
- 22. Talati AN, Hackney DN, Mesiano S. Pathophysiology of preterm labor with intact membranes. Semin Perinatol 2017; 41:420–426. [DOI] [PubMed] [Google Scholar]
- 23. Rauk PN, Chiao JP. Interleukin-1 stimulates human uterine prostaglandin production through induction of cyclooxygenase-2 expression. Am J Reprod Immunol 2000; 43:152–159. [DOI] [PubMed] [Google Scholar]
- 24. Chevillard G, Derjuga A, Devost D, Zingg HH, Blank V. Identification of interleukin-1beta regulated genes in uterine smooth muscle cells. Reproduction 2007; 134:811–822. [DOI] [PubMed] [Google Scholar]
- 25. Zaragoza DB, Wilson RR, Mitchell BF, Olson DM. The interleukin 1beta-induced expression of human prostaglandin F2alpha receptor messenger RNA in human myometrial-derived ULTR cells requires the transcription factor, NFkappaB. Biol Reprod 2006; 75:697–704. [DOI] [PubMed] [Google Scholar]
- 26. Nadeau-Vallée M, Quiniou C, Palacios J, Hou X, Erfani A, Madaan A, Sanchez M, Leimert K, Boudreault A, Duhamel F, Rivera JC, Zhu T et al.. Novel noncompetitive IL-1 receptor-biased ligand prevents infection- and inflammation-induced preterm birth. J Immunol 2015; 195:3402–3415. [DOI] [PubMed] [Google Scholar]
- 27. Nadeau-Vallée M, Chin PY, Belarbi L, Brien M, Pundir S, Berryer MH, Beaudry-Richard A, Madaan A, Sharkey DJ, Lupien-Meilleur A, Hou X, Quiniou C et al.. Antenatal suppression of IL-1 protects against inflammation-induced fetal injury and improves neonatal and developmental outcomes in mice. J Immunol 2017; 198:2047–2062. [DOI] [PubMed] [Google Scholar]
- 28. Flower L, Gray R, Pinkney J, Mohamed-Ali V. Stimulation of interleukin-6 release by interleukin-1beta from isolated human adipocytes. Cytokine 2003; 21:32–37. [DOI] [PubMed] [Google Scholar]
- 29. Sironi M, Breviario F, Proserpio P, Biondi A, Vecchi A, Van Damme J, Dejana E, Mantovani A. IL-1 stimulates IL-6 production in endothelial cells. J Immunol 1989; 142:549–553. [PubMed] [Google Scholar]
- 30. Kniss DA, Zimmerman PD, Garver CL, Fertel RH. Interleukin-1 receptor antagonist blocks interleukin-1-induced expression of cyclooxygenase-2 in endometrium. Am J Obstet Gynecol 1997; 177:559–567. [DOI] [PubMed] [Google Scholar]
- 31. Molnár M, Romero R, Hertelendy F. Interleukin-1 and tumor necrosis factor stimulate arachidonic acid release and phospholipid metabolism in human myometrial cells. Am J Obstet Gynecol 1993; 169:825–829. [DOI] [PubMed] [Google Scholar]
- 32. JR C, DM O. Parturition In: Knobil E, Neill J, (eds.), The Physiology of Reproduction. New York: Raven Press; 1988:2177–2216. [Google Scholar]
- 33. Hirst JJ, Teixeira FJ, Zakar T, Olson DM. Prostaglandin endoperoxide-H synthase-1 and -2 messenger ribonucleic acid levels in human amnion with spontaneous labor onset. J Clin Endocrinol Metab 1995; 80:517–523. [DOI] [PubMed] [Google Scholar]
- 34. Hirst JJ, Mijovic JE, Zakar T, Olson DM. Prostaglandin endoperoxide H synthase-1 and -2 mRNA levels and enzyme activity in human decidua at term labor. J Soc Gynecol Investig 1998; 5:13–20. [DOI] [PubMed] [Google Scholar]
- 35. Mijovic JE, Zakar T, Nairn TK, Olson DM. Prostaglandin-endoperoxide H synthase-2 expression and activity increases with term labor in human chorion. Am J Physiol 1997; 272:E832–840. [DOI] [PubMed] [Google Scholar]
- 36. Mijovic JE, Zakar T, Nairn TK, Olson DM. Prostaglandin endoperoxide H synthase (PGHS) activity and PGHS-1 and -2 messenger ribonucleic acid abundance in human chorion throughout gestation and with preterm labor. J Clin Endocrinol Metab 1998; 83:1358–1367. [DOI] [PubMed] [Google Scholar]
- 37. Mijovic JE, Zakar T, Angelova J, Olson DM. Prostaglandin endoperoxide H synthase mRNA expression in the human amnion and decidua during pregnancy and in the amnion at preterm labour. Mol Hum Reprod 1999; 5:182–187. [DOI] [PubMed] [Google Scholar]
- 38. Slater DM, Berger LC, Newton R, Moore GE, Bennett PR. Expression of cyclooxygenase types 1 and 2 in human fetal membranes at term. Am J Obstet Gynecol 1995; 172:77–82. [DOI] [PubMed] [Google Scholar]
- 39. Slater DM, Dennes WJ, Campa JS, Poston L, Bennett PR. Expression of cyclo-oxygenase types-1 and -2 in human myometrium throughout pregnancy. Mol Hum Reprod 1999; 5:880–884. [DOI] [PubMed] [Google Scholar]
- 40. Poore KR, Young IR, Hirst JJ. Efficacy of the selective prostaglandin synthase type 2 inhibitor nimesulide in blocking basal prostaglandin production and delaying glucocorticoid-induced premature labor in sheep. Am J Obstet Gynecol 1999; 180:1244–1253. [DOI] [PubMed] [Google Scholar]
- 41. Senior J, Marshall K, Sangha R, Baxter GS, Clayton JK. In vitro characterization of prostanoid EP-receptors in the non-pregnant human myometrium. Br J Pharmacol 1991; 102:747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Senior J, Sangha R, Baxter GS, Marshall K, Clayton JK. In vitro characterization of prostanoid FP-, DP-, IP- and TP-receptors on the non-pregnant human myometrium. Br J Pharmacol 1992; 107:215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Senior J, Marshall K, Sangha R, Clayton JK. In vitro characterization of prostanoid receptors on human myometrium at term pregnancy. Br J Pharmacol 1993; 108:501–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Goupil E, Tassy D, Bourguet C, Quiniou C, Wisehart V, Pétrin D, Le Gouill C, Devost D, Zingg HH, Bouvier M, Saragovi HU, Chemtob S et al.. A novel biased allosteric compound inhibitor of parturition selectively impedes the prostaglandin F2alpha-mediated Rho/ROCK signaling pathway. J Biol Chem 2010; 285:25624–25636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hirst JJ, Parkington HC, Young IR, Palliser HK, Peri KG, Olson DM. Delay of preterm birth in sheep by THG113.31, a prostaglandin F2alpha receptor antagonist. Am J Obstet Gynecol 2005; 193:256–266. [DOI] [PubMed] [Google Scholar]
- 46. Ulug U, Goldman S, Ben-Shlomo I, Shalev E. Matrix metalloproteinase (MMP)-2 and MMP-9 and their inhibitor, TIMP-1, in human term decidua and fetal membranes: the effect of prostaglandin F2alpha and indomethacin. Mol Hum Reprod 2001; 7:1187–1193. [DOI] [PubMed] [Google Scholar]
- 47. Parkington HC, Tonta MA, Davies NK, Brennecke SP, Coleman HA. Hyperpolarization and slowing of the rate of contraction in human uterus in pregnancy by prostaglandins E 2 and F2alpha: involvement of the Na + pump. J Physiol 1999; 514 (Pt 1):229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ibrahim SA, Ackerman WE, Summerfield TL, Lockwood CJ, Schatz F, Kniss DA. Inflammatory gene networks in term human decidual cells define a potential signature for cytokine-mediated parturition. Am J Obstet Gynecol 2016; 214:284.e1–284.e47. [DOI] [PubMed] [Google Scholar]
- 49. Bartlett SR, Sawdy R, Mann GE. Induction of cyclooxygenase-2 expression in human myometrial smooth muscle cells by interleukin-1beta: involvement of p38 mitogen-activated protein kinase. J Physiol 1999; 520(Pt 2):399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zaragoza DB, Wilson R, Eyster K, Olson DM. Cloning and characterization of the promoter region of the human prostaglandin F2alpha receptor gene. Biochim Biophys Acta 2004; 1676:193–202. [DOI] [PubMed] [Google Scholar]
- 51. Aguilar HN, Tracey CN, Zielnik B, Mitchell BF. Rho-kinase mediates diphosphorylation of myosin regulatory light chain in cultured uterine, but not vascular smooth muscle cells. J Cell Mol Med 2012; 16:2978–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mosher AA, Rainey KJ, Bolstad SS, Lye SJ, Mitchell BF, Olson DM, Wood SL, Slater DM. Development and validation of primary human myometrial cell culture models to study pregnancy and labour. BMC Pregnancy Childbirth 2013; 13(Suppl 1):S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Friel AM, O’Reilly MW, Sexton DJ, Morrison JJ. Specific PGF(2alpha) receptor (FP) antagonism and human uterine contractility in vitro. BJOG 2005; 112:1034–1042. [DOI] [PubMed] [Google Scholar]
- 54. Puchner K, Iavazzo C, Gourgiotis D, Boutsikou M, Baka S, Hassiakos D, Kouskouni E, Economou E, Malamitsi-Puchner A, Creatsas G. Mid-trimester amniotic fluid interleukins (IL-1β, IL-10 and IL-18) as possible predictors of preterm delivery. In Vivo 2011; 25:141–148. [PubMed] [Google Scholar]
- 55. Yin N, Takeda J, Fang X, Qi H, Olson D. An In Vitro model for studying the regulation of uterine chemotactic factor. In: Society for Reproductive Investigation Annual Meeting, vol. 22 San Francisco, CA, USA: Reproductive Sciences; 2015: 141–142A. [Google Scholar]
- 56. Yin N, Wang H, Zhang H, Ge H, Tan B, Yuan Y, Luo X, Olson DM, Baker PN, Qi H. IL-27 induces a pro-inflammatory response in human fetal membranes mediating preterm birth. Int Immunopharmacol 2017; 50:361–369. [DOI] [PubMed] [Google Scholar]
- 57. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001; 29:45e–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Georgiou EX, Lei K, Lai PF, Yulia A, Herbert BR, Castellanos M, May ST, Sooranna SR, Johnson MR. The study of progesterone action in human myometrial explants. Mol Hum Reprod 2016; 22:877–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Presicce P, Senthamaraikannan P, Alvarez M, Rueda CM, Cappelletti M, Miller LA, Jobe AH, Chougnet CA, Kallapur SG. Neutrophil recruitment and activation in decidua with intra-amniotic IL-1beta in the preterm rhesus macaque. Biol Reprod 2015; 92:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Huang Y, Smith DE, Ibáñez-Sandoval O, Sims JE, Friedman WJ. Neuron-specific effects of interleukin-1β are mediated by a novel isoform of the IL-1 receptor accessory protein. J Neurosci 2011; 31:18048–18059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Smith DE, Lipsky BP, Russell C, Ketchem RR, Kirchner J, Hensley K, Huang Y, Friedman WJ, Boissonneault V, Plante MM, Rivest S, Sims JE. A central nervous system-restricted isoform of the interleukin-1 receptor accessory protein modulates neuronal responses to interleukin-1. Immunity 2009; 30:817–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Guo CM, Kasaraneni N, Sun K, Myatt L. Cross talk between PKC and CREB in the induction of COX-2 by PGF2α in human amnion fibroblasts. Endocrinology 2012; 153:4938–4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sales KJ, Grant V, Jabbour HN. Prostaglandin E2 and F2alpha activate the FP receptor and up-regulate cyclooxygenase-2 expression via the cyclic AMP response element. Mol Cell Endocrinol 2008; 285:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schönbeck U, Mach F, Libby P. Generation of biologically active IL-1 beta by matrix metalloproteinases: a novel caspase-1-independent pathway of IL-1 beta processing. J Immunol 1998; 161:3340–3346. [PubMed] [Google Scholar]
- 65. Press OU. The Concise Oxford English Dictionary. In: Pearsall J. (ed.), 10th, rev. ed New York: Oxford University Press; 2002:1728. [Google Scholar]
- 66. Gijsbers K, Gouwy M, Struyf S, Wuyts A, Proost P, Opdenakker G, Penninckx F, Ectors N, Geboes K, Van Damme J. GCP-2/CXCL6 synergizes with other endothelial cell-derived chemokines in neutrophil mobilization and is associated with angiogenesis in gastrointestinal tumors. Exp Cell Res 2005; 303:331–342. [DOI] [PubMed] [Google Scholar]
- 67. Gouwy M, Struyf S, Catusse J, Proost P, Van Damme J. Synergy between proinflammatory ligands of G protein-coupled receptors in neutrophil activation and migration. J Leukoc Biol 2004; 76:185–194. [DOI] [PubMed] [Google Scholar]
- 68. Ding L, Buckwalter JA, Martin JA. DAMPs synergize with cytokines or fibronectin fragment on inducing chondrolysis but lose effect when acting alone. Mediators Inflamm 2017; 2017:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Conde J, Lazzaro V, Scotece M, Abella V, Villar R, López V, Gonzalez-Gay M, Pino J, Gómez R, Mera A, Gualillo O. Corticoids synergize with IL-1 in the induction of LCN2. Osteoarthritis Cartilage 2017; 25:1172–1178. [DOI] [PubMed] [Google Scholar]
- 70. Foucquier J, Guedj M. Analysis of drug combinations: current methodological landscape. Pharmacol Res Perspect 2015; 3:e00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ackerman WE, Rovin BH, Kniss DA. Epidermal growth factor and interleukin-1beta utilize divergent signaling pathways to synergistically upregulate cyclooxygenase-2 gene expression in human amnion-derived WISH cells. Biol Reprod 2004; 71:2079–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bernard C, Merval R, Lebret M, Delerive P, Dusanter-Fourt I, Lehoux S, Créminon C, Staels B, Maclouf J, Tedgui A. Oncostatin M induces Interleukin-6 and Cyclooxygenase-2 expression in human vascular smooth muscle cells. Circ Res 1999; 85:1124–1131. [DOI] [PubMed] [Google Scholar]
- 73. Matsusaka T, Fujikawa K, Nishio Y, Mukaida N, Matsushima K, Kishimoto T, Akira S. Transcription factors NF-IL6 and NF-kappa B synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc Natl Acad Sci USA 1993; 90:10193–10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Faggioli L, Costanzo C, Donadelli M, Palmieri M. Activation of the Interleukin-6 promoter by a dominant negative mutant of c-Jun. Biochim Biophys Acta 2004; 1692:17–24. [DOI] [PubMed] [Google Scholar]
- 75. Belt AR, Baldassare JJ, Molnár M, Romero R, Hertelendy F. The nuclear transcription factor NF-kappaB mediates interleukin-1beta-induced expression of cyclooxygenase-2 in human myometrial cells. Am J Obstet Gynecol 1999; 181:359–366. [DOI] [PubMed] [Google Scholar]
- 76. Duggan SV, Lindstrom T, Iglesias T, Bennett PR, Mann GE, Bartlett SR. Role of atypical protein kinase C isozymes and NF-kappaB in IL-1beta-induced expression of cyclooxygenase-2 in human myometrial smooth muscle cells. J Cell Physiol 2007; 210:637–643. [DOI] [PubMed] [Google Scholar]
- 77. Sooranna SR, Engineer N, Loudon JA, Terzidou V, Bennett PR, Johnson MR. The mitogen-activated protein kinase dependent expression of prostaglandin H synthase-2 and interleukin-8 messenger ribonucleic acid by myometrial cells: The differential effect of stretch and interleukin-1{beta}. J Clin Endocrinol Metab 2005; 90:3517–3527. [DOI] [PubMed] [Google Scholar]
- 78. Xu C, You X, Liu W, Sun Q, Ding X, Huang Y, Ni X. Prostaglandin F2α regulates the expression of uterine activation proteins via multiple signalling pathways. Reproduction 2015; 149:139–146. [DOI] [PubMed] [Google Scholar]
- 79. Vichai V, Suyarnsesthakorn C, Pittayakhajonwut D, Sriklung K, Kirtikara K. Positive feedback regulation of COX-2 expression by prostaglandin metabolites. Inflamm Res 2005; 54:163–172. [DOI] [PubMed] [Google Scholar]
- 80. Cahill CM, Rogers JT. Interleukin (IL) 1beta induction of IL-6 is mediated by a novel phosphatidylinositol 3-kinase-dependent AKT/IkappaB kinase alpha pathway targeting activator protein-1. J Biol Chem 2008; 283:25900–25912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tazawa R, Xu XM, Wu KK, Wang LH. Characterization of the genomic structure, chromosomal location and promoter of human prostaglandin H synthase-2 gene. Biochem Biophys Res Commun 1994; 203:190–199. [DOI] [PubMed] [Google Scholar]
- 82. Fang X, Wong S, Mitchell BF. Relationships among sex steroids, oxytocin, and their receptors in the rat uterus during late gestation and at parturition. Endocrinology 1996; 137:3213–3219. [DOI] [PubMed] [Google Scholar]
- 83. Madsen G, Zakar T, Ku CY, Sanborn BM, Smith R, Mesiano S. Prostaglandins differentially modulate progesterone receptor-A and -B expression in human myometrial cells: evidence for prostaglandin-induced functional progesterone withdrawal. J Clin Endocrinol Metab 2004; 89:1010–1013. [DOI] [PubMed] [Google Scholar]
- 84. Amini P, Michniuk D, Kuo K, Yi L, Skomorovska-Prokvolit Y, Peters GA, Tan H, Wang J, Malemud CJ, Mesiano S. Human parturition involves phosphorylation of progesterone Receptor-A at Serine-345 in myometrial cells. Endocrinology 2016; 157:4434–4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Peters GA, Yi L, Skomorovska-Prokvolit Y, Patel B, Amini P, Tan H, Mesiano S. Inflammatory stimuli increase progesterone Receptor-A stability and transrepressive activity in myometrial cells. Endocrinology 2017; 158:158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Osman I, Young A, Ledingham MA, Thomson AJ, Jordan F, Greer IA, Norman JE. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod 2003; 9:41–45. [DOI] [PubMed] [Google Scholar]
- 87. Robertson SA, Christiaens I, Dorian CL, Zaragoza DB, Care AS, Banks AM, Olson DM. Interleukin-6 is an essential determinant of on-time parturition in the mouse. Endocrinology 2010; 151:3996–4006. [DOI] [PubMed] [Google Scholar]
- 88. Romero R, Parvizi ST, Oyarzun E, Mazor M, Wu YK, Avila C, Athanassiadis AP, Mitchell MD. Amniotic fluid interleukin-1 in spontaneous labor at term. J Reprod Med 1990; 35:235–238. [PubMed] [Google Scholar]
- 89. Opsjłn SL, Wathen NC, Tingulstad S, Wiedswang G, Sundan A, Waage A, Austgulen R. Tumor necrosis factor, interleukin-1, and interleukin-6 in normal human pregnancy. Am J Obstet Gynecol 1993; 169:397–404. [DOI] [PubMed] [Google Scholar]
- 90. Sierra EM, Quiniou C, Nadeau-Vall ée M, Hou X, Hales B, Chemtob S. An Anti-IL-6R peptide as a potential therapeutic agent in inflammation- and infection-induced preterm birth. In: Taylor HS. (ed.) Society for Reproductive Investigation, vol. 24 Orlando, FL, USA: SAGE Publishing; 2017: 109A–110A. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.