Abstract
Background:
Immune-based therapy for metastatic breast cancer has had limited success, particularly in molecular subtypes with low somatic mutations rates. Strategies to augment T cell infiltration of tumors include vaccines targeting established oncogenic drivers like the genomic amplification of HER2. We constructed a vaccine based on a novel alphaviral vector encoding a portion of HER2 (VRP-HER2).
Methods:
In preclinical studies, mice were immunized with VRP-HER2 before or after implantation of hHER2+ tumor cells and HER2-specific immune responses and anti-tumor function were evaluated. We tested VRP-HER2 in a Phase I clinical trial where subjects with advanced HER2-overexpressing malignancies in cohort 1 received VRP-HER2 every 2 weeks for a total of three doses. In cohort 2, subjects received the same schedule concurrently with a HER2-targeted therapy.
Results:
Vaccination in preclinical models with VRP-HER2 induced HER2-specific T cells and antibodies while inhibiting tumor growth. VRP-HER2 was well tolerated in patients and vaccination induced HER2-specific T cells and antibodies. Although a phase I study, there was one partial response and two patients with continued stable disease. Median OS was 50.2 months in cohort 1 (n=4) and 32.7 months in cohort 2 (n=18). Perforin expression by memory CD8 T cells post-vaccination significantly correlated with improved PFS.
Conclusions:
VRP-HER2 increased HER2-specific memory CD8 T cells and had anti-tumor effects in preclinical and clinical studies. The expansion of HER2-specific memory CD8 T cells in vaccinated patients was significantly correlated with increased PFS. Subsequent studies will seek to enhance T cell activity by combining with anti-PD-1.
Keywords: Breast Cancer, Immunotherapy, Cancer vaccines, Clinical trials, Immune responses to cancer
Introduction
HER2 is overexpressed in 20–30% of breast cancers, and is associated with more aggressive tumor behavior (1). Treatment of HER2 overexpressing tumors has been revolutionized by therapies targeting HER2. Treatment with single agent HER2-targeted therapy (trastuzumab and T-DM1), combinations of chemotherapy and HER2-targeted therapy, and more recently, combinations of HER2-targeted therapies (trastuzumab plus pertuzumab or trastuzumab plus lapatinib) have lengthened progression free (PFS) and overall survival (OS) in the metastatic setting (2–4). However, additional therapies are clearly needed as the majority of metastatic HER2+ patients will eventually progress on these agents. While treatment with immune checkpoint blockade (ICB) has shown activity in other molecular subtypes of breast cancer like triple negative breast cancer (5, 6), HER2 overexpressing tumors have lower response rates to ICB (7). This may be due to fewer somatic mutations and therefore fewer tumor neoepitopes, but even treatment-refractory tumors generally continue to overexpress HER2 (8). It is also recognized that HER2-specific immunity is lost throughout the progression of HER2+ disease (9). Accordingly, there is great interest in enhancing adaptive immune responses to HER2, including cancer vaccines that activate broad, HER2-specific T cell and antibody responses, and how such therapeutics can be integrated into the current management of metastatic HER2+ breast cancer.
A variety of vaccines targeting HER2, based on proteins, peptides, modified tumor cells, viral vectors, pDNA and dendritic cells (DC) have been developed (10). Results from phase I and II studies of HER2-targeting cancer vaccines have demonstrated that HER2 is immunogenic, and that immune responses against HER2 may be associated with an improved clinical outcome (11–15). In our previous studies, vaccine-induced antibodies elicited various anti-tumor activities, including increased internalization of the target antigen, antiproliferative effects, and antibody dependent cellular cytotoxicity (ADCC) (16, 17).
Among viral vectors, alphavirus-like replicon particles (VRP), based on attenuated Venezuelan equine encephalitis (VEE) virus, are especially attractive because of their high expression of heterologous proteins, targeted expression in and maturation of dendritic cells, and induction of robust humoral and cellular immune responses to the vectored gene products (18–21). Foreign genes can be inserted in place of the VEE structural protein gene region in the cDNA plasmid, and an RNA transcript, when introduced into dendritic cells, will replicate. This self-amplifying replicon RNA will direct the synthesis of large amounts of the foreign gene product within the cell, typically reaching levels of 15–20% of total cell protein (22). They also activate immune responses against the encoded foreign gene product despite the development of neutralizing antibodies against the viral particle (18).
We previously performed a phase I clinical trial of a novel alphavirus vector encoding the tumor-associated antigen carcinoembryonic antigen (CEA) (VRP-CEA(6D)), in metastatic cancer patients (23). There was no dose limiting toxicity and the highest dose tested (4×108 IU) was determined to be the maximal feasible dose. CEA-specific T cell and antibody responses following VRP-CEA(6D) vaccination were observed regardless of the development of neutralizing antibodies. We chose a dose level of 4×108 IU viral replicon particles for the current study because it induced consistent activation of T cell and antibody responses in our previous study (23).
Here, we developed an alphaviral vector-based vaccine encoding the extracellular (ECD) and transmembrane (TM) domains of HER2 (VRP-HER2). We tested the safety, immunogenicity, and efficacy of this vaccine in preclinical murine models and in patients with advanced HER2-overexpressing breast cancers either alone or with concurrent HER2-targeted therapy. We demonstrated that the vaccine was safe, tolerable, and successfully induced T cell and polyfunctional antibody responses in our preclinical model and in humans with advanced HER2+ breast cancer. Strikingly, we identified an expanded perforin expressing memory CD8 T cell population that was significantly associated with improved PFS, indicating a possible biomarker for future studies.
Materials and Methods
Cell Lines
Tumor cell lines MM3MG, NMUMG, EPH4, Vero and U2OS were obtained from and maintained as recommended by the American Tissue Culture Collection (ATCC). MM3MG, NMUMG, and EPH4 cells were stably transfected with a lentivirus expressing full-length human HER2 or a mutated version of human HER2 as controls. CEM.NKR cells (NIH AIDS Reagent Program cat# 458) are CEM cells that are resistant to NK cell-mediated cell lysis. This reagent was obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: from Dr. Alexandra Trkola (24–26).
Mouse Experiments
Human HER2-transgenic mice (kindly provided by Dr. Wei-Zen Wei, Wayne State University, Detroit, MI; (27)) were crossed with BALB/c mice (000651, Jackson Labs) and the 6–10 weeks old F1 generation was used for immunogenicity testing. MM3MG-HER2 cells were orthotopically implanted into the mammary fat pad of female 6–10 week old BALB/c mice (1 × 106 cells/animal) as previously described and measured biweekly (28). Tumors were measured with calipers and volumes calculated using [v = width × width × (length / 2)]. Vaccinations of VRP-HER2 and control VRP-CEA were given by footpad injection of 1×107 particles/mouse. All mouse experiments were done in accordance with Duke Institutional Animal Care and Use Committee–approved protocol (A198–18-08).
Analysis of mouse anti-HER2 antibodies by Cell based ELISA
Parental or hHER2-expressing NMUMG cells were plated overnight in 96-well plates (Corning). Serum was diluted and added to plates for 1 hour. Plates were washed and cells were fixed with 1% formalin. A secondary anti-mouse IgG-HRP conjugated antibody (Cell Signaling) was used prior to developing with TMB substrate (Biolegend) and absorbance determined using a Bio-Rad Model 680 microplate reader (Bio-Rad).
ELISPOT
Mouse IFN-γ ELISPOT assay (Mabtech Inc.) was performed according to manufacturer’s instructions. Briefly, splenocytes (500,000 cells/well) were incubated in RPMI-1640 medium (Invitrogen) with 10% FBS for 24 hours. Cells were stimulated with HER2 pooled peptides (1 μg/ml; JPT, Germany), empty VRP vector, or irrelevant HIV-gag peptide mix (1 μg/ml: JPT, Germany). PMA (50 ng/ml) and Ionomycin (1μg/ml) (Sigma) were used as positive controls. All values are shown corrected for background, with spots from HIV control wells subtracted.
Flow Cytometry and peptide restimulation
For flow cytometry, cells were isolated from mouse spleens and mechanically dissociated with a 40-µm cell strainer (Greiner Bio-One). Red blood cells were lysed with RBC lysing buffer (Sigma). Cells were plated in 96-well plates (Corning) (1×106 cells/well) with brefeldin A (Biolegend), monensin (Biolegend), CD107a (1D4B), and CD107b (ABL-93; both BD Biosciences at 0.1 μg/well) in the presence or absence of a pool of HER2 peptides for 6 hours as previously described (29). Cells were stained with Fixable viability dye (Invitrogen) and additional fluorochrome-conjugated antibodies. For intracellular staining, a FoxP3 Fix/Perm kit was used according to the manufacturer’s instructions (eBioscience). Antibodies used include: CD45 (30F11), CD8β (YTS156.7.7), CD4 (RM4–5), CD44 (IM7), FoxP3 (FJK-16S), IFNγ (XMG1.2), and granzyme B (GB11) (all Biolegend). Data were collected using an LSR II flow cytometer (BD Bioscience) and analyzed with FlowJo software (Tree Star).
Patients and study drug administration
The phase I clinical trial was performed under an FDA-approved Investigational New Drug Exemption and registered at ClinicalTrials.gov (NCT01526473). Participants were recruited from medical oncology clinics at Duke University Medical Center, Durham, NC and provided written consent under a protocol approved by the Duke University Medical Center Institutional Review Board. This study was conducted in accordance with all recognized institutional and federal guidelines including those put forth in the Declaration of Helsinki.
Eligibility requirements included age >18, stage IV, HER2-overexpressing (2+, 3+) or FISH+ malignancy, and progressive or locally recurrent disease despite having received at least 1 prior FDA-approved HER2-targeted therapy (e.g. trastuzumab, trastuzumab plus pertuzumab, T-DM1, or lapatinib), ECOG status of 0 or 1, adequate hematologic counts, hepatic and renal function, and a left ventricular ejection fraction (LVEF) of >50%. Known autoimmune disease, immunosuppressive therapies or HIV, significant cardiovascular disease or arrhythmias were exclusionary criteria.
There were two treatment cohorts (Sup Figure S1). Cohort 1 evaluated vaccination with VRP-HER2 alone and cohort 2 evaluated vaccination with VRP-HER2 during concurrent anti-HER2 therapy chosen by their attending medical oncologist. Concurrent bisphosphonates and hormonal therapy were permitted in both cohorts. Discontinuation of prior chemotherapy or targeted therapy was required for at least three weeks before the first VRP-HER2 administration in cohort 1; however, the lack of concurrent anti-HER2 therapy severely limited enrollment and therefore after 4 patients had completed therapy in cohort 1 and there were no dose limiting toxicities, the protocol was amended to permit the opening of cohort 2. VRP-HER2 was administered at 4 × 108 IU intramuscularly into the deltoid every 2 weeks for 3 administrations (arms were alternated with each injection). Chest, abdominal, pelvic CT or MRI scans were performed as part of a patient’s standard management at baseline and after the final vaccination (e.g., week 8). Cardiac monitoring included 12-lead ECG at screening, and a MUGA or echocardiogram evaluation of LVEF at screening and in week 8 of study.
CEM-NKR Cell Lines – Antibody Dependent Cellular Cytotoxicity
Parental or HER2-expressing CEM.NKR cells (targets) were incubated overnight with normal donor PBMC (effector cells) and 2,000 U/mL of IL-2 (Prometheus Laboratories Inc). These cells were mixed at a 5:1 or 25:1 effector:target ratio along with pre- or post-vaccination patient serum or Herceptin (positive control) for 2–3 hours at 37 °C. Cells were stained with Annexin V-APC (BD Biosciences), 7-AAD (Beckman Coulter), and anti-HER2 (PE; Clone NEU 24.7; BD Biosciences) and analyzed by flow cytometry to determine total percent of cell death.
HER2 Fluorogen Activating Peptide (FAP) Internalization Assay
Synthetic construct encoding Homo sapiens v-erb-b2 avian erythroblastic leukemia viral oncogene homolog 2 (ERBB2, RefSeq mRNA: NM_004448.2) was inserted into FAP-EGFP plasmid (30). The resulting construct (FAP-HER2) was transfected in U2OS cells. Stably expressing FAP-HER2 cell line was maintained in DMEM+10%FCS+G418. Parental and FAP-HER2-expressing U2OS cells were seeded at 105 cells/well in 96 well plate overnight. Cells were incubated with patient serum pre- and post-vaccination, control mouse serum, or Herceptin for 4 hours and washed. Serum free media + the membrane impermeant fluorogen Sci1 (kind gift from the Waggoner Lab (31)) were added and incubated for 20 minutes at room temperature. Plates were washed with PBS and scanned at 700 nm on an Odyssey CLx (LI-COR Biosciences).
VRP neutralization assay
VRP vector expressing a different target protein (CEA) (MOI 10) was mixed with pre- and post-vaccination patient sera and incubated at 4°C for 24 hours. The VRP-CEA/serum mixture was added to Vero cells. Following incubation, the Vero cells were stained for CEA expression and analyzed by flow cytometry to measure neutralization of VRP-CEA infection.
EPH4 Cell-Based HER2 ELISA
EPH4 (HER2-), EPH4-HER2wt (wild type HER2+) and EPH4-HER2mut (mutant HER2+ (7 mutations within the Herceptin binding domain (557–603), specifically P557S, V563A, P571S, P572S, F573S, F594Y, and C601A to rat homolog counterparts)) cells were used to test patient serum for HER2-specific antibodies. Dilutions of patient sera pre- and post-VRP-HER2 vaccination were added in a 96 well plate and incubated for 1 hour on ice. The plates were washed with PBS twice, and fixed with 1% formalin at room temperature. After washing with PBS, HRP-conjugated goat anti-human IgG (BioRad) was added to the wells prior to incubation with TMB substrate (Biolegend). The color development was stopped by adding 50 µL of 1M H2SO4 buffer. Absorbance at 650 nm was read using a BioRad Microplate Reader (Model 680).
CYTOF Flow Cytometry analysis
PBMC stimulation.
Frozen vials were thawed at 37°C, diluted and washed 2x with warm RPMI-1640 medium (Hyclone) supplemented with 10% FBS, (Atlanta Biologicals), 100 U/ml penicillin, 100 μg/ml streptomycin, 29.2 mg/ml L-glutamine (Hyclone) and 25 U/ml benzonase (Sigma-Aldrich). Cells were washed once and plated at a concentration of 4×106/well in 2 ml of complete media in a cell culture-treated 12 well plate (Corning) at 37°C for 4 hours in 10-ng/ml PMA and 1 μg/ml ionomycin (both from Sigma-Aldrich)) or 20 hours in PBS or 0.5 µg/ml HER2 ECD peptide mix (JPT, Germany). 5 μg/ml Brefeldin A (Sigma-Aldrich), 2 μM monensin (eBioscience), and a titered concentration of CD107a antibody conjugate were added for the final 4 hours. After the incubation, cells were washed with PBS and further extracted with 15 mM EDTA (Hoefer) for 15 min at 37°C. Cells were washed and stained for mass cytometry.
CYTOF antibody panel.
Sup table S1 lists the 34 labels with the corresponding antibodies. Antibodies were purchased from BD Biosciences, Biolegend, eBioscience, Fluidigm, and R&D Systems. Primary antibodies were conjugated to their corresponding metals using the Maxpar antibody labeling protocol and kits (Fluidigm).
Mass Cytometry.
All samples were washed in CyFACS, 1x CyPBS (Rockland) with 0.1% BSA (Sigma-Aldrich), 2 mM EDTA (Hoefer) and 0.05% NaAzide (Teknova); made in MilliQ water, and resuspended on ice for one hour in a master mix of cell-surface staining antibodies. Samples were resuspended in 100 μl of 5 mg/ml 115-DOTA maleimide (Fluidigm) at a 1:3000 dilution and kept overnight in 2% para-formaldehyde (PFA) at 4°C. Samples were washed in 1x saponin permeabilization buffer (eBioscience) and incubated in the intracellular antibody master mix in permeabilization buffer on ice. Iridium intercalator (Fluidigm) was added at 1:5000 dilution in 2% PFA. Three additional water washes were performed immediately before running on the mass cytometer and samples were resuspended in 0.1X EQ four-element calibration beads (Fluidigm) at a concentration of 5×105/ml. Samples were run on a CyTOF version 1 (Fluidigm) within three days of staining and were analyzed using Spanning-tree Progression Analysis of Density-normalized Events (SPADE) (32) in Cytobank (Mountain View, CA). FCS files were normalized through median bead intensity and beads were removed prior to analysis (33).
SPADE Analysis.
The spanning-tree progression analysis of density-normalized events (SPADE) algorithm was used to identify clusters of cells that were organized into a SPADE tree (Sup Figure S3). Cell surface markers were used to create the SPADE clusters and were pregated on live, intact singlets. Once clusters had been made, effector molecule expression pre- and post-vaccination within these clusters was analyzed. Significant differences in median expression levels were determined using a two-sided paired t test with multiple hypothesis test correction with a family-wise error rate (FWER). For each stimulation condition, the random permutation was performed 100 times, and all significance tests were repeated for each permutation. For each permutation, the minimum p value from all of these significance tests was recorded, and the 5th percentile of this distribution of minimum p values was used as a significance cutoff for the stimulation condition and clustering method.
Statistical analyses
Data are presented as mean ± standard error of the mean (SEM). Tumor volumes, flow cytometry, ELISA, and ELISPOT data from experiments with 3 or more treatment groups were analyzed by 1-way ANOVA with Bonferroni’s multiple comparisons test. A 2-tailed, unpaired Student’s t test was used for experiments with only 2 groups. Tumor volumes were analyzed at the terminal endpoint only, unless otherwise indicated. Statistical analysis was performed using Prism (GraphPad). P values of 0.05 or less were considered statistically significant. Not all significant differences are shown in every graph. *p < 0.05; **p < 0.01; ***p < 0.001
For clinical studies, PFS interval was defined as the time from trial enrollment to disease progression or death, whichever came first. OS was defined from the time of enrollment until death due to any cause. PFS and OS were calculated using the Kaplan-Meier product limit method. Radiographic response was determined according to RECIST criteria 1.1. A paired Student’s t test was used to determine differences pre- and post-vaccination.
Results
VRP-HER2 generates robust anti-HER2 response
We developed a VRP-based vaccine expressing the ECD and TM domains of human HER2 (VRP-HER2). Standard toxicology analysis detected no systemic or organ specific toxicities in VRP-HER2 vaccinated, HER2-transgenic mice (data not shown). Following vaccination, induction of both VRP- and HER2-specific T cell responses was detected in splenocytes using an IFN-γ-ELISPOT assay after restimulation with empty VRP vector or HER2 peptides (Figure 1A). Similarly, high titers of HER2-specific antibodies (Figure 1B) that bound HER2-expressing cells were induced by VRP-HER2 vaccination. These data demonstrate that the VRP-HER2 vaccine breaks tolerance to HER2 and induces potent anti-HER2 immune responses.
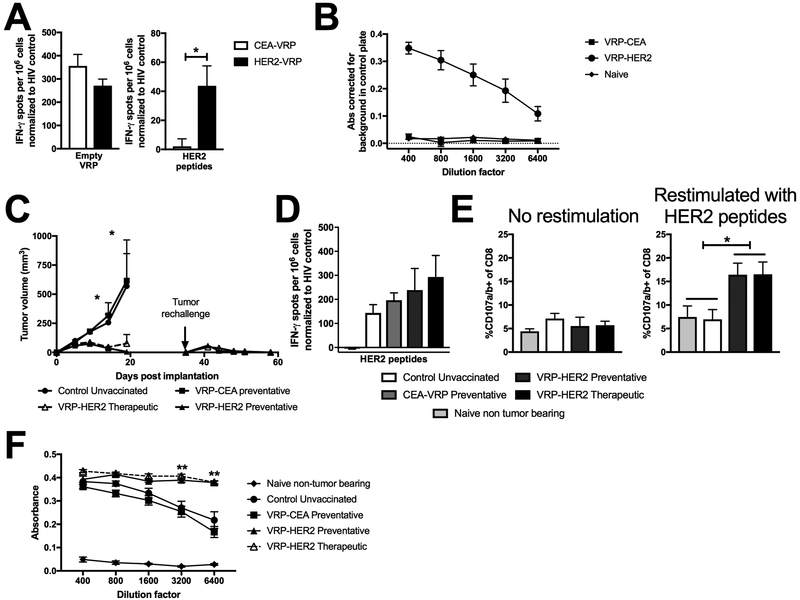
Figure 1. Immunogenicity and efficacy of VRP-HER2 in HER2-transgenic mouse model.
Mice were vaccinated with 1×107 IU in the footpad. Two weeks post-vaccination spleen and serum was taken and analyzed. (N=4 mice/group) A.) Splenocytes were stimulated with empty VRP vector or a pool of HER2 peptides and IFN-γ producing cells were analyzed by ELISPOT. B.) Serum was analyzed for HER2-specific antibodies using a cell-based ELISA. Absorbances are corrected for signal seen in a negative control plate. C.) MM3MG-HER2 tumor cells were implanted into the mammary fat pad of mice. Preventative vaccines were given 2 weeks prior to implantation and therapeutic vaccines were given 1 day post tumor implantation. Mice that completely cleared tumors were rechallenged on the opposite side in the mammary fat pad on day 35. Tumors were measured biweekly. (n=5 mice/group) D.) Splenocytes from mice in (C) were stimulated with a pool of HER2 peptides and IFN-γ producing cells were analyzed by ELISPOT. E.) Splenocytes from mice in (C) following rechallenge were incubated with brefeldin A alone or a mixture of HER2 peptides and CD107a/b antibodies to measure degranulation by flow cytometry. Cells were pregated on live CD45+ CD8+ CD44hi prior to analysis of CD107a/b expression. F.) Serum from mice in (C) was analyzed for HER2-specific antibodies using a cell-based ELISA.
We tested the antitumor effect of VRP-HER2 immunization in vivo. In both prevention (vaccine given 2 weeks prior to tumor implantation) and treatment (vaccine given the day after tumor implantation) models, there was sustained control of tumor growth compared with control VRP-CEA vaccination (vaccine given 2 weeks prior) and unvaccinated mice (Figure 1C). In addition, mice receiving VRP-HER2 that had cleared their primary tumor challenge were all resistant to subsequent tumor rechallenge (Figure 1C). Given this evidence for a sustained memory response, we analyzed splenocytes for responsiveness to restimulation with HER2 peptides by IFN-γ-ELISPOT (Figure 1D) and found that HER2-specific T cell responses were present in all mice that were implanted with a HER2-expressing tumor due to immune responses against HER2 presented by tumor cells in these mice. We further evaluated the quality of long term memory CD8 T cells induced by VRP-HER2 vaccination by measuring their ability to rapidly degranulate following HER2 peptide stimulation ex vivo >45 days post vaccination. Indeed, we found that only the VRP-HER2 vaccinated mice contained a systemic population of expanded, activated HER2-specific cytolytic CD8+ T cells (Figure 1E). No changes in CD4 memory T cell frequency or functionality in response to HER2 peptide restimulation was observed (data not shown).
Serum from VRP-HER2 vaccinated mice contained a significantly higher titer (P<0.005) of anti-HER2 antibodies than unvaccinated or control VRP-CEA vaccinated mice (Figure 1F), further supporting the formation of long lasting HER2-specific memory responses. These data demonstrate that VRP-HER2 is able to provide significant anti-tumor protection by eliciting both T and B cell responses to HER2. These data further supported the initiation of a phase I study in humans with advanced HER2 over-expressing malignancies.
Patient demographics
Patients (n=22) were enrolled at one center from 10/21/2012 to 10/19/2015. Four patients were enrolled and received treatment in cohort 1 (no concurrent HER2-targeted therapy) and eighteen in cohort 2 (combined with HER2 targeted therapy) (Sup Figure S1). Demographics for cohorts 1 and 2 are listed for each patient (Table 1). The majority of patients had substantial metastatic disease burden (median of 2 sites including lung and liver) and had been heavily pretreated (median of 3.5 prior lines of therapy), all having progressed on prior trastuzumab, 13/22 (59.1%) having progressed on a lapatinib-containing regimen, 8/22 (36.3%) having progressed on a pertuzumab-containing regimen, and 9/22 (40.9%) having progressed on a T-DM1-containing regimen (Table 1). Additionally, the median duration of metastatic disease at the time of enrollment was 31 months. In cohort 2, concurrent regimens during the immunization schedule included: TDM-1 (6/18; 33.3%) patients, trastuzumab/pertuzumab (5/18; 27.8%) patients, trastuzumab/lapatinib (3/18; 16.7%), single agent trastuzumab (3/18; 11.1%) and single agent lapatinib (1/18; 5.6%). All 22 enrolled patients completed all 3 vaccinations. In addition, in cohort 2, two patients were receiving concurrent anti-endocrine therapy, one of these patients was receiving the aromatase inhibitor Letrozole and the other was receiving the SERD Faslodex.
Table 1:
Demographics and baseline characteristics of enrolled patients
| Characteristic | Cohort 1 VRP- HER2 (N = 4) |
Cohort 2 VRP- HER2 + Anti-HER2 therapy (N = 18) |
Total (N = 22) |
|---|---|---|---|
| Median Age at trial entry (Range) | 58.5 (53-64) | 59.0 (25–76) | 59.0 (25–76) |
| Sex | No. (%) | No. (%) | No. (%) |
| Male | 1 (25.0) | 0 (0.0) | 1 (4.5) |
| Female | 3 (75.0) | 18 (100.0) | 21 (95.4) |
| Race | |||
| White | 4 (100.0) | 14 (77.8) | 18 (81.8) |
| Nonwhite | 0 (0.0) | 4 (22.2) | 4 (18.2) |
| HER2+ Disease Type | |||
| Breast Cancer | 3 (75.0) | 18 (100.0) | 21 (95.4) |
| Esophageal Cancer | 1 (25.0) | 0 (0.0) | 1 (4.5) |
| Hormone Receptor Status (BCa only) | |||
| HR+ (ER+ and or PR+ | 1 (33.3) | 11 (61.1) | 12 (57.1) |
| HR− (ER / PR−) | 2 (66.7) | 7 (38.9) | 9 (42.9) |
| Years with metastatic disease | |||
| ≤2 yrs | 1 (25.0) | 5 (27.8) | 6 (27.2) |
| >2 yrs | 3 (75.0) | 13 (72.2) | 16 (72.7) |
| Site(s) of metastasis (prior or current) | |||
| 1-2 | 3 (75.0) | 11 (61.1) | 14 (63.6) |
| ≥ 3 | 1 (25.0) | 7 (38.9) | 8 (36.3) |
| Prior Lines of Tx | |||
| 1-2 | 1 (25.0) | 8 (44.4) | 9 (40.9) |
| 3-5 | 2 (50.0) | 4 (22.2) | 6 (27.3) |
| >5 | 1 (25.0) | 6 (33.3) | 7 (31.8) |
| Prior Anti-HER2 Therapy | |||
| Trastuzumab | 4 (100.0) | 18 (100.0) | 22 (100.0) |
| Pertuzumab + Trastuzumab | 0 (0.0) | 8 (44.4) | 8 (36.3) |
| TDM-1 | 0 (0.0) | 9 (50.0) | 9 (40.9) |
| Lapatinib | 3 (75%) | 10 (55.6) | 13 (59.1) |
VRP-HER2 vaccination alone (cohort 1) and in combination with anti-HER2 therapies (cohort 2) was well tolerated and with no dose limiting toxicity (Table 2). There were four grade 3 adverse events (AEs) but none were deemed to be related to VRP-HER2. Two Grade 2 AEs (fatigue and decreased white blood cell count) were deemed possibly related to VRP-HER2. The remainder of the AEs deemed related to the VRP-HER2 were Grade 1 and included: diarrhea (definite), pustular rash (definite), oral mucositis (definite), malaise (possible), nausea (possible), dry mouth (possible), sore throat (possible), decreased neutrophil count (possible), and decreased white blood cell count (possible). The remaining AEs were felt to be unrelated. One patient withdrew consent for participation after receiving injection #3 on week 4, but did not report any AEs.
Table 2:
Possible, probable, and definite VRP-HER2 related adverse events
| Non-Hematologic Adverse Events | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1- Mild | 2- Moderate |
3/4 - Severe/Life Threatening |
Total | |||||
| Cohort | n | (%) | n | (%) | n | (%) | N | |
| General disorders and administration site conditions | ||||||||
| Fatigue | 1 | 0 | 0% | 0 | 0% | 0 | 0% | 4 |
| 2 | 0 | 0% | 1 | 6% | 0 | 0% | 18 | |
| Fever | 1 | 0 | 0% | 0 | 0% | 0 | 0% | 4 |
| 2 | 2 | 11% | 0 | 0% | 0 | 0% | 18 | |
| Injection site reaction | 1 | 0 | 0% | 0 | 0% | 0 | 0% | 4 |
| 2 | 2 | 11% | 0 | 0% | 0 | 0% | 18 | |
| Malaise | 1 | 0 | 0% | 0 | 0% | 0 | 0% | 4 |
| 2 | 1 | 6% | 0 | 0% | 0 | 0% | 18 | |
| Infections and infestations | ||||||||
| Rash pustular | 1 | 1 | 20% | 0 | 0% | 0 | 0% | 4 |
| 2 | 0 | 0% | 0 | 0% | 0 | 0% | 18 | |
| Musculoskeletal and connective tissue disorders | ||||||||
| Musculoskeletal and connective tissue disorder - Other, specify | 1 | 0 | 0% | 0 | 0% | 0 | 0% | 4 |
| 2 | 1 | 6% | 1 | 6% | 0 | 0% | 18 | |
| Pain in extremity | 1 | 0 | 0% | 0 | 0% | 0 | 0% | 4 |
| 2 | 5 | 28% | 0 | 0% | 0 | 0% | 18 | |
| Renal and urinary disorders | ||||||||
| Urinary retention | 1 | 0 | 0% | 0 | 0% | 0 | 0% | 4 |
| 2 | 1 | 6% | 0 | 0% | 0 | 0% | 18 | |
| Summary | Cohort | n | (%) | n | (%) | n | (%) | N |
| Maximum Non-Hematologic AE | 1 | 1 | 20% | 0 | 0% | 0 | 0% | 4 |
| 2 | 7 | 39% | 2 | 11% | 0 | 0% | 18 | |
| Maximum Overall AE | 1 | 1 | 20% | 0 | 0% | 0 | 0% | 4 |
| 2 | 7 | 39% | 2 | 11% | 0 | 0% | 18 | |
There were three grade 3 AEs but these AEs were not attributable to the VRP-HER2 vaccine.
Because the anti-HER2 antibody trastuzumab can be associated with cardiotoxicity, left ventricular ejection fraction (LVEF) was measured prior to starting vaccination and at week eight. No symptomatic heart failure or significant decrease in LVEF of 16 or more percentage points from baseline or 10 to 15 percentage points from baseline to below the lower limit of normal was observed for any enrolled patient at any time point on trial.
Induction of T cell responses by VRP-HER2
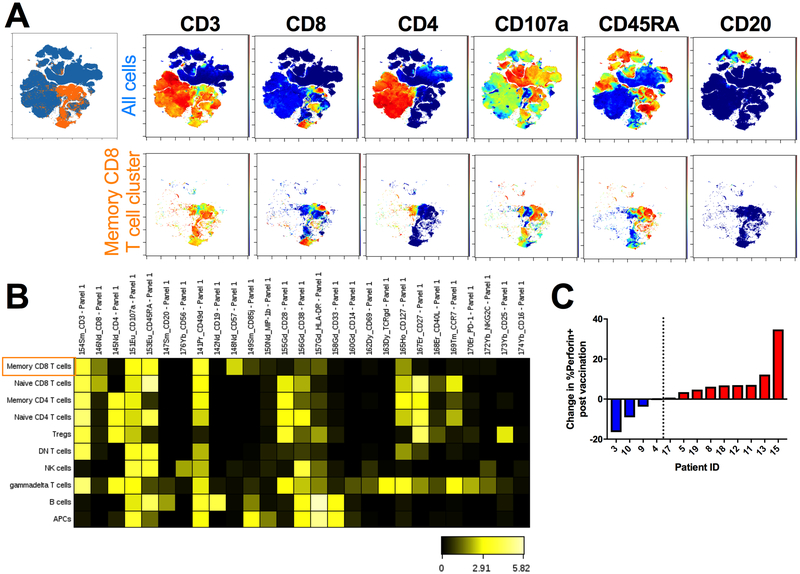
To evaluate any systemic changes in T cell responses post-vaccination, peripheral blood mononuclear cells (PBMCs) from baseline and six weeks after the initial vaccination were evaluated by multiparameter mass cytometry in 13 of the patients in cohort 2. This technique allowed us to maximize our analysis of these samples by staining with a panel of 34 surface markers and cytokines (Sup Table S1). Cells were restimulated with a pool of HER2 peptides and stained to evaluate the impact of vaccination on HER2-specific immunity. Live, intact single cells were clustered using cell surface markers into a SPADE tree that identified major immune cell subsets (Sup Figure S3). Expression of effector molecules following HER2 peptide restimulation of PBMCs taken pre- and post-vaccination within these clusters was analyzed and one cluster had a significant increase in the expression of perforin following vaccination. This cluster was identified as containing effector/effector memory CD8 T cells. viSNE plots colored by various cell surface markers confirm the identify of this cluster by the high expression of CD3, CD8, CD107a, and the low expression of CD4 and CD20 (Figure 2A). Additionally, a subset of the cluster expresses high levels of CD45RA, whereas the rest do not, indicating a mixture of CD8 effector and memory T cells (Figure 2A). A heat map of expression of all surface markers by each of the identified SPADE clusters further shows that this cluster (shown in the top row) is negative for CCR7 and CD28 while expressing high levels of CD57, further distinguishing it from naïve CD8 T cells (Figure 2B).
Figure 2. Identification of expanded memory CD8 T cell population following vaccination using CYTOF.
PBMCs were collected pre-vaccination and 6 weeks post the first vaccination. Cells were stimulated with a pool of HER2 peptides and stained for analysis by CYTOF mass cytometry. SPADE analysis was performed and clusters of cells were identified using surface markers. A. viSNE plots showing expression of select cell surface markers by all cells (Top, blue) or the memory CD8 T cell SPADE cluster that was expressing significantly more perforin post-vaccination (Bottom, orange). B. Heatmap of expression of all surface markers by each SPADE cluster. C. Change in the percent of perforin expression by cells in the identified memory CD8 T cell SPADE cluster from HER2 peptide restimulation PBMCs post-vaccination for each individual patient.
Within this cluster, median expression of perforin was significantly higher in restimulated PBMCs post-vaccination (p=0.0056). The change seen in each individual patient is shown, with two groups identified: those with any decrease in median perforin expression by memory CD8 T cells following vaccination (blue; 4/13; 30.8%) and those with an increase following vaccination (red; 9/13; 69.2%) (Figure 2C and Sup Figure S4). The same analysis performed on unstimulated PBMC samples did not reveal any significant changes in perforin expression highlighting the HER2 specificity of this response post-vaccination (data not shown). Furthermore, analysis of the other effector molecules in all clusters post-vaccination did not reveal any additional significant changes. Of note, one of our clusters was identified as a potential CD4+ Treg population based on high CD25 expression (Figure 2B). When the number of cells within this cluster was evaluated pre- and post-vaccination, there were patients that had no change, an increase, or a decrease in Tregs following vaccination (Sup Figure S5A).
Induction of antibody responses by VRP-HER2
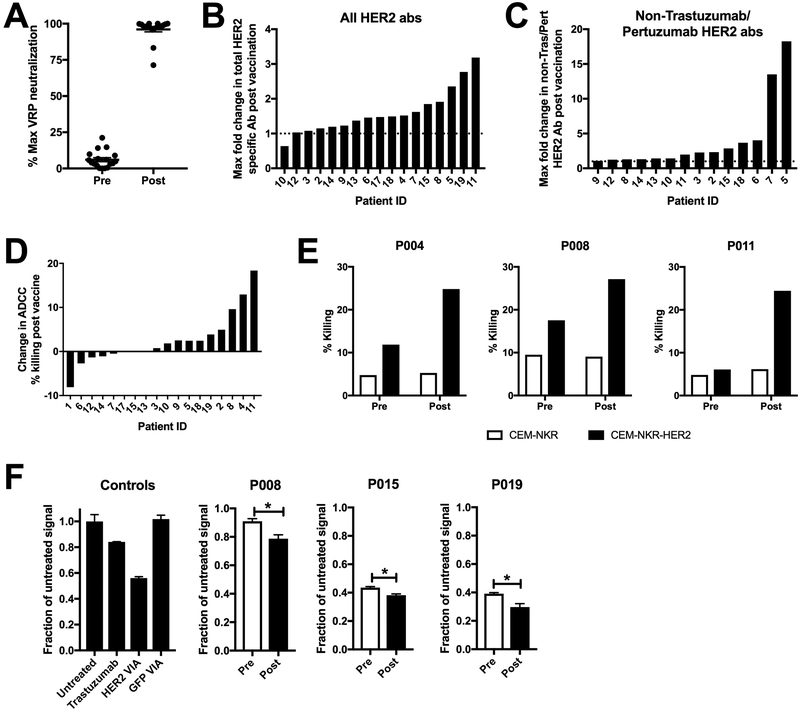
In our previous VRP-CEA study (34), we observed viral replicon particle (VRP-induced neutralizing antibodies as defined by the ability of serum from vaccinated patients to prevent VRP from infecting a permissive cell line. In the current study, neutralizing antibodies against VRP were again detected at high levels after immunization (Figure 3A). Despite the presence of neutralizing antibody, we were able to also detect HER2-specific antibodies in the serum of vaccinated patients. Although the overall levels of HER2-specific antibodies were low, VRP-HER2 induced a detectable increase in HER2-specific antibodies in 15/17 (88.2%) patients with available serum specimens post-vaccination (Figure 3B). Because of the extensive use of trastuzumab in this patient population, we modified our cell-based ELISA to allow us to assess antibody responses to HER2 epitopes other than the trastuzumab and pertuzumab binding sites. We stably transfected EPH4 cells, a murine breast epithelial cell line that lacks endogenous HER2 expression, with a form of hHER2 containing 7 point mutations within the trastuzumab and pertuzumab binding sites. This mutant form of HER2 is no longer bound by trastuzumab or pertuzumab but is still recognized by antibodies generated in mice following vaccination against hHER2 (Sup Figure S2). Using this cell line, we also observed an increase in non-trastuzumab/pertuzumab HER2-specific antibodies in 14/17 (82.4%) patients tested post vaccination (Figure 3C). The overall levels of antibodies present were low, but indicate an expansion of HER2-specific humoral responses above background trastuzumab/pertuzumab levels.
Figure 3. Induction of poly-functional VRP and HER2-specific antibodies following vaccination.
A.) Patient serum pre and post VRP-HER2 vaccination was tested for VRP vector specific antibodies in a VRP neutralization assay. VRP vector expressing a control transgene was incubated with patient serum and plated with Vero cells. Expression of transgene was determined by staining of cells with fluorochrome labeled antibody for transgene and analyzed by flow cytometry. B and C.) Serum was analyzed for HER2-specific antibodies using a cell-based ELISA. Cells expressing full length HER2 (B) or cells expressing a mutated form of HER2 that is not bound by Trastuzumab or Pertuzumab (C) were used. Absorbances are corrected for signal seen in a negative control non HER2 expressing plate. Several dilutions were assessed and the maximum observed fold change in the level of HER2-specific antibody signal post-vaccination for each patient is reported. If values were below the level of detection for a particular patient, they are not shown. D.) CEM.NKR cell line expressing HER2 (targets) were incubated with serum from patients vaccinated with VRP-HER2 and normal donor PBMC (effectors) at an E:T ratio of 1:25 in an ADCC assay for 2-4 hours. Cells were stained with annexinV and 7-AAD to determine total lysis of CEM.NKR-HER2 targets. Shown as percent change in maximum killing resulting from serum pre verses post VRP-HER2 vaccination. E.) Individual killing results for patients 4, 8, and 11 from (D) are shown. F.) internalization of HER2 was measured using a Mars1-tagged form of HER2. Background signal from non-HER2 expressing cells was subtracted. Level of Mars1 signal was then normalized to untreated cells. VIA= Vaccine-induced antibodies. Individual results from patients 8, 15, and 19 are shown. *p<0.05
Functionality of antibodies induced by VRP-HER2
Many standard of care (SOC) treatments for advanced HER2+ breast cancer include the use of monoclonal antibodies against HER2. An advantage to our vaccine approach is that in addition to stimulating a T cell response, we can also induce polyclonal, polyfunctional anti-HER2 antibodies. In order to study the functionality of these vaccine-induced antibodies, we first determined whether they can mediate ADCC. To study ADCC, we combined patient serum with healthy donor PBMC and a CEM.NKR cell line expressing HER2, which is resistant to nonspecific NK killing (24). In 10/18 (55.6%) subjects, the serum from post VRP-HER2 immunization induced a greater degree of ADCC than prior to vaccination (Figure 3D). Individual killing data for the subjects that induced the greatest degree of ADCC following immunization is shown (Figure 3E). Despite the presence of trastuzumab and/or pertuzumab, which can both mediate ADCC (35, 36), in the serum of these patients, the increase in ADCC activity following the immunizations suggests that additional ADCC capable antibodies were induced by vaccination.
Antibodies can also mediate other anti-tumor functions such as internalization of surface-expressed HER2, inhibition of HER2/HER3 heterodimerization and/or prevention of signaling thus resulting in reduced proliferation of tumor cells (37, 38). Indeed, we have shown that vaccine-induced HER2-specific antibodies can cause internalization and degradation of HER2 on tumor cells in murine models (39). Mars1 is a fluorogen activating peptide (FAP) that binds to the membrane impermeant fluorogen SCi1 and induces its fluorescence (40). Mars1-tagged membrane protein trafficking can be quantitatively assessed through the addition of SCi1 and imaging on a near-infrared plate reader (41). To assess the ability of VRP-HER2 vaccine-induced antibodies to mediate internalization of HER2 in the current study, sera from vaccinated patients were mixed with U2OS cells expressing a Mars1-tagged form of HER2. Trastuzumab induces a modest internalization of HER2 and is used as a control to account for patients being treated with trastuzumab (Figure 3F). Other controls included serum from mice that were vaccinated against hHER2 (positive control) or GFP (negative control) (Figure 3F). Following immunization, serum from 3/18 (16.8%) patients induced a greater amount of internalization as demonstrated by loss of HER2 surface expression following vaccination (Figure 3F). We noted variable levels of internalization between patients even prior to immunization as demonstrated by the individual data shown in Figure 3F. These data taken together indicate that VRP-HER2 immunization induces antibodies with multiple effector functions, including ADCC and receptor internalization, and thus with multiple potential mechanisms for anti-tumor activity.
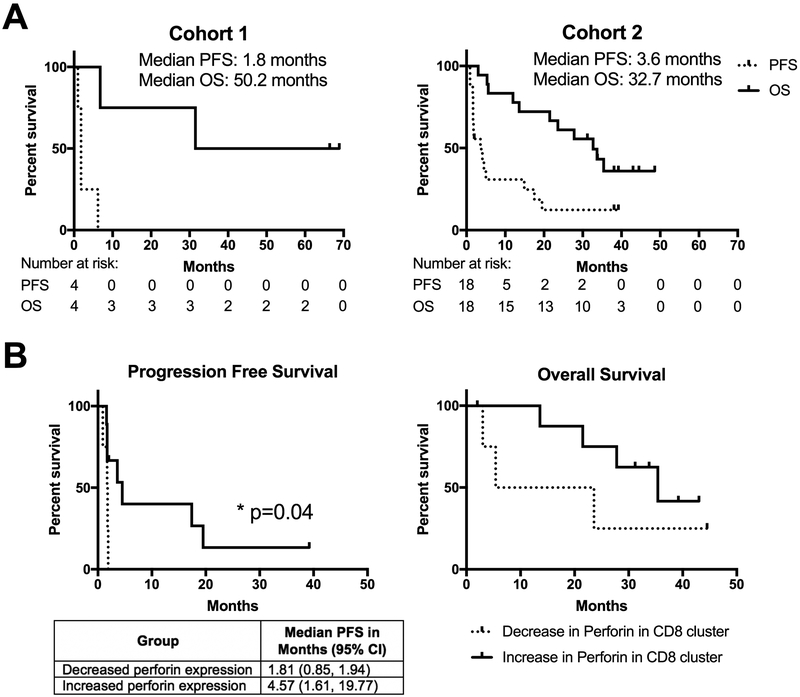
Clinical Outcomes
In cohort 1, there were no responses and the median PFS was 1.8 months and the median OS was 50.2 months (Figure 4A). In cohort 2, there was one partial response (PR) and 7 with initially stable disease (SD), two of whom had continued SD at time of manuscript submission. The median PFS for cohort 2 was 3.6 months and the median OS was 32.7 months. Among the subgroup of patients in cohort 2 who demonstrated an increase in perforin expression by memory CD8 T cells following vaccination (Figure 2C) there was a significantly longer PFS than among those who did not (Figure 4B). These data demonstrate that the induction of a HER2-specific memory CD8 T cell population following vaccination may represent a biomarker for immune responsiveness and beneficial clinical outcomes. The small sample size did not allow for any trends to be identified in the relationship between the induction of this perforin expressing population and the specific concurrent HER2 targeted therapies each patient was being given (Sup Table S2). This is a relationship that we will follow up on in our future studies. We attempted to find a correlation between any of the antibody responses and clinical outcome, but none were statistically significant, possibly due to the small number of patients and concurrent therapy with trastuzumab in the majority of the patients which made detection of small clinical changes difficult to identify. Interestingly, when we evaluated the impact of changes in Treg populations post-vaccination, patients that had a decrease in Tregs had a significantly improved overall survival (Sup Figure S5B). This is another potential biomarker that can be further validated in larger studies.
Figure 4. Expansion of perforin expressing memory CD8 T cell population is significantly associated with improved progression free survival.
A. Progression free and overall survival curves for each cohort. Number at risk for each timepoint is shown below. B. Progression free and overall survival of patients grouped according to change in perforin expression (Decrease, dotted; increase, solid) by memory CD8 T cells following vaccination.
Discussion
Patients with metastatic HER2+ breast cancer have multiple FDA approved anti-HER2 therapies available, yet no curative options have been developed and the majority of patients will eventually die of progressive disease. While ICB has shown clinical activity in many tumor types, including a few long term survivors, responses in HER2+ breast cancer are modest, perhaps reflecting a less robust adaptive immune response in HER2 driven breast cancer (9). Rather than relying on enhancing the naturally occurring adaptive immune response to tumor neoepitopes, we developed an immunization strategy that would expand HER2-specific T cells and antibodies to provide effective anti-tumor responses that complement current HER2 targeting SOC regimens.
It will be of significance to better understand how different SOC therapies for patients with HER2+ BC may interact with VRP-HER2 vaccination. Multiple studies have documented the ability of HER2 antibodies to elicit different types of immune responses (42), which may synergize with HER2 vaccination. Additionally, many HER2+ BC patients are also ER+ and undergoing various anti-endocrine therapies, but while some studies have suggested that that estrogen signaling can alter immunity (43, 44), other studies suggest that endocrine therapies may have a more modest effect on vaccine-mediated immunity (45). In our trial, all patients in cohort 2 were receiving concurrent HER2 targeted treatments, while only 2 had received endocrine therapies. Intriguingly, all patients receiving T-DM1 that were analyzed by CyTOF (n=3, Sup Table S2) were in the group with increased perforin expression following VRP-HER2 vaccination. Concurrent T-DM1 has been shown to improve responses to ICB pre-clinically (46), thus assessing this trend will be of significant interest in our future studies.
Despite the difficulty of measuring HER2-specific antibodies in a population of patients receiving trastuzumab treatment, we developed assays that demonstrate the presence of vaccine-induced HER2-specific antibodies with multiple effector functions (Figure 3). As single agent trastuzumab does not provide benefit to all patients with HER2+ breast cancer (47) and the combination of two HER2 targeted antibodies provides greater clinical benefit (3), a vaccine strategy that broadens the anti-HER2 antibody response to increase both the epitopes that are recognized and the effector functions elicited, as we have demonstrated here, has the potential to limit immune escape and resistance. While we were able to detect functional antibodies in vaccinated patients, high background and patient specific variability in addition to our small sample sizes limit our ability to extrapolate these results and link them to survival advantages. We will continue to monitor the relationship between polyfunctional antibody responses and favorable clinical outcomes in our future studies.
Clinically, VRP-HER2 was well tolerated and there were no dose limiting toxicities when it was given alone or in conjunction with other HER2-targeting therapies. The majority of AEs that occurred during this trial were grade 1 or 2. There were three grade 3 AEs, though none were felt related to VRP-HER2. There was one partial response and stable disease in seven patients in cohort 2, with continued stable disease in two patients at the time of manuscript submission. Median PFS in both the VRP-HER2 only cohort and the VRP-HER2 + anti-HER2 therapy cohort was modest at 1.8 and 3.6 months respectively; however, median OS was 50.2 months in cohort 1 and 32.7 months in cohort 2. This dichotomy in early progression but long-term survival may suggest that the immune response to vaccination which eventually results in clinical benefit is slow to develop and that overall survival is a better metric of efficacy of this vaccine strategy. In considering how our clinical results compare against similar published studies, the most comparable published study is the TH3RESA study (TDM-1 vs physicians’ choice) (48). Both studies enrolled HER2+ patients with a median of >3 prior lines of HER2 directed systemic therapy. The median OS of 32.7 months for our study compares favorably to the median OS of 22.7 months seen in the TH3RESA study. Importantly, the subset of patients with the greatest expansion of functional HER2-specific T cells in response to vaccination correlated with improved PFS (p=0.04), suggesting that readouts of immune activation may also be a useful marker for efficacy (Figure 4B). In addition, there was an overall survival advantage seen in patients that had a decrease in peripheral Tregs following vaccination (Sup Figure S5B). The small sample size of this clinical trial limits its extrapolation and prompted us to begin a larger Phase II study to validate and extend these findings.
This phase I study supported the hypothesis that we can expand HER2-specific T cells in patients with advanced HER2 overexpressing breast cancer. Both our pre-clinical and clinical data using this vaccine demonstrated the expansion of a HER2-specific, cytolytic CD8 T cell population (Figures 1E and 2C). Importantly, the expansion of the perforin+ subset also correlates with improved PFS (Figure 4B). It is now well-appreciated that many cancer immunotherapies are hampered by an extensive immunoregulatory tumor microenvironment. The generation of an activated CD8 T cell population with a vaccine alone may be insufficient to demonstrate dramatic tumor killing of bulky tumors. Combination with ICB strategies to enhance the induction of antigen specific T cells and/or the effector functionality of these T cells may enhance the clinical benefit seen with our vaccine (49, 50). We are currently initiating a clinical trial (NCT03632941) combining VRP-HER2 with checkpoint blockade in advanced HER2+ breast cancer patients receiving concurrent trastuzumab + pertuzumab.
Supplementary Material
Translational relevance.
HER2-targeting monoclonal antibody therapies are widely used to treat HER2-overexpressing breast cancers, but therapeutic resistance is common, leading to progressive disease and recurrence. We have constructed a vaccine based on a novel alphaviral vector encoding HER2 and tested it in a Phase I clinical trial, where we demonstrate that it was well tolerated with no dose limiting toxicity. Preclinical models additionally demonstrate immunogenicity and anti-tumor activity of the vaccine. Pre- and post-vaccination analysis of PBMCs revealed the expansion of a HER2-specific, perforin expressing memory CD8 T cell population in a subset of patients and an increase in the presence of HER2-specific, polyfunctional antibodies. The presence of the expanded HER2-specific memory T cell population correlated with improved progression free survival (p= 0.04). These data support the further testing of this vaccine platform in combination with immune checkpoint inhibitors like anti-PD1 to better engage the expanded T cell populations.
Acknowledgements
This work was supported by the Department of Defense (Contract # W81XWH-06–1-0585, for H. Lyerly; BC113107, for H. Lyerly; ), Susan G. Komen Foundation (KG081026, for M. Morse; CCR14299200, for Z. Hartman; PDF17481657, for E. Crosby), the National Center for Research Resources (UL1RR033176, for A. Rogatko), the National Center for Advancing Translational Sciences (UL1TR000124, for A. Rogatko), NCI (1K22CA212058–01, for J. Snyder) and the NIH (S10RR027582, for H. Maecker; 5T32CA009111–37, for E. Crosby). The following reagent was obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: CEM.NKR CCR5+ Cells from Dr. Alexandra Trkola. We would like to acknowledge Tao Wang, Delila Serra, Amanda Bradshaw, Karrie Comatas, and Chris Rabiola for their technical assistance with animal models, immune monitoring, and flow cytometry. We would also like to acknowledge the tireless efforts of the phase I and breast oncology coordinators (Paula Lee, Denise Morin, Shawna Savage, and Wanda Honeycutt) in assisting with patient management on the clinical trial.
Footnotes
Conflict of Interest statement: H. Kim Lyerly holds an equity interest in AlphaVax. All other authors declare no potential conflicts of interest.
References
- 1.Paik S, Hazan R, Fisher ER, Sass RE, Fisher B, Redmond C, et al. Pathological findings from the National Surgical Adjuvant Breast and Bowel Project - Prognostic-Significance of ErbB-2 Protein Overexpression in Primary Breast Cancer. Journal of Clinical Oncology. 1990. January;8(1):103–12. [DOI] [PubMed] [Google Scholar]
- 2.Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer. New England Journal of Medicine. 2012. November;367(19):1783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, Trastuzumab, and Docetaxel in HER2-Positive Metastatic Breast Cancer. New England Journal of Medicine. 2015. February;372(8):724–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. New England Journal of Medicine. 2006. December 28;355(26):2733–43. [DOI] [PubMed] [Google Scholar]
- 5.Adams S, Schmid P, Rugo HS, Winer EP, Loirat D, Awada A, et al. Phase 2 study of pembrolizumab (pembro) monotherapy for previously treated metastatic triple-negative breast cancer (mTNBC): KEYNOTE-086 cohort A. Journal of Clinical Oncology. 2017. 2017/October/11;35(15_suppl):1008-. [Google Scholar]
- 6.Nanda R, Chow LQM, Dees EC, Berger R, Gupta S, Geva R, et al. Pembrolizumab in Patients With Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. Journal of Clinical Oncology. 2016;34(21):2460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dirix LY, Takacs I, Jerusalem G, Nikolinakos P, Arkenau HT, Forero-Torres A, et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Research and Treatment. 2018. February;167(3):671–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ritter CA, Perez-Torres M, Rinehart C, Guix M, Dugger T, Engelman JA, et al. Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB Ligands and remain dependent on the ErbB receptor network. Clinical Cancer Research. 2007. August 15;13(16):4909–19. [DOI] [PubMed] [Google Scholar]
- 9.Datta J, Rosemblit C, Berk E, Showalter L, Namjoshi P, Mick R, et al. Progressive loss of anti-HER2 CD4(+) T-helper type 1 response in breast tumorigenesis and the potential for immune restoration. Oncoimmunology. 2015 2015;4(10):e1022301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cimino-Mathews A, Foote JB, Emens LA. Immune Targeting in Breast Cancer. Oncology-New York. 2015. May;29(5):375–85. [PubMed] [Google Scholar]
- 11.Peoples GE, Gurney JM, Hueman MT, Woll MW, Ryan GB, Storrer CE, et al. Clinical trial results of a HER2/neu (E75) vaccine to prevent recurrence in high-risk breast cancer patients. Journal of Clinical Oncology. 2005. October 20;23(30):7536–45. [DOI] [PubMed] [Google Scholar]
- 12.Disis ML, Schiffman K, Guthrie K, Salazar LG, Knutson KL, Goodell V, et al. Effect of dose on immune response in patients vaccinated with an HER-2/neu intracellular domain protein-based vaccine. Journal of Clinical Oncology. 2004. May 15;22(10):1916–25. [DOI] [PubMed] [Google Scholar]
- 13.Murray JL, Gillogly ME, Przepiorka D, Brewer H, Ibrahim NK, Booser DJ, et al. Toxicity, immunogenicity, and induction of E75-specific tumor-lytic CTLs by HER-2 peptide E75 (369–377) combined with granulocyte macrophage colony-stimulating factor in HLA-A2+patients with metastatic breast and ovarian cancer. Clinical Cancer Research. 2002. November;8(11):3407–18. [PubMed] [Google Scholar]
- 14.Salazar LG, Fikes J, Southwood S, Ishioka G, Knutson KL, Gooley TA, et al. Immunization of cancer patients with HER-2/neu-derived peptides demonstrating high-affinity binding to multiple class II alleles. Clinical Cancer Research. 2003. November 15;9(15):5559–65. [PubMed] [Google Scholar]
- 15.Morse MA, Hobeika A, Osada T, Niedzwiecki D, Marcom PK, Blackwell KL, et al. Long term disease-free survival and T cell and antibody responses in women with high-risk Her2+ breast cancer following vaccination against Her2. Journal of Translational Medicine. 2007. September 6;5(42). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren XR, Wei JP, Lei GJ, Wang JB, Lu JY, Xia WL, et al. Polyclonal HER2-specific antibodies induced by vaccination mediate receptor internalization and degradation in tumor cells. Breast Cancer Research. 2012;14(3):R89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei JP, Hartman Z, Osada T, Yang XY, Lei GJ, Jiang HX, et al. Adenovirus-human HER2 vaccine inhibits breast cancer growth and vaccine induced antibodies (VIA) are efficacious against Herceptin-refractory human breast cancer. Journal of Immunology. 2007. April;178:S81. [Google Scholar]
- 18.Durso RJ, Andjelic S, Gardner JP, Margitich DJ, Donovan GP, Arrigale RR, et al. A novel alphavirus vaccine encoding prostate-specific membrane antigen elicits potent cellular and humoral immune responses. Clinical Cancer Research. 2007. July 1;13(13):3999–4008. [DOI] [PubMed] [Google Scholar]
- 19.Thompson JM, Whitmore AC, Staats HF, Johnston RE. Alphavirus replicon particles acting as adjuvants promote CD8(+) T cell responses to co-delivered antigen. Vaccine. 2008. August 5;26(33):4267–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis NL, West A, Reap E, MacDonald G, Collier M, Dryga S, et al. Alphavirus replicon particles as candidate HIV vaccines. Iubmb Life. 2002. April-May;53(4–5):209–11. [DOI] [PubMed] [Google Scholar]
- 21.Avogadri F, Merghoub T, Maughan MF, Hirschhorn-Cymerman D, Morris J, Ritter E, et al. Alphavirus Replicon Particles Expressing TRP-2 Provide Potent Therapeutic Effect on Melanoma through Activation of Humoral and Cellular Immunity. Plos One. 2010. September;5(9):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pushko P, Parker M, Ludwig GV, Davis NL, Johnston RE, Smith JF. Replicon-helper systems from attenuated venezuelan equine encephalitis virus: Expression of heterologous genes in vitro and immunization against heterologous pathogens in vivo. Virology. 1997. December 22;239(2):389–401. [DOI] [PubMed] [Google Scholar]
- 23.Morse MA, Hobeika A, Gwin W, Osada T, Gelles J, Rushing C, et al. Phase I study of alphaviral vector (AVX701) in colorectal cancer patients: comparison of immune responses in stage III and stage IV patients. Journal for ImmunoTherapy of Cancer. 2015;3(2):P444. [Google Scholar]
- 24.Howell DN, Andreotti PE, Dawson JR, Cresswell P. Natural killing target antigens as inducers of interferon: studies with an immunoselected, natural killing-resistant human T lymphoblastoid cell line. Journal of Immunology. 1985;134(2):971–6. [PubMed] [Google Scholar]
- 25.Lyerly HK, Reed DL, Matthews TJ, Langlois AJ, Ahearne PA, Petteway SR, et al. Anti-GP-120 antibodies from HIV seropositive individuals mediate broadly reactive anti-HIV ADCC. Aids Research and Human Retroviruses. 1987;3(4):409–22. [DOI] [PubMed] [Google Scholar]
- 26.Trkola A, Matthews J, Gordon C, Ketas T, Moore JP. A cell line-based neutralization assay for primary human immunodeficiency virus type 1 isolates that use either the CCR5 or the CXCR4 coreceptor. Journal of Virology. 1999. November;73(11):8966–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piechocki MP, Ho YS, Pilon S, Wei WZ. Human ErbB-2 (Her-2) transgenic mice: a model system for testing Her-2 based vaccines. J Immunol. 2003. December 1;171(11):5787–94. [DOI] [PubMed] [Google Scholar]
- 28.Crosby EJ, Wei JPP, Yang XY, Lei GJ, Wang T, Liu CX, et al. Complimentary mechanisms of dual checkpoint blockade expand unique T-cell repertoires and activate adaptive anti-tumor immunity in triple-negative breast tumors. Oncoimmunology. 2018;7(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruitenberg JJ, Ghanekar SA, Brockstedt DG, Maecker HT. Simultaneous detection of murine antigen-specific intracellular cytokines and CD107a/CD107b by flow cytometry. Protocol Exchange. 2007. [Google Scholar]
- 30.Snyder JC, Pack TF, Rochelle LK, Chakraborty SK, Zhang M, Eaton AW, et al. A rapid and affordable screening platform for membrane protein trafficking. Bmc Biology. 2015. December;13:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang M, Chakraborty SK, Sampath P, Rojas JJ, Hou WZ, Saurabh S, et al. Fluoromodule-based reporter/probes designed for in vivo fluorescence imaging. Journal of Clinical Investigation. 2015. October;125(10):3915–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qiu P, Simonds EF, Bendall SC, Gibbs KD, Bruggner RV, Linderman MD, et al. Extracting a cellular hierarchy from high-dimensional cytometry data with SPADE. Nature Biotechnology. 2011. October;29(10):886–U181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finck R, Simonds EF, Jager A, Krishnaswamy S, Sachs K, Fantl W, et al. Normalization of mass cytometry data with bead standards. Cytometry Part A. 2013. May;83A(5):483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morse MA, Hobeika AC, Osada T, Berglund P, Hubby B, Negri S, et al. An alphavirus vector overcomes the presence of neutralizing antibodies and elevated numbers of Tregs to induce immune responses in humans with advanced cancer. Journal of Clinical Investigation. 2010. September;120(9):3234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scheuer W, Friess T, Burtscher H, Bossenmaier B, Endl J, Hasmann M. Strongly Enhanced Antitumor Activity of Trastuzumab and Pertuzumab Combination Treatment on HER2-Positive Human Xenograft Tumor Models. Cancer Research. 2009. December 15;69(24):9330–6. [DOI] [PubMed] [Google Scholar]
- 36.Diessner J, Bruttel V, Becker K, Pawlik M, Stein R, Haesler S, et al. Targeting breast cancer stem cells with HER2-specific antibodies and natural killer cells. American Journal of Cancer Research. 2013 2013;3(2):211–20. [PMC free article] [PubMed] [Google Scholar]
- 37.Pedersen MW, Jacobsen HJ, Koefoed K, Dahlman A, Kjaer I, Poulsen TT, et al. Targeting Three Distinct HER2 Domains with a Recombinant Antibody Mixture Overcomes Trastuzumab Resistance. Molecular Cancer Therapeutics. 2015. March;14(3):669–80. [DOI] [PubMed] [Google Scholar]
- 38.Szymanska M, Fosdahl AM, Nikolaysen F, Pedersen MW, Grandal MM, Stang E, et al. A combination of two antibodies recognizing non-overlapping epitopes of HER2 induces kinase activity-dependent internalization of HER2. Journal of Cellular and Molecular Medicine. 2016. October;20(10):1999–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ren XR, Wei JP, Lei GJ, Wang JB, Lu JY, Xia WL, et al. Polyclonal HER2-specific antibodies induced by vaccination mediate receptor internalization and degradation in tumor cells. Breast Cancer Research. 2012;14(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szent-Gyorgyi C, Schmidt BF, Creeger Y, Fisher GW, Zakel KL, Adler S, et al. Fluorogen-activating single-chain antibodies for imaging cell surface proteins. Nature Biotechnology. 2008. April;26(4):470. [DOI] [PubMed] [Google Scholar]
- 41.Snyder JC, Pack TF, Rochelle LK, Chakraborty SK, Zhang M, Eaton AW, et al. A rapid and affordable screening platform for membrane protein trafficking. Bmc Biology. 2015. December;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bianchini G, Gianni L. The immune system and response to HER2-targeted treatment in breast cancer. Lancet Oncol. 2014. February;15(2):e58–68. [DOI] [PubMed] [Google Scholar]
- 43.Kovats S Estrogen receptors regulate innate immune cells and signaling pathways. Cellular immunology. 2015. April;294(2):63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cunningham M, Gilkeson G. Estrogen receptors in immunity and autoimmunity. Clin Rev Allergy Immunol. 2011. February;40(1):66–73. [DOI] [PubMed] [Google Scholar]
- 45.Coveler AL, Goodell V, Webster DJ, Salazar LG, Fintak PA, Childs JS, et al. Common adjuvant breast cancer therapies do not inhibit cancer vaccine induced T cell immunity. Breast cancer research and treatment. 2009. January;113(1):95–100. [DOI] [PubMed] [Google Scholar]
- 46.Muller P, Kreuzaler M, Khan T, Thommen DS, Martin K, Glatz K, et al. Trastuzumab emtansine (T-DM1) renders HER2+ breast cancer highly susceptible to CTLA-4/PD-1 blockade. Sci Transl Med. 2015. November 25;7(315):315ra188. [DOI] [PubMed] [Google Scholar]
- 47.Hudis CA. Drug therapy: Trastuzumab - Mechanism of action and use in clinical practice. New England Journal of Medicine. 2007. July;357(1):39–51. [DOI] [PubMed] [Google Scholar]
- 48.Krop IE, Kim S-B, Martin AG, LoRusso PM, Ferrero J-M, Badovinac-Crnjevic T, et al. Trastuzumab emtansine versus treatment of physician’s choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): final overall survival results from a randomised open-label phase 3 trial. The Lancet Oncology. 2017;18(6):743–54. [DOI] [PubMed] [Google Scholar]
- 49.Grenier JM, Yeung ST, Khanna KM. Combination Immunotherapy: Taking cancer vaccines to the next level. Frontiers in Immunology. 2018. March 22;9:610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Osada T, Morse MA, Hobeika A, Diniz MA, Gwin WR, Hartman Z, et al. Vaccination targeting human HER3 alters the phenotype of infiltrating T cells and responses to immune checkpoint inhibition. Oncoimmunology. 2017 2017;6(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.