Abstract
Much progress has happened in understanding developmental vulnerability to preventable environmental hazards. Along with the improved insight, the perspective has widened, and developmental toxicity now involves latent effects that can result in delayed adverse effects in adults or at old age and additional effects that can be transgenerationally transferred to future generations. Although epidemiology and toxicology to an increasing degree are exploring the adverse effects from developmental exposures in human beings, the improved documentation has resulted in little progress in protection, and few environmental chemicals are currently regulated to protect against developmental toxicity, whether it be neurotoxicity, endocrine disruption or other adverse outcome. The desire to obtain a high degree of certainty and verification of the evidence used for decision-making must be weighed against the costs and necessary duration of research, as well as the long-term costs to human health because of delayed protection of vulnerable early-life stages of human development and, possibly, future generations. Although two-generation toxicology tests may be useful for initial test purposes, other rapidly emerging tools need to be seriously considered from computational chemistry and metabolomics to CLARITY-BPA-type designs, big data and population record linkage approaches that will allow efficient generation of new insight; epigenetic mechanisms may necessitate a set of additional regulatory tests to reveal such effects. As reflected by the Prenatal Programming and Toxicity (PPTOX) VI conference, the current scientific understanding and the timescales involved require an intensified approach to protect against preventable adverse health effects that can harm the next generation and generations to come. While further research is needed, the main emphasis should be on research translation and timely public health intervention to avoid serious, irreversible and perhaps transgenerational harm.
1 |. LIFELONG CONSEQUENCES OF DEVELOPMENTAL TOXICITY
When two Boston paediatricians tracked down twenty children who had allegedly recovered from lead poisoning in the 1940s, they discovered that the young patients had not at all recovered, but suffered from severe learning or behavioural problems and performed poorly in school.1 Elevated early-life exposure to inorganic lead had long-term consequences that much later were found to include psychopathies, delinquency and increased risk of cardiovascular disease in adulthood.2–4 In the late 1950s, a Japanese fishing town was plagued by a mysterious illness that was at first thought to be infectious. Of note, a pregnant woman who ate seafood heavily contaminated with methylmercury might be unscathed herself but would give birth to a poisoned child with spastic paresis and intellectual disability.5 Again, the infants’ condition did not improve with time, and patients with congenital mercury poisoning now show increased incapacitation as older adults.6 A third remarkable event happened in France in the 1960s, where a paediatrician noted the preponderance of alcohol-dependent women among the mothers of mentally disabled children at an institution.7 As with lead and mercury, the discovery, later named foetal alcohol syndrome, was at first met with scepticism and disbelief, but adverse effects were subsequently documented at much lower maternal alcohol intake levels, with additional problems, such as behavioural problems and delinquency, emerging at later ages.8
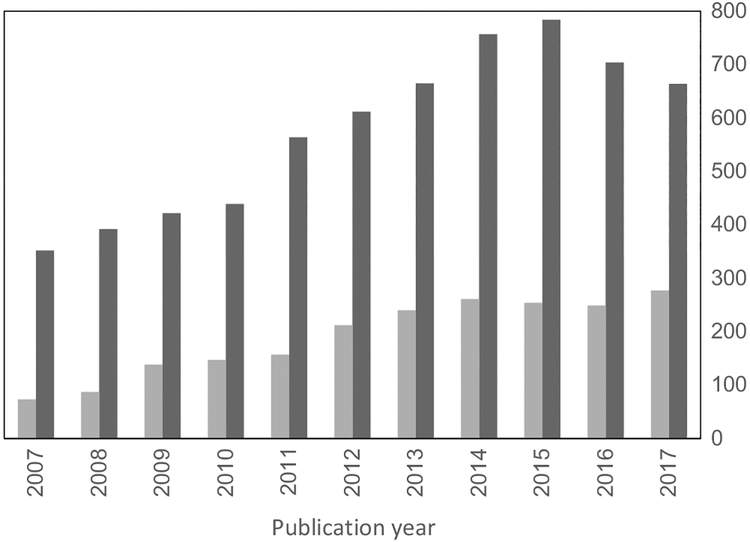
While scientists characterized the long-term effects of early-life exposures to these neurotoxicants, other researchers in social medicine and epidemiology discovered, in the mid-1980s, that low birthweight was associated with excess risk of cardiovascular disease and mortality in adulthood.9,10 These findings led to the formation of a research field coined the “developmental origins of health and disease” (DOHaD). In parallel, environmental toxicologists and epidemiologists began to explore the mechanisms, the occurrence and the implications throughout the lifespan of toxicity incurred during early development.11 The discovery of the multigenerational effects of diethylstilboestrol (DES) in offspring of males and females exposed in utero during the 1940s–70s is a prime example, but it will not be known for decades if there will be transmittal of these effects to future generations. Also, the broader risk of developing non-communicable diseases, such as cardiovascular disease, diabetes, obesity and cancer, now seems to be affected by a variety of stressors during foetal or post-natal development.13 Such early-life exposures can permanently change physiology, metabolism and/or functions of tissues and organs, as supported by epidemiological studies and animal experiments.14 During the most recent decade, the number of research publications in this field has substantially increased (Figure 1).
FIGURE 1.
Number of publications retrieved from the PubMed literature database from 2007, where the first PPTOX conference was held, and the following ten years. Dark grey columns reflect the total number of journal articles identified under the Medical Subject Heading “pre-natal exposure delayed effects,” while pale grey shows the articles within this category classified as epidemiology. Although the total number seems to have levelled off in recent years, the proportion of epidemiology reports has doubled from 21% in 2007 to 42% in 2017
With time, science-informed regulatory efforts have resulted in a gradually tightened regulation for some chemicals that can cause developmental toxicity. Thus, many initial exposure limits later appeared too high for proper protection, and decreases have subsequently occurred in response to better information on health consequences of early-life exposures.15 The delay in regulatory response owes in part to the lack of internationally accepted guidelines for testing of developmental toxicity. Still, a test guideline for developmental neurotoxicity is available,16 but it is seldom used. Also, such tests may be required only for chemicals produced at very high tonnages or when triggered by results from routine studies in adult laboratory animals. Thus, examination of developmental toxicity often is lacking and therefore cannot be considered in regulatory decisions.17 Even the tightening of regulations for well-understood agents, such as lead and methylmercury, has been unable to keep pace with the increasing knowledge on low-level toxicity and the complexity of human exposures to environmental chemicals.
Ten years after the Faroes statement,18 researchers again gathered at Torshavn, Faroe Islands, for the Prenatal Programming and Toxicity (PPTOX VI) conference. The unique location of the conference venue surrounded by the Atlantic Ocean was supported by on-the-spot measurements of ambient black carbon that showed an average of 0.17 μg/m3, which is lower than most background levels in the EU of about 1 μg/m3 (T. Nawrot, results to be published). The purpose of this MiniReview, written by researchers who participated in the conference, is to summarize new insight, lessons learned for research and the public health implications, now that the timescales involved in developmental toxicity have become more evident.
2 |. VULNERABLE DEVELOPMENT
The pre-natal vulnerability to environmental toxicants has recently been highlighted.19,20 Thus, systematic reviews and meta-analyses have related maternal exposure during pregnancy to air pollution and specific toxicants to indicators of impaired foetal growth, such as low birthweight or small for gestational age.21–23 The increased developmental vulnerability to toxicants during development may involve all organ systems, but is probably most evident regarding the nervous system. For example, experimentally reducing oxygen and nutrients to rabbits during the last third of gestation resulted in poorer neurobehavioral performance,24 an effect not seen in adults. In utero exposure to numerous neurotoxicants results in lasting brain deficits in children,25,26 as also first shown for lead, methylmercury and ethanol. Pre-natal exposure to air pollutants can result in white matter impairment, as seen in follow-up studies of children,27,28 and school performance improved more slowly in children exposed to elevated levels of air pollution.29 Other organ systems, such as the cardiovascular,30 the respiratory,31 the reproductive32 and the immune systems,33 are also known to be affected by early-life exposures.
Puberty also may represent an additional vulnerable time window.34 Moreover, adverse health outcome may develop after a substantial delay, as has been suggested for early-life exposures to certain pesticides that may trigger subsequent development of degenerative nervous system disease at a younger age than anticipated.35 Similar observations refer to cancer development. For example, rats treated with aspartame at low doses from pre-natal life have been reported to develop higher incidence of malignant tumours compared with rats first treated at maturity. In agreement with the low-dose hypothesis of carcinogenesis, rats exposed to 50 Hz magnetic fields during pre-natal have been shown to later exhibit enhanced carcinogenic-induced responses to formaldehyde and gamma radiation.37,38 These experimental examples demonstrate how early-life exposures may potentially impact disease risks in later life, sometimes after a substantial latency period. Parallel studies in human beings are developing at a much slower rate that emphasizes the importance of the timescale.
3 |. MECHANISTIC INSIGHTS
Early-life cues can induce metabolic and other phenotypic modifications of the offspring that aim to shape the progeny according to the anticipated environment.9 This developmental plasticity may, however, be maladaptive and lead to the development of an inappropriate phenotype with increased susceptibility to disease.39 The placenta, a largely understudied organ, may mediate some of these phenotype modifications.40 Thus, impaired placental function can disrupt foetal growth, which in turn may affect, for example, neurodevelopment in animals and neurobehavioral development in children.41 Thus, in a study that included several birth cohorts, exposure to air pollution was associated with changes in placental mitochondrial DNA (mtDNA) content that mediated the association between pre-natal air pollution exposure and foetal growth.42 Toxicant effects on the placenta can also result in sex-dimorphic functional changes in the offspring.43
Epigenetics is a potential mechanism through which the environment can influence development of the organism. Thus, patterns of epigenetic markers, such as DNA methylation, histone modification and non-coding RNAs, which ultimately regulate chromatin structure or gene activity, can be influenced by a variety of exposures including environmental toxicants.44 Epigenetic marks, by design, undergo profound changes during development with the establishment of the different cell lineages, and this phenomenon may account for the high vulnerability to insults in this developmental period.
There is increasing evidence that environmental stressors as well as other chemicals can modify epigenetic patterns.13 Among examples mentioned in the introduction, there is now strong evidence for the involvement of epigenetic regulation in the long-term toxicity of lead45 as well as in the toxicity related to the foetal alcohol syndrome.46 Epigenetic markers can be the targets of individual stressors or reflect interactions between multiple stressors.
Telomere length is considered a biomarker of biological ageing and has been associated with age-related diseases and premature mortality.47 While telomere lengths in newborns are highly variable, mothers exposed to higher levels of air pollution within EU limits gave birth to newborns with shorter telomere length.48 The implications of changes in telomere lengths for long-term health deserve further scrutiny.
Growing evidence suggests that effects of environmental contaminants are not limited to the exposed individual but may persist in unexposed descendants.49 These effects are possible only if exposure-induced changes are transmitted through the germline, making it essential to understand how and when exposures impact the developing germline in males and females. By now, considerable documentation is available for such transgenerational effects in mammals (ie, effects seen in subsequent generations that had no direct exposure).50 Experimental studies in non-mammalian systems are providing crucial mechanistic insight.51 Thus, a recent study, presented at PPTOX VI, showed that transgenerational inheritance of obesity was observed from peri-natal tributyltin exposure, apparently mediated through altered higher-order chromatin organization.52 Reorganized chromatin, transmissible through meiosis and mitosis, can in turn influence DNA methylation at accessible sites that modified transcription, suggesting that this is a proximal event. These new mechanistic insights indicate that a revision of research paradigms and interpretation is required.
In order to rationally implicate particular mechanisms in developmental toxicity, it is critical to satisfy appropriate causal criteria. These would include a detailed characterization of the stressors, the determination of the window of exposure with highest vulnerability, the determination of the target tissue and the function that is altered in that tissue, the specificity of the changes and the biological plausibility of the linkage between those changes and health outcome.49 While this research is crucial, the need to avoid substantial delays in prudent interventions suggests that appropriate changes in prevention policies should be considered already when existence of developmental toxicity has been demonstrated.
4 |. THE ROLE OF RESERVE CAPACITY
Because early-life toxicity may prevent optimal organ development and thereby result in a latent deficiency, deficits or disease progression may later be unmasked by ageing or by toxicant exposure later in life.53 A structural reserve must exist for pulmonary, renal and cognitive function, as well as for bone mass. Initial exposures, whether from tobacco smoking or cadmium contamination, may pave the way for subsequent exposures to cause disease in vulnerable organs with diminished remaining capacity.54 On the other hand, an optimal reserve capacity may counteract age-related cell loss and acute disease sequelae, as is known from studies of pancreatic beta cells,55 dopaminergic cells of the brain56 and the cells that determine the so-called renal functional reserve.57 This reserve capacity may, in part, be determined by the extent of the stem cells remaining in the target organs.58
As a consequence, early-life effects on organ development may, in combination with the impact of subsequent exposures to toxicants and normal ageing, result in an increased risk of later life disease. Thus, these exposures that occur later in life may also unmask a decreased reserve capacity of vulnerable organ systems. Such findings have led to the two-hit hypothesis, that is, that pre-natal insults increase the vulnerability to impacts of later life exposures.53
Accordingly, early-life toxicity that generates or leads to later life organ dysfunction, disease or vulnerability to other insults, may be subtle and hard to detect in the individual seemingly healthy child.53 These effects may appear to be silent, as they may barely affect standardized, routine clinical health measures, and changes in function may be too subtle or may change too slowly to be detected in prospective studies. Nonetheless, we are facing massive pandemics of non-communicable diseases and many other signs of ill health likely due in part to preventable developmental insults that may affect functional reserve capacities. Accordingly, research in this field is crucial, though fraught with difficulties. Due to the implications of reduced reserve capacity, any changes that, at the beginning, remain within the reference range should not necessarily be considered innocuous.
5 |. RESEARCH APPROACHES
Prospective studies in human beings, best when relying on birth cohorts, are crucial for understanding the impact of developmental stressors. There are limits to the duration of most research projects, however, given the time span of most research grants, perhaps also exacerbated by the ageing of the researchers themselves. Accordingly, only in the very long term will such epidemiology research be able to document the full lifespan impacts of developmental exposure stressors.
Most studies in this field are observational, because experimental studies are rarely possible. However, dietary or other interventions to reduce exposures may be possible, thereby in part compensating for the lack of an unexposed control population. For example, changing from conventional to organic foods will substantially decrease pesticide exposures,59 and such studies have found a reduction in the risk of eczema at two years of age60 and pre-eclampsia61 in mother-child cohorts. Similar interventions are possible regarding marine food intake by avoiding high-mercury species62 and for plastics additives by avoiding certain food contact materials.63,64 This research approach needs to be promoted in the future.
Cross-sectional epidemiological studies are faster and cheaper to conduct but more limited in terms of causal inference. Residual confounding and other uncertainties are frequently raised as major concerns, but available evidence15 suggests that their impact is often exaggerated, and reverse confounding often is neglected.65 Reaching formal statistical significance usually requires large study groups and wide ranges of exposures. Accurate and valid estimates of exposures may be difficult to obtain,66,67 especially if they are meant to reflect the exposure during a vulnerable life stage. Also, many birth cohorts are relatively small and concentrate on only a few outcome measures or chemicals. The possibility of joining studies of thousands of mother-child pairs with potential for the same types of exposure and outcome assessments would be crucial for expanding our knowledge base on developmental toxicity. In a more general sense, cord blood should be routinely collected and stored for future research purposes whenever possible.18
Attention to newer test approaches to examine chemicals for developmental toxicity is needed. Although the standard two-generation test sometimes is used, developmental neurotoxicity and endocrine disruption may be easily missed. Development in methods that can identify epigenetic mechanism, molecular “omics” technologies and computational chemistry approaches should be exploited and will necessitate the development of and acceptance by regulatory entities of criteria for such additional regulatory tests that can reliably reveal or predict relevant mechanisms of toxicity.
Prominent environmental toxicants have attracted the most attention, and during the first decade of this millennium, the top ten substances covered in public health journals were all metals.68 An important reason for such inertia—both in science and in science-informed decision-making—relates to the tradition of requiring replication and verification as justification for reliable conclusions. Thus, funding agencies69 and journals70 have announced their intention of increasing reproducibility of research. Although the credibility and accuracy of research must be emphasized,71 emphasis on replication can also have untoward effects, as certain environmental chemicals already trigger over 1000 publications per year,68 while emerging chemicals have attracted much less attention. Accordingly, despite the increasing number of publications in the field (Figure 1) and the growing insight into the impact of developmental exposures to toxicants on subsequent health, the trend of innovation may be difficult to maintain, unless a sustained balance is achieved between innovation and replication. These findings suggest that choices of research plans and research topics need to be less conservative, more explorative and less risk averse, as also recommended in a recent report from the National Research Council.72 The field of developmental toxicology would clearly benefit from more visionary and innovative science.
The Consortium Linking Academic and Regulatory Insights on Bisphenol A Toxicity (CLARITY-BPA) model, highlighted separately in this issue,73 illustrates a new approach to address uncertainties in experimental research. To resolve current controversies on BPA safety, U.S. federal agencies established the project in which all NCTR Sprague-Dawley rats were centrally treated by gavage with BPA over a 10 000-fold dose range during the developmental period or throughout life. The Food and Drug Administration performed standard toxicology analysis and also distributed the treated target organs/cells in a coded manner to 14 academic laboratories for detailed analysis. The core studies have been completed by now and are available in a draft report.74 This co-ordinated effort has identified the disruption of several outcome across different organ systems, with a majority of positive findings identified in the low-dose range (<250 μg/kg-BW/day). Responses were frequently non-monotonic, as may be expected for an endocrine disruptor. The CLARITY-BPA co-ordinated approach between independent laboratories and regulatory agencies provides a useful model for future toxicology studies.
6 |. RESEARCH TRANSLATION
The present report is by itself a product of research translation, that is, a document that lays out the societal implications of the research documentation. Developmental toxicity information must also target prospective parents. Although policymakers may take decades to enact legislation to reduce toxic pollutants in the environment, men and women planning pregnancies often ask their physicians whether there are any precautions they should consider today. The history of such well-established hazards as pre-natal exposure to lead, mercury and second-hand smoke shows many years of epidemiological and laboratory research before the weight of the evidence compels a consensus. While the evidence is accumulating, what should a prospective parent do? Prudently avoid exposure after the first published study suggesting problems? At what point should the physician advocate a specific action? There are no easy answers to these questions. Issues of value, scientific understanding and cost are involved. Each hazardous exposure must be considered in the context of other problems facing the prospective parents and the financial, emotional and intellectual resources available to surmount them. An environmental exposure history, taken at a pre-conception visit, can help to identify exposures of potential concern. Although professional societies75,76 recommend taking an environmental history, it is rarely undertaken.77 Nonetheless, reproductive health care providers and paediatricians can play an essential role in helping parents to understand the importance of avoiding pre-conception and pre-natal exposures to toxic chemicals, whether from parental work, environmental pollution or otherwise.78–80
7 |. PUBLIC HEALTH CONSEQUENCES
Public health intervention first focused on environmental hazards such as lead as acute exposures. The timescale has changed, so that now the main focus is on the toxic imprinting during early development that may be latent and which only reveals itself later in life. The science needed to identify and document critical environmental hazards has its own pace for each field, and the research on lead, mercury and ethanol shows that it may take decades to generate evidence that was considered sufficient to justify action. Thus far, a single experimental study has explored the consequences of successive generations of exposure showing and that effects of the exposure may intensify with repeated generations of exposure.81 Because these findings have potentially severe implications that may affect future generations, procedures must be generated to allow appropriate responses regarding the consequences for research planning and for prudent decisions on related policy decisions.
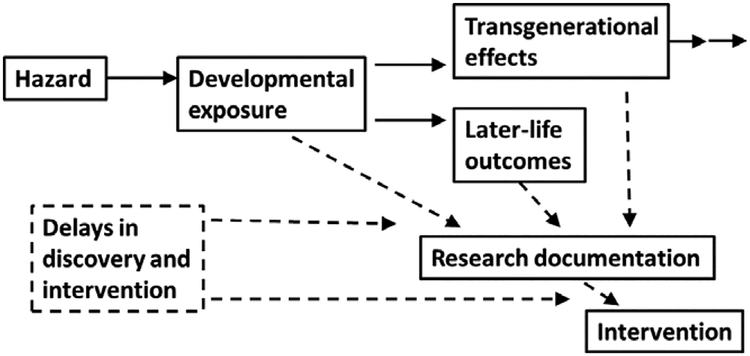
A major obstacle in translating research evidence was addressed several years ago by a U.S. National Research Council committee, which highlighted the erroneous inference that chemicals can be considered inert or safe, unless proven otherwise.82 Thus, inconclusive studies have sometimes been labelled as “negative” or were thought to represent “no risk” rather than “uncertain information”.83 Accordingly, translation into public policy can be delayed, even by decades, as illustrated in Figure 2.
FIGURE 2.
The timescale for developmental toxicity involves delayed lifespan consequences and transgenerational transfer of dysfunctions and disease risks. In addition, delays occur in research documentation and subsequently in decision-making, both of which will have long-term consequences that will call for a precautionary approach to prudent intervention
One defensive strategy, initially used by the tobacco industry, is to argue, “Doubt is our product” to compete with the “body of scientific facts” developed by more impartial researchers and to create “a controversy” about the scientific issues.84 This strategy has been widely adopted by other industries whose products are threatened by legal action to protect the health of children and others.85 Often strong opposition has succeeded, challenging the science, undermining political will by legislators and public health personnel and possibly confusing the wider public.
Another public relations strategy is to argue that before legal action can occur, there must be “ideal evidence,” including human studies with large samples, corroborated by animal data at exposures to which children and others are exposed, and further supported by mechanistic data that explain the biological connection between exposures and diseases.86 Although appropriate for some purposes, this is quite excessive for protecting the public, but can appear persuasive. If such broad and diverse data were required before any public health protections could be initiated, few would be implemented because each would need to be developed appropriate to the highest internal quality standards of each field, which would take considerable time and money, further failing to protect the public. Both of these strategies have created a need to verify, replicate and confirm toxicity documentation, thus generating substantial inertia also in research, with a preference for repeated studies, rather than exploration of new aspects of toxicity.
Developmental vulnerability should give social and legal institutions greater urgency to protect this susceptible subpopulation due to the long-term consequences, but social and legal responses have been quite sluggish. For fifty years, powerful economic interests resisted restrictions to the use of lead additives in gasoline, insisting that documentation did not exist that lead pollution was dangerous.87 For other toxicants, research results obtained by the private sector were not shared with the public, thereby resulting in necessary risk assessment being delayed by decades,88 not because of lack of effort by public health agencies, but because the opponents of regulation have found ways to slow the process.89
The legal environment in which potentially toxic substances are assessed is another concern. The REACH legislation in the EU requires some toxicological information before chemicals and products can be marketed, while chemicals in the United States are mostly subject to post-market evaluation of any risks, unless there is evidence that a substance poses an unreasonable threat to health or the environment. Consequently, the overwhelming number of substances in commerce has not been tested for their toxicity to human beings, not to speak about developmental toxicity. Even when tests have been carried out, this is not a safeguard that the product is safe. Epigenetic mechanisms necessitate a set of additional regulatory tests to reveal epigenetic mechanisms of toxicity. An improved battery of tests should also include testing for such effects as developmental neurotoxicity and endocrine disruption.
8 |. A NEW STRATEGY
As already emphasized by the Faroes statement18 and subsequent recommendations,13,90,91 a greater attention to research is warranted regarding pre-natal and early post-natal exposures to environmental hazards and their consequences. Although new findings of adverse effects of developmental origin would need to be replicated and confirmed, the unique timescale issue regarding long-term implications demands that a desire for more detailed documentation should not inappropriately delay prudent action that can benefit the health of the youngest generation, or future generations. In this field, and given the current status of the documentation, the emphasis needs to be on translation and intervention, not just on research expansion.
This fundamental approach contrasts with a previous interpretation of developmental toxicity that was summarized about 25 years ago as follows: “Differences in sensitivity between children and adults are chemical-specific and must be studied and evaluated on a case-by-case basis”.92 However, the National Research Council (NRC) report on pesticide residues in the food of infants and children93 challenged that view and recommended the use of a 10-fold safety factor to protect the health of this vulnerable subpopulation. Still, this precautionary approach has not been generally applied and indeed not internationally. Current evidence suggests that the NRC conclusion needs to be revived and extended. Thus, in the absence of convincing evidence one way or another, it is vital to address the supplementary question whether it is responsible to expose the next generation to potential toxicants, in particular when effects can be irreversible and transferred to future generations.
An improved public health strategy should take into consideration that the present epidemiological dimension of chronic diseases in older adults regards people born in the 1970s or before, when the toxic developmental exposures were different and less complicated than today. Thus, current epidemiological evidence on early-life impacts on degenerative disease and cancer later on most likely underestimates the real impact of developmental stressors.94,95 Multi- and transgenerational effects make it clear that the consequences of exposure cannot be understood by merely assessing exposure and outcome in the exposed individual. Thus, as part of the timescale concern, when new and more convincing research emerges over the coming years or decades, developmental toxicity will have adversely affected many more children and perhaps even additional generations. Given the uncertainties in research, the delays in decision-making, and the timescale for lifespan consequences of developmental stressor exposures, we recommend that this field of study and its public health implications be given high priority, as follows:
Research strategies and support should include developmental toxicity as a priority field, especially regarding the long-term consequences for human health. Follow-up of child or birth cohorts is crucial. Collaboration between birth cohorts, as done by the Environment and Child Health International Birth Cohort Group82 and in the Environmental influences on Child Health Outcomes (ECHO) Program of the U.S. National Institutes of Health, should be extended. Meta-analyses should be facilitated and supported.
Support for generating a clearing house for developmental toxicity research is needed, as has already been proposed for developmental neurotoxicity.25 This function would entail systematic compilation of evidence, as successfully done at the International Agency for Research on Cancer, with evaluation of the weight of the evidence to derive science-based conclusions that can facilitate translation into policy development to control preventable environmental hazards that cause developmental toxicity.
The United Nations Sustainable Development Goals have important implications for human health and environmental protection. The goals require better research support, as outlined in a recent report.96 However, the UN goals do not specifically mention adverse impacts on future generations, and neither does the research report specify the need for research on developmental toxicity. The present recommendations serve to support the research needed for fulfilment of the UN sustainable development goals.
9 |. CONCLUSIONS
Progress in understanding developmental vulnerability to environmental hazards has resulted in a widening perspective, in which developmental toxicity now involves latent effects that can result in delayed adverse effects in adults or the elderly and effects that can be transferred to future generations. Although epidemiology to an increasing degree is documenting the harmful effects of developmental exposures in human beings, the improved evidence and the widened perspective have resulted in little progress in protection, and few environmental chemicals are currently explicitly regulated to protect against developmental toxicity, whether neurotoxicity, endocrine disruption or other adverse outcomes. The desire to obtain a high degree of certainty and verification of the evidence must be weighed against the costs of research and the time delay, as demonstrated by the expensive and multiyear CLARITY-BPA project, and against the costs to human health due to delayed protection of vulnerable early-life stages of human development and, possibly, future generations.
ACKNOWLEDGEMENTS
The PPTOX VI conference was held in Torshavn, Faroe Islands, in May 2018, and was supported by Basic & Clinical Pharmacology & Toxicology and by a grant from the National Institute of Environmental Health Sciences (NIEHS, R13ES029385). PG is supported by the STEEP Superfund Center (P42ES027706) also from NIEHS.
Funding information
STEEP Superfund Center, Grant/Award Number: P42ES027706; National Institute of Environmental Health Sciences, Grant/Award Number: R13ES029385
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interests.
REFERENCES
- 1.Byers R, Lord E. Late effects of lead poisoning on mental development. Am J Dis Child. 1943;66:471–494. [Google Scholar]
- 2.Beckley AL, Caspi A, Broadbent J, et al. Association of childhood blood lead levels with criminal offending. JAMA Pediatr. 2018;172:166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beckwith TJ, Dietrich KN, Wright JP, Altaye M, Cecil KM. Reduced regional volumes associated with total psychopathy scores in an adult population with childhood lead exposure. Neurotoxicology. 2018;67:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Toxicology Program. NTP monograph on health effects of low-level lead. NTP Monogr. 2012;xiii:xv–148. [PubMed] [Google Scholar]
- 5.Harada M Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995;25:1–24. [DOI] [PubMed] [Google Scholar]
- 6.Yorifuji T, Takaoka S, Grandjean P. Accelerated functional losses in ageing congenital Minamata disease patients. Neurotoxicol Teratol. 2018;69:49–53. [DOI] [PubMed] [Google Scholar]
- 7.Koren G, Navioz Y. Historical perspective: the original description of fetal alcohol spectrum disorder in France, 1967. Ther Drug Monit. 2003;25:131. [DOI] [PubMed] [Google Scholar]
- 8.Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O’Malley K, Young JK. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. J Dev Behav Pediatr. 2004;25:228–238. [DOI] [PubMed] [Google Scholar]
- 9.Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990;301:1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–1081. [DOI] [PubMed] [Google Scholar]
- 11.Landrigan PJ, Goldman LR. Children’s vulnerability to toxic chemicals: a challenge and opportunity to strengthen health and environmental policy. Health Aff. 2011;30:842–850. [DOI] [PubMed] [Google Scholar]
- 12.Goodson WH 3rd, Lowe L, Carpenter DO, et al. Assessing the carcinogenic potential of low-dose exposures to chemical mixtures in the environment: the challenge ahead. Carcinogenesis. 2015;36(Suppl 1):S254–S296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barouki R, Gluckman PD, Grandjean P, Hanson M, Heindel JJ. Developmental origins of non-communicable disease: implications for research and public health. Environ Health. 2012;11:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanson MA, Gluckman PD. Developmental origins of health and disease: new insights. Basic Clin Pharmacol Toxicol. 2008;102:90–93. [DOI] [PubMed] [Google Scholar]
- 15.Grandjean P Science for precautionary decision-making In: Gee D, Grandjean P, Hansen SF, van den Hove S, MacGarvin M, Martin J, Nielsen G, Quist D, Stanners D, eds. Late Lessons from Early Warnings. Copenhagen: European Environment Agency; 2013:517–535. [Google Scholar]
- 16.OECD. Developmental Neurotoxicity Study. OECD Guidelines for the Testing of Chemicals, Section 4: Health Effects Test No. 426. Paris: OECD; 2007. [Google Scholar]
- 17.Scientific Committee on Occupational Exposure Limits (SCOEL). Methodology for the Derivation of Occupational Exposure Limits. Luxembourg: European Commission; 2013. [Google Scholar]
- 18.Grandjean P, Bellinger D, Bergman A, et al. The Faroes statement: human health effects of developmental exposure to chemicals in our environment. Basic Clin Pharmacol Toxicol. 2008;102:73–75. [DOI] [PubMed] [Google Scholar]
- 19.Di Renzo GC, Conry JA, Blake J, et al. International federation of gynecology and obstetrics opinion on reproductive health impacts of exposure to toxic environmental chemicals. Int J Gynaecol Obstet. 2015;131:219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heindel JJ. The developmental basis of disease: update on environmental exposures and animal models Basic Clin Pharmacol Toxicol. 2018. 10.1111/bcpt.13118 [DOI] [PubMed] [Google Scholar]
- 21.Li X, Huang S, Jiao A, et al. Association between ambient fine particulate matter and preterm birth or term low birth weight: An updated systematic review and meta-analysis. Environ Pollut. 2017;227:596–605. [DOI] [PubMed] [Google Scholar]
- 22.Casas M, Nieuwenhuijsen M, Martinez D, et al. Prenatal exposure to PCB-153, p, p’-DDE and birth outcomes in 9000 mother-child pairs: exposure-response relationship and effect modifiers. Environ Int. 2015;74:23–31. [DOI] [PubMed] [Google Scholar]
- 23.Bach CC, Bech BH, Brix N, Nohr EA, Bonde JP, Henriksen TB. Perfluoroalkyl and polyfluoroalkyl substances and human fetal growth: a systematic review. Crit Rev Toxicol. 2015;45:53–67. [DOI] [PubMed] [Google Scholar]
- 24.Illa M, Eixarch E, Muñoz-Moreno E, et al. Neurodevelopmental effects of undernutrition and placental underperfusion in fetal growth restriction rabbit models. Fetal Diagn Ther. 2017;42:189–197. [DOI] [PubMed] [Google Scholar]
- 25.Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014;13:330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grandjean P Only One Chance How Environmental Pollution Impairs Brain Development – and How to Protect the Brains of the Next Generation. New York: Oxford University Press; 2013. [Google Scholar]
- 27.Peterson BS, Rauh VA, Bansal R, et al. Effects of prenatal exposure to air pollutants (polycyclic aromatic hydrocarbons) on the development of brain white matter, cognition, and behavior in later childhood. JAMA Psychiatr. 2015;72:531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guxens M, Lubczyńska MJ, Muetzel RL, et al. Air pollution exposure during fetal life, brain morphology, and cognitive function in school-age children. Biol Psychiatry. 2018;S0006–3223: 30064–30067. [DOI] [PubMed] [Google Scholar]
- 29.Sunyer J, Esnaola M, Alvarez-Pedrerol M, et al. Association between traffic-related air pollution in schools and cognitive development in primary school children: a prospective cohort study. PLoS Med. 2015;12:e1001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breton CV, Mack WJ, Yao J, et al. Prenatal air pollution exposure and early cardiovascular phenotypes in young adults. PLoS ONE. 2016;11:e0150825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gauderman WJ, Urman R, Avol E, et al. Association of improved air quality with lung development in children. N Engl J Med. 2015;372:905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Juul A, Almstrup K, Andersson AM, et al. Possible fetal determinants of male infertility. Nat Rev Endocrinol. 2014;10:553–562. [DOI] [PubMed] [Google Scholar]
- 33.DeWitt JC, Peden-Adams MM, Keil DE, Dietert RR. Current status of developmental immunotoxicity: early-life patterns and testing. Toxicol Pathol. 2012;40:230–236. [DOI] [PubMed] [Google Scholar]
- 34.Mantovani A, Fucic A. Puberty dysregulation and increased risk of disease in adult life: possible modes of action. Reprod Toxicol. 2014;44:15–22. [DOI] [PubMed] [Google Scholar]
- 35.Yan D, Zhang Y, Liu L, Yan H. Pesticide exposure and risk of Alzheimer’s disease: a systematic review and meta-analysis. Sci Rep. 2016;6:32222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soffritti M, Belpoggi F, Tibaldi E. Degli Esposti D, Lauriola M. Life-span exposure to low doses of aspartame beginning during prenatal life increases cancer effects in rats. Environ Health Perspect. 2007;115:1293–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soffritti M, Tibaldi E, Padovani M, et al. Synergism between sinusoidal-50 Hz magnetic field and formaldehyde in triggering carcinogenic effects in male Sprague-Dawley rats. Am J Ind Med. 2016;59:509–521. [DOI] [PubMed] [Google Scholar]
- 38.Soffritti M, Tibaldi E, Padovani M, et al. Life-span exposure to sinusoidal-50 Hz magnetic field and acute low-dose gamma radiation induce carcinogenic effects in Sprague-Dawley rats. Int J Radiat Biol. 2016;92:202–214. [DOI] [PubMed] [Google Scholar]
- 39.Gluckman PD, Hanson MA, Beedle AS. Early life events and their consequences for later disease: a life history and evolutionary perspective. Am J Hum Biol. 2007;19:1–19. [DOI] [PubMed] [Google Scholar]
- 40.Bronson SL, Bale TL. The placenta as a mediator of stress effects on neurodevelopmental reprogramming. Neuropsychopharmacology. 2016;41:207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Figueras F, Cruz-Martinez R, Sanz-Cortes M, et al. Neurobehavioral outcomes in preterm, growth-restricted infants with and without prenatal advanced signs of brain-sparing. Ultrasound Obstet Gynecol. 2011;38:288–294. [DOI] [PubMed] [Google Scholar]
- 42.Clemente DB, Casas M, Vilahur N, et al. Prenatal ambient air pollution, placental mitochondrial DNA content, and birth weight in the INMA (Spain) and ENVIRONAGE (Belgium) birth cohorts. Environ Health Perspect. 2016;124:659–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adibi JJ, Buckley JP, Lee MK, et al. Maternal urinary phthalates and sex-specific placental mRNA levels in an urban birth cohort. Environ Health. 2017;16:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Faulk C, Dolinoy DC. Timing is everything: the when and how of environmentally induced changes in the epigenome of animals. Epigenetics. 2011;6:791–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Faulk C, Liu K, Barks A, Goodrich JM, Dolinoy DC. Longitudinal epigenetic drift in mice perinatally exposed to lead. Epigenetics. 2014;9:934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ehrhart F, Roozen S, Verbeek J, et al. Review and gap analysis: molecular pathways leading to fetal alcohol spectrum disorders Mol Psychiatry. 2018. 10.1038/s41380-018-0095-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Meyer T, Nawrot T, Bekaert S, De Buyzere ML, Rietzschel ER, Andres V. Telomere length as cardiovascular aging biomarker: JACC review topic of the week. J Am Coll Cardiol. 2018;72:805–813. [DOI] [PubMed] [Google Scholar]
- 48.Martens DS, Cox B, Janssen BG, et al. Prenatal air pollution and newborns’ predisposition to accelerated biological aging. JAMA Pediatr. 2017;171:1160–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barouki R, Melen E, Herceg Z, et al. Epigenetics as a mechanism linking developmental exposures to long-term toxicity. Environ Int. 2018;114:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Skinner MK. Endocrine disruptors in 2015: Epigenetic transgenerational inheritance. Nat Rev Endocrinol. 2016;12:68–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klosin A, Casas E, Hidalgo-Carcedo C, Vavouri T, Lehner B. Transgenerational transmission of environmental information in C. elegans. Science. 2017;356:320–323. [DOI] [PubMed] [Google Scholar]
- 52.Chamorro-Garcia R, Diaz-Castillo C, Shoucri BM, et al. Ancestral perinatal obesogen exposure results in a transgenerational thrifty phenotype in mice. Nat Commun. 2017;8:2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kraft AD, Aschner M, Cory-Slechta DA, Bilbo SD, Caudle WM, Makris SL. Unmasking silent neurotoxicity following developmental exposure to environmental toxicants. Neurotoxicol Teratol. 2016;55:38–44. [DOI] [PubMed] [Google Scholar]
- 54.Ben-Shlomo Y, Cooper R, Kuh D. The last two decades of life course epidemiology, and its relevance for research on ageing. Int J Epidemiol. 2016;45:973–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cersosimo E, Solis-Herrera C, Trautmann ME, Malloy J, Triplitt CL. Assessment of pancreatic beta-cell function: review of methods and clinical applications. Curr Diabetes Rev. 2014;10:2–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Booij J, Bergmans P, Winogrodzka A, Speelman JD, Wolters EC. Imaging of dopamine transporters with [123I]FP-CIT SPECT does not suggest a significant effect of age on the symptomatic threshold of disease in Parkinson’s disease. Synapse. 2001;39:101–108. [DOI] [PubMed] [Google Scholar]
- 57.Palsson R, Waikar SS. Renal functional reserve revisited. Adv Chronic Kidney Dis. 2018;25:e1–e8. [DOI] [PubMed] [Google Scholar]
- 58.Prins GS, Calderon-Gierszal EL, Hu WY. Stem cells as hormone targets that lead to increased cancer susceptibility. Endocrinology. 2015;156:3451–3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu C, Barr DB, Pearson MA, Waller LA. Dietary intake and its contribution to longitudinal organophosphorus pesticide exposure in urban/suburban children. Environ Health Perspect. 2008;116:537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thijs C, Muller A, Rist L, et al. Fatty acids in breast milk and development of atopic eczema and allergic sensitisation in infancy. Allergy. 2011;66:58–67. [DOI] [PubMed] [Google Scholar]
- 61.Torjusen H, Brantsaeter AL, Haugen M, et al. Reduced risk of pre-eclampsia with organic vegetable consumption: results from the prospective Norwegian Mother and Child Cohort Study. BMJ Open. 2014;4:e006143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weihe P, Grandjean P, Jorgensen PJ. Application of hair-mercury analysis to determine the impact of a seafood advisory. Environ Res. 2005;97:200–207. [DOI] [PubMed] [Google Scholar]
- 63.Ackerman JM, Dodson RE, Engel CL, Gray JM, Rudel RA. Temporal variability of urinary di(2-ethylhexyl) phthalate metabolites during a dietary intervention study. J Expo Sci Environ Epidemiol. 2014;24:595–601. [DOI] [PubMed] [Google Scholar]
- 64.Carwile JL, Luu HT, Bassett LS, et al. Polycarbonate bottle use and urinary bisphenol A concentrations. Environ Health Perspect. 2009;117:1368–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Choi AL, Cordier S, Weihe P, Grandjean P. Negative confounding in the evaluation of toxicity: the case of methylmercury in fish and seafood. Crit Rev Toxicol. 2008;38:877–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bach CC, Henriksen TB, Bossi R, et al. Perfluoroalkyl acid concentrations in blood samples subjected to transportation and processing delay. PLoS ONE. 2015;10:e0137768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grandjean P, Budtz-Jorgensen E. An ignored risk factor in toxicology: The total imprecision of exposure assessment. Pure Appl Chem. 2010;82:383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grandjean P, Eriksen ML, Ellegaard O, Wallin JA. The Matthew effect in environmental science publication: a bibliometric analysis of chemical substances in journal articles. Environ Health. 2011;10:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Collins FS, Tabak LA. Policy: NIH plans to enhance reproducibility. Nature. 2014;505:612–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anon. Journals unite for reproducibility. Nature. 2014;515:7. [DOI] [PubMed] [Google Scholar]
- 71.Ioannidis JPA. All science should inform policy and regulation. PLoS Med. 2018;15:e1002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.National Academies of Sciences Engineering and Medicine (U.S.). Committee on Incorporating 21st Century Science into Risk-Based Evaluations Using 21st Century Science to Improve Risk-Related Evaluations. Washington, DC: National Academies Press; 2017. [PubMed] [Google Scholar]
- 73.Prins GS, Patisaul HB, Belcher SM, Vandenberg LN. CLARITY-BPA Academic laboratory studies identify consistent low-dose bisphenol A effects on multiple organ systems. Basic Clin Pharmacol Toxicol. 2018. [Epub ahead of print]. 10.1111/bcpt13125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.National Toxicology Program. Draft NTP Research Report on the CLARITY-BPA Core Study: A Perinatal and Chronic Extended-Dose-Range Study of Bisphenol A in Rats. Raleigh, NC: National Institute for Environmental Health Sciences; 2018. [PubMed] [Google Scholar]
- 75.American Academy of Pediatrics AAP. Pediatric Environmental Health, 4th edn. Etzel RA, ed. Itasca IL: American Academy of Pediatrics; 2018. [Google Scholar]
- 76.The American College of Obstetricians and Gynecologists Committee ACOG, American Society for Reproductive Medicine Practice ASRMP, The University of California SFPoRHatE, U.C.S.F.P.R.H.E. Exposure to toxic environmental agents; 2013. Available from: https://www.acog.org/-/media/Committee-Opinions/Committee-on-Health-Care-for-Underserved-Women/ExposuretoToxic.pdf. Accessed October 24, 2018. [Google Scholar]
- 77.Grindler NM, Allshouse AA, Jungheim E, Powell TL, Jansson T, Polotsky AJ. OBGYN screening for environmental exposures: A call for action. PLoS ONE. 2018;13:e0195375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sathyanarayana S, Focareta J, Dailey T, Buchanan S. Environmental exposures: how to counsel preconception and prenatal patients in the clinical setting. Am J Obstet Gynecol. 2012;207:463–470. [DOI] [PubMed] [Google Scholar]
- 79.Mori C, Todaka E. For a healthier future: a virtuous cycle for reducing exposure to persistent organic pollutants. J Epidemiol Community Health. 2017;71:660–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grason HA, Misra DP. Reducing exposure to environmental toxicants before birth: moving from risk perception to risk reduction. Public Health Rep. 2009;124:629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Horan TS, Marre A, Hassold T, Lawson C, Hunt PA. Germline and reproductive tract effects intensify in male mice with successive generations of estrogenic exposure. PLoS Genet. 2017;13: e1006885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.National Research Council. Science and Decisions: Advancing Risk Assessment. Washington, DC: National Academy Press; 2009. [PubMed] [Google Scholar]
- 83.Grandjean P Seven deadly sins of environmental epidemiology and the virtues of precaution. Epidemiology. 2008;19:158–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Michaels D Doubt is Their Product: How Industry’s Assault on Science Threatens Your Health. Oxford; New York: Oxford University Press; 2008. [Google Scholar]
- 85.Cranor CF. Tragic Failures: How and Why Are We Are Harmed by Toxic Chemicals. Oxford University Press; 2017. [Google Scholar]
- 86.Furst A Yes, but is it a human carcinogen? J Am Coll Toxicol. 1990;9:1–8. [Google Scholar]
- 87.Needleman HL. The removal of lead from gasoline: historical and personal reflections. Environ Res. 2000;84:20–35. [DOI] [PubMed] [Google Scholar]
- 88.Grandjean P Delayed discovery, dissemination, and decisions on intervention in environmental health: a case study on immunotoxicity of perfluorinated alkylate substances. Environ Health. 2018;17:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Center for Progressive Reform CPR. The costs of regulatory delay: Halting pace of regulation takes lives, wastes billions of dollars. 2013. Available from: http://www.progressivereform.org/regdelay.cfm. Accessed October 24, 2018. [Google Scholar]
- 90.Darney S, Fowler B, Grandjean P, Heindel J, Mattison D, Slikker W Jr. Prenatal programming and toxicity II (PPTOX II): role of environmental stressors in the developmental origins of disease. Reprod Toxicol. 2011;31:271. [DOI] [PubMed] [Google Scholar]
- 91.Grandjean P, Barouki R, Bellinger DC, et al. Life-long implications of developmental exposure to environmental stressors: New perspectives. Endocrinology. 2015;156:3408–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guzelian PS, Henry CJ, Olin SS. Proceedings of the ILSI Conference on Similarities and Differences between Children and Adults: Implications for Risk Assessment. Washington, DC: ILSI Press; 1992. [Google Scholar]
- 93.National Research Council (U.S.). Committee on Pesticides in the Diets of Infants and Children Pesticides in the Diets of Infants and Children. Washington, DC: National Academy Press; 1993. [PubMed] [Google Scholar]
- 94.Slotkin TA, Seidler FJ. Developmental exposure to organophosphates triggers transcriptional changes in genes associated with Parkinson’s disease in vitro and in vivo. Brain Res Bull. 2011;86:340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wilson WW, Shapiro LP, Bradner JM, Caudle WM. Developmental exposure to the organochlorine insecticide endosulfan damages the nigrostriatal dopamine system in male offspring. Neurotoxicology. 2014;44:279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Van den Brink PJ, Boxall ABA, Maltby L, et al. Toward sustainable environmental quality: Priority research questions for Europe. Environ Toxicol Chem. 2018;37:2281–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]