Abstract
Aberrant activation of the Hedgehog (Hh) signaling pathway has been linked to the formation of numerous cancer types, including the myogenic soft tissue sarcoma, embryonal rhabdomyosarcoma (eRMS). Here, we report PCG2, a novel mouse model in which human GLI2A, a constitutive activator of Hh signaling, induced undifferentiated sarcomas that were phenotypically divergent from eRMS. Rather, sarcomas arising in PCG2 mice featured some characteristics that were reminiscent of Ewing sarcoma (ES). Even though it is widely understood that ES formation is driven by EWS-ETS gene fusions, a genetically-defined mouse model is not well-established. While EWS-ETS gene fusions were not present in PCG2 sarcomas, precluding their designation as ES, we did find that GLI2A induced expression of known EWS-ETS gene targets essential to Ewing pathogenesis, most notably, Nkx2.2. Moreover, we found that naive mesenchymal progenitors originate tumors in PCG2 mice. Altogether, our work provides a novel genetic mouse model, which directly connects oncogenic Hh activity to the etiology of undifferentiated soft tissue sarcomas for the first time.
Keywords: Hedgehog, GLI2A, soft tissue sarcoma, mesenchymal progenitor
Introduction
Small round cell sarcomas are a category of phenotypically diverse, often rare, but clinically aggressive cancers. The relative rareness of these soft tissue or bone-localized tumors versus other solid tumor types (lung, GI, skin, etc) and limited access to prospectively-isolated clinical specimens needed for laboratory investigation has caused them to be less-well-studied by comparison (1). This deficit in accessibility may have hindered progress in testing potential diagnostic markers and development of new therapeutic interventions for cancers that can be difficult to manage clinically especially when not accurately diagnosed. Fortunately, for several sarcoma entities genetic mouse models exist as a tool that can mitigate the problem of specimen access. Models for alveolar rhabdomyosarcoma (aRMS), synovial sarcoma, clear cell sarcoma, and numerous embryonal rhabdomyosarcoma (eRMS) models have been well described, and often have succeeded owing to incorporation of a defining genetic lesion into their design.
For example, several fusion-positive sarcoma models have utilized clinically-relevant gene fusions, such as PAX43-FKHR in aRMS, SYT-SSX in synovial sarcoma, and EWS-ATF1 in clear cell sarcoma (2–4). Additionally, an array of models has been created for the fusion-negative sarcoma, eRMS, utilizing numerous genetic mechanisms, but haven often incorporated induction of the developmental signaling pathway (5,6), Hedgehog (Hh), which alone can be sufficient to induce formation of eRMS in mice (7,8). As more data accumulates related to gene fusions identified in other sarcoma variants, such as CIC-DUX4, FUS-NFATc2, and BCOR-CCNB3 for example, it will be important to test their respective activities in mouse model design. Although, one test case for this modeling approach that has consistently underwhelmed, despite novel and clever design approaches from many groups worldwide, is that of Ewing sarcomas (ES) (9), which are clinically defined by the presence of EWS and ETS gene fusions, most notably EWS-FLI1 (10).
In this manuscript we endeavor to describe a novel genetic mouse model called PCG2, in which induction of Hh signaling was achieved using an activated human GLI2 (GLI2A). Much to our surprise, tumors formed by these mice diverged phenotypically from eRMS, and instead displayed pathological, ultrastructural, immuno-phenotypic, and gene expression patterns reported by others in human ES. Although key differences were noted as well, such as the absence of a relevant EWS-ETS gene fusion – the most critical diagnostic criterion for ES. We determined that PCG2 sarcomas emerged from Pcp2-cre-expressing multipotent mesenchymal progenitor cells, which were not transformed by ectopic activation of EWS-FLI1 in separate experiments. We also observed a dramatic sensitivity to Gli1 gene dosage, as partial loss diminished the PCG2 phenotype. Altogether, we found that GLI2A is sufficient to induce undifferentiated soft tissue sarcomas in a new genetically-defined mouse model, adding to the overall understanding of the transformative role for Hh in sarcoma pathogenesis.
Materials and Methods
Animals
All experiments were performed using embryos from timed pregnancies or young neonatal and adult animals (ages P3 to 1.5 years) according to the NIH and VUMC Division of Animal Care. Mice with the following alleles, of either sex, were used for this study and were maintained on a mixed genetic background. Animals were either obtained from Jackson or the originating laboratory. Ai9 [Gt(ROSA)26Sortm9(CAG-tdTomato)Hze], CLEG2, EWSEWS-FLI1, Gli1nlacZ [Gli1tmAlj], Myf5Cre [Myf5tm1(cre)Mrc], Pax7-creER [Pax7tm2.1(cre/ERT2)Fan], Pcp2-cre [Tg(Pcp2-cre)1Amc], PtchlacZ [Ptch1tm1Mps], R26ReYFP [Gt(ROSA)26Sortm1(EYFP)Cos], SmoM2 [Gt(ROSA)26Sortm1(Smo/EYFP)Amc], and SpiB−/−.
Tamoxifen (Sigma) was administered to Pax7-creER; CLEG2 neonates at P3 or P7, and young adults at P21. A stock solution of 2mg/ mL was prepared in corn oil (Sigma), and a dose of 100 μg or 600 μg was administered to neonates or young adults, respectively.
Tumor volume was determined using digital calipers (Electron Microscopy Sciences) to measure the largest and smallest diameter, from the earliest signs of tumor palpability until reaching a largest diameter measuring no larger than 3 cm.
Human Tumor Specimens
De-identified human sarcoma blocks and slides were retrieved from pathology archives at Vanderbilt University Medical Center.
Cell lines
The PCG2M226 tumor cell line was generated in our laboratory in 2011 and authenticated using cell grafting, immunohistochemical and RNAseq-based comparisons with parent tumor tissue. PCG2M226 tumor cells were isolated from freshly resected tumor tissue from PCG2 animals under sterile conditions. Small samples (1–2 mm2) were transferred to 60 mm culture dishes and minced with microdissection scissors. Cells were allowed to adhere to plastic prior to media change (24–48 hours), and were in culture 1.5–2 weeks until large colonies were observed. Colonies were dissociated with 0.05% trypsin, spun down, washed and plated for passaging. Following two passages (7–10 days) near homogeneous cultures were obtained, which could then be passaged every 3 days. Cell lines were maintained in standard culture conditions (37°C, 5%CO2) in DMEM (Gibco™) supplemented with 10% fetal bovine serum and Pen-Strep. PCG2M226 tumor cells have been kept as ‘low-passage’ (<6) or ‘high passage’ (>6).
Grafting experiments
Sarcoma cells (either mouse or human) were injected unilaterally into gastrocnemius muscle of NOD-SCID mice under anesthesia. By pressing the muscle of the lower hind limb together with thumb and index finger below the knee joint, the gastrocnemius muscle becomes readily conspicuous after removal of fur. Approximately 2×10^6 cells re-suspended in HBSS were be injected using 1 ml syringes capped with 30 gauge needles.
Tissue processing, protein isolation, immunohistochemistry, and western blotting
Tissues were dissected and fixed in 4% paraformaldehyde for either 4–6 hours or O/N at 4° C, and were either processed for frozen embedding in OCT compound or processed for paraffin embedding. Frozen tissues were sectioned on a Leica cryostat at 10 um, paraffin embedded tissues were cut at 5 um. Specimens containing portions of bone were decalcified in 0.4M EDTA at 4° C for two weeks prior to dehydration. Protein was isolated from fresh or snap-frozen tumor and tibialis anterior muscle using standard lysis buffer, and a BCA assay (Thermo) was used for measuring concentration. Immunohistochemistry (IHC) and immunocytochemistry (ICC) were performed with standard protocols, 1 mM EDTA pH 9.0 was used for antigen retrieval with tumor sections. Mouse on mouse blocking reagent (VECTOR) was used with mouse primary antibodies.
Antibodies
The following primary antibodies were used to perform IHC on frozen and/ or paraffin tissue sections: mouse α-β-Catenin (Vanderbilt Antibody and Protein Resource), rabbit α-Cd99 (Dr. Dietmar Vestweber, Max Plank), rabbit α-cleaved Caspase3 (CST), mouse α-CyclinD1 (DSHB), rabbit α-Desmin (Thermo), mouse α-EZH2 (CST), rabbit α-Foxd3 (Dr. Patricia Labosky, NIH), chicken α-GFP (Aaves), rabbit α-Gli1 (CST), rabbit α-γH2AX (BETHYL), rabbit α-Keratin (Sigma), rabbit α-Ki67 (NeoMarkers), mouse α-Laminin (Thermo), mouse α-Myc (DSHB), mouse α-Myc Tag 9B11 (CST), mouse α-Myogenin (DSHB), rabbit α-Myogenin (EPITOMICS), mouse α-Myf5 (DSHB), mouse α-MyoD (DAKO), mouse α-Nkx2.2 (DSHB), mouse α-Pax7 (DSHB), mouse α-Pax3 (DSHB), rabbit α-p-histone H3 (Millipore), rabbit α-PPARγ (CST), rabbit α-SpiB (CST), rabbit α-Tnc (Dr. Herald Erickson, DUKE), mouse α-tubulin (DSHB). Species-specific HRP-conjugated secondary antibodies (Invitrogen) were used followed by incubation in DAB reaction (Invitrogen). Double-labeling fluorescence immunohistochemistry was performed using species-specific, AlexaFluor secondary antibodies (Invitrogen) followed by counterstaining with To-pro3 iodide (Invitrogen).
Chromatin Immunoprecipitation (ChIP) and quantitative PCR
ChIP was carried out on PCG2M226 cells of ~3 × 106 cells per epitope. Chromatin from 1% formaldehyde-fixed was fragmented to a range between 200–700 bases using a BioRuptor (Diagenode). Solubilized chromatin (5μg) was immunoprecipitated with the following antibodies; mouse α-Myc Tag 9B11 (Cell Signaling #2267) and rabbit α-H3K4me3 (Cell Signaling #9751). The latter antibody was used for trouble-shooting purposes. Antibody-chromatin complexes were pulled down with either protein G-Dynabeads or protein A-Dynabeads (LifeTech), for mouse and rabbit primaries, respectively, then wash and eluted. Following crosslink reversal with proteinase K treatment overnight at 65°C, the immunoprecipitated DNA was extracted using the PCR purification kit from Qiagen.
Real-time quantitative PCR was performed using specific primers (for Ptch1, Gli1, and Nkx2.2) and SYBR Green PCR Master Mix in a CFX96 Thermocycler (BioRad). % Input was used to calculate enrichment, and species-specific IgG antibodies were used as controls. Primers were designed based on regulatory regions described previously (11), sequences are as follows:
Ptch1 Forward TTCTTTGGAGCTCAATTTCC, Ptch1 Reverse GTTTTCCCCGATTTTAAGGT
Gli1 Forward AAGCCAGATGTGATGTAGCA, Gli1 Reverse TTATGGAAGGACAGACAGCA
Nkx2.2 Forward ATCCAAGCTCTGAGTTGAGG, Nkx2.2 Reverse AAGTGCCAAACAGGTCTCTC
Gene Expression Analysis
a) RNAseq data analysis
We first performed quality control on raw data to identify potential outliers before doing any advanced analysis by applying tool FastQC. RNA data alignment was performed by TopHat, gene quantification was done by Cufflinks. Unsupervised cluster analysis based on Pearson’s correlation coefficient on all genes shows clear separation between muscle and tumor samples. RPKM (reads per kilobase per million reads) based approaches with Cuffdiff were used to detect differential expressed genes. False discovery rate (FDR < 0.05) was used for multiple test correction.
b) Gene Set Enrichment Analysis (GSEA)
Gene Set Enrichment Analysis (GSEA) was performed using software and datasets made available by the Broad Institute (http://software.broadinstitute.org/gsea/index.jsp), as well as several published modules as indicated. A ranked was generated of the PCG2 transcriptome (average of three tumors) for GSEA, which included 20, 461 gene values. Gene sets that were significant at FDR < 25% and significantly enriched at nominal pvalue < 1% or < 5% were considered significantly enriched.
c) Principal Components Analysis (PCA) and Hierarchal Clustering Analysis
RNAseq data for PCG2 and PCG2M226 cells were generated as described at VANTAGE. We obtained normalized RNAseq data for Ewing sarcoma (n=66) from the NCI (12), and data for other human cancers, including synovial sarcoma (n=10), myxofibrosarcoma (n=25), undifferentiated pleomorphic sarcoma (n=21), and diffuse large B cell lymphoma (n=48), were obtained from TCGA. For each data set we removed genes whose median expression is zero and log-transformed the gene expression after filtering. We manually inspected the distribution of the expression data in all data sets and confirmed that all have similar distribution. After centering and quantile normalization of the data we ran principal component analysis and plotted all samples based on their first three principal components.
d) RNA isolation, reverse transcription, and quantitative PCR
Total RNA was isolated from paraffin-imbedded tissue using the RecoverAll™ Total Nucleic Acid Isolation Kit (ambion by life technologies). cDNAs were synthesized with 300 ng total RNA input for all samples using a high-capacity cDNA reverse transcription kit (Applied Biosciences). Real-time quantitative PCR was performed using specific primers for Gli1 and Gapdh and SYBR Green PCR Master Mix in a CFX96 Thermocycler (BioRad), and fold change was calculated using the ΔCt method. Sequences for Gli1 primers are as follows:
Gli1 Forward CCTGAGCCTGAGTCTGTGTATG, Gli1 Reverse CGTGGATATGCTCACTGTTGAT
Microscopy
a) Bright-field and Confocal Imaging
Bright-field images were collected on an Olympus BX51 upright microscope and a Leica M165 FC stereoscope. Fluorescent images were acquired using a Leica TCS SP5 laser-scanning confocal.
b) Transmission Electron Microscopy
Specimens were processed for TEM and imaged in the Vanderbilt Cell Imaging Shared Resource-Research Electron Microscopy facility. TEM was performed on a Philips/FEI T-12.
Embedding:
Samples were fixed in 2.5% gluteraldehyde in 0.1M cacodylate buffer, pH7.4 at room temperature (RT) 1 hour then transferred to 4°C, overnight. The samples were washed in 0.1M cacodylate buffer, then incubated 1 hour in 1% osmium tetraoxide at RT then washed with 0.1M cacodylate buffer. Subsequently, the samples were dehydrated through a graded ethanol series and then 3 exchanges of 100% ethanol, followed by 2 exchanges of pure propylene oxide (PO). Samples were then infiltrated with 25% Epon 812 resin and 75% PO for 30 minutes at RT. Next, they were infiltrated with Epon 812 resin and PO [1:1] for 1 hour at RT then overnight at RT. The samples are subsequently infiltrated with resin for 48 hours then allowed to polymerize at 60°C for 48 hours.
Sectioning and Imaging:
500nm to 1-micron thick sections were collected using a Leica Ultracut microtome. Thick sections were contrast stained with 1% toluidine blue and imaged with a Nikon AZ100 microscope. 70–80nm ultra-thin sections were cut and collected on 300-mesh copper grids and post-stained with 2% uranyl acetate and then with Reynold’s lead citrate. Samples were subsequently imaged on the Philips/FEI Tecnai T12 electron microscope at various magnifications.
Cell Counting
For counting, entire tumor sections were imaged on a Leica SCN400 slide scanner. Cell counts were obtained from all intact tissue for Ki67, cleaved-Caspase3, and phospho-Histone H3, and were quantified as positive cells per mm2. Slides from at least five animals per genotype were used for counting.
Transcript detection
For RNA in situ hybridization, Gli1 cDNA was used as a template for synthesizing digoxygenin-labeled riboprobes.
Spectral Karyotyping (SKY)
Spectral karyotyping (SKY) was performed by Roswell Park Cancer Institute’s SKY laboratory (Buffalo, NY) on metaphase PCG2M226 and PCG2M656 cells. https://www.roswellpark.edu/shared-resources/pathology-resource-network/services-and-fees/sky/fish
RNA isolation and sequencing
Total RNA from PCG2 tumor, tibialis anterior muscle, and PCG2M226 cell lines, was isolated using a two-step protocol in which tissue/ cells were homogenized in Trizol and RNA was collected by phenol-chloroform extraction and column elution with RNeasy Mini kit (Qiagen). RNA was reverse transcribed to create a cDNA library by the VANTAGE core at VUMC prior to paired-end sequencing on Illumina Hiseq 2500.
Statistics
Bar charts and Survival curves were generated using Prism software (GraphPad). Clustering and PCA were performed with R.
Accession Numbers
The data accompanying this manuscript were deposited into the GEO database under accession number GSE89419.
Results
PCG2 mice form undifferentiated soft tissue sarcomas
Initially, we sought to activate Hh signaling in mouse cerebellar Purkinje neurons, which produce, but do not respond to, the ligand, Sonic hedgehog (Shh). We bred Pcp2-cre (13) and CLEG2 transgenic mice (14) to conditionally express a constitutively activated human GLI2 (GLI2A) (Figure 1A, C). After cre-mediated excision of an upstream GFP coding sequence and stop signal, Purkinje neurons expressing GLI2A appeared qualitatively normal (Figure S1A). However, to our surprise, these Pcp2-cre; CLEG2 (PCG2) animals developed soft tissue tumors (Figure 1A) similar to human small round cell sarcomas, displaying cohesive sheets of small round cells with high nuclear to cytoplasmic (n/c) ratio and geographic necrosis (Figures 1A and S1B). 98% of male and female mice evaluated (n>50) formed fast growing, encapsulated tumors by 8 weeks of life on average that targeted multiple anatomical sites (p < 0.0001) (Figure 1D, E). Invasion of tumor cells into adjacent muscle and bone marrow was found in long bones of ipsilateral limbs, but was not apparent in other tissues, likely owing to rapid growth kinetics that necessitated timely sacrifice (Figure S1B, C). Intriguingly, mitotic figures were rare in PCG2 tumors (Figure S1D).
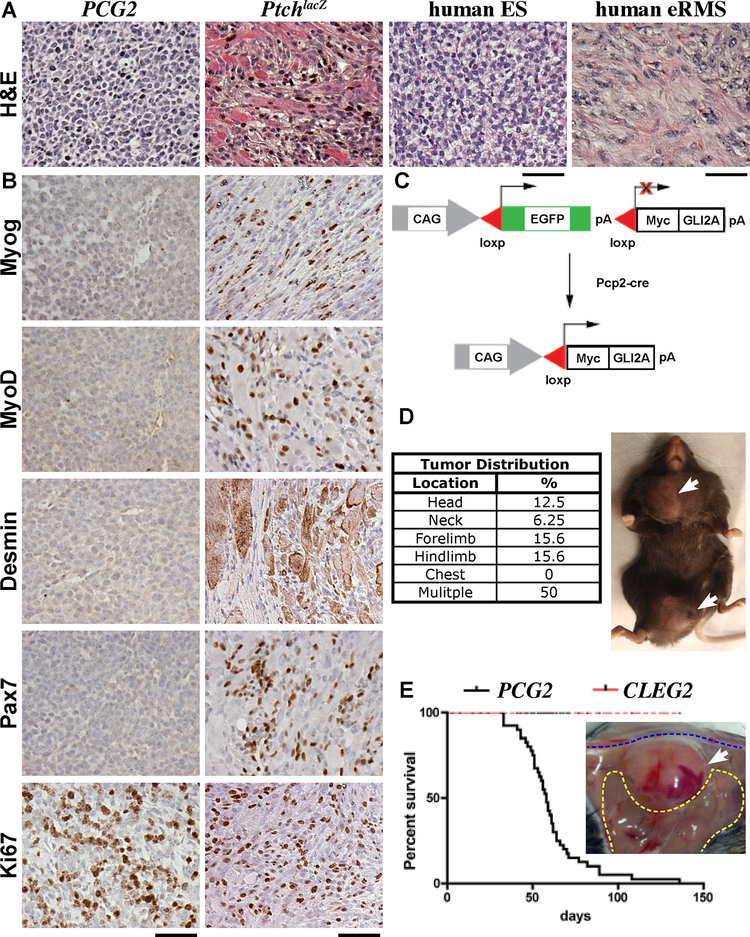
Figure 1. GLI2A induced undifferentiated sarcomas distinct from PtchlacZ eRMS.
(A) H&Es of PCG2 and PtchlaZ mouse tumors, and human ES and eRMS. (B) Immunostaining as indicated, PCG2 tumors lacked myogenic markers Myogenin MyoD, Desmin, and Pax7, which were present in PtchlaZ RMS. Both tumors broadly expressed proliferation marker Ki67. (C) Schematic showing CLEG allele and breeding strategy with Pcp2-cre mice. (D) Frequency of tumor foci at various anatomical sites and external view of tumor masses located on the right hip/ thigh and ipsilateral shoulder/ chest (white arrows). (E) Kaplan-Myer survival plot for PCG2 and CLEG2 mice, tumor margin on right thigh muscle of a separate mouse. Scale bars are 50 μm.
Since oncogenic Hh signaling has previously been linked to the formation of eRMS, we compared PCG2 tumors with those from PtchlacZ mice. Distinctive features seen in PtchlacZ eRMS (i.e., rhabdomyoblasts and entrapped myofibers) were absent from PCG2 tumors (Figure 1A). Comparison of immuno-phenotypes consistently indicated that PCG2 tumors lacked myogenic differentiation, although Myogenin, MyoD, Desmin, and Pax7 were readily detected in PtchlacZ eRMS (Figure 1B). Both tumors displayed a large fraction of cycling cells indicated by Ki67+ status (Figure 1B). We measured the mitotic and apoptotic indices of PCG2 tumors using phospho-histone H3 and cleaved-Caspase3, respectively, and found on average ~479 cells/ mm2 were mitotic and ~337 cells/ mm2 apoptotic.
We evaluated GLI2A expression using the Myc epitope tag encoded by CLEG2, which revealed widespread nuclear staining (S1E). Persistent Hh signaling was evident in PCG2 tumors, indicated by expression of target gene Gli1, which was not detected in adjacent myofibers (Figure S1F). Gli1 and Cyclin D1 protein levels were also high in PCG2 tumor lysates compared to tibialis anterior (TA) muscle, further confirming persistent Hh pathway activity (Figure S1G). We crossed Pcp2-cre and SmoM2 mice, to evaluate whether the upstream acting oncogene could differentially influence tumor phenotype. Unfortunately, these mice succumbed at weaning with severe brain malformations not seen in PCG2 animals (Figure S1H). These findings indicate that GLI2A is sufficient to drive the formation of soft tissue sarcomas distinct from eRMS.
Evaluating the gene expression profile of PCG2 sarcomas
Next, RNA Sequencing (RNAseq) was conducted for comparative gene expression profiling of PCG2 sarcomas. Hierarchal clustering of the top 100 most differentially expressed genes between tumor and unaffected TA muscle revealed distinct gene profiles (Figure 2A and Table S1). As expected, Hh target genes (Gli1, Ptch1, Hhip) were highly upregulated in tumors. Unexpectedly, we also detected induction of the pro-neural transcription factor and known Hh target Nkx2.2 in PCG2 tumors (Figure 2A’). Immunostaining confirmed positivity for Nkx2.2 protein in all tumors analyzed (n > 25), while PtchlacZ eRMS were consistently negative (Figure 2B). Since Nkx2.2 is well documented as an essential target gene of EWS-FLI1 in ES pathogenesis (15,16), but is not frequently seen in other sarcomas (15), we chose to incorporate additional characteristic elements of ES described by other groups elsewhere into our analyses.
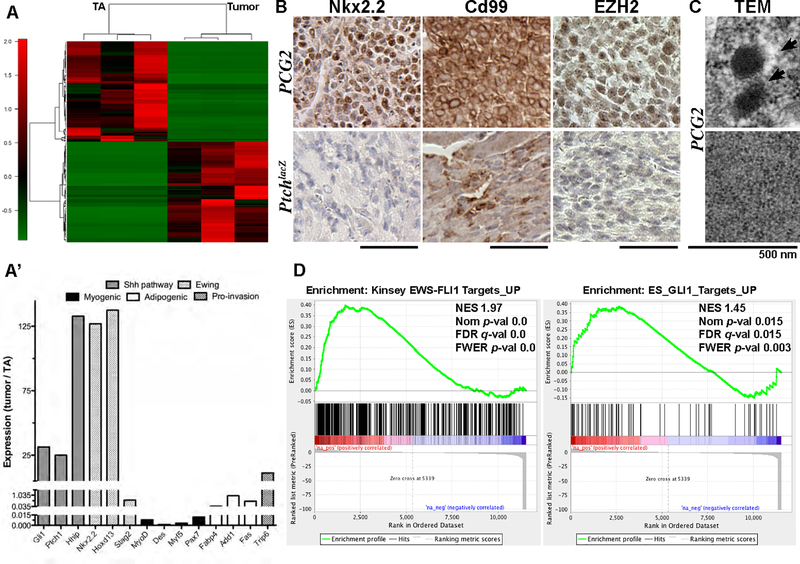
Figure 2. Exploring the PCG2 tumor transcriptome.
(A) Hierarchal clustering analysis of RNA-Seq data shows top 100 most differentially expressed genes from tumor versus unaffected tibialis anterior muscle in PCG2 mice (n=3). (A’) RPKM values show induction of Hh targets, ES-related genes, and select developmental genes in PCG2 tumors versus TA muscle. (B) Immuno-phenotype indicates PCG2 tumors, but not PtchlaZ eRMS, robustly express ES markers Nkx2.2 and Cd99, as well as methyltransferase EZH2. (C) TEM on ultra-thin PCG2 tumor sections. (D) GSEA shows enrichment of indicated gene sets. Scale bars are 50 μm.
In line with previous animal model studies (17,18), we attempted immunostaining against Cd99, which revealed striking membranous localization in all PCG2 sarcomas tested, while staining in PtchlacZ eRMS was clearly diffuse (Figure 2B). This result was intriguing at first, given findings by others and that conservation of the latter is limited at the sequence level in mice, but is reconciled in that functionally of the protein may be somewhat well conserved (19–21). The histone methyltransferase, Enhancer of Zest Homologue 2 (EZH2), another known EWS-FLI1 target (22), was detected in PCG2 tumors, but not in PtchlacZ eRMS (Figure 2B). Transmission electron microscopy (TEM) revealed neurosecretory granule-like particles, abundant cytoplasmic glycogen, a high n/c ratio, and close cellular contacts in PCG2 sarcomas, all of which have been observed previously in ES but not in other sarcomas to our knowledge (23) (Figures 2C and S2A, A’).
We utilized gene set enrichment analysis (GSEA) (24) to explore the activity of previously defined gene modules in the PCG2 transcriptome (containing 20,461 ranked genes). Significantly enriched gene sets included Epithelial to Mesenchymal Transition (NES 1.68, Nom p-val 0.0, FDR q-val <0.03, FWER p-val 0.0) and G2M Checkpoint modules (NES 2.5, Nom p-val 0.0, FDR q-val 0.0, FWER p-val 0.0) (not shown), consistent with the fast-growing nature of PCG2 tumors. Meanwhile, genes upregulated in other cancers, such as mesenchymal glioblastoma multiforme (GBM), alveolar RMS, and malignant fibrous histiocytoma, did not show significant enrichment (NES −0.53, Nom p-val 0.97, FDR q-val 0.97, FWER p-val 0.92; NES −0.58, Nom p-val 0.33, FDR q-val 0.33, FWER p-val 0.28; NES 1.34, Nom p-val 0.09, FDR q-val 0.09, FWER p-val 0.03, respectively) (25–27). However, 53 genes upregulated in mouse Hh-driven medulloblastoma were significantly enriched in PCG2 tumors (NES 1.96, Nom p-val 0.0, FDR q-val 0.0, FWER p-val 0.00) (28).
Other tumor gene modules with significant enrichment included 500 genes upregulated by EWS-FLI1 in ES cells (NES 1.97, Nom p-val 0.0, FDR q-val 0.0, FWER p-val 0.0) (29), and the EWS-FLI1-core module (NES 1.38, Nom p-val 0.0, FDR q-val 0.0, FWER p-val 0.0)(30), (Figures 2D and S2B). Of the 293 genes downregulated by EWS-FLI1 in the latter module, 219 were strongly downregulated in PCG2 tumors. The ES GLI1 gene module, consisting of 86 genes upregulated by GLI1 (31), also showed significant enrichment (NES 1.46, Nom p-val 0.023, FDR q-val 0.023, FWER p-val 0.004) (Figure 2D). Keratin 17, whose function is necessary for transformation and cellular adhesion in ES (31), was the leading gene. E2F targets (NES 2.7, Nom p-val 0.0, FDR q-val 0.0, FWER p-val 0.0) (Figure S2B), which ETS fusions are also known to deregulate, were upregulated (32). The PCG2 transcriptome showed upregulation of additional ES-associated genes, including Hoxd13 (33), and pro-invasion gene Trip6 (34), while down-regulation of Stag2, whose loss confers poor prognosis in ES, was observed (Figure 2A’) (12). Many genes commonly activated by EWS-FLI1 were not upregulated in PCG2 tumors, like Prkcb, while others reversed trend, such as Gstm4, Parp1 and Cav1 (Figure S2C). Nr0b1 did not appear in the PCG2 dataset, and therefore could not be evaluated.
To test whether EWS-FLI1 could induce tumors in our system, and to have a true point of comparison for PCG2 mice, we crossed Pcp2-cre mice with an allele expressing EWS-FLI1 from the endogenous EWS gene locus (35). However, EWS-FLI1 did not induce tumors in Pcp2-cre; EWSEWS-FLI1 mice (n=7), indicating that this oncogene alone is not sufficient to transform Pcp2-lineage cells. However, activity of the oncogene was apparent, as all Pcp2-cre; EWSEWS-FLI1 mice displayed ataxia and exopthalmia (not shown), consistent with cerebellar and retinal expression of Pcp2-cre, and apoptosis induced by EWS-FLI1 in certain tissues (35,36). We also checked for expression of Fli1 and other ETS genes in the PCG2 transcriptome, but found that only SpiB was differentially upregulated (Figure S2D). Immunostaining confirmed SpiB expression, but ultimately tumors indistinguishable from PCG2 formed in PCG2; SpiB−/− animals (not shown).
GLI2A-mediated induction of Nkx2.2 expression, genomic instability, and a distinctive gene profile
We isolated primary cells with serial passaging of a dissociated PCG2 tumor (Figure S3A). Expression of mitotic markers and Nkx2.2 was robust in the resulting PCG2M226 cell line, which, when injected into gastrocnemius muscle of NOD/ SCID recipient mice, propagated aggressive tumors within 10–14 days (Figure S3A, B). These tumors showed a robust Nkx2.2+, Cd99+ signature and Myc expression, similar to parent PCG2 sarcomas (Figure 3A and S3B). In an effort to understand Nkx2.2 induction, chromatin immunoprecipitation (ChIP) was performed with chromatin from PCG2M226 cells (Figure S3C). By targeting the GLI2A-MYC epitope tag, we found enrichment at previously described regulatory elements in Hh target genes Gli1 and Ptch1, and importantly, in Nkx2.2 (Figure S3C). GLI2A occupancy in this Nkx2.2 regulatory element is consistent with studies of Hh-dependent patterning in the neural tube (11), and provides a mechanism for Nkx2.2 induction, which appears specific to PCG2, given that Nkx2.2 is absent in all other reported Hh-driven mouse tumors.
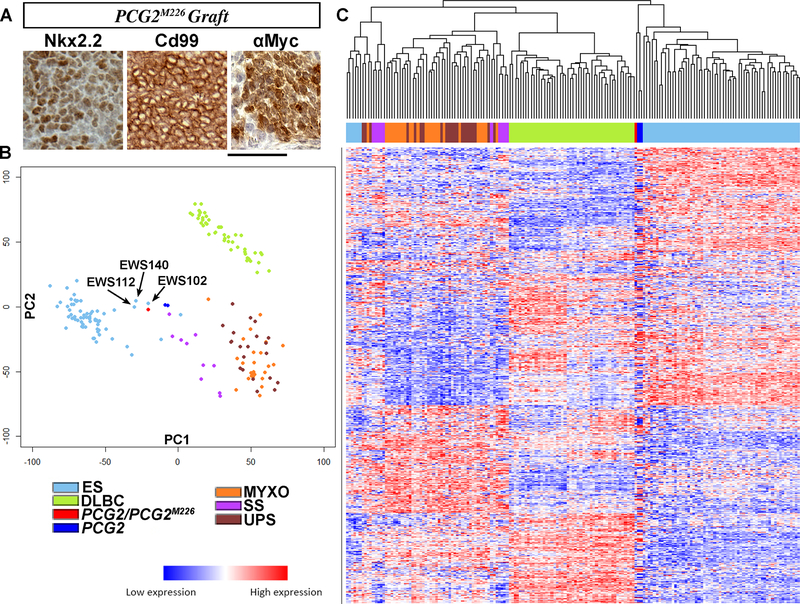
Figure 3. Induction of Nkx2.2 expression and a distinctive gene signature.
(A) Immunostaining of allografted PCG2M226 cells shows Cd99 and Nkx2.2 localization in resultant tumor. (B) PCA of transcriptomes of indicated human tumors and PCG2 tumors/ cells. (C) A heatmap and dendrogram of the same human tumors and PCG2 tumors/ cells (x axis) is shown by hierarchal clustering analysis of 13,345 genes (y axis). Scale bars indicate 50 μm.
Since many sarcomas are defined by chromosomal translocation, we tested whether such changes accompany tumorigenesis in PCG2 mice. PCG2 sarcomas showed greater positivity for double-stranded break marker γH2AX than PtchlacZ eRMS (Figure S3D), indicative of heightened genomic instability (GI) (37). Spectral karyotyping (SKY) of PCG2M226 cells revealed amplifications, deletions, and a t(1;7) translocation (Figure S3E). These findings indicate that Hh activation through GLI2A, but not Ptch1 loss-of-function, induces persistent GI, which might explain the more aggressive features of PCG2 tumors and is consistent with previous studies of GLI2A (38).
We used RNAseq to profile the transcriptome of PCG2M226 cells, which demonstrated a 0.98 correlation coefficient with a re-sequenced parent tumor. Principal components analysis (PCA) was used to compare transcriptomes of PCG2 tumors/ PCG2M226 cells (average of 3; red/ n=2; royal blue), and five different small round cell human tumor types with data from The Cancer Genome Atlas (TCGA). The latter group included synovial sarcoma (SS) (n=10; purple), myxofibrosarcoma (MFS) (n=25; orange), undifferentiated pleomorphic sarcoma (UPS) (n=21; brown), diffuse large B cell lymphoma (DLBCL) (n=48; green), and clinically and experimentally defined ES (n=61; light blue) (12). The results showed dissimilarity between PCG2 tumors/ PCG2M226 cells and DLBCL, MFS, and UPS, but similarity with certain human ES samples (Figure 3C). Separation of PCG2 into three individual tumors (red), and ES into extraosseous (eoES) (n=6; light blue) and osseous (oES) (n=55; dark green) did not alter these trends (Figure S3F).
We chose to focus on the three ES samples that fell nearest to the PCG2 domain. EWS112 was a soft tissue tumor of the neck harboring an EWS-FLI1 type 1 fusion and expression loss of STAG2. EWS102 was a tumor of the femur with a novel FUS-NFATc2 fusion, and EWS140 was a soft tissue tumor of the thigh with no detected gene fusion (Figure 3B), but both were clinically diagnosed as ES (12). In hierarchal clustering analysis with the above tumor groups, PCG2 clustered with the bulk of primary ES samples when 13,345 gene expression values per sample were compared, indicated by the accompanying dendrogram (Figure 3C).
Gli1 gene dosage influenced sarcoma phenotypic outcome
To localize ectopic Hh signals in PCG2 tissues, we utilized heterozygous Gli1nlacZ knock-in reporter mice, which carry only one functional Gli1 allele. The resulting PCG2; Gli1nlacZ animals formed soft tissue tumors with histological features that were divergent from PCG2 tumors, showing instead nested cells with peripherally arranged nuclei, collagen-rich matrix (blue) and eosinphilic cysts (Figures 4A and S4A). Fewer PCG2; Gli1nlacZ mice developed tumors than PCG2 (27% versus 98%), with an average onset of 6 months, while the remaining animals succumbed to old age (n=10, p < 0.0001) (Figure 4B). Along with tumor latency, penetrance, and pathology, the immuno-phenotype of PCG2; Gli1nlacZ tumors was also markedly different. Although myogenic marker expression was still absent in PCG2; Gli1nlacZ tumors, which were still highly proliferative, the Nkx2.2+, Cd99+ signature was not maintained (Figure 4A, C and S4B). To determine whether phenotypic outcome can be associated with level of Hh pathway activation, we compared Gli1 abundance between tumors from PCG2, PCG2; Gli1nlacZ and PtchlacZ mice. We found that Gli1 induction in PCG2; Gli1nlacZ tumors was less than half the level detected in PCG2. Surprisingly, induction in PCG2 tumors did not differ considerably from PtchlacZ eRMS using this method (Figure S4C). These findings indicate that a threshold of Hh induction must be reached for PCG2 features to be expressed, and that the level of pathway induction alone does not likely distinguish undifferentiated and myogenic sarcomas.
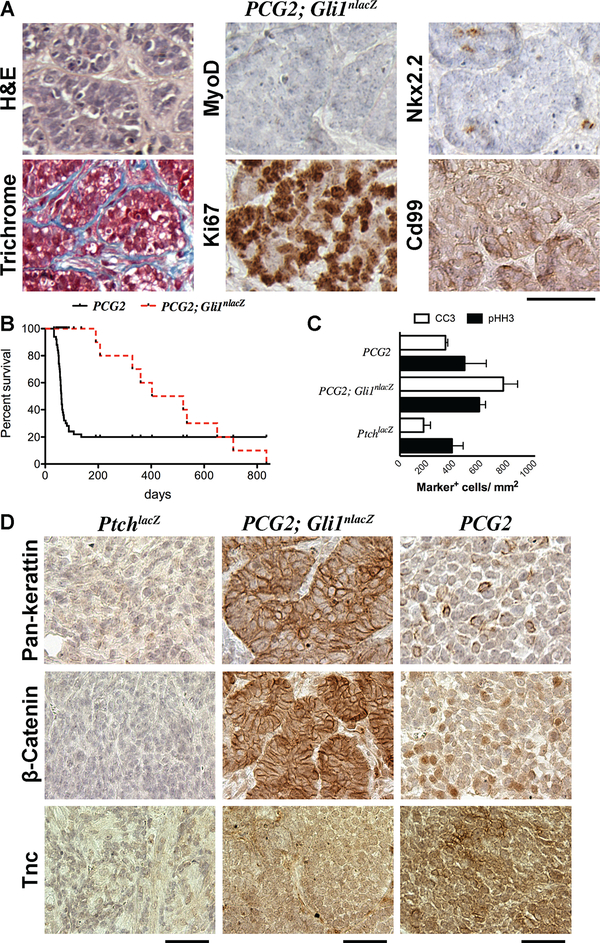
Figure 4. Gli1 gene dosage influenced tumor phenotype.
(A) H&E, Trichrome staining, and immunostaining on PCG2; Gli1nlaZ tumors for indicated markers. (B) Kaplan-Myer survival curves for PCG2 and PCG2; Gli1nlaZ mice. (C) Mitotic and apoptotic indices for PtchlacZ, PCG2; Gli1nlacZ, and PCG2 tumors. Bars indicate average ± SEM. (D) Immunostaining on tumors from PtchlacZ, PCG2; Gli1nlacZ, and PCG2 mice for indicated markers. Scale bars are 50 μm.
We chose to further characterize the epithelial-like histology of PCG2; Gli1nlacZ tumors. Although absent in PtchlacZ eRMS, keratins had strong membranous localization in PCG2; Gli1nlacZ tumors, consistent with epithelial organization. Cytoplasmic keratin was seen occasionally in PCG2 tumor cells (Figure 4D). For corroboration we evaluated β-Catenin localization. Similar to Keratins, expression of β-Catenin was below the detection limit in PtchlacZ tumors, but showed strong enrichment at cell borders in PCG2; Gli1nlacZ tumors (Figure 4D). In contrast, PCG2 tumors showed intermittent nuclear enrichment of β-Catenin (Figure 4D). Interestingly, aggressiveness in a subset of ES with more frequent nuclear β-Catenin appears to be driven through activation of Wnt/β-Catenin signaling concomitant with upregulation of the extracellular matrix component and known EWS-FLI1 target gene, Tenascin-C (TNC) (39,40). Scattered enrichment of Tnc was detected in PCG2 tumors, but not in those from PtchlacZ or PCG2; Gli1nlacZ mice (Figure 4D). Together these findings suggest a possible mechanism supporting the aggressive growth kinetics of PCG2 sarcomas, but not a likely parallel connecting them with the broader collection of ES.
PCG2 sarcomas emerge from a naïve mesenchymal ancestor
Knowledge of the ancestral cell type for a given tumor provides key insight into the specific molecular landscape initially permissive to oncogenic transformation, details that could be powerful in the design of more effective treatment options (41). Therefore, we sought to elucidate a prospective cellular origin for PCG2 sarcomas. To achieve this end, we performed genetically-inducible fate-mapping (GIFM) to mark Pcp2-lineage cells with indelible reporters that express either cytoplasmic eYFP (R26ReYFP) or tdTomato (Ai9). At embryonic day 11.5 (e11.5), eYFP reporter activity (red) was largely detected in forelimb level dermamyotome cells that expressed Pax7/3 (green) (Figure 5A–D). Lineage cells were less frequently seen in Myf5 or Myogenin expression domains (green) (Figure 5A–D). Additionally, we evaluated neural crest marker Foxd3, but found no overlap with lineage cells (Figure S5A, A’). These findings suggest that Pcp2-lineage cells are multipotent mesenchymal progenitors that predominantly generate skeletal muscle, adipocytes, and satellite cells.
Figure 5. Prospective cell-of-origin for PCG2 sarcomas.
(A-D) Immunostaining on transverse sections from Pcp2-cre; R26ReYFP embryos (e11.5) shows Pcp2 lineage cells in the transient dermamyotome (white outline, A and C) at the forelimb level express Pax7, Pax3 more frequently than Myogenin or Myf5, as indicated. (E, E’) Direct fluorescence for tdTomato indicates Pcp2 lineage cells (red) contribute extensively to musculature (blue outline) in adult Pcp2-cre; Ai9 mice. (F, F’) Immunostaining of cross sections from proximal hind limb (black dotted line) revealed that Pcp2 lineage cells become both mature myofibers (F) and PPARγ+ adipocytes (F’). (G) H&E, immunophenotype, and survival for Myf5Cre; CLEG2 (M5G2) mice. White arrowheads indicate double labeled cells. Scale bars represent distances indicated.
Whole-mount imaging of skeletal muscle in young adult Pcp2-cre; Ai9 mice showed that myofibers of the head, neck, and proximal limbs were often tdTomato+ (Figure 5E, E’). In tissue sections, myofibers and PPARγ+ adipocytes were clearly labeled, consistent with Pax7 expression in Pcp2-lineage cells at e11.5 (Figure 5F, F’). In postnatal TA muscle, which was used in RNAseq studies, only few interstitial cells were tdTomato+, indicating limited cre activity in this tissue (Figure S5B, B’). We also targeted GLI2A to precursors of muscle and fat using Myf5Cre mice. ~50% of the resulting M5G2 animals formed aggressive soft tissue tumors by four weeks of age that were indistinguishable from PCG2 tumors in terms of pathology and molecular marker profile (n=11, p = 0.0016) (Figure 5G). However, when activated in postnatal satellite cells at P3 or P7 using Pax7-creER; Ai9 mice, GLIA did not induce tumor formation by 18 months, even though recombination was detected with the Ai9 reporter allele (n=10, not shown). Altogether, these findings argue that the undifferentiated soft tissue sarcomas seen in PCG2 mice likely originate in common progenitors of myogenic and adipogenic cells.
Discussion
Collectively, our description of PCG2 mice demonstrates that GLI2A is sufficient to induce formation of sarcomas that are distinctly different from Hh-driven eRMS. Instead, PCG2 tumors displayed an unexpected assortment of pathological, immuno-phenotypic, transcriptomic, and ultrastructural features typical of human ES and Ewing-like tumors, the latter of which receive the same standard of care treatment as bone fide ES. Our work argues that PCG2 sarcomas emerge from a multipotent mesenchymal origin cell, which, in our hands, could not be transformed by EWS-FLI1, and demonstrates that Gli1 dosage can determine sarcoma phenotypic outcome. Altogether, we have provided a novel genetically-defined mouse model with potential preclinical utility. Despite the perceived similarity shared by ES and Ewing-like tumors with those formed by PCG2 mice, it is essential to emphasize that the latter are decidedly not a mouse equivalent to ES, as they are not defined by an EWS-ETS gene fusion. However, PCG2 mice do have utility to inform more generally about the etiology of undifferentiated soft tissue sarcomas, especially those that display Ewing-like features but lack expected gene fusions and representative animal models.
A clear impediment to furthering knowledge of sarcoma biology, particularly in the case of ES, has been limited availability of faithful mouse models. Difficulty with producing a true, genetically-defined ES mouse may stem from non-conservation of microsatellites, which determine functional output of EWS-FLI1, and/ or lethality induced by the oncogene (9,30,42). Promising work with flank grafting of isolated progenitor cells ectopically expressing EWS-FLI1 has yielded mouse ‘Ewing-like’ tumors (17,18), which leaves hope that the right design may ultimately yield a true ‘Ewing’ mouse. Future approaches should consider harnessing direct activation of known downstream effectors of EWS-FLI1 as an alternative or in combination with the oncogene. GLI1 (43–45), one of three zinc-finger transcription factors that regulate vertebrate Hh target genes (46), is an established target and effector of EWS-FLI1, hence the sensitivity of ES cell lines to GLI1/2 inhibitor arsenic trioxide (ATO) (47).
It is likely that the subset of features shared by PCG2 sarcomas and ES is attributable to this core connection, utilization of GLI activator function, which has not been observed in any other sarcoma variant beyond eRMS. Even though PCG2 mice leverage activity of a truncated human GLI2, GLI1 and GLI2 are known to operate through a consensus binding sequence to regulate common target genes such as PTCH1(46). Since ubiquitous activation of Hh signaling in mice, due to either widespread deactivation of receptor Patched1 (Ptch1) (5,6) or constitutively activated Smoothened (SmoM2), is so far only known to cause eRMS and not other sarcoma types (7,8), suggesting these different modes of activating the pathway induce different target gene modules and ultimately very different tumor phenotypes.
However, it is critical to emphasize that in addition to the noted similarities, differences between genuine Ewing tumors and those formed by PCG2 mice were also observed. The most significant of which is that the latter do not appear to harbor any gene fusions, definitely not a fusion relevant in the context of the human sarcoma variant. Given this important difference, it is understandable that the resulting gene expression profiles different in a meaningful way; several notable ‘Ewing’ genes were strongly down-regulated or not detected in in our model, such as Prkcb and Nr0b1. Whether the epigenetic landscape in PCG2 tumors would be reflective of what has been documented in Ewing is to be determined, but given that EWS-FLI1 is thought to be a major driver of establishing that signature (42), there may not be much similarity in that regulatory dimension. When considering these distinguishing differences, it becomes clear why PCG2 specimens would group most closely with a subset of Ewing and ‘Ewing-like’ tumors in PCA of their respective transcriptomic profiles. Furthermore, the most telling comparison will be testing the efficacy of therapeutic agents in PCG2 mice that one day may be considered for Ewing treatment.
Our work also demonstrated that Pcp2-lineage tumor origin cells are naïve mesenchymal progenitors of muscle and fat, not neural crest. Interestingly, the prospective cellular origin for ES is thought to be either mesenchymal stem/progenitor cells, possibly from bone marrow, or cells in the neural crest lineage. This thinking is driven by ES tumor pathology that can exhibit mesenchymal and/ or neuroectodermal elements. The latter being more prevalent in peripheral neuroectodermal tumors (PNETs) that share the hallmark t(11; 22) rearrangement and therefore the ES classification. Whether originating cell type is an influential factor that helps shape tumor phenotype (osseous, extra-osseous, or PNETs in the case of ES) is unclear. Why EWS-FLI1 failed to induce tumors in Pcp2-cre; EWSEWS-FLI1 mice is also not apparent, but may suggest that an additional genetic alteration is needed to drive transformation over lethality in Pcp2-lineage mesenchymal progenitor cells.
Altogether, PCG2 represents the first genetically-inducible, Hh-driven mouse model of undifferentiated sarcoma and is therefore a unique tool for exploring tumor initiation/ progression, and perhaps even for pre-clinical studies. Our data argue that future studies should evaluate the efficacy of targeting Hh signaling at the transcriptional level. For example, use of inhibitors of BET bromodomain proteins (48) in the clinical management of ES warrants consideration. Indeed, work from our lab and others has already demonstrated efficacy for these compounds in suppressing EWS-FLI1-mediated transcriptional regulation and ES tumor growth (49–52).
Supplementary Material
Implications.
The finding that activation of Gli2 transcription factor is sufficient to induce Ewing-like sarcomas provides a direct transformative role of the Hh signaling pathway in undifferentiated soft tissue sarcoma.
Acknowledgements
We wish to extend many thanks to those who shared their reagents or assistance during the course of this study, including Javed Khan (NCI), Patricia Labosky (NIH), Dietmar Vestweber (Max Plank), Herald Erickson (Duke), and Leighton Stein (RPCI), and Vanderbilt colleagues: Patrick Grohar (VARI), Scott Borinstein, and Cheryl Coffin. We thank several VUMC core resources, TPSR, DHSR, CISR, and VANTAGE. We are also grateful to the DSHB, established in part through generosity from the NIH. This study was supported by grants to C.C. from the Vanderbilt-Ingram Cancer Center Support Grant P30 CA068485 and Alex Lemonade Foundation.
Footnotes
The authors declare no competing financial interests.
References
- 1.Post SM. Mouse models of sarcomas: critical tools in our understanding of the pathobiology. Clin Sarcoma Res. BioMed Central; 2012;2:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keller C Alveolar rhabdomyosarcomas in conditional Pax3:Fkhr mice: cooperativity of Ink4a/ARF and Trp53 loss of function. Genes & Development. 2004;18:2614–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haldar M, Hancock JD, Coffin CM, Lessnick SL, Capecchi MR. A Conditional Mouse Model of Synovial Sarcoma: Insights into a Myogenic Origin. Cancer Cell. 2007;11:375–88. [DOI] [PubMed] [Google Scholar]
- 4.Straessler KM, Jones KB, Hu H, Jin H, van de Rijn M, Capecchi MR. Modeling Clear Cell Sarcomagenesis in the Mouse: Cell of Origin Differentiation State Impacts Tumor Characteristics. Cancer Cell. Elsevier Inc; 2013;23:215–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hahn H, Wojnowski L, Zimmer AM, Hall J, Miller G, Zimmer A. Rhabdomyosarcomas and radiation hypersensitivity in a mouse model of Gorlin syndrome. Nature Medicine. 1998;4:619–22. [DOI] [PubMed] [Google Scholar]
- 6.Nitzki F, Zibat A, Frommhold A, Schneider A, Schulz-Schaeffer W, Braun T, et al. Uncommitted precursor cells might contribute to increased incidence of embryonal rhabdomyosarcoma in heterozygous Patched1-mutant mice. Oncogene. Nature Publishing Group; 2011;:1–9. [DOI] [PubMed] [Google Scholar]
- 7.Mao J, Ligon KL, Rakhlin EY, Thayer SP, Bronson RT, Rowitch D, et al. A Novel Somatic Mouse Model to Survey Tumorigenic Potential Applied to the Hedgehog Pathway. Cancer Research. 2006;66:10171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajurkar M, Huang H, Cotton JL, Brooks JK, Sicklick J, McMahon AP, et al. Distinct cellular origin and genetic requirement of Hedgehog-Gli in postnatal rhabdomyosarcoma genesis. Nature Publishing Group; 2013;:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minas TZ, Surdez D, Javaheri T, Tanaka M, Howarth M, Kang H-J, et al. Combined experience of six independent laboratories attempting to create an Ewing sarcoma mouse model. Oncotarget. Impact Journals; 2017;8:34141–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delattre O, Zucman J, Plougastel B, Desmaze C, Melot T, Peter M, et al. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature. Nature Publishing Group; 1992;359:162–5. [DOI] [PubMed] [Google Scholar]
- 11.Vokes SA, Ji H, McCuine S, Tenzen T, Giles S, Zhong S, et al. Genomic characterization of Gli-activator targets in sonic hedgehog-mediated neural patterning. Development. 2007;134:1977–89. [DOI] [PubMed] [Google Scholar]
- 12.Brohl AS, Solomon DA, Chang W, Wang J, Song Y, Sindiri S, et al. The genomic landscape of the Ewing Sarcoma family of tumors reveals recurrent STAG2 mutation Horwitz MS, editor. PLoS Genet. Public Library of Science; 2014;10:e1004475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis PM, Gritli-Linde A, Smeyne R, Kottmann A, McMahon AP. Sonic hedgehog signaling is required for expansion of granule neuron precursors and patterning of the mouse cerebellum. Developmental Biology. 2004;270:393–410. [DOI] [PubMed] [Google Scholar]
- 14.Pasca di Magliano M, Sekine S, Ermilov A, Ferris J, Dlugosz AA, Hebrok M. Hedgehog/Ras interactions regulate early stages of pancreatic cancer. Genes & Development. 2006;20:3161–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shibuya R, Matsuyama A, Nakamoto M, Shiba E, Kasai T, Hisaoka M. The combination of CD99 and NKX2.2, a transcriptional target of EWSR1-FLI1, is highly specific for the diagnosis of Ewing sarcoma. Virchows Arch. 2014;465:599–605. [DOI] [PubMed] [Google Scholar]
- 16.Smith R, Owen LA, Trem DJ, Wong JS, Whangbo JS, Golub TR, et al. Expression profiling of EWS/FLI identifies NKX2.2 as a critical target gene in Ewing’s sarcoma. Cancer Cell. 2006;9:405–16. [DOI] [PubMed] [Google Scholar]
- 17.Riggi N, Cironi L, Provero P, Suvà M-L, Kaloulis K, Garcia-Echeverria C, et al. Development of Ewing’s sarcoma from primary bone marrow-derived mesenchymal progenitor cells. Cancer Research. 2005;65:11459–68. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka M, Yamazaki Y, Kanno Y, Igarashi K, Aisaki K-I, Kanno J, et al. Ewing’s sarcoma precursors are highly enriched in embryonic osteochondrogenic progenitors. J Clin Invest. American Society for Clinical Investigation; 2014;124:3061–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bixel MG, Li H, Petri B, Khandoga AG, Khandoga A, Zarbock A, et al. CD99 and CD99L2 act at the same site as, but independently of, PECAM-1 during leukocyte diapedesis. Blood. 2010;116:1172–84. [DOI] [PubMed] [Google Scholar]
- 20.Nasdala I A Transmembrane Tight Junction Protein Selectively Expressed on Endothelial Cells and Platelets. Journal of Biological Chemistry. 2002;277:16294–303. [DOI] [PubMed] [Google Scholar]
- 21.Bixel G, Kloep S, Butz S, Petri B, Engelhardt B, Vestweber D. Mouse CD99 participates in T-cell recruitment into inflamed skin. Blood. American Society of Hematology; 2004;104:3205–13. [DOI] [PubMed] [Google Scholar]
- 22.Richter GHS, Plehm S, Fasan A, Rössler S, Unland R, Bennani-Baiti IM, et al. EZH2 is a mediator of EWS/FLI1 driven tumor growth and metastasis blocking endothelial and neuro-ectodermal differentiation. Proc Natl Acad Sci USA. National Acad Sciences; 2009;106:5324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Llombart-Bosch A, Peydro-Olaya A. Scanning and transmission electron microscopy of Ewing’s sarcoma of bone (typical and atypical variants). An analysis of nine cases. Virchows Arch A Pathol Anat Histopathol. 1983;398:329–46. [DOI] [PubMed] [Google Scholar]
- 24.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences. National Acad Sciences; 2005;102:15545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verhaak RGW, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ren Y-X, Finckenstein FG, Abdueva DA, Shahbazian V, Chung B, Weinberg KI, et al. Mouse mesenchymal stem cells expressing PAX-FKHR form alveolar rhabdomyosarcomas by cooperating with secondary mutations. Cancer Research. American Association for Cancer Research; 2008;68:6587–97. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen TO, West RB, Linn SC, Alter O, Knowling MA, O’Connell JX, et al. Molecular characterisation of soft tissue tumours: a gene expression study. Lancet. 2002;359:1301–7. [DOI] [PubMed] [Google Scholar]
- 28.Lee Y, Kawagoe R, Sasai K, Li Y, Russell HR, Curran T, et al. Loss of suppressor-of-fused function promotes tumorigenesis. Oncogene. 2007;26:6442–7. [DOI] [PubMed] [Google Scholar]
- 29.Kinsey M, Smith R, Lessnick SL. NR0B1 Is Required for the Oncogenic Phenotype Mediated by EWS/FLI in Ewing’s Sarcoma. Molecular Cancer Research. 2006;4:851–9. [DOI] [PubMed] [Google Scholar]
- 30.Hancock JD, Lessnick SL. A transcriptional profiling meta-analysis reveals a core EWS-FLI gene expression signature. Cell Cycle. 2014;7:250–6. [DOI] [PubMed] [Google Scholar]
- 31.Sankar S, Tanner JM, Bell R, Chaturvedi A, Randall RL, Beckerle MC, et al. A novel role for keratin 17 in coordinating oncogenic transformation and cellular adhesion in Ewing sarcoma. Molecular and Cellular Biology. American Society for Microbiology; 2013;33:4448–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwentner R, Papamarkou T, Kauer MO, Stathopoulos V, Yang F, Bilke S, et al. EWS-FLI1 employs an E2F switch to drive target gene expression. Nucleic Acids Research. 2015;43:2780–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Svoboda LK, Harris A, Bailey NJ, Schwentner R, Tomazou E, Levetzow von C, et al. Overexpression of HOX genes is prevalent in Ewing sarcoma and is associated with altered epigenetic regulation of developmental transcription programs. Epigenetics. Taylor & Francis; 2014;9:1613–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grunewald TGP, Willier S, Janik D, Unland R, Reiss C, Prazeres da Costa O, et al. The Zyxin-related protein thyroid receptor interacting protein 6 (TRIP6) is overexpressed in Ewing’s sarcoma and promotes migration, invasion and cell growth. Biol Cell. 2013;105:535–47. [DOI] [PubMed] [Google Scholar]
- 35.Sohn EJ, Li H, Reidy K, Beers LF, Christensen BL, Lee SB. EWS/FLI1 oncogene activates caspase 3 transcription and triggers apoptosis in vivo. Cancer Research. American Association for Cancer Research; 2010;70:1154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ivanova E, Hwang GS, Pan ZH. Characterization of transgenic mouse lines expressing Cre recombinase in the retina. NSC. Elsevier Inc; 2010;165:233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Celeste A, Petersen S, Romanienko PJ, Fernandez-Capetillo O, Chen HT, Sedelnikova OA, et al. Genomic instability in mice lacking histone H2AX. Science. American Association for the Advancement of Science; 2002;296:922–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pantazi E, Gemenetzidis E, Trigiante G, Warnes G, Shan L, Mao X, et al. GLI2 induces genomic instability in human keratinocytes by inhibiting apoptosis. Cell Death and Disease. Nature Publishing Group; 2014;5:e1028–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pedersen EA, Menon R, Bailey KM, Thomas DG, Van Noord RA, Tran J, et al. Activation of Wnt/β-Catenin in Ewing Sarcoma Cells Antagonizes EWS/ETS Function and Promotes Phenotypic Transition to More Metastatic Cell States. Cancer Research. American Association for Cancer Research; 2016;76:5040–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watanabe G, Nishimori H, Irifune H, Sasaki Y, Ishida S, Zembutsu H, et al. Induction of tenascin-C by tumor-specific EWS-ETS fusion genes Genes Chromosomes Cancer. Wiley Subscription Services, Inc., A Wiley Company; 2003;36:224–32. [DOI] [PubMed] [Google Scholar]
- 41.Wang ZA, Mitrofanova A, Bergren SK, Abate-Shen C, Cardiff RD, Califano A, et al. Lineage analysis of basal epithelial cells reveals their unexpected plasticity and supports a cell-of-origin model for prostate cancer heterogeneity. Nature Publishing Group. 2013;15:274–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riggi N, Knoechel B, Gillespie SM, Rheinbay E, Boulay G, Suvà ML, et al. EWS-FLI1 utilizes divergent chromatin remodeling mechanisms to directly activate or repress enhancer elements in Ewing sarcoma. Cancer Cell. 2014;26:668–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zwerner JP, Joo J, Warner KL, Christensen L, Hu-Lieskovan S, Triche TJ, et al. The EWS/FLI1 oncogenic transcription factor deregulates GLI1. Oncogene. Nature Publishing Group; 2008;27:3282–91. [DOI] [PubMed] [Google Scholar]
- 44.Beauchamp E, Bulut G, Abaan O, Chen K, Merchant A, Matsui W, et al. GLI1 is a direct transcriptional target of EWS-FLI1 oncoprotein. J Biol Chem. American Society for Biochemistry and Molecular Biology; 2009;284:9074–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joo J, Christensen L, Warner K, States L, Kang H-G, Vo K, et al. GLI1 is a central mediator of EWS/FLI1 signaling in Ewing tumors. Jones C, editor. PLoS ONE. Public Library of Science; 2009;4:e7608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hooper JE, Scott MP. Communicating with Hedgehogs. Nat Rev Mol Cell Biol. Nature Publishing Group; 2005;6:306–17. [DOI] [PubMed] [Google Scholar]
- 47.Beauchamp EM, Ringer L, Bulut G, Sajwan KP, Hall MD, Lee Y-C, et al. Arsenic trioxide inhibits human cancer cell growth and tumor development in mice by blocking Hedgehog/GLI pathway. J Clin Invest. 2011;121:148–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang Y, Gholamin S, Schubert S, Willardson MI, Lee A, Bandopadhayay P, et al. Epigenetic targeting of Hedgehog pathway transcriptional output through BET bromodomain inhibition. Nature Medicine. Nature Research; 2014;20:732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jacques C, Lamoureux F, Baud’huin M, Rodriguez Calleja L, Quillard T, Amiaud J, et al. Targeting the epigenetic readers in Ewing sarcoma inhibits the oncogenic transcription factor EWS/Fli1. Oncotarget. Impact Journals; 2016;7:24125–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hensel T, Giorgi C, Schmidt O, Calzada-Wack J, Neff F, Buch T, et al. Targeting the EWS-ETS transcriptional program by BET bromodomain inhibition in Ewing sarcoma. Oncotarget. Impact Journals; 2016;7:1451–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bid HK, Phelps DA, Xaio L, Guttridge DC, Lin J, London C, et al. The Bromodomain BET Inhibitor JQ1 Suppresses Tumor Angiogenesis in Models of Childhood Sarcoma. Mol Cancer Ther. American Association for Cancer Research; 2016;15:1018–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Loganathan SN, Tang N, Fleming JT, Ma Y, Guo Y, Borinstein SC, et al. BET bromodomain inhibitors suppress EWS-FLI1-dependent transcription and the IGF1 autocrine mechanism in Ewing sarcoma. Oncotarget. Impact Journals; 2016;7:43504–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.