Abstract
Objective:
To investigate the effect of timing and magnitude of horizontally directed propulsive forces to the center of mass (COM) on the metabolic cost of walking for individuals following stroke.
Design:
Repeated measures, within-subjects design
Setting:
Research laboratory
Participants:
Nine individuals with chronic hemiparesis post-stroke and seven unimpaired similarly aged controls
Intervention:
Individuals walked on a treadmill in two separate studies. First, we compared the metabolic cost of walking with an anterior force applied to the COM that 1) coincided with paretic propulsion or 2) was applied throughout the gait cycle. Next, we compared the metabolic cost of walking with anterior (assistive) or posterior (resistive) forces applied during paretic propulsion.
Main Outcome Measure:
metabolic cost of walking
Results:
The cost of walking was significantly greater in the Stroke group. Anterior (propulsive) assistance reduced the cost of walking differently based on group. The Stroke group exhibited a 12% reduction in cost of walking when assistance was provided only during paretic propulsion, but not when assistance was provided throughout the gait cycle. In contrast, the Control group demonstrated reduced cost of walking during both anterior assistance conditions. In addition, we observed that resistance during paretic propulsion (simulated hemiparesis for Control group) significantly increased the cost of walking.
Conclusions:
Systematically manipulating propulsive forces at the body’s COM had a profound influence on metabolic cost. The timing of propulsive forces to the COM are important and need to coincide with paretic terminal stance. Additional internally or externally generated propulsive forces applied to the body’s COM after stroke may produce a lower metabolic cost of walking.
Keywords: stroke, gait, energy cost, propulsion
Hemiparetic gait can require up to two times more metabolic energy than unimpaired walking.1-5 A substantial amount of this energy may be due to the mechanical work required to redirect the body’s center of mass (COM) with each step.4, 6 The redirection of the COM occurs in both vertical and anterior directions,1 however, most of the available literature has focused on control of the vertical COM displacement during walking.7, 8 Although vertical forces are larger, it is the anteriorly directed propulsive force that acts on the COM to translate the body forward, and thus represents a key determinant of walking function.9, 10
Following stroke, individuals exhibit varying levels of unilateral muscle weakness, which contributes to reduced paretic propulsion and disrupted forward progression during walking. Specifically, unilateral ankle plantarflexor weakness contributes to decreased propulsion in the latter half of stance 1,11, 12 and has therefore been targeted to optimize walking recovery.13, 14 The higher metabolic energy costs associated with hemiparetic gait are not due to decreased efficiency of work production, but rather an increase in mechanical work done by the active muscles.1, 4,15 Thus, the reduction in paretic ankle power requires increased work by other muscles to maintain anterior COM velocity throughout the gait cycle.4, 16, 17 In agreement with this, walking following stroke exhibits pronounced mechanical asymmetries, with the non-paretic limb producing more positive mechanical power than the paretic limb, regardless of functional recovery.18 This mechanical imbalance between limbs induces unequal accelerations and decelerations throughout the gait cycle, interrupting the expected symmetric forward progression of the COM that is characteristic of healthy human walking.19
Individuals following stroke require effective solutions to reduce the large energy cost of walking. Altering walking mechanics via internally or externally imposed forces to the COM may influence the energy cost of walking. For example, the application of additional anteriorly directed forces to the COM throughout the gait cycle is well known to reduce metabolic cost in unimpaired individuals.20-22 These externally imposed, anteriorly directed forces to the COM may be capable of compensating for the known reduction in paretic limb propulsion.10, 18, 23 A constant anteriorly directed force, however, may exaggerate the already large braking forces for an individual post-stroke.24 The timing of any additional internal or external force application should therefore be carefully considered due to the influence of magnitude 1, 17, 23 and timing 17, 25 of propulsion on COM movement for forward progression. In particular, the resulting hemiparesis that persists following stroke suggests that a unilateral solution is needed to overcome the mechanical work asymmetries observed during walking.1, 18 As such, the production of additional anteriorly directed forces to the COM, whether produced internally or externally, may only be needed during paretic propulsion, rather than during the entire gait cycle for individuals with hemiparesis. The knowledge regarding how externally generated forces to the COM influence metabolic cost is necessary and important to consider how similar internally generated forces to the COM might influence the metabolic cost of walking for people following stroke.
The purpose of this study was twofold: to determine 1) how the timing of an applied anteriorly directed force to the body’s COM affects the metabolic cost of walking for individuals following stroke and 2) to determine if externally enhancing/reducing paretic propulsion at the whole body level would influence the metabolic cost of walking. It was hypothesized that an anteriorly directed force applied to the COM, which coincides with paretic propulsion, would reduce the metabolic cost of walking more than an imposed anteriorly directed force that is applied throughout the entire gait cycle. Likewise, we hypothesized that we would observe an increase in metabolic energetics when participants received propulsion resistance and a decrease in metabolic energetics when participants received propulsion assistance.
METHODS
Participants
A group of nine individuals with chronic (>6 months) stroke and a group of seven similarly aged unimpaired control participants were recruited for two separate studies (Table 1). All Control subjects participated in both studies, whereas seven subjects in the Stroke group participated in both. All subjects post-stroke presented with lower extremity hemiparesis resulting from an ischemic or hemorrhagic unilateral brain lesion. Subjects were excluded from this study if they could not walk without therapist assistance, self-reported a preexisting cardiovascular, metabolic, or musculoskeletal condition(s) that prohibited strenuous activity, a separate neurologic condition that could affect walking ability, or a history of balance deficits or unexplained falls prior to stroke onset. Participants used their typical shoes, and one individual post-stroke used an ankle-foot orthosis during testing. We used the lower extremity portion of the Fugl-Meyer 26 to assess sensorimotor coordination for each subject in the Stroke group. All participants signed an informed consent form approved by the University of North Carolina at Chapel Hill IRB before participating.
Table 1.
Subject Demographics
| Control | Stroke | ||
|---|---|---|---|
| Sex | 6 F / 1 M | 4 F / 4 M | p=0.282 |
| Age (years) | 49±14 years | 56±14 | p=0.354 |
| Stroke Onset (months) | 76±63 | ||
| Paretic Side | 3 L / 5 R | ||
| LE Fugl-Meyer | 27±3 | ||
| Height (inches) | 66.1±1.3 | 67.4±3.3 | p=0.379 |
| Weight | 163±58 | 184±46 | p=0.450 |
| Comfortable Overground speed (m/s) | 1.38±0.24 | 0.73±0.29 | p<0.001 |
| Treadmill speed (m/s) | 1.23±0.18 | 0.63±0.25 | p<0.001 |
Procedures
To address our dual questions, we devised two related studies. In Study 1, we sought to determine how the timing of externally imposed anteriorly directed forces impacts metabolic energetics. In Study 2, we explicitly tested the effect of whole body propulsive mechanics on metabolic energetics. For both Study 1 and 2, all walking conditions were performed on a dual-belt instrumented treadmill.a Participants wore a safety harness attached overhead that did not restrict lower extremity movement or provide unweighting. Prior to treadmill walking, all participants performed two passes of overground walking at their self-selected comfortable gait speed across a 20-ft walkwayb The treadmill speed was then set for each participant to be ~80-100% of the overground gait speed. We often selected slower speeds to account for the challenge associated with the Posteriorparetic condition (described below). Participants were permitted to use a side-mounted handrail as needed, but researchers did not provide any physical assistance. Handrail use was maintained constant across all conditions. For all conditions, in both studies, individuals in the Stroke group walked for four minutes and the Control group walked for five minutes to reach steady state. A minimum of a five minute seated rest break was provided between each condition for all participants.
Study 1 Conditions
Participants performed three randomly ordered conditions, using a random number generator, to assess how the timing of anteriorly directed forces to the COM affects metabolic cost.
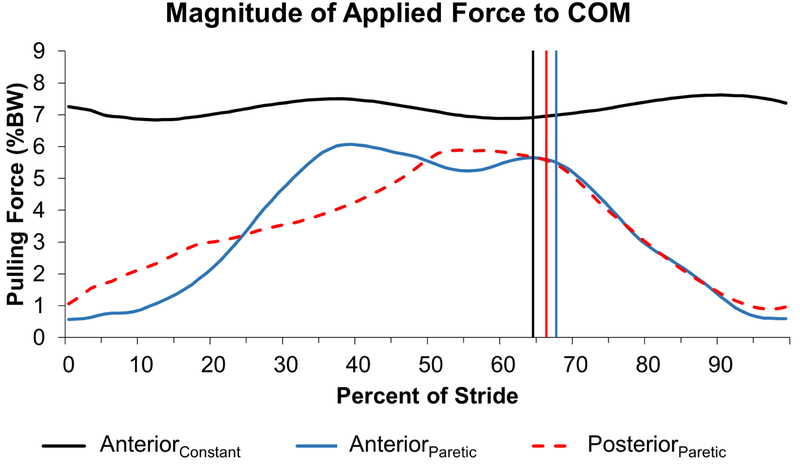
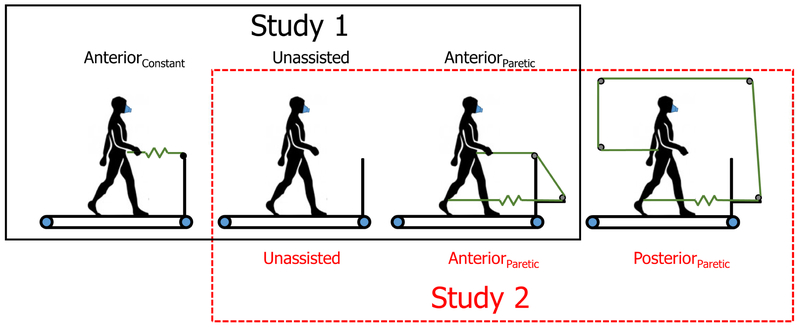
Constant anterior assistance (AnteriorConstant): While walking on the treadmill, participants received a constant anterior pull force to the pelvis (i.e., COM) with a magnitude of 5-10% body weight (BW), 20, 27 measured continuously by a load-cell c The anterior pull was provided by stretched elastic tubing d fixed anteriorly to the handrail of the treadmill and to a standard gait belt wrapped around the participant’s pelvis. Notably, the assistance force remained nearly constant (Figure 1) and was present during the entire gait cycle, including both limb’s propulsive phases.
Anterior assistance during paretic propulsion (AnteriorParetic): In this condition, we modified the timing of the anterior pull force on the COM by using a novel design that applied the anterior force during paretic push-off. To achieve this, the elastic tubing was extended anteriorly from the paretic (for Stroke) or right (for Control) ankle, looped over a pulley in front of the treadmill and attached to the anterior pelvis (Figure 2). As the paretic limb began stance and was drawn into extension by the treadmill, the anteriorly directed force on the COM increased until the end of stance. As the foot came off the ground, the force diminished (Figure 1). Because the force is time-varying we set the peak of the anteriorly directed force to 5-10% BW. Thus, the AnteriorParetic and AnteriorConstant conditions received equivalent peak anteriorly directed forces.
Unassisted: Participants walked on the treadmill without receiving any applied forces to the COM.
Figure 1:
Magnitude of applied force to the COM during the Bilateral (black), unilateral/anterior (blue), and posterior (red dashed) conditions. Values represent the mean of all participants from the Stroke group only. Note that the anterior/unilateral and posterior conditions apply peak force to the COM during late stance. Vertical lines indicate toe-off.
Figure 2:
Schematic of test conditions. Study one is depicted by the black solid box and tests the timing effect of anterior assistance applied throughout the gait cycle (Bilateral) or coinciding with just one limb (Unilateral). Study two is depicted by the red dashed box and tests the effect of unilateral propulsive assistance (Anterior) and resistance (Posterior).
Study 2 Conditions
Similar to Study 1, the order of conditions for each participant was randomly selected. Here, our goal was to determine the presence of an association between metabolic energy expenditure and paretic propulsion at the COM level. We therefore designed conditions that would manipulate the direction (anterior vs posterior) of forces to the COM, so that we could measure the resulting metabolic energetics.
Anterior assistance during paretic propulsion: This condition is identical to that described above as AnteriorParetic. Notably, this condition provides anterior assistance to the COM during only half of the gait cycle, and coincides with paretic propulsion in the Stroke group.
Posterior assistance during paretic propulsion (PosteriorParetic): The elastic tubing was attached to the front of the paretic (for Stroke) or right (for Controls) ankle and looped through a series of four pulleys to attach to the posterior pelvis (Figure 2). This condition stretches the tubing with limb extension (like the AnteriorParetic condition), but induces a posteriorly-directed force on the COM. As a result, there is an impeding force to the COM that coincides with paretic (or right, for the Control group) propulsion. This allows us to test the influence of an impairment in unilateral propulsion. Similar to the AnteriorParetic condition, the peak of the imposed force was ~5-10% of body weight.
Unassisted: Participants walked on the treadmill without receiving any applied forces to the COM.
During all conditions requiring imposed forces to the COM, the investigators provided verbal cues about positioning on the treadmill, which allowed participants to maintain the appropriate force from the tubing.
Data Collection and Processing
Throughout each walking condition, we sampled ground reaction forces from the treadmill and the imposed force to the COM from the load cell at 1200Hz. Gas exchange was measured for each condition using a portable metabolic cart e which was calibrated prior to each session using a known concentration of gas. All participants began testing with 5 minutes of quiet sitting to determine baseline energy cost at rest. Throughout each condition, oxygen consumption (VO2; mL·kg−1·min−1) and carbon dioxide production (VCO2) were collected on a breath-by-breath basis. The mask was removed during each rest break for participant comfort.
Net metabolic data from the fourth (Stroke group) or fifth (Control group) minute of walking were normalized to BW and speed (m/s) to yield cost of walking (ml O2/kg/m). Ensemble curves of braking and propulsive forces were calculated for the final minute of each condition. We then extracted the peaks from the ensemble curves for analysis. The peak pull force on the COM associated with each stride was extracted and averaged separately for each condition using custom-written LabVIEW software.f
Data Analysis
Shapiro-Wilks tests and Q-Q plots indicated no deviations of normality in our data. We then performed within-subject statistical analyses g separately for each study. Specifically, we compared the cost of walking (COW), peak propulsion, and peak braking forces between groups using a repeated-measure ANOVA (repeated for condition) with Bonferroni-corrected paired samples t-tests used as post-hoc tests, as necessary. Effect sizes are included as η2p or Cohen’s d, as appropriate. An a-priori power analysis for COW estimated that a sample of 6 participants would be sufficient to detect a difference between the Unassisted and AnteriorParetic conditions for the Stroke group with an effect size of 0.5 (Cohen’s f),14 power of 0.8 and a significance level of α= 0.05. Nevertheless, we increased the sample size somewhat to be consistent with prior literature using similar techniques.20, 27
RESULTS
Effect of timing of anteriorly directed force on metabolic energetics
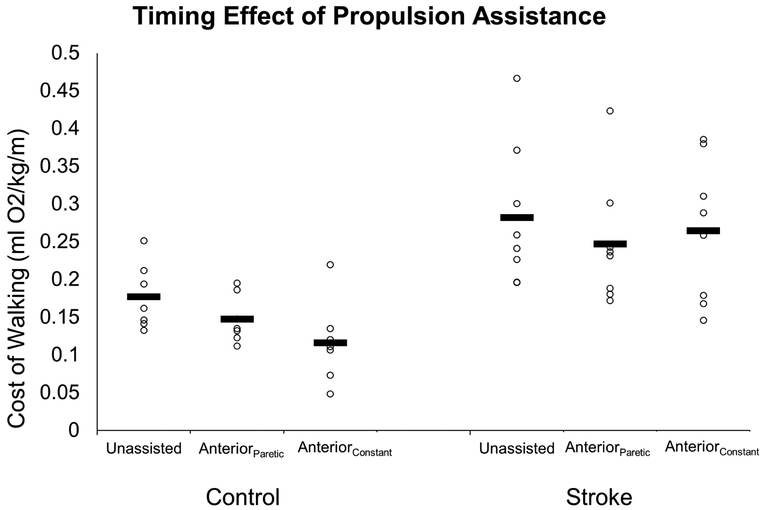
Demographic data were comparable between the two groups (Table 1). The Stroke group walked significantly slower (0.73±0.29 m/s) overground than the Control group (1.38±0.24 m/s; p<0.001), but the treadmill speeds represented a comparable percentage of their comfortable overground speed (Stroke: 87±5 % of CGS; Control: 90±10 % of CGS; p=0.481). During treadmill walking, we observed a significant condition by group interaction (p=0.050; η2p=0.224; Figure 3) for the metabolic COW, indicating that the groups did not respond similarly to the anterior conditions. Nevertheless, we observed that the Stroke group exhibited a significantly greater COW across all conditions compared to the Control group (p=0.006; η2p=0.452). Within the Stroke group, only the AnteriorParetic condition demonstrated a significant reduction compared to the Unassisted condition (p=0.006; d=1.67) representing a 12±5% decrease. The AnteriorConstant assistance, however, did not alter COW in the Stroke group (p=0.907; d=0.39). In contrast, the Control group exhibited their largest reduction in COW with the AnteriorConstant condition (p=0.018; d=1.58), but also demonstrated reduced COW with AnteriorParetic assistance (p=0.032; d=1.38).
Figure 3:
Cost of walking for the Control (left) and Stroke (right) groups during unassisted walking, walking with unilateral propulsive assistance (Anteriorparetic), or bilateral propulsive assistance (AnteriorConstant). Open circles represent individual subjects and horizontal bars represent the condition means.
The anteriorly directed force during the AnteriorParetic condition peaked at 60±13 % of the gait cycle, coinciding with terminal stance. The peak anteriorly directed force magnitude applied to the COM was not different between conditions (p=0.322; ηp2 = 0.075) for the Stroke group (AnteriorConstant: 7.8±1.8 %BW; AnteriorParetic: 7.0±2.5 %BW) or the Control group (AnteriorConstant: 8.4±1.9 %BW; AnteriorParetic: 8.3±2.6 %BW). The applied force influenced the peak propulsive and braking forces of both groups (Table 2).
Table 2:
Peak Anterior-Posterior Ground Reaction Forces for Study 1
| Stroke | Control | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Unassisted | Anteriorconstant | Anteriorparetic | Unassisted | Anteriorconstant | Anteriorparetic | Main effect: Group |
Main effect: Condition |
Interaction effect |
|
| Paretic/Tested Limb | |||||||||
| Peak Propulsion (%BW) | 0.08±0.02 | 0.04±0.03* | 0.00±0.02* | 0.19±0.04 | 0.14±0.04* | 0.07±0.03* | p<0.001 | p<0.001 | p=0.032 |
| Peak Braking (%BW) | −0.11±0.10 | −0.16±0.11* | −0.13±0.10 | −0.19±0.05 | −0.29±0.09* | −0.26±0.08* | p=0.031 | p<0.001 | p=0.019 |
| Non-Paretic/Non-Tested Limb | |||||||||
| Peak Propulsion (%BW) | 0.10±0.06 | 0.06±0.04* | 0.08±0.06 | 0.20±0.05 | 0.14±0.04* | 0.16±0.05* | p=0.006 | p<0.001 | p=0.179 |
| Peak Braking (%BW) | −0.07±0.04 | −0.13±0.06* | −0.12±0.04* | −0.19±0.05 | −0.29±0.08* | −0.28±0.07* | p<0.001 | p<0.001 | p=0.017 |
indicates significantly different from Unassisted condition (p<0.05).
Effect of the direction of the applied force on metabolic energetics
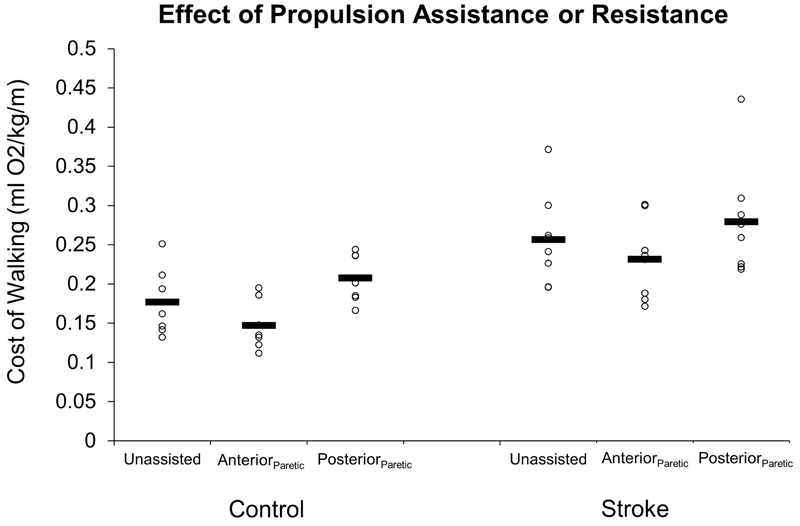
With regards to the COW, we observed no interaction effect (group × condition; p=0.747; ηp2=0.022), but did observe a significant main effect for group (p=0.007; ηp2=0.443) and a significant main effect for condition (p<0.001; ηp2=0.621). In particular, the AnteriorParetic assistance significantly reduced the COW (p=0.005; d=1.00), whereas the PosteriorParetic resistance significantly increased the COW (p=0.012; d=0.89) compared to the Unassisted walking condition (Figure 4). Participants were exposed to equivalent peak forces during each condition (p=0.516; ηp2=0.033) for both the Stroke group (AnteriorParetic: 7.3±2.1 % BW; PosteriorParetic: 6.2±2.1 %BW) and the Control group (AnteriorParetic: 8.3±2.6 % BW; PosteriorParetic: 8.8±3.1 %BW). Alterations to both peak propulsion and peak braking forces were apparent for both groups (see Table 3).
Figure 4:
Cost of walking for the Control (left) and Stroke (right) groups during unassisted walking, and walking with unilateral propulsive assistance (Anteriorparetic), or resistance (Posteriorparetic). Open circles represent individual subjects and horizontal bars represent the condition means.
Table 3:
Peak Anterior-Posterior Ground Reaction Forces for Study 2
| Stroke | Control | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Unassisted | Anteriorparetic | Posteriorparetic | Unassisted | Anteriorparetic | Posteriorparetic | Main effect: Group |
Main effect: Condition |
Interaction effect |
|
| Paretic/Tested Limb | |||||||||
| Peak Propulsion (%BW) | 0.06±0.02 | 0.00±0.02* | 0.02±0.03* | 0.19±0.04 | 0.07±0.03* | 0.11±0.04* | p<0.001 | p<0.001 | p=0.041 |
| Peak Braking (%BW) | −0.12±0.09 | −0.14±0.10 | −0.14±0.10 | −0.19±0.05 | −0.26±0.08* | −0.19±0.07 | p=0.019 | p=0.001 | p=0.090 |
| Non-Paretic/Non-Tested Limb | |||||||||
| Peak Propulsion (%BW) | 0.11±0.05 | 0.08±0.06 | 0.13±0.06 | 0.20±0.05 | 0.16±0.05* | 0.19±0.05 | p=0.017 | p<0.001 | p=0.081 |
| Peak Braking (%BW) | −0.06±0.03 | −0.11±0.04* | −0.03±0.03* | −.019±0.05 | −0.28±0.07* | −0.14±0.05* | p<0.001 | p<0.001 | p=0.003 |
indicates significantly different from Unassisted condition (p<0.05).
DISCUSSION
These data support our hypotheses that (1) propulsive forces influence metabolic energetics after stroke and that (2) there are important timing effects associated with restoring propulsive forces. In particular, we observed that an anterior force applied to the body’s COM that coincides with paretic propulsion was able to reduce the metabolic COW for individuals with chronic hemiparesis due to stroke. Further, the fact that COW was reduced with propulsion assistance (Anteriorparetic), whereas propulsion resistance (Posteriorparetic) caused an increase in COW, suggests that rehabilitation strategies that ameliorate the reduced paretic propulsion will result in an overall reduction in COW.14
An anteriorly directed force that is applied to the COM acts as a substitute for the limb’s propulsive forces (as seen in Tables 2 and 3), and thus contributes to reduced metabolic costs of walking in unimpaired populations.20, 27 Our findings extend this knowledge to suggest that there is an important timing component to the horizontal force application for individuals post-stroke. In particular, the timing and coordination of paretic propulsion are believed to play an important factor in hemiparetic gait.17 Indeed, we observed that when force was applied throughout the gait cycle, it did not reduce the COW in individuals post-stroke, whereas, when the anteriorly directed force coincided with paretic propulsion the excessive energy cost was substantially reduced. The lack of metabolic benefit during the Anteriorconstant condition may be due to the Stroke group’s already higher braking forces 28 being exaggerated during non-paretic propulsion of the Anteriorconstant condition.29 Indeed, we observed that the peak braking force was exaggerated during Anteriorconstant, but not during Anteriorparetic in the Stroke group. Thus, the paretic limb’s propulsion deficit appears to represent a suitable rehabilitation target for reducing the high COW following stroke.
Our findings provide compelling evidence that the high energy COW after stroke can be reduced by targeting the forces applied to the COM. Prior work related to propulsion mechanics, however, has focused at the joint or muscle level.13, 14, 30 Specifically, these interventions have been largely limited to addressing altered ankle mechanics through functional electrical stimulation14 or exoskeletons.13, 30 Although such joint- or muscle-level approaches represent indirect means of manipulating the whole body COM mechanics and energy cost, these approaches may be most effective for individuals with a single impairment. In contrast, directly addressing the body’s COM mechanics may serve an important complementary approach for individuals with multiple joint or muscle deficits. Indeed, the variety of muscle and joint responses following stroke31, 32 may limit the generalizability of approaches that target a single joint. Thus, we propose that a focus on the mechanics of the COM will result in more meaningful gains because the functional goal of smooth forward progression allows the individual to select the most appropriate neuromuscular response within their available repertoire. Indeed, our focus on COM mechanics yielded an immediate 12% reduction in COW, whereas approaches that target the ankle joint using exoskeletons/suits produce somewhat smaller changes.13, 30 Future work is needed, however, to definitively compare COM approaches with joint-level approaches for improving metabolic cost of walking. Furthermore, because the COW is a whole body estimate, it makes sense that interventions would target the COM, which is also a whole body estimate. In support of this idea, prior work that used visual feedback to minimize fluctuations in vertical COM height during walking after stroke successfully reduced the energy cost of walking,8 despite the fact that minimizing vertical COM height is not desirable.7 Considering this, clinical interventions designed to reduce the energy cost of walking may be effective if they include anterior manipulation of the COM at a time in the gait cycle when smooth forward progression is disrupted: the latter half of the paretic stance phase. Our ongoing work seeks to elucidate methods to encourage participants to modulate the forces applied to the COM by internally generating limb forces. For example, feedback of COM anterior acceleration should provide a suitable surrogate for limb propulsive forces during walking and would promote subject-specific changes in muscle function that are appropriate given the present impairments. This is fundamentally different than approaches that target individual joints (i.e., robotics/exoskeletons) or muscles (i.e., FES), and represents our ongoing work.
Appropriate timing of the anterior assistance produced a substantial (i.e., ~12%) decrease in the energy cost of walking. The additional assistance would likely allow a patient to walk longer or further without fatigue, which may be beneficial for increasing stepping training. Nevertheless, we are not advocating that this technique be used as a rehabilitation intervention. Because the anterior assistance acts as a substitute for the deficient paretic propulsive force, as seen in Tables 2 and 3, an individual would require less muscle activity.20 Training under this condition would therefore occur at a low intensity, making it less optimal for eliciting long-term neuromuscular changes.33 Instead, the Posteriorparetic resistance represents an error augmentation strategy34 and may be capable of generating greater propulsion through adaptive feed-forward processes.35 In chronic stroke, exaggeration of error is thought to be beneficial because it provides a deviation substantial enough for the nervous system to detect and therefore, correct altered movements.36, 37 In the Control subjects, this unilateral reduction in propulsive force at the COM mimicked the reduced paretic propulsion commonly seen post-stroke.10, 18 Consequently, they experienced a significant increase in metabolic cost of walking. Thus, our paradigm simulated reduced paretic propulsion in our Control subjects and exaggerated the existing paretic propulsive deficits in our Stroke group. Our results therefore provide compelling evidence regarding the importance of improving paretic propulsive forces for reducing metabolic expenditure. Furthermore, these results indicate our Posteriorparetic condition effectively challenged forward progression.
Study Limitations
A potential limitation to this work is that we did not quantify handrail use. Although we were careful to ensure consistency of handrail use within a subject (between conditions), we did not document which participants used or did not use the handrail. Thus, variations in handrail use between subjects could have influenced the effects of the imposed COM force on gait mechanics and metabolic cost. Additionally, whereas propulsive forces clearly have an important role in manipulating the energy cost of walking, there are other components to abnormal gait that need to be acknowledged. For example, leg swing provides a small (< 5% per leg) energy cost of walking.29 The role of leg swing is particularly critical here, however, because it is frequently impaired following stroke.38, 39 Notably, both the Anteriorparetic and Posteriorparetic conditions also helped initiate leg swing on the paretic/tested limb of our subjects. Specifically, the applied force to the ankle increased as the treadmill translated the foot posteriorly during stance (Figure 1) and was briefly present as the foot was lifted off the treadmill into the beginning of swing. Because the assistance to initiate swing was comparable between conditions, we do not believe that small amount of leg swing assistance influenced the interpretation of the results, especially given the magnitude of change attributed to the COM propulsive forces. Additionally, BW support requires metabolic cost and is estimated to constitute ~25-30% of the net metabolic cost of walking.40 Importantly, our protocol did not provide BW support for any of our participants. Clearly, propulsive forces are a key determinant in metabolic cost and thus the deficits in propulsion following stroke are energetically costly.
Conclusions
Future studies should investigate the role of self-generated internal forces from the legs applied to the COM on the forward progression and metabolic cost of hemiparetic walking. The decrease in energetic cost of walking associated with anterior COM motion found in this study suggest that hemiparetic gait can be improved with a focus on propulsive forces applied to the body’s COM. Importantly, these favorable energetic effects can be elicited with interventions that target only the portion of the gait cycle during which the paretic limb is providing propulsion.
Acknowledgments
Funding: The project described was supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award Number UL1TR002489. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
List of Abbreviations:
- ANOVA
analysis of variance
- COM
center of mass
- COW
cost of walking
- VCO2
volume of carbon dioxide
- VO2
volume of oxygen
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors have no conflict of interest to declare.
Prior Presentation: This work was presented at the annual meeting of the American Society of Biomechanics, July 2016, Raleigh, NC (Study 1) and at the APTA Combined Sections Meeting, February 2017, San Antonio, TX (Study 2)
Supplier List
Bertec Corp., Columbus, OH, USA
Zeno, Protokinetics, Havertown, PA, USA
MLP-100; Transducer Techniques, Temecula, CA, USA
Theratube; Theraband, Akron, OH, USA
K4b2; Cosmed, Chicago, IL, USA
National Instruments, Austin, TX, USA
SPSS, ver 24, Chicago, IL, USA
REFERENCES
- 1.Farris DJ, Hampton A, Lewek MD, Sawicki GS. Revisiting the mechanics and energetics of walking in individuals with chronic hemiparesis following stroke: from individual limbs to lower limb joints. J Neuroeng Rehabil 2015;12:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ganley KJ, Herman RM, Willis WT. Muscle metabolism during overground walking in persons with poststroke hemiparesis. Top Stroke Rehabil 2008;15(3):218–26. [DOI] [PubMed] [Google Scholar]
- 3.Platts MM, Rafferty D, Paul L. Metabolic cost of over ground gait in younger stroke patients and healthy controls. Med Sci Sports Exerc 2006;38(6):1041–6. [DOI] [PubMed] [Google Scholar]
- 4.Stoquart G, Detrembleur C, Lejeune TM. The reasons why stroke patients expend so much energy to walk slowly. Gait & posture 2012;36(3):409–13. [DOI] [PubMed] [Google Scholar]
- 5.Zamparo P, Francescato MP, De Luca G, Lovati L, di Prampero PE. The energy cost of level walking in patients with hemiplegia. Scandinavian journal of medicine & science in sports 1995;5(6):348–52. [DOI] [PubMed] [Google Scholar]
- 6.Kuo AD, Donelan JM, Ruina A. Energetic consequences of walking like an inverted pendulum: step-to-step transitions. Exerc Sport Sci Rev 2005;33(2):88–97. [DOI] [PubMed] [Google Scholar]
- 7.Gordon KE, Ferris DP, Kuo AD. Metabolic and mechanical energy costs of reducing vertical center of mass movement during gait. Archives of physical medicine and rehabilitation 2009;90(1):136–44. [DOI] [PubMed] [Google Scholar]
- 8.Massaad F, Lejeune TM, Detrembleur C. Reducing the energy cost of hemiparetic gait using center of mass feedback: a pilot study. Neurorehabilitation and neural repair 2010;24(4):338–47. [DOI] [PubMed] [Google Scholar]
- 9.Balasubramanian CK, Bowden MG, Neptune RR, Kautz SA. Relationship between step length asymmetry and walking performance in subjects with chronic hemiparesis. Archives of physical medicine and rehabilitation 2007;88(1):43–9. [DOI] [PubMed] [Google Scholar]
- 10.Bowden MG, Balasubramanian CK, Neptune RR, Kautz SA. Anterior-posterior ground reaction forces as a measure of paretic leg contribution in hemiparetic walking. Stroke; a journal of cerebral circulation 2006;37(3):872–6. [DOI] [PubMed] [Google Scholar]
- 11.Hsiao H, Knarr BA, Higginson JS, Binder-Macleod SA. Mechanisms to increase propulsive force for individuals poststroke. J Neuroeng Rehabil 2015;12:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peterson CL, Hall AL, Kautz SA, Neptune RR. Pre-swing deficits in forward propulsion, swing initiation and power generation by individual muscles during hemiparetic walking. J Biomech 2010;43(12):2348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahashi KZ, Lewek MD, Sawicki GS. A neuromechanics-based powered ankle exoskeleton to assist walking post-stroke: a feasibility study. J Neuroeng Rehabil 2015;12:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Awad LN, Reisman DS, Pohlig RT, Binder-Macleod SA. Reducing The Cost of Transport and Increasing Walking Distance After Stroke: A Randomized Controlled Trial on Fast Locomotor Training Combined With Functional Electrical Stimulation. Neurorehabilitation and neural repair 2016;30(7):661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Detrembleur C, Dierick F, Stoquart G, Chantraine F, Lejeune T. Energy cost, mechanical work, and efficiency of hemiparetic walking. Gait & posture 2003;18(2):47–55. [DOI] [PubMed] [Google Scholar]
- 16.Huang TW, Shorter KA, Adamczyk PG, Kuo AD. Mechanical and energetic consequences of reduced ankle plantar-flexion in human walking. The Journal of experimental biology 2015;218(Pt 22):3541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soo CH, Donelan JM. Coordination of push-off and collision determine the mechanical work of step-to-step transitions when isolated from human walking. Gait & posture 2012;35(2):292–7. [DOI] [PubMed] [Google Scholar]
- 18.Mahon CE, Farris DJ, Sawicki GS, Lewek MD. Individual limb mechanical analysis of gait following stroke. J Biomech 2015;48(6):984–9. [DOI] [PubMed] [Google Scholar]
- 19.Whittle MW. Three-dimensional motion of the center of gravity of the body during walking. Hum Mvt Sci 1997;16:347–55. [Google Scholar]
- 20.Gottschall JS, Kram R. Energy cost and muscular activity required for propulsion during walking. J Appl Physiol (1985) 2003;94(5):1766–72. [DOI] [PubMed] [Google Scholar]
- 21.Minetti AE, Ardigo LP, Saibene F. Mechanical determinants of gradient walking energetics in man. J Physiol 1993;472:725–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Margaria R. Positive and negative work performances and their efficiencies in human locomotion. Internationale Zeitschrift fur angewandte Physiologie, einschliesslich Arbeitsphysiologie 1968;25(4):339–51. [DOI] [PubMed] [Google Scholar]
- 23.Awad LN, Binder-Macleod SA, Pohlig RT, Reisman DS. Paretic Propulsion and Trailing Limb Angle Are Key Determinants of Long-Distance Walking Function After Stroke. Neurorehabilitation and neural repair 2015;29(6):499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hurt CP, Burgess JK, Brown DA. Limb contribution to increased self-selected walking speeds during body weight support in individuals poststroke. Gait & posture 2015;41(3):857–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donelan JM, Kram R, Kuo AD. Simultaneous positive and negative external mechanical work in human walking. J Biomech 2002;35(1):117–24. [DOI] [PubMed] [Google Scholar]
- 26.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med 1975;7(1):13–31. [PubMed] [Google Scholar]
- 27.Zirker CA, Bennett BC, Abel MF. Changes in kinematics, metabolic cost and external work during walking with a forward assistive force. J Appl Biomech 2013;29(4):481–9. [DOI] [PubMed] [Google Scholar]
- 28.Turns LJ, Neptune RR, Kautz SA. Relationships between muscle activity and anteroposterior ground reaction forces in hemiparetic walking. Archives of physical medicine and rehabilitation 2007;88(9): 1127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gottschall JS, Kram R. Energy cost and muscular activity required for leg swing during walking. J Appl Physiol (1985) 2005;99(1):23–30. [DOI] [PubMed] [Google Scholar]
- 30.Awad LN, Bae J, O'Donnell K, De Rossi SMM, Hendron K, Sloot LH et al. A soft robotic exosuit improves walking in patients after stroke. Science translational medicine 2017;9(400). [DOI] [PubMed] [Google Scholar]
- 31.De Quervain IA, Simon SR, Leurgans S, Pease WS, McAllister D. Gait pattern in the early recovery period after stroke. J Bone Joint Surg Am 1996;78(10):1506–14. [DOI] [PubMed] [Google Scholar]
- 32.Mulroy S, Gronley J, Weiss W, Newsam C, Perry J. Use of cluster analysis for gait pattern classification of patients in the early and late recovery phases following stroke. Gait & posture 2003;18(1):114–25. [DOI] [PubMed] [Google Scholar]
- 33.Israel JF, Campbell DD, Kahn JH, Hornby TG. Metabolic costs and muscle activity patterns during robotic- and therapist-assisted treadmill walking in individuals with incomplete spinal cord injury. Physical therapy 2006;86(11):1466–78. [DOI] [PubMed] [Google Scholar]
- 34.Lewek MD, Braun CH, Wutzke C, Giuliani C. The role of movement errors in modifying spatiotemporal gait asymmetry post stroke: a randomized controlled trial. Clin Rehabil 2018;32(2):161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bastian AJ. Understanding sensorimotor adaptation and learning for rehabilitation. Current opinion in neurology 2008;21(6):628–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Helm EE, Reisman DS. The Split-Belt Walking Paradigm: Exploring Motor Learning and Spatiotemporal Asymmetry Poststroke. Physical medicine and rehabilitation clinics of North America 2015;26(4):703–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wutzke CJ, Faldowski RA, Lewek MD. Individuals Poststroke Do Not Perceive Their Spatiotemporal Gait Asymmetries as Abnormal. Physical therapy 2015;95(9):1244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Savin DN, Tseng SC, Morton SM. Bilateral adaptation during locomotion following a unilaterally applied resistance to swing in nondisabled adults. J Neurophysiol 2011;104(6):3600–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yen SC, Schmit BD, Wu M. Using swing resistance and assistance to improve gait symmetry in individuals post-stroke. Human movement science 2015;42:212–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grabowski A, Farley CT, Kram R. Independent metabolic costs of supporting body weight and accelerating body mass during walking. J Appl Physiol 2005;98(2):579–83 [DOI] [PubMed] [Google Scholar]