Abstract
The current study evaluated a mechanistic pathway by which prenatal stress increases the risk of postpartum depressive (PPD) symptoms via observed dyadic emotional, behavioral, and attentional dysregulation and associated cortisol responses during mother-infant interactions.
Methods.
Participants included 322 low-income Mexican American mother-infant dyads. Depressive symptoms, economic hardship, and negative life events were assessed at a prenatal visit. Dysregulation in dyadic (mother-infant) interactions and cortisol responses to mother-infant interaction were evaluated at 12 weeks after the birth. Twenty-four weeks after the birth, PPD symptoms were predicted from prenatal stress (negative life events and economic hardship) and prenatal depressive symptoms, mediated through dyadic dysregulation and maternal and infant cortisol responses.
Results.
More negative life events in the prenatal period predicted more dyadic dysregulation at 12 weeks postpartum. Dyadic dysregulation and economic hardship predicted elevated 12-week infant cortisol total response and reactivity, and higher total infant cortisol response predicted higher maternal PPD symptoms at 24 weeks. Maternal cortisol response was not associated with dyadic dysregulation, either form of prenatal stress, or PPD symptoms.
Conclusion.
The results indicate the salience of early psychosocial processes and mother-infant relationship challenges for subsequent maternal affective wellbeing.
Keywords: Postpartum depression, dyadic dysregulation, cortisol, prenatal stress
Introduction
Postpartum depression (PPD) represents a sizeable public health concern, with wide-ranging negative consequences for women and their children. Ethnic minority and low-income women, particularly recent immigrants, are at substantially elevated risk for PPD, and are less likely to receive treatment (Institute of Medicine, 2001; Liu & Tronick, 2013). Given that the Hispanic population is the largest ethnic minority group in the U.S. (U.S. Census Bureau, 2017), the majority of whom (approximately 64%) are of Mexican origin (Gonzales-Barrera & Lopez, 2013), research focused on Mexican-origin women and their infants is necessary to reduce the public health burden of PPD. PPD occurs within a complex sociocultural context, aspects of which can either increase or decrease depression, yet few studies of perinatal mental health have been conducted with Mexican-origin women and their infants, and little is known about how prenatal psychosocial risk factors might influence the emerging mother-infant relationship, associated physiological regulation, and maternal well-being in the postpartum period.
Mothers carry a level of risk for depression into the postpartum period stemming from prior history and hardship in the prenatal period (Yim, Stapleton, Guardino, Hahn-Holbrook, & Dunkel Schetter, 2015). Well-known psychosocial risk factors for PPD include a history of depression, prenatal stress, low socioeconomic status (SES), low education, lack of health insurance, and low social support (Beck, 2001; Segre et al., 2007; Yim et al., 2015). Prenatal stressors, conceptualized a variety of ways, are commonly associated with greater risk of PPD symptoms. Major life event stress, including stressors such as death of a loved one or housing difficulties, are positively correlated with PPD symptoms in general population samples (Milgrom et al., 2008; Dennis, Janssen, & Singer, 2004) and among Hispanic women specifically (Zayas, Jankowski, & McKee, 2005; Lara-Cinisomo et al., 2017). For low-income women, financial or economic stressors are especially salient, and have been strongly linked to the risk of PPD (Ritter et al., 2000; Rich-Edwards et al., 2006; Segre, O’Hara, Arndt, & Stuart, 2007). These risks are particularly salient for Mexican origin women in the U.S. who, compared to non-Hispanic white women, are more likely to live in poverty, less likely to have graduated from high school, more likely to be unemployed or underemployed, and less likely to have health insurance (U.S. Census Bureau, 2017).
Biological models of risk for PPD development generally focus on prenatal hormones and the impact of dramatic alterations in reproductive and stress hormone levels following childbirth (Yim et al., 2015). In the context of maternal stress and depression, neuroendocrine activity is particularly relevant. Stress and affective disorders have been associated with dysregulated cortisol in general population samples (McEwen & Wingfield, 2003; Powers et al., 2016), and among pre- and postpartum women (Diego et al., 2006; Glynn, Davis & Sandman, 2013; Jolley, Elmore, Barnard, & Carr, 2007; Taylor, Glover, Marks, & Kammerer, 2009).
Postpartum depression poses significant risks for women as well as for their newborn infants (Goodman et al., 2011; Field, 2010; Gress-Smith, Luecken, Lemery-Chalfant, & Howe, 2012). One route through which PPD may negatively impact maternal and child health is through disruption of sensitive parenting and impaired quality of mother-infant interactions (Diener et al., 2003; Goodman & Gotlib, 2002). Mothers with elevated postpartum depressive symptoms are less affirming and more negating of infant experience (Murray, Fiori-Cowley, & Hooper, 1996), limited in their capacity to engage positively with their infant during social interactions (Murray et al., 2003), and struggle to read their infant’s affective communication and respond appropriately (Tronick, 2007). Psychosocial adversity also negatively affects the quality of mother-infant interactions (Boyd, Zayas, & McKee, 2006; Cmic et al., 2005; Field, 1998).
During the early infancy period, the quality of mother-infant interactions is largely based on the mother’s capacity to modulate arousal and promote attunement with her infant (Tronick, 2007). Despite infants being limited in their ability to modulate their own arousal, they are sensitive to their mother’s communicative behaviors (Csibra, 2010; Nakata & Trehub, 2004). However, as Cole et al. (2004) note, in mother-infant interactions, each partner’s emotions regulates the other’s and are regulated by the other’s. Similar to states of regulation and dysregulation of the mother and the infant separately, the dyad they form is also a functional unit with its own state of regulation and dysregulation. Well-regulated dyads are characterized by high levels of mutual attunement, allowing the dyad to recover smoothly and quickly from breaks in synchrony that may occur. Conversely, dyads may be considered dysregulated to the degree that they have difficulty modulating the frequency, intensity, lability, and duration (Thompson, 1994) of their collective levels of dyadic emotional, behavioral, and attentional arousal.
A cycle of mutual regulatory problems may become established between distressed mothers and their children, particularly during challenging social contexts (Weinberg, Olson, Beeghly, & Tronick, 2006). Maternal prenatal distress has been linked with lower quality dyadic interaction during the infancy period (Goldstein & Diener, 1996). In the postpartum, maternal distress, economic stress, and negative life events have similarly been associated with more disturbed, negative, and/or less synchronous mother-infant interactions (Boyd, et al., 2006; Coyl, Roggman, & Newland, 2002; Kaitz & Maytal, 2005; Murray, Fiori-Cowley, & Hooper, 1996). Among low-income Mexican-American families, maternal prenatal stress predicts problematic regulatory behaviors of infants (Lin, Crnic, Luecken, & Gonzales, 2018) and has been linked with more problematic dyadic interaction during the infancy period (Coburn, Crnic, & Ross, 2015). Mothers who are not struggling with depressive symptoms or stress may be better able to maintain connection and communication with their infants in the face of challenge, but distressed mothers may impair the functioning of the dyadic unit through failure to recover from their own negative arousal or failure to regulate the arousal of their infant.
Despite broad empirical support for an elevated risk of dysregulation in mother-infant interactions in the context of maternal distress (Broth et al., 2004; Feldman et al., 2004; Goodman & Gotlib, 1999; Lovejoy, Graczyk, O’Hare, & Neuman, 2000), we know little about the impact of dyadic dysregulation on women’s PPD symptoms. Difficulty co-regulating an aroused infant may foster negative maternal cognitions (e.g., low maternal self-efficacy;Navarro, Navarrete, & Lara, 2011), undermine mother-infant bonding, and minimize the affective boost that can result from mutually positive interactions between mother and infant (Feldman, 2007). For Latina mothers, who place a high value on the maternal role (Gress-Smith et al., 2013), difficulties establishing a well-regulated relationship with their new baby may be especially distressing. Dysregulated mother-infant interactions across the early infancy period may play an integral role in PPD symptoms, particularly among ethnic minority and low-income women facing significant socioeconomic and/or psychosocial adversity.
Physiological stress responses resulting from behaviorally and emotionally dysregulated mother-infant interactions may also help explain the development of PPD symptoms. Less commonly evaluated than behavioral or emotional factors, but equally important within the framework of dyadic dysregulation, is the influence of mother-infant interaction on physiological stress processes. Prior studies demonstrate that external factors such as SES can directly affect both mother and infant neuroendocrine stress responses (Chen et al., 2010; Cohen, Doyle, & Baum, 2006; Clearfield, Carter-Rodriguez, Merali, & Shober, 2014). Further, the quality of mother-infant interaction may affect biological stress responses. In a recent correlational study, lower maternal positive engagement synchrony and higher maternal intrusiveness in motherinfant interaction at six months were correlated with higher maternal hair cortisol, suggestive of chronic physiological stress (Tarullo, St. John, & Meyer, 2017). As a result of poorly coregulated interactions, children may become overly reliant on underdeveloped self-regulatory strategies, which tax developing stress response systems and can lead to exaggerated HPA responsivity (Feldman et al., 2009; Gunnar & Donzella, 2002; Vanska et al., 2016). The adverse effects of maternal stress and depressive symptoms on infant cortisol regulation are well documented (e.g., Pratt et al., 2017; Letourneau et al., 2011; Luecken et al., 2013), especially among infants who use more independent regulatory strategies (Khoury et al., 2016). However, little is known about the effect of infant physiological arousal on their mothers’ affective state. As a result, we know little about the implications of infant cortisol regulation for the development and course of maternal PPD symptomatology.
The current study is informed by a biopsychosocial model in which maternal prenatal stress and depressive symptoms are theorized to negatively impact maternal and infant dyadic regulation during interactions, leading to elevated maternal and infant cortisol responses to the interactions and ultimately, higher maternal PPD symptoms (see Figure 2). A large number of risk factors for PPD have been identified, but surprisingly little research has focused explicitly on the process by which the mother’s relationship with her new baby affects her symptoms of PPD, despite the fact that childbirth makes PPD unique relative to depression at other times in a woman’s life. We theorize that prenatal risk factors directly contribute to the onset of PPD symptoms in Mexican-origin women, but emerging patterns of dyadic regulation and physiological responses to dyadic dysregulation also play a role, and serve to mediate the effects of prenatal stressors on postpartum depressive symptoms. We hypothesized that higher prenatal stress (operationalized as negative life events and economic hardship) would predict more dyadic emotional, attentional, and behavioral dysregulation and higher maternal and infant cortisol responses to dysregulated dyadic interaction at 12 weeks postpartum. Higher cortisol responses to the interaction were hypothesized to contribute to elevated symptoms of depression at 24 weeks postpartum. Importantly, we posit that it is not just the mother’s biological response to dysregulation that affects her PPD risk, but also that of her baby. That is, we propose that infant biological functioning, although typically evaluated with respect to infant outcomes, may also contribute to mother’s affective well-being. In a sample of low-income Mexican-origin women and infants, we evaluated a longitudinal mediation model in which prenatal stress and depressive symptoms predict dyadic dysregulation during mother-infant interactions and higher maternal and infant cortisol responses at 12 weeks postpartum, and higher maternal depressive symptoms at 24 weeks postpartum.
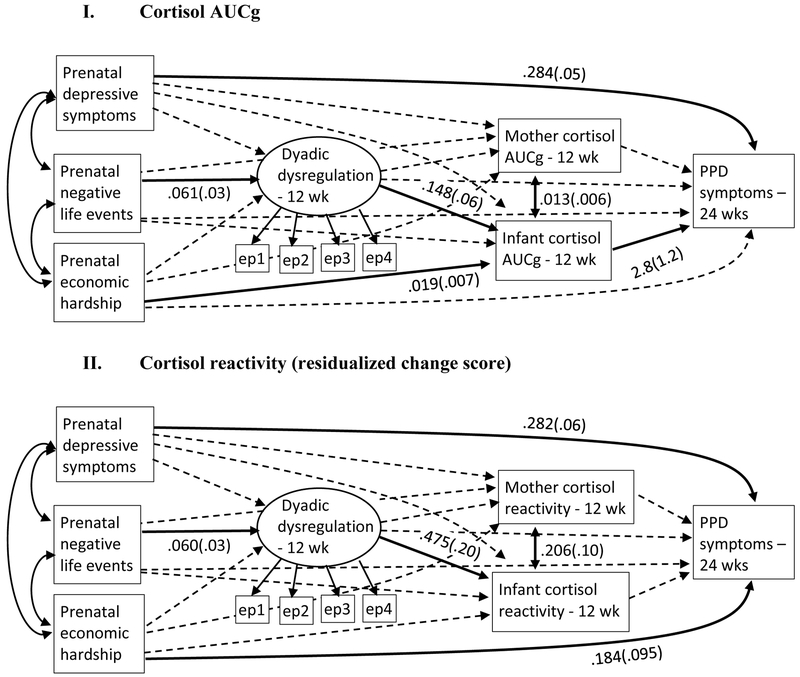
Figure 2. Mediational Models1.
1 Solid lines are statistically significant; dashed lines are non-significant (unstandardized estimates and SEs are shown for significant paths only); “PPD” = Postpartum depression; “ wk” = weeks; “ep1” = observed free play episode; “ep2” = observed soothing episode; “ep3” = observed teaching episode; “ep4” = observed peekaboo episode; Error terms and covariates (time of day at 12 weeks, maternal country of birth) not shown.
Materials and Methods
Participants.
Participants included 322 Mexican-origin mother-infant dyads (maternal mean age = 27.8, SD = 6.5, range 18-42). Participants were part of the larger Las Madres Nuevas study, which conducted home visits with mothers and infants at five time points: prenatal (26-38 weeks gestation; mean 35.4 weeks, SD = 2.8), and at 6-, 12-, 18-, and 24-weeks postpartum. The current analyses include data from the prenatal, 12-week, and 24-week visits. Pregnant women were recruited from prenatal clinics in Maricopa County, Arizona that serve low-income, uninsured, and/or undocumented women. During prenatal care appointments, potentially eligible pregnant women were approached by a female bilingual interviewer who explained the study and conducted an assessment of eligibility using the following criteria: 1) self-identification as Mexican or Mexican American, (2) fluency in English or Spanish, (3) age 18 or older, (4) low-income status (family income below $25,000 or eligibility for Medicaid or Federal Emergency Services coverage for the birth), (5) no prenatal evidence in the medical records of a serious infant health or developmental problem (e.g., Down’s Syndrome), and (6) anticipated delivery of a singlet baby. See Table 1 for sample demographics.
Table 1:
Sample demographics
| Maternal age; range, M (SD) | 18-42; 27.8 (6.5) |
|---|---|
| Maternal country of birth; N (%) | |
| Mexico | 278 (86%) |
| U.S. | 44 (14%) |
| Marital status; N (%) | |
| Married/living together | 249 (77%) |
| Single, never married | 49 (15%) |
| Separated/divorced | 24 (7%) |
| Maternal education; N (%) | |
| Less than high school | 190 (59%) |
| High school diploma/GED | 86 (27%) |
| Some college/technical school/college degree | 45 (14%) |
| Annual family income; N (%) | |
| < $5,000 | 44 (14%) |
| $5,000-$10,000 | 61 (19%) |
| $10,000-$15,000 | 87 (28%) |
| $15,000-$20,000 | 37 (12%) |
| $20,000-$25,000 | 40 (13%) |
| $25,000 and above | 45 (14%) |
| Missing/did not answer (n=8) | |
| Gestational age at birth (weeks); range, M (SD) | 26-42; 39.3 (1.4) |
| Premature birth (<37 weeks gestation), N (%) | 6 (1.8%) |
| Infant birthweight (g); range, M (SD) | 1190-4935; 3391 (461) |
| Low birthweight (<2500g), N (%) | 7 (2.2%) |
| Prenatal depressive symptoms; range, M (SD) | 0 -24; 5.91 (5.4) |
| Negative life events; range, M (SD) | 0 -11; 2.72 (2.2) |
| Economic hardship (z-score); range, M (SD) | −6.48 -10.06; 0 (3.0) |
| Dyadic dysregulation; range, M (SD) | 1 -4.5; 1.88 (.84) |
| Infant cortisol AUCg; range, M (SD) | .54 -2.20; 1.18 (.35) |
| Maternal cortisol AUCg; range, M (SD) | .06 -1.85; .783 (.28) |
| Infant cortisol reactivity (difference score), range, M (SD) | −1.13 -1.19; −.026 (.27) |
| Maternal cortisol reactivity (difference score), range, M (SD) | −.16 -.151; −.02 (.04) |
| Postpartum depressive symptoms; range, M (SD) | 0 – 22; 3.95 (4.4) |
Procedure.
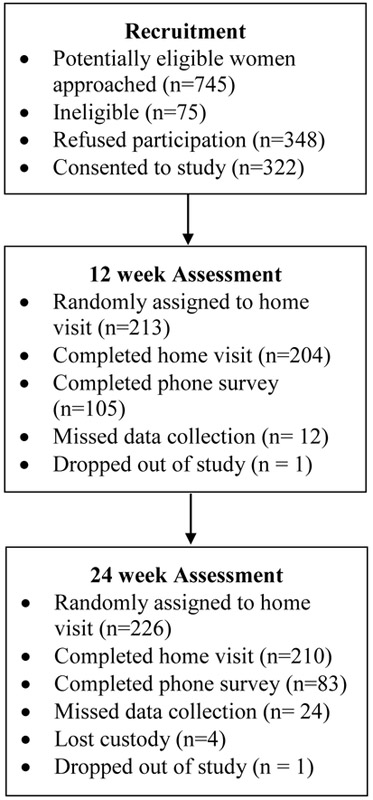
The study was approved by the Internal Review Boards at Arizona State University and Maricopa Integrated Health System. All interviews were conducted at participants’ homes, and were scheduled between 8 AM and 4:30 PM. To minimize participant burden while retaining statistical power, the study followed a “planned missingness” design (Enders, 2010) in which all women completed the prenatal visit, but were randomly assigned to complete two of three remaining home visits. The planned missingness design produces data missing completely at random, thus the missing data resulting from this technique are benign and do not introduce bias into parameter estimates (Enders, 2010). Random assignment to a missed visit was determined by a computer algorithm prior to the prenatal data collection. Of the 322 women who completed the prenatal visit, 204 (out of a randomly assigned 213; 96%) completed the 12 week visit, and 210 (out of a randomly assigned 226; 93%) completed the 24 week home visit. Women not assigned to a home visit were instead assigned to telephone surveys of postpartum depressive symptoms; at 24-weeks postpartum 83 women completed phone surveys, for a total 24-week retention of 91% (293 surveys out of 322). See Figure 1 for a flowchart of recruitment, retention, and data collection for the current analyses.
Figure 1.
Flowchart of recruitment and participation
Interviews were conducted in the participants’ choice of Spanish (82%) or English (18%). Informed consent and all survey questions were read aloud, and participants were provided with written and graphic descriptions of item response options. At the 12-week visit, saliva samples were collected from mother and infant before and after videorecorded interaction tasks that began approximately 30 minutes after arrival in the home. Interviews lasted approximately two hours. Women were compensated with $75 and small gifts (e.g., bath oils) at the prenatal interview, and $50 and small gifts (e.g., bath toys) at the 12- and 24-week visits.
Measures
Prenatal Stress:
At the prenatal visit, women completed the 20-item Economic Hardship Scale (EHS; Barrera et al., 2001; Cronbach’s α = .72), developed for low-income families as a subjective measure of economic hardship. Sample items include: “We had enough money to afford the kind of food we should have,” “A family member didn’t go to see the doctor or dentist when he or she needed to because we had to save money,” and “We fell far behind in paying bills.” Responses were converted to z-scores and summed. Higher scores indicate more economic hardship. Negative life events during pregnancy were obtained using 13 items from the Pregnancy Risk Assessment Monitoring System (PRAMS; CDC, 2009–2011). A Negative Life Event total was obtained by summing events that women endorsed experiencing since becoming pregnant. Sample items and the percent of women who endorsed them include: “Someone very close to you died” (22.7%), “You were homeless” (9%), “Husband/partner lost his job” (33.5%), and “Someone close to you had a bad problem with drinking or drugs” (16.1%).
Maternal Depressive Symptoms.
The 10-item Edinburgh Postnatal Depression Scale (EPDS; Cox et al., 1987) was given at the prenatal visit and 24 week visit (prenatal Cronbach’s α = .86; 24 week Cronbach’s α = .86).
Covariates.
The following factors were considered for inclusion in statistical models as covariates given their potential relation to maternal postpartum depression or mother/infant cortisol levels: maternal age, country of birth, number of children, and marital status; time of day; infant birthweight, gestational age at birth, and sex; and breastfeeding status at 12 weeks postpartum (coded 0 = formula-feeding only (49%), 1 = partial or exclusive breastfeeding (51%)). Infant birth outcomes were obtained from medical records at the hospital of birth; all other potential covariates were obtained from maternal report. These variables are not expected to explain relations between prenatal factors, dyadic dysregulation, maternal/infant cortisol, and PPD, but inclusion of covariates may improve model estimation (Sauer, Brookhart, Roy, & Vanderweele, 2013). Maternal country of birth was included in statistical models as an a priori covariate due to previous evidence of higher PPD symptoms among Mexican-origin women born in the U.S. (Cobum, Gonzales, Cmic & Luecken, 2016; Davila, McFall, & Cheng, 2009). Time of day of cortisol sampling (calculated as minutes past midnight) was a covariate due to diurnal influences on cortisol output (Nicolson, 2008). Additional covariates were included if they were identified in preliminary analyses as statistically significant correlates of primary study variables.
Dyadic dysregulation.
At the 12-week home visit, structured mother-infant observational episodes were conducted to provide a context for mother-infant dyadic regulation/dysregulation. Episodes included 1) Free play (5 mins), 2) Arm restraint (2 mins; Goldsmith & Rothbart, 1993) followed by maternal soothing (3 mins), 3) Teaching task (5 mins; mothers are asked to “teach” her child a task from the Bayley Scales of Infant Development II [Bayley, 1993] that reflects a skill 1–2 months beyond the infant’s capabilities), and 4) Peek-a-boo (3 mins; Goldsmith & Rothbart, 1993). The degree to which the mother-child dyad showed signs of dyadic dysregulation during the video-recorded observational episodes was assessed using the dysregulation coding system (Lin, Crnic, Luecken, & Gonzales, 2014). The dysregulation coding system was modified from a system previously used by Crnic and colleagues (Hoffman, Crnic, & Baker, 2006) and conceptually informed by relevant research (Thompson, 1994; Cole, Michel, & O’Donnell Teti, 1994). A dyad was considered dysregulated if mother and infant were unable to modulate their collective levels of dyadic emotional, attentional, and behavioral arousal. Scores ranged from 1 to 5, with 5 signifying a high degree of dyadic dysregulation. Undergraduate research assistants were trained to a standard by a graduate student and required to maintain an inter-rater reliability above 70% exact match and 100% within one rating point of the master codes. Dyadic dysregulation was represented in statistical analyses by a latent variable, with coded dysregulation during each of the four episodes as indicators. The measurement model showed good fit (χ2(1) = 04, p = .84, CFI = 1.0, RMSEA = 0 (90% Cl: 0, .110}, SRMR = .002), and all indicators were statistically significant (p’s < .001).
Cortisol Sampling.
At the 12-week visit, saliva samples were obtained from mothers and infants before and after the mother-infant observational episodes. Samples were collected immediately before the first episode, and at 0, 20, and 40 minutes after the final episode, using Salivette (mother) and Sorbette (infant) sampling devices (Sarstedt, Rommelsdorf, Germany; Salimetrics Inc, State College, PA). Saliva samples were frozen and mailed to Salimetrics where they were assayed for free cortisol. Cortisol levels at each of the four time points were used to calculate area under the curve with respect to ground (AUCg) for mothers and infants with at least three of four viable cortisol samples, adjusted for total task time. AUCg reflects total hormonal output over a discrete period of time, and thus captures both sensitivity (e.g., time course) and intensity (e.g., amount of output) of cortisol response (Fekedulegn et al., 2007). Because cortisol is sampled over a discrete period of time, it reflects a response to an environmental provocation. As a measure of total hormonal secretion over a specified time period, AUCg reflects both pre-task cortisol (T1) and task-induced change (changes from T1 to T5; Morris, Rao, Wang & Garber, 2014). AUCg was log-transformed to correct for deviations from normality. Five infants and three mothers had AUCg values > 3 SD from the sample mean; these values were removed a priori from analyses. We also calculated residualized change scores reflecting specific reactivity the task, calculated as the change in cortisol from baseline to the first sample after conclusion of mother-infant interactions.
Data Analyses.
Preliminary analyses.
Zero-order correlations were examined between primary study variables and potential covariates. Time of day was the only variable significantly correlated with maternal and infant cortisol (see Table 2). Therefore, only time of day and country of birth (selected a priori, as described above) were selected as covariates for final models. Zero-order correlations between primary study variables are shown in Table 2. Maternal and infant cortisol AUCg were significantly correlated, as were maternal and infant cortisol reactivity. Dyadic dysregulation was positively correlated with infant cortisol AUCg and reactivity, and infant cortisol AUCg was positively correlated with maternal 24-week PPD symptoms.
Table 2.
Correlations among primary study variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7. | 8. | 9. | 10. | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Prenatal depressive symptoms | 1.0 | |||||||||
| 2. Prenatal economic hardship | .346** | 1.0 | ||||||||
| 3. Prenatal negative life events | .332** | .319** | 1.0 | |||||||
| 4. Mother’s birth country1 | −.089 | .092 | −182** | 1.0 | ||||||
| 5. Time of day (12 weeks) | −.085 | .058 | −.001 | .066 | 1.0 | |||||
| 6. Dyadic dysregulation2 | .114 | .037 | .212** | .041 | .061 | 1.0 | ||||
| 7. Infant cortisol AUCg | .052 | .188* | .104 | −.015 | −.225** | .219** | 1.0 | |||
| 8. Maternal cortisol AUCg | .006 | .035 | −.044 | −.022 | −.539** | −.048 | .317** | 1.0 | ||
| 9. Infant cortisol reactivity3 | .129 | .101 | .149* | .086 | .000 | .275** | .558** | .040 | 1.0 | |
| 10. Maternal cortisol reactivity3 | .035 | .072 | .016 | .067 | .000 | .002 | .101 | .304** | .206** | 1.0 |
| 11. Postpartum depressive symptoms at 24 weeks | .394** | .241** | .140* | −.057 | −.042 | .060 | .200** | .097 | .109 | .076 |
Coded 0 = U.S., 1 = Mexico
Mean across all four tasks
Residualized change score from regression of pre-task cortisol on first post-task cortisol
p < .05
p < .01
One infant was identified as an outlier based on low birthweight (1190g; the next closest birthweight = 2360g) and premature birth (26 weeks; the next closest gestational age = 36 weeks). Exclusion of the case from analyses had no significant effects on results; therefore, the case was retained in the dataset. Additionally, exclusion of infants born before 37 weeks gestation (n=6) had no significant effects on results; therefore all were retained for analyses.
Given the planned missingness design of the study, the general analysis program MPlus (Muthén & Muthén, 2012) was used for statistical analyses in a structural equation modeling framework because of its advanced capability for handling missing data with full information maximum likelihood (FIML) estimation (Enders, 2010).
For the primary analyses, the effects of prenatal stressors and depressive symptoms on postpartum depressive symptoms at 24 weeks were evaluated with mediation through degree of dyadic dysregulation and maternal and infant cortisol responses (AUCg and residualized change in separate models) to the mother-infant interaction tasks at 12 weeks (see Figure 2). Covariates included maternal country of birth and time of day of the 12-week visit. Bootstrap resampling with 5,000 bootstrap samples was applied. Model fit was examined using Chi-square (χ2), cumulative fit index (CFI), root mean square error of approximation (RMSEA), and standard root mean square residual (SRMR), interpreted based on criteria outlined by Hu and Bender (1999). Statistical significance of model paths was determined by examination of 95% bootstrap confidence intervals: If the confidence interval does not include zero, the path is considered statistically significant.
Mediation of the effect of prenatal stressors on maternal PPD symptoms by dyadic dysregulation and mother/infant cortisol was tested by examining the statistical significance of the indirect effect using bias-corrected bootstrap confidence intervals because of the optimal statistical properties of this test over examination of p-values from normal-theory tests of mediation (MacKinnon, 2008; MacKinnon, Lockwood, & Williams, 2004). The indirect effects of the models indicate the effect of prenatal stressors on PPD symptoms through the mediators.
Results
Model 1: Cortisol AUCg. The mediation model shown in Figure 2 was evaluated for the prediction of 24-week PPD symptoms from prenatal stress and depressive symptoms, mediated through dyadic dysregulation and maternal and infant cortisol AUCg response. Country of mother’s birth and time of day at the 12-week visit were included as covariates. Model fit was good, χ2(31) = 59.5, p = .002, CFI = .92, RMSEA = .053 (90% CI .032, .074), SRMR = .056. Model results are shown in Figure 2. Higher negative life events predicted more dyadic dysregulation (estimate = .061, 95% CI {.013, .116}), more dysregulation predicted higher infant cortisol (estimate = .148, 95% CI {.048, .281}), and higher infant cortisol predicted higher PPD symptoms (estimate = 2.81, 95% CI {.355, 5.22}). Higher economic hardship also predicted higher infant cortisol (estimate = .019, 95% CI {.004, .033}). Prenatal depressive symptoms predicted higher PPD symptoms (estimate = .284, 95% CI {.181, .390}), but did not predict dyadic dysregulation, maternal cortisol, or infant cortisol. Maternal cortisol was only associated with infant cortisol (estimate = .013, 95% CI {.002, .028}).1
Tests of mediation revealed a statistically significant indirect effect of negative life events on PPD through dyadic dysregulation and infant cortisol (estimate = .025, 95% CI {.003, .093}): more negative life events predicted higher dyadic dysregulation at 12 weeks, which predicted higher infant cortisol response, which predicted higher PPD symptoms at 24 weeks, after adjusting for prenatal depressive symptoms, economic hardship, and maternal cortisol. The indirect effect of economic hardship on PPD symptoms through infant cortisol was also statistically significant (estimate = .052, 95% CI {.006, .142}): higher economic hardship predicted higher infant cortisol, which predicted higher PPD symptoms at 24 weeks postpartum.
Model 2: Cortisol reactivity. This model predicted 24-week PPD symptoms from prenatal stress and depressive symptoms, mediated through dyadic dysregulation and maternal and infant cortisol reactivity to the interaction (resi dualized change scores), with mother’s country of birth and time of day at the 12-week visit as covariates. Model fit was comparable to the prior model: χ2 (31) = 67.8, p = .0001, CFI = .939, RMSEA = .061 (90% CI .041, .080), SRMR = .062. Model results are shown in Figure 2. Higher negative life events predicted higher dyadic dysregulation (estimate = .060, 95% CI {.009, .113}), and higher dyadic dysregulation predicted higher infant cortisol reactivity (estimate = .475, 95% CI {.130, .834}). However, infant cortisol reactivity was not significantly predicted by economic hardship (estimate = .018, 95% CI {−.039, .072}), or predictive of PPD symptoms at 24 weeks (estimate = .269, 95% CI {−.540, 1.111}). Maternal cortisol reactivity was significantly correlated with infant reactivity (estimate = .206, 95% CI {.024, .403}), but was not predicted by any of the prenatal variables or dyadic dysregulation, and did not significantly predict PPD symptoms at 24 weeks. PPD symptoms at 24 weeks were only significantly predicted by prenatal depressive symptoms (estimate = .282, 95% CI {.159, .386}), and economic hardship (estimate = .184, 95% CI {.001, .374}). No indirect paths were statistically significant mediators of the relation of prenatal economic hardship or depressive symptoms to PPD symptoms at 24 weeks postpartum in this model.2
Discussion
Although it is commonly acknowledged that postpartum depression evolves from a complex interplay of biological and psychological factors operating across the perinatal period, the biopsychosocial processes that underlie the development or maintenance of maternal depressive symptoms remain poorly understood, particularly among low-SES and ethnic minority populations. Further, the role of dyadic and infant contributing factors to maternal affective well-being is often overlooked. The goal of the current study was to explore potential behavioral and physiological mechanisms through which maternal prenatal stress and depressive symptoms influence the development of maternal postpartum depressive symptoms across the early infancy period in a sample of low-income Mexican-origin mothers. Our hypothesis, that mother-infant dyadic regulatory behavior and neuroendocrine responses to dysregulation at 12 weeks postpartum would mediate the connections between prenatal distress and PPD symptomatology at 24 weeks postpartum, was partially confirmed.
Prenatal stress and depressive symptoms emerged as important contributors to PPD symptoms, both directly and indirectly. We evaluated two types of prenatal stressors, economic hardship and negative life events, that are especially salient to low-income women. Both types of stressors showed indirect pathways to later PPD symptoms even after adjusting for prenatal depressive symptoms. Negative life events reported prenatally predicted more behaviorally and emotionally dysregulated mother-infant interaction when infants were 12 weeks of age, which was associated with higher infant cortisol reactivity and overall cortisol response to the interaction tasks, adjusted for maternal cortisol. Maternal and infant cortisol were positively correlated across the sample, but only higher infant overall cortisol AUCg predicted higher maternal PPD symptoms at 24 weeks. Infant cortisol reactivity, calculated as a change from baseline to the first post-task sample, did not predict later maternal PPD symptoms. Although there is a lack of consensus in the literature regarding the aspects of cortisol response captured by AUCg compared to change scores, our results suggest that AUCg (encompassing both reactivity and recovery components of a response) better captures the implications of infant physiological arousal for maternal adjustment than the initial reactivity measure. These findings highlight the salience of early coregulatory behavior in the mother-infant relationship, and infant HPA activity to mothers’ well-being in early infancy.
Mother-infant regulatory interactions have long been identified as important contributors to infant developmental competence (Calkins & Perry, 2016), but rarely has research on dyadic regulation focused on consequences for maternal behavior and well-being. Multiple findings in the literature assume a parent-effect model which suggests that maternal distress promotes problematic coregulated interaction that adversely affects child development (e.g., Granat, Gadassi, Gilboa-Schechtman, & Feldman, 2017; Lunkenheimer, Albrecht, & Kemp, 2013). Our results, however, suggest that child-effect associations (i.e., impact of the child and mother-child interactions on maternal depressive symptoms) also merit close attention. Higher prenatal negative life events predicted more dyadic dysregulation in interactions between infants and mothers, which was linked with greater infant biological arousal over and above maternal biological arousal, which was linked to more maternal depressive symptoms 12 weeks later. The results suggest that coregulated interaction may serve primarily to help young children learn to become competent self-regulators over time, but the functional implications of dyadic dysregulation may also contribute, at least indirectly, to compromised maternal well-being.
It was unexpected that dysregulation in the dyadic interaction was only significantly predictive of elevated infant cortisol, with nonsignificant effects on maternal cortisol. Additionally, neither total maternal cortisol response nor maternal cortisol reactivity was predictive of later maternal depressive symptoms after adjustment for infant cortisol. The context for sampling cortisol was a mild-to-moderately challenging series of interaction tasks. For a well-regulated dyad, these tasks would not be expected to result in activation of the HPA stress response system, but for a dysregulated dyad, the interaction may be stressful and result in cortisol reactivity. For mothers, dysregulation may have been too mild to challenge their own self-regulatory abilities. Alternatively, persistent life stress may have led to blunted responsivity (e.g., Lovallo et al., 2012). However, infants have minimal self-regulatory ability, and dyadic dysregulation during negatively arousing events may overwhelm their ability to regulate physiological responses (Stifter & Braungart, 1995). That is, cortisol responses appears to be more closely tied to disruption in dyadic regulation for young infants than for mothers.
Interpretation of elevated cortisol responses as dysregulated or maladaptive is complicated. A well-regulated biological response involves a degree of reactivity appropriate to the demands of the challenge. Both blunted and exaggerated cortisol responses can indicate maladaptation (Shirtcliff et al., 2014). The current study provides evidence that dyadic dysregulation is biologically challenging for infants. Young infants have limited agency to influence their social world, but they are nonetheless keenly attuned to their mothers’ behavior (Csibra, 2010; Nakata & Trehub, 2004). When infants perceive maternal unavailability, they may become reliant on less sophisticated and less effective regulatory strategies, which can tax the developing HPA axis (Feldman et al., 2004; Khoury et al., 2016; Vanska et al., 2016).
Infant physiological arousal may not only reflect a ways in which infants are influenced by their social world, but may also reflect a mechanism through which infants influence their social world. The question arises as to how an aroused physiological state in an infant might influence maternal affective state later in the postpartum period. One possibility is through observable infant behaviors associated with negative arousal. In the absence of sensitive caregiving to assist in regulating arousal, infants are nonetheless able to invoke some primitive independent/autonomous means of arousal regulation (e.g., gaze aversion, non-nutritive sucking; Field, 2002; Rothbart & Derryberry, 1981). These infant behaviors, displayed overtime, may disrupt the mother-infant bond and the affective boost and oxytocin release that can result from synchronous, warm mother-infant interactions (Feldman et al., 2010). The current study did not evaluate infant behaviors outside of the dyadic context, and we cannot determine if such behaviors are correlated with cortisol output or reactivity.
Alternatively, mothers may be directly responding affectively and physiologically to an altered physiological state in their infant. Elevated cortisol output is one component of a complex set of physiological changes during a stress response. From an evolutionary standpoint, it is likely that consciously or unconsciously, mothers are able to detect physiological changes in their infant associated with potential threats to survival. Our finding of positively correlated maternal and infant cortisol responses to dyadic behavioral dysregulation suggests potential biological attunement. However, mothers differ in their effectiveness at recognizing and responding to infant cues (Dix & Meunier, 2009). Persistent difficulties recognizing infant distress and co-regulating an aroused infant may weaken a mother’s sense of parental competence, contributing to depressive symptoms. Women at risk of depression may be especially prone to negative biases in their sense of competence (Weaver, Shaw, Dishion, & Wilson, 2008). Dyadic behavioral and infant biological dysregulation may each reflect difficult infant temperament and presage behavioral problems in the child, both of which may further impact maternal psychological adaptation (NICHD Early Child Care Research Network, 2004; Luecken, MacKinnon, & Jewell, 2015).
Consistent with hypotheses and prior research, exposure to significant life stressors in the prenatal period increased the risk of dyadic dysregulation in mother-infant interactive context. The impact of prenatal stress on dysregulation was only statistically significant for specific negative life events, and not for stress from economic hardship. The current sample of impoverished ethnic minority families reported a large number of prenatal negative life events (e.g., close family member sick/hospitalized, separated/divorced from husband/partner, husband/partner in jail, family was homeless). Many of these events would be considered uncontrollable or unpredictable, and involve significant disruptions to the social support network that could otherwise help smooth the transition to motherhood and promote a healthy mother-child bond (Crnic, Greenberg, Ragozin, Robinson, & Basham, 1983). The restricted range of economic hardship in this very low-income sample, however, may have limited the ability to identify effects on dyadic regulation, which may be evident only in comparison to higher SES dyads. It is also possible that chronic, predictable economic hardship may not exert the same level of disruption in the mother-infant interaction context as major life event stressors.
Economic hardship was, however, directly associated with elevated infant overall cortisol response, a finding consistent with prior research on the impact of socioeconomic status on the development of children’s physiological stress response system (Clearfield, Carter-Rodriguez, Merali, & Shober, 2014; Lupien, King, Meaney, & McEwen, 2000; Saridjan et al., 2010). The current findings demonstrate that the impact of economic strain, reported prenatally by mothers, is evidenced biologically in infants as early as 3 months of age. Growing literature demonstrates the potential for maternal stress in the prenatal period to affect fetal development of the HPA axis (Glover, O’Connor, & O’Donnell, 2010). The chronic stress of economic hardship, especially during pregnancy, may have altered infant cortisol responsivity. This impact of economic hardship may also be engendered through broader ecological threats associated with economic instability. Threats associated with low-income status may manifest in neighborhood and home environments that undermine developing biological regulatory capacities (e.g., through food insecurity, unstable housing or overcrowded homes, unsafe neighborhoods). These types of problems are typically pronounced for immigrants, who may lack access to the resources for assistance raising a child that are available to non-immigrant low-income families.
In contrast to prenatal stressors, which showed indirect associations with PPD symptoms, mothers’ prenatal depressive symptoms were directly associated with higher PPD symptoms 24 weeks after childbirth, even when other contributing factors such as economic hardship and negative life events were accounted for in the model. However, we did not find evidence of a mediational pathway from prenatal to postpartum depressive symptoms through mother-infant dyadic dysregulation. The subclinical levels of depressive symptoms in the current sample may not have been of sufficient magnitude to impact dyadic mother-infant interactions. Prior studies have also failed to find a direct effect of maternal PPD symptoms on mother-infant interactions in low-income ethnic minority families (Boyd et al., 2006). The cultural context of the current study’s sample is worth considering in this regard. Culturally-specific goals and values affect parenting practices and responses to child emotions and behavior (Calzada, Fernandez, & Cortes, 2010). Mexican cultural factors such as familismo (i.e., strong family values and extended family social and emotional support; Campos et al., 2008) may operate to protect the developing mother-child bond so that it is not significantly impaired by maternal depressive symptoms. The current sample of Mexican-origin mothers, most of whom were bom in Mexico and primarily speak Spanish, strongly endorsed cultural values such as familismo. The Mexican cultural value maricmismo, which highlights idealized gender role expectations for Latina women encompassing care and nurturance of children and sacrifice for the sake of their family (Castillo, Perez, Castillo, & Ghosheh, 2010), may also function to minimize the impact of maternal depressive symptoms on the mother-infant bond. Future studies may want to evaluate the protective effect of cultural values with samples endorsing more variability in acculturation.
This study is strengthened by longitudinal examination of the development of PPD symptoms in a sample of women at elevated risk due to economic hardship and ethnic minority status. The results expand existing knowledge by evaluating a mechanistic pathway operating in the postpartum period, with a focus on the processes by which infants influence their mother’s emotional well-being. Although much has been learned from prior research about how maternal depression affects child behavior, little is known about the child’s role in affecting maternal depressive symptoms (Dix & Meunier, 2009). Additionally, this study examines the impact of prenatal factors on dyadic dysregulation early in infancy, while prior studies have primarily focused on risk factors present later in development. An additional strength includes the examination of observed dysregulation and associated biological responses during mother-infant interaction as mediators, minimizing the impact of potential self-report biases.
Several limitations of this study should be considered. On average infants and mothers did not show increases from baseline cortisol in response to the task. However, the task was not designed to elicit a cortisol stress response across the sample; rather, it provided a context in which to observe the coregulatory qualities of the dyad. In the absence of behavioral or emotional dysregulation, cortisol would be expected to decline in a normal diurnal fashion across the episodes. A more challenging task that elicits arousal across the sample may reveal different links between infant and maternal cortisol reactivity and later maternal depressive symptoms. The response of other biological systems (e.g., heart rate rhythms; Feldman et al., 2011) may also be implicated in the development of maternal PPD symptoms. We did not recruit women at elevated risk of distress, and the sample as a whole did not exhibit high levels of PPD symptoms, despite considerable prior research suggesting elevated rates of PPD in the population from which this sample was drawn. We did not evaluate clinical diagnoses, thus our results primarily demonstrate dyadic and infant influences on subclinical symptoms of depression, and may not generalize to women with clinical depression. The negative life events scale does not specify the exact timing of the event, and we cannot address when during pregnancy stress exposure may post the greatest risk. Finally, our study sample only included low-income, Mexican-origin women. The results may not generalize to women from other ethnic or socioeconomic backgrounds. Hispanics account for approximately 23% of births in the U.S. and 40% of births in Arizona in 2016 (Martin, Hamilton, Osterman, Driscoll, & Drake, 2018); Mexican-origin families account for the largest percentage of births in the southwest. However, even within this specific national origin group (Mexican), there is significant diversity, and more in depth evaluation of culturally relevant values, beliefs, and behaviors is warranted.
Conclusions.
The current investigation with low-income, Mexican-origin mothers offers support for a biopsychosocial mechanistic model of risk factors for PPD symptoms. Unique to this study is our evaluation of the regulatory qualities of early mother-infant dyadic interaction. A path was identified by which prenatal stressors influence maternal well-being up to 6 months postpartum through dysregulated mother-infant interactions and infant biological response to the interactions. This pathway contributed to the prediction of PPD symptoms over and above prenatal depressive symptoms. In particular, infant biological response to dysregulated mother-infant interaction may have significant consequences for maternal affective symptoms. This study builds on prior research largely focused on maternal effects on infant well-being, overlooking the potential for infants to influence their mother’s biological and emotional wellbeing. Future research in the study of maternal PPD may benefit from moving away from a parent-effect model to a model that gives due credit to infant influences on their mothers.
Article Highlights.
Prenatal major life stressors predict dyadic dysregulation in mother-infant interactions at infant age 12 weeks
Higher prenatal economic stress and dyadic dysregulation are associated with elevated infant cortisol response
Elevated infant cortisol response predicts higher maternal postpartum depressive symptoms at 24 weeks
12-week infant cortisol response mediates the impact of prenatal stress on postpartum depression.
Acknowledgements:
Funded by the National Institute of Mental Health (R01 MH083173-01). The funding source had no role in study design, collection, analysis, interpretation, or writing of the report; or in the decision to submit the article for publication. We thank the mothers and infants for their participation; Anne Mauricio, Kirsten Letham, and Monica Gutierrez for their assistance with data collection and management; Dr. Dean Coonrod and the Maricopa Integrated Health Systems for their assistance with recruitment; and the interviewers for their commitment and dedication to this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Infant baseline cortisol sample alone is not a significant predictor of maternal postpartum PPD symptoms, suggesting that the results are not accounted for by “anticipatory” cortisol. Further, baseline cortisol is not correlated with any of the independent variables.
Results are comparable for a model that includes maternal and infant cortisol AUCg and reactivity in the same model: Both infant cortisol AUCg and reactivity are statistically significantly predicted by dyadic dysregulation, but only infant cortisol AUCg was predictive of maternal PPD symptoms at 24 weeks. The indirect path to PPD symptoms from prenatal negative life events through dyadic dysregulation and infant cortisol AUCg is statistically significant, as is the path from economic stress to PPD symptoms through infant cortisol AUCg.
Declarations of interest: none.
REFERENCES
- Barrera M, Caples EL, & Tein JY (2001). The psychological sense of economic hardship: Measurement models, validity, and cross-ethnic equivalence for urban families. American journal of community psychology, 29(3), 493–517. [DOI] [PubMed] [Google Scholar]
- Bayley N (1993). Bayley scales of infant development -2nd edition (BSID-II). Psychological Corporation, San Antonio, TX. [Google Scholar]
- Beck CT (2001). Predictors of postpartum depression: an update. Nursing research, 50(5), 275–285. [DOI] [PubMed] [Google Scholar]
- Boyd RC, Zayas LH, & McKee MD (2006). Mother-infant interaction, life events and prenatal and postpartum depressive symptoms among urban minority women in primary care. Maternal and Child Health .Journal, 10(2), 139. [DOI] [PubMed] [Google Scholar]
- Broth MR, Goodman SH, Hall C, & Raynor LC (2004). Depressed and well mothers’ emotion interpretation accuracy and the quality of mother—infant interaction. Infancy, 6(1), 37–55. [Google Scholar]
- Calkins SD, & Perry NB (2016). The development of emotion regulation: Implications for child adjustment In Cicchetti D (Ed.), 3rd ed.; developmental psychopathology: Maladaptation and psychopathology (vol. 3, 3rd ed.) (3rd ed. ed., pp. 187–242, Chapter xv, 1225 Pages) John Wiley & Sons Inc, Hoboken, NJ. [Google Scholar]
- Calzada EJ, Fernandez Y, & Cortes DE (2010). Incorporating the cultural value of respeto into a framework of Latino parenting. Cultural Diversity and Ethnic Minority Psychology, 76(1), 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos B, Dunkel Schetter C, Abdou CM, Hobel CJ, Glynn LM, & Sandman CA (2008). Familialism, social support, and stress: Positive implications for pregnant Latinas. Cultural Diversity and Ethnic Minority Psychology, 14, 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo LG, Perez FV, Castillo R, & Ghosheh MR (2010). Construction and initial validation of the marianismo beliefs scale, Counseling Psychology Quarterly, 23, 163–175. [Google Scholar]
- CDC: Center for Disease Control and Prevention (2009). PRAMS Questionnaires Phase 6 (2009–2011). Retrieved from https://www.cdc.gov/prams/questionnaire.htm
- Chen E, Cohen S, & Miller GE (2010). How low socioeconomic status affects 2-year hormonal trajectories in children. Psychological Science, 21(1), 31–37. [DOI] [PubMed] [Google Scholar]
- Clearfield MW, Carter-Rodriguez A, Merali AR, & Shober R (2014). The effects of SES on infant and maternal diurnal salivary cortisol output. Infant Behavior and Development, 37(3), 298–304. [DOI] [PubMed] [Google Scholar]
- Coburn S, Gonzales NA, Crnic K, & Luecken LJ (2016). Multiple domains of stress predict postpartum depressive symptoms in low-income Mexican American women: The moderating effect of social support. Archives of Women’s Mental Health, 19, 1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn SS, Crnic KA, & Ross EK (2015). Mother-infant dyadic state behaviour: Dynamic systems in the context of risk. Infant and Child Development, 24(3), 274–297. [Google Scholar]
- Cohen S, Doyle WJ, & Baum A (2006). Socioeconomic status is associated with stress hormones. Psychosomatic medicine, 68(3), 414–420. [DOI] [PubMed] [Google Scholar]
- Cole PM, Michel MK, & Teti LOD (1994). The development of emotion regulation and dysregulation: A clinical perspective. Monographs of the society for research in child development, 59(2–3), 73–102. [PubMed] [Google Scholar]
- Cole PM, Martin SE, & Dennis TA (2004). Emotion regulation as a scientific construct: Methodological challenges and directions for child development research. Child Development, 75(2), 317–333. [DOI] [PubMed] [Google Scholar]
- Cox JL, Holden JM, & Sagovsky R(1987). Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal Depression Scale. British Journal of Psychiatry, 150, 782–786. [DOI] [PubMed] [Google Scholar]
- Coyl DD, Roggman LA, & Newland LA (2002). Stress, maternal depression, and negative mother-infant interactions in relation to infant attachment. Infant Mental Health Journal: Official Publication of The World Association for Infant Mental Health, 23(1– 2), 145–163. [Google Scholar]
- Crnic KA, Gaze C, & Hoffman C (2005). Cumulative parenting stress across the preschool period: Relations to maternal parenting and child behaviour at age 5. Infant and Child Development, 14(2), 117–132. [Google Scholar]
- Crnic KA, Greenberg MT, Ragozin AS, Robinson NM, & Basham RB (1983). Effects of stress and social support on mothers and premature and full-term infants. Child Development, 54, 209–217. [PubMed] [Google Scholar]
- Csibra G (2010). Recognizing communicative intentions in infancy. Mind & Language, 25(2), 141–168. [Google Scholar]
- Davila M, McFall S, & Cheng D (2009). Acculturation and depressive symptoms among pregnant and postpartum Latinas. Maternal and Child Health Journal, 13(3), 318–325. [DOI] [PubMed] [Google Scholar]
- Dennis CL, Janssen PA, & Singer J (2004). Identifying women at-risk for postpartum depression in the immediate postpartum period. Acta Psychiatrica Scandinavica, 110(5), 338–346. [DOI] [PubMed] [Google Scholar]
- Diego MA, Jones NA, Field T, Hernandez-Reif M, Schanberg S, Kuhn C, & Gonzalez-Garcia A (2006). Maternal psychological distress, prenatal cortisol, and fetal weight. Psychosomatic Medicine, 68(5), 747–753. [DOI] [PubMed] [Google Scholar]
- Diener ML, Casady MA, & Wright C (2003). Attachment security among mothers and their young children living in poverty: Associations with maternal, child, and contextual characteristics. Merrill-Palmer Quarterly, 49(2), 154–182. [Google Scholar]
- Dix T, & Meunier LN (2009). Depressive symptoms and parenting competence: An analysis of 13 regulatory processes. Developmental Review, 29, 45–68. [Google Scholar]
- Enders CK(2010). Applied Missing Data Analysis. Guilford Press, New York. [Google Scholar]
- Fekedulegn DB, Andrew ME, Burchfiel CM, Violanti JM, Hartley TA, Charles LE, & Miller DB (2007). Area under the curve and other summary indicators of repeated waking cortisol measurements. Psychosomatic Medicine, 69, 651–659. [DOI] [PubMed] [Google Scholar]
- Feldman R, Eidelman AI, & Rotenberg N (2004). Parenting stress, infant emotion regulation, maternal sensitivity, and the cognitive development of triplets: A model for parent and child influences in a unique ecology. Child Development, 75(6), 1774–1791. [DOI] [PubMed] [Google Scholar]
- Feldman R (2007). Parent-infant synchrony: Biological foundations and developmental outcomes. Current Directions in Psychological Science, 16(6), 340–345. [Google Scholar]
- Feldman R, Granat A, Pariente C, Kanety H, Kuint J, & Gilboa-Schechtman E (2009). Maternal depression and anxiety across the postpartum year and infant social engagement, fear regulation, and stress reactivity. Journal of the American Academy of Child & Adolescent Psychiatry, 48(9), 919–927. [DOI] [PubMed] [Google Scholar]
- Field TM (2002). Early interactions between infants and their postpartum depressed mothers. Infant Behavior and Development, 25(1), 25–29. [Google Scholar]
- Feldman R, Magori-Cohen R, Galili G, Singer M & Louzon Y (2011). Mother and infant coordinate heart rhythms through episodes of interaction synchrony. Infant Behavior and Development, 34, 569–577. [DOI] [PubMed] [Google Scholar]
- Field T (1998). Maternal depression effects on infants and early interventions. Preventive Medicine, 27(2), 200–203. [DOI] [PubMed] [Google Scholar]
- Field TM (2002). Early interactions between infants and their postpartum depressed mothers. Infant Behavior and Development, 25(1), 25–29. [Google Scholar]
- Field T (2010). Postpartum depression effects on early interactions, parenting, and safety practices: a review. Infant Behavior and Development, 33(1), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn LM, Davis EP, & Sandman CA (2013). New insights into the role of perinatal HPA-axis dysregulation in postpartum depression. Neuropeptides, 47(6), 363–370. [DOI] [PubMed] [Google Scholar]
- Glover V, O’Connor TG, & O’Donnell K (2010). Prenatal stress and the programming of the HPA axis. Neuroscience & Biobehavioral Reviews, 35, 17–22. [DOI] [PubMed] [Google Scholar]
- Goldstein LH, Diener ML, & Mangelsdorf SC (1996). Maternal characteristics and social support across the transition to motherhood: Associations with maternal behavior. Journal of Family Psychology, 10(1), 60 [Google Scholar]
- Goldsmith HH, & Rothbart MK (1993). The laboratory temperament assessment battery (LAB-TAB). University of Wisconsin, Madison, WI. [Google Scholar]
- Gonzalez-Barrera A, & Lopez MH (2013). A demographic portrait of Mexican-origin Hispanics in the United States. Washington, DC: Pew Hispanic Center. [Google Scholar]
- Goodman SH, & Gotlib IH (1999). Risk for psychopathology in the children of depressed mothers: a developmental model for understanding mechanisms of transmission. Psychological Review, 106, 458–490. [DOI] [PubMed] [Google Scholar]
- Goodman SH, & Gotlib IH (2002). Children of depressed parents: Mechanisms of risk and implications for treatment. American Psychological Association. [Google Scholar]
- Goodman SH, Rouse MH, Connell AM, Broth MR, Hall CM, & Heyward D (2011). Maternal depression and child psychopathology: A meta-analytic review. Clinical child and family psychology review, 14(1), 1–27. [DOI] [PubMed] [Google Scholar]
- Gress-Smith JL, Luecken LJ, Lemery-Chalfant K, & Howe R (2012). Postpartum depression prevalence and impact on infant health, weight, and sleep in low-income and ethnic minority women and infants. Maternal and Child Health Journal, 16, 887–893. [DOI] [PubMed] [Google Scholar]
- Gress-Smith JL, Roubinov DS, Tanaka R, Cirnic K, Gonzales N, Enders C, & Luecken LJ (2013). Prenatal expectations in Mexican American women: development of a culturally sensitive measure. Archives of women’s mental health, 16(4), 303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granat A, Gadassi R, Gilboa-Schechtman E, & Feldman R (2017). Maternal depression and anxiety, social synchrony, and infant regulation of negative and positive emotions. Emotion, 17(1), 11–27. doi:http://dx.doi.org.ezproxyl.lib.asu.edu/10.1037/emo0000204 [DOI] [PubMed] [Google Scholar]
- Gunnar MR, & Donzella B (2002). Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology, 27(1), 199–220. [DOI] [PubMed] [Google Scholar]
- Hoffman C, Crnic KA, & Baker JK (2006). Maternal depression and parenting: Implications for children’s emergent emotion regulation and behavioral functioning. Parenting: Science and Practice, 6(4), 271–295. [Google Scholar]
- Hu L, & Bentler PM (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling, 6(1), 1–55. [Google Scholar]
- Institute of Medicine (2001). Coverage Matters: Insurance and Health Care. National Academy Press. [PubMed] [Google Scholar]
- Jolley SN, Elmore S, Barnard KE, & Carr DB (2007). Dysregulation of the hypothalamic-pituitary-adrenal axis in postpartum depression. Biological research for nursing, 8(3), 210–222. [DOI] [PubMed] [Google Scholar]
- Kaitz M, & Maytal H (2005). Interactions between anxious mothers and their infants: An integration of theory and research findings. Infant Mental Health Journal: Official Publication of The World Association for Infant Mental Health, 26(6), 570–597 [DOI] [PubMed] [Google Scholar]
- Khoury JE, Gonzalez A, Levitan R, Masellis M, Basile V, & Atkinson L (2016). Infant emotion regulation strategy moderates relations between self-reported maternal depressive symptoms and infant HPA activity. Infant and Child Development, 25, 64–83. [Google Scholar]
- Lara-Cinisomo S, Grewen KM, Girdler SS, Wood J, & Meltzer-Brody S (2017). Perinatal depression, adverse life events, and hypothalamic-adrenal-pituitary axis response to cold pressor stress in Larinas: an exploratory study. Women’s Health Issues, 27(6), 673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letoueneau N, Watson B, Duffett-Leger L, Hegadoren K, & Tryphonopoulos P (2011). Cortisol patterns of depressed mothers and their infants are related to maternal-infant interactive behaviours. Journal of Reproductive and Infant Psychology, 29(5), 439–459. [Google Scholar]
- Lin B, Crnic K, Luecken L, & Gonzales N (in press; ). Ontegeny of emotional and behavioral problems in a low-income, Mexican American sample Developmental Psychology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, Crnic K, Luecken LJ, & Gonzales NA (2014). Maternal prenatal stress and infant regulatory capacity in Mexican Americans. Infant Behavior and Development, 37, 571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CH, & Tronick E (2013). Rates and predictors of postpartum depression by race and ethnicity: Results from the 2004 to 2007 New York City PRAMS survey (Pregnancy Risk Assessment Monitoring System). Maternal and Child Health Journal, 17, 1599–1610. doi:http://dx.doi.org.ezproxyl.lib.asu.edu/10.1017/S003329171600283X [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Farag NH, Sorocco KH, Cohoon AJ, & Vincent AS (2012). Lifetime adversity leads to blunted stress axis reactivity: Studies from the Oklahoma Family Health Patterns Project. Biological Psychiatry, 71, 344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovejoy MC, Graczyk PA, O’Hare E, & Neuman G (2000). Maternal depression and parenting behavior: A meta-analytic review. Clinical Psychology Review, 20(5), 561–592. [DOI] [PubMed] [Google Scholar]
- Luecken LJ, Lin B, Coburn S, MacKinnon DP, Gonzales N, & Cmic KA (2013). Prenatal stress, partner support, and infant cortisol reactivity in low-income Mexican American families. Psychonenroendocrinology, 38, 3092–3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunkenheimer ES, Albrecht EC, & Kemp CJ (2013). Dyadic flexibility in early parent-child interactions: Relations with maternal depressive symptoms and child negativity and behaviour problems. Infant and Child Development, 22(3), 250–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, & McEwen BS (2000). Child’s stress hormone levels correlate with mother’s socioeconomic status and depressive state. Biological psychiatry, 48(10), 976–980. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, & Williams J (2004). Confidence limits for the indirect effect: Distribution of the product and resampling methods. Multivariate Behavioral Research, 39(1), 99–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP (2008). Introduction to statistical mediation analysis. NY: Erlbaum; 2008. [Google Scholar]
- Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Drake P. Births: Final data for 2016. National Vital Statistics Reports; vol 67 no 1 Hyattsville, MD: National Center for Health Statistics; 2018. [PubMed] [Google Scholar]
- McEwen BS, & Wingfield JC (2003). The concept of allostasis in biology and biomedicine. Hormones and Behavior, 43(1), 2–15. [DOI] [PubMed] [Google Scholar]
- Milgrom J, Gemmill AW, Bilszta JL, Hayes B, Barnett B, Brooks J, Ericksen J, Ellwood D, & Buist A (2008). Antenatal risk factors for postnatal depression: a large prospective study. Journal of affective disorders, 108(1), 147–157. [DOI] [PubMed] [Google Scholar]
- Morris MC, Rao U, Wang L, & Garber J (2014). Cortisol reactivity to experimentally manipulated psychosocial stress in young adults at varied risk for depression. Depression and anxiety, 31(1), 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L, Fiori-Cowley A, Hooper R, & Cooper P (1996). The impact of postnatal depression and associated adversity on early mother-infant interactions and later infant outcome. Child Development, 67(5), 2512–2526. [PubMed] [Google Scholar]
- Murray L, Cooper P, & Hipwell A (2003). Mental health of parents caring for infants. Archives of Women’s Mental Health, 6, s71–s77. [DOI] [PubMed] [Google Scholar]
- Muthen LK, & Muthen BO Mplus User’s Guide (7th ed.). Los Angeles, CA: Muthen & Muthen; 1998–2012. [Google Scholar]
- Nakata T, & Trehub SE (2004). Infants’ responsiveness to maternal speech and singing. Infant Behavior and Development, 27(4), 455–464. [Google Scholar]
- Navarro C, Navarrete L, & Lara MA (2011). Factores asociados a la perception de eficacia materna durante el posparto. Salad mental, 34(1), 37–43. [Google Scholar]
- NICHD Early Child Care Research Network. (2004). Affect dysregulation in the mother-child relationship in the toddler years: Antecedents and consequences. Development and Psychopathology, 16(1), 43–68. [DOI] [PubMed] [Google Scholar]
- Nicolson NC (2008). Measurement of cortisol In Luecken LJ & Gallo LC (Eds), Handbook of Physiological Research Methods in Health Psychology, Sage Publications: Thousand Oaks, CA. [Google Scholar]
- Powers SI, Laurent HK, Gunlicks-Stoessel M, Balaban S, & Bent E (2016). Depression and anxiety predict sex-specific cortisol responses to interpersonal stress. Psychonenroendocrinology, 69, 172–179. [DOI] [PubMed] [Google Scholar]
- Pratt M, Apter-Levi Y, Vakart A, Kanat-Maymon Y, Zagoory-Sharon O, & Feldman R (2017). Mother-child adrenocortical synchrony; Moderation by dyadic relational behavior. Hormones and Behavior, 89, 167–175. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, & Derryberry D (1981). Theoretical issues in temperament In Developmental disabilities (pp. 383–400). Springer, Dordrecht. [Google Scholar]
- Rich-Edwards JW, Kleinman K, Abrams A, Harlow BL, McLaughlin TJ, Joffe H, & Gillman MW (2006). Sociodemographic predictors of antenatal and postpartum depressive symptoms among women in a medical group practice. Journal of Epidemiology & Community Health, 60(3), 221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter C, Hobfoll SE, Lavin J, Cameron RP, & Hulsizer MR (2000). Stress, psychosocial resources, and depressive symptomatology during pregnancy in low-income, inner-city women. Health Psychology, 19(6), 576. [DOI] [PubMed] [Google Scholar]
- Saridjan NS, Huizink AC, Koetsier JA, Jaddoe VW, Mackenbach JP, Hofman A, Kirschbaum C, Verhulst FC & Tiemeier H (2010). Do social disadvantage and early family adversity affect the diurnal cortisol rhythm in infants? The Generation R Study. Hormones and Behavior, 57(2), 247–254. [DOI] [PubMed] [Google Scholar]
- Sauer B, Brookhart MA, Roy JA, & Vanderweele TJ, (2013). Covariate selection In Venentgas P, & Torchia MM, (Eds.), Developing a protocol for observational comparitive effectiveness research: A user’s guide. Rockville, MD: Agency for Healthcare Research and Quality. [PubMed] [Google Scholar]
- Segre LS, O’Hara MW, Arndt S, & Stuart S (2007). The prevalence of postpartum depression. Social Psychiatry and Psychiatric Epidemiology, 42(4), 316–321. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Peres JC, Dismukes AR, Lee Y, & Phan JM (2014). Riding the physiological roller coaster: Adaptive significance of cortisol stress reactivity to social contexts. Journal of Personality Disorders, 28, 40–51. [DOI] [PubMed] [Google Scholar]
- Stifter CA, & Braungart JM (1995). The regulation of negative reactivity in infancy: function and development. Developmental Psychology, 31(3), 448. [Google Scholar]
- Tarullo AR, John AMS, & Meyer JS (2017). Chronic stress in the mother-infant dyad: Maternal hair cortisol, infant salivary cortisol and interactional synchrony. Infant Behavior and Development, 47, 92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A, Glover V, Marks M, & Kammerer M (2009). Diurnal pattern of cortisol output in postnatal depression. Psychoneuroendocrinology, 34(8), 1184–1188. [DOI] [PubMed] [Google Scholar]
- Thompson RA (1994). Emotion regulation: A theme in search of definition. Monographs of the Society for Research in Child Development, 59(2–3), 25–52. [PubMed] [Google Scholar]
- Tronick E (2007). The neurobehavioral and social-emotional development of infants and children. WW Norton & Company. [Google Scholar]
- Vanska M, Punamaki RL, Lindblom J, Tolvanen A, Flykt M, Unkila-Kallio L, & Tiitinen A (2016). Timing of early maternal mental health and child cortisol regulation. Infant and Child Development, 25(6), 461–483. [Google Scholar]
- Weaver CM, Shaw DS, Dishion TJ, & Wilson MN (2008). Parenting self-efficacy and problem behavior in children at high risk for early conduct problems: The mediating role of maternal depression. Infant Behavior and Development, 31, 594–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg MK, Olson KL, Beeghly M, & Tronick EZ (2006). Making up is hard to do, especially for mothers with high levels of depressive symptoms and their infant sons. Journal of Child Psychology and Psychiatry, 47(1), 670–683. [DOI] [PubMed] [Google Scholar]
- Yim IS, Stapleton LRT, Guardino CM, Hahn-Holbrook J, & Schetter CD (2015).Biological and psychosocial predictors of postpartum depression: systematic review and call for integration. Annual review of clinical psychology, 11, 99–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayas LH, Jankowski KR, & McKee MD (2005). Parenting competency across pregnancy and postpartum among urban minority women. Journal of Adult Development, 12, 53–62. [Google Scholar]