Abstract
Introduction:
Biology of ageing is focused on elucidating the biochemical and genetic pathways that contribute to cellular damage accumulation over time. Thirty years of research are beginning to bear fruit as the first pharmacological interventions based on biology of ageing go through clinical trials. Evolutionary theories of ageing suggest that naturally selected traits believed to impart fitness in young organisms may be damaging in later life. Three major areas of focus in biology of ageing are lifespan, healthspan, and rejuvenation.
Areas Covered:
Ageing research has produced several validated pharmacological interventions currently in clinical trials. Herein, the authors consider two representative case studies: 1) rapamycin analogues and their effect on the mTORC1 pathway, and 2) small molecules that target and kill senescent cells. The authors also provide their expert current and future perspectives on ageing targeting drug discovery.
Expert opinion:
Ageing-related therapeutic interventions will continue to emerge at an accelerating pace, both from research in biology of ageing, as well as from coordinated biomedical research in ageing-related chronic conditions.
Keywords: age-related, ageing, aging, drug discovery, geroscience, healthspan, lifespan, longevity, rapamycin, rejuvenation, senolytics
1. Introduction
As a research science, the biology of ageing is relatively new. It has been 30 years since the discovery of age-1, a recessive mutant allele that produced a mean lifespan increase of 40–65% in the nematode animal model Caenorhabditis elegans [1*]. This discovery, that is, the identification of the life-extending properties of the mutant age-1 gene, is considered by many to be the unofficial debut of the biology of ageing as a tractable research science [2]. Since that time, significant progress has been made in both elucidating the drivers of ageing as well as identifying and validating potential pharmacological targets to slow, halt, or reverse the effects of ageing. This paper will provide a brief overview of ageing as a biomedical research science as well as a description of the drug discovery process behind a limited selection of ageing-related pharmacological interventions currently in clinical trials. It is worth noting that the list of proposed ageing-related targets and geroprotective substances is growing rapidly; a comprehensive review of all potential therapies is beyond the scope of this paper. Case studies included herein were chosen based on their clinical trial status and representation of lifespan- and healthspan-related research, as described below.
2. What is Ageing and How Do We Research It?
2.1. Defining Ageing
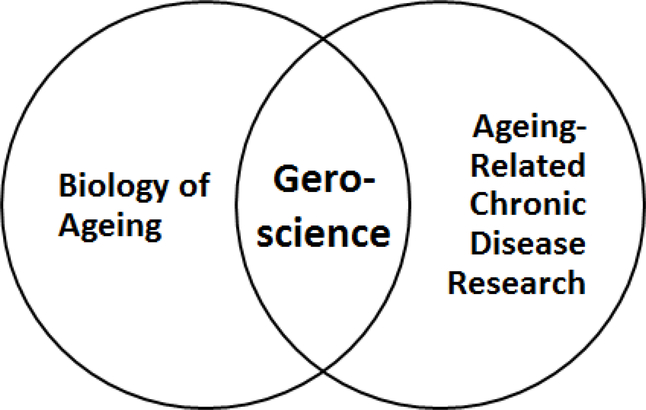
Broadly speaking, ageing can be defined as the time-dependent functional decline observed in nearly all living organisms. It is believed that ageing is a result of accumulated cellular damage resulting in hallmarks such as telomere attrition, epigenetic alterations, loss of protein homeostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, and stem cell exhaustion, among others [3**]. Research in the biology of ageing focuses on identifying and elucidating the genetic and biochemical pathways that cause or contribute to this damage accumulation (Figure 1).
Figure 1.
Geroscience – the connection between Ageing and Ageing-Related Diseases. Initially, the ageing research community viewed its work as distinct from indication-specific biomedical research in ageing-related diseases. Over time, however, this view evolved with the understanding that not only do ageing-related chronic diseases share a commonality amongst themselves, but ageing itself is ultimately a contributor to these conditions. The interdisciplinary connection between biology of ageing and ageing-related disease research is known as geroscience. [6]
2.2. What Causes Ageing?
Theories of evolutionary biology of ageing seek to address the question of why ageing happens. Two major non-exclusive schools of thought have emerged: 1) that there is a tradeoff between resources, such as energy, spent on biological processes driving cell growth and proliferation, versus those driving cell repair and maintenance (the ‘Disposable Soma’ theory) and 2) certain genetic traits that impart an advantage in early life become disadvantageous in later life (the ‘Antagonistic Pleiotropy’ theory). Both theories suggest that ageing is not a programmed event like cell cycle traverse but rather the consequence of natural selection’s focus on fitness in early life. Conversely, when a mutation confers an extension in lifespan, it is often detrimental to early life growth or fertility [1*,4,5]. As we begin to think about targeting ageing processes pharmacologically, we should be aware that the target is likely involved in multiple pathways with multiple consequences of intervention.
2.3. Longevity as Proxy for Slowing Ageing
Early researchers in biology of ageing did not have well-defined hallmarks or theories, but rather adopted life-extension as a proxy for slowing of ageing due to the ease of phenotypic analysis [2], asking the question, ‘Is it possible to manipulate an animal model to extend its average and/or maximal lifespan?’ On this basis the search began for factors that extend lifespan and thus presumably slow ageing. Longevity research in the 1980s and 90s took advantage of emergent molecular and genomic techniques and made significant progress in identifying genes that affect lifespan in short-lived animal models, mainly yeast (Saccharomyces cerevisiae), worms (Caenorhabditis elegans), and flies (Drosophila melanogaster). These models have reproductive cycles and lifespans that are days or weeks long, compared to rodent models with lifespans of two to three years; thus they provide a relatively time- and cost-effective means to identify potential leads through screening for life-extension in phenotypic assays. The phenotypic hits can then be used to guide deconvolution of the underlying targets through additional research, for example, using RNAi or genetic knockins and knockouts to identify relevant genes and the proteins they encode [7].
2.4. Lifespan, Healthspan, Rejuvenation
Lifespan extension is still an important phenotypic assay for identifying pathways of interest, but there is a growing emphasis on extending the proportion of lifespan that is relatively free from disease and morbidity, commonly referred to as healthspan [8], as well as interest in the possibility of reversing ageing-related damage accumulation, that is, rejuvenation. Thus far, successful pharmacological lead identification in ageing has emerged mainly from research into lifespan extension, with some progress in identifying leads that extend healthspan, and rejuvenation research still in the basic science phase (Figure 2).
Figure 2.
Areas of Focus in Biology of Ageing Research: Lifespan, Healthspan, Rejuvenation. Early research on lifespan extension has evolved to take into consideration the extension of healthy lifespan, with rejuvenation a forward-looking goal for therapeutic intervention.
3. Case Study – Extending Lifespan via Inhibition of mTORC1
3.1. Dietary Restriction is Life-Extending and Health-Promoting
One of the most significant modulators of longevity to emerge from phenotypic screening is neither pharmacological nor genetic in nature, but rather behavioural. Dietary restriction (‘DR’) is a highly reproducible means of extending lifespan in animal models (under the right genetic and environmental conditions), with the first scientific results published in 1935 showing that a diet containing 20% indigestible cellulose increased both mean and maximum lifespan in rodents, versus controls fed ad libitum [9*]. Since then both lifespan extension and reduction in disease pathology have been demonstrated in yeast, worms, rodents, and non-human primates [10], through various forms of DR such as reduced overall caloric consumption, restriction of a particular class of nutrients, variation in timing of food intake (intermittent fasting), or some combination thereof [5].
Animals with increased lifespan through DR often display a concomitant reduction in fertility/fecundity. It is hypothesized that, in line with the disposable soma evolutionary theory of ageing, DR triggers a redirection of cellular energy expenditure from growth and reproduction to somatic maintenance during times of stress in order to increase the chances of survival [4].
Human clinical trials of DR show improved health markers similar to those in animal models, with the proviso that adequate nutrition must be maintained as calories are reduced. The complexity of structuring a nutritionally adequate calorie-restricted human diet, together with the behavioural difficulty of maintaining a restrictive dietary regime, highlights the desirability of deconvoluting the underlying target(s) of DR in order to develop pharmacological interventions that mimic these beneficial effects [11]. A handful of biochemical pathways have been shown to be both mediated by dietary restriction and also relevant for ageing. One of these, the mTOR pathway, is a druggable target and has been the focus of much of the successful clinical work in ageing to date.
3.2. The mTOR Metabolic Pathway
mTOR is a serine/threonine kinase belonging to the phosphatidylinositol kinase-related kinase (PIKK) family. mTOR was first identified in a 1991 study exploring the toxic effects of the antibiotic rapamycin in yeast. The study uncovered the binding of rapamycin to a protein whose mechanism of toxic action was not understood. For this reason the protein was named simply TOR, for ‘target of rapamycin’ [12], later modified to mTOR, for ‘mechanistic target of rapamycin.’ Since that time, mTOR has been shown to be highly conserved across all eukaryotic species studied, including mammals. mTOR can be found in two distinct complexes that have different functions, one of which, the mTORC1 complex, is sensitive to and inhibited by rapamycin [5].
mTORC1 is a nutrient-sensing protein complex that monitors and responds to intra- and extracellular parameters and appears to act as a control center for various downstream pathways that control cellular growth, proliferation, and lifespan. mTORC1 is released from its inhibited state in times of low stress and nutritional and oxygen abundance, triggering metabolism, RNA translation, and ribosome biogenesis, essentially giving a green light to cell growth and reproduction. Conversely, in times of nutrient stress, for example, under DR, mTORC1 is inhibited, which turns off cell proliferation and enables stress resistance functions and autophagy of damaged cells [5].
3.3. Both Dietary Restriction and Rapamycin inhibit mTORC1
Inhibiting the mTOR signaling pathway through genetic modulation causes lifespan-extension in worms [13], flies [14], and yeast [15]. Furthermore, DR for mTOR knockdown mutants fails to further increase lifespan in flies [14] and yeast [15], suggesting at least a partial overlap between lifespan-extension mechanisms for both mTOR inhibition and DR. From an evolutionary biology perspective, consistent with the disposable soma theory, it is hypothesized that mTORC1 is a mediator of the cellular energy redirection observed under DR [5].
Following the 1991 yeast studies, further studies uncovered the mechanism of rapamycin’s toxicity: rapamycin binds with FK506 binding protein (FKBP12), and in turn this complex binds with and allosterically inhibits the mTORC1 complex. In both cases, i.e. via rapamycin and via DR, inhibition of mTORC1 arrests cell cycle growth by failing to activate downstream kinases necessary for ribosomal synthesis and mRNA translation for key proteins required for G1 to S phase traverse [16]. It is this downregulation of cell growth that makes mTORC1 a target for cancer treatment, immunosuppression, and life extension.
3.4. Rapamycin is a Multifaceted Drug
Rapamycin belongs to the antibiotic class macrolide and was first discovered in samples of soil from Rapa Nui, Easter Island, collected in 1964 by a team of microbiologists from the University of Montreal, who later gave the samples to Ayerst pharmacological researchers searching for fungicides. In 1972, the Ayerst scientists isolated a fungicidal compound produced by the Streptomyces hygroscopicus bacterium and named it rapamycin, after the island on which it was discovered [17]. Initially of interest as an antifungal, rapamycin was later discovered to have immunosuppressant properties [18], and the rapamycin analog sirolimus was cleared by the FDA in 1999 for use in reducing rejection of transplanted kidneys.
Dr Suren Seghal, a researcher who was part of the original group that isolated the compound, believed that rapamycin possessed more than just antifungal and immunosuppressant properties and sent a sample to the National Cancer Institute with a request for screening. His hunch was validated: rapamycin was found to be effective at tumor suppression in a number of cell lines including mammary, colon, and melanocarcinoma [19]. In 2007 a second derivative of rapamycin (temsirolimus) was approved to treat kidney cancer.
Based on genetic studies demonstrating a role for the mTOR pathway in lifespan of worms, flies, and yeast, there was interest in whether rapamycin could also extend lifespan in mammals. In a landmark study published in 2009, Jackson Laboratories in Bar Harbor, Maine, the University of Michigan, and University of Texas Health Science Center in San Antonio together conducted the first controlled studies of rapamycin and lifespan in mice, funded by the National Institute on Ageing Interventions Testing Program. They found that rapamycin induced lifespan extensions of 9–14% in genetically heterogeneous mice at all three testing sites [20**]. Followup studies confirmed that the effects of rapamycin were indeed age-slowing according to a number of markers, rather than merely delaying onset of disease [21].
3.5. Rapamycin Analogues (‘Rapalogs’) in Clinical Studies
Encouraged by the results from the rapamycin mouse longevity studies, scientists at Novartis began exploring whether rapamycin could have age-related effects in humans. Rather than targeting ageing itself, they chose several categories of age-related functional decline, initially focusing on immune decline, to guide their studies. The Novartis rapalog everolimus, also known as RAD001 in clinical studies, is a partial allosteric inhibitor of mTORC1. Everolimus was given to 216 elderly participants together with the 2012 flu vaccine and subsequently shown to improve immune response by 20%. The results were published in 2014 [22].
In 2017, Novartis chose to spin out its mTORC1 inhibitor technology into a biotech JV with PureTech called resTORbio. The spinout included licensing for use of both everolimus and BEZ235 (now called RTB101), a competitive inhibitor of mTORC1. resTORbio conducted a follow-on phase 2a study in 2018, with 264 participants. The active arm participants were given a combination of RAD001 and RTB101. These participants showed not only boosted antibody response to influenza vaccines versus placebo, but also an overall decrease of other infections (mostly respiratory tract): 1.5 per year vs 2.4 in the placebo arm [23]. A phase 2b study concluded in the second half of 2018, and phase 3 trials (of RTB101 only) are currently underway [J. Mannick, personal communication, December 3, 2018].
3.6. Future Directions for mTORC1 as a Pharmacological Target
The resTORbio website also lists several other intended clinical trials making use of RTB101 and/or everolimus technology to inhibit mTORC1 with an expectation of improved health outcomes for several pathologies including other infections (not necessarily respiratory tract), heart failure, and autophagy-related diseases. A clinical trial for RTB001 plus sirolimus is also listed as being underway, with a target indication of Parkinson’s disease [24].
Other companies, such as Mount Tam Biotechnologies, are also developing mTORC1 inhibitors. Per Mount Tam’s website, their rapamycin analogue TAM-01 is in pre-clinical testing with a target indication of systemic lupus erythematosus [25]. Interestingly, Navitor Pharmaceuticals is focusing on selective mTORC1 agonists, as opposed to inhibitors, and its mTORC1 agonist NV-5138 is in recruitment for phase 1A safety trials as an antidepressant. Thus, mTORC1 may require upregulation rather than downregulation for certain indications, underscoring the importance of context and specificity when targeting metabolic pathways. Overall, there appears to be significant potential for future development of pharmacological leads targeting the mTORC1 pathway.
4. Case Study – Extending Healthspan by Targeting Cellular Senescence
4.1. Cellular Senescence is a Tradeoff
Cellular senescence (hereinafter, ‘senescence’) refers to a permanent state of arrested cell proliferation wherein a cell remains alive but fails to divide and reproduce. Senescent cells can be distinguished, both morphologically and through biomarkers, from either cells undergoing transient growth arrest (‘quiescence’) or terminally differentiated cells, which lose their ability to replicate once they have taken on a specialised function (e.g. red blood cells, neurons, etc.) [26]. Senescence was discovered by Leonard Hayflick and Paul Moorhead during experiments in the 1960s that uncovered the Hayflick Limit, i.e. the inability for cells to divide and reproduce more than a certain number of times in culture [27*, 28]. It was later determined that this type of senescence (termed ‘replicative senescence’) is a result of a DNA damage response triggered by telomere attrition during repeated replication that cannot be repaired by endogenous DNA repair machinery. Cellular senescence can also be induced in other ways, for example through mutations that produce activated oncogenes, elevated levels of reactive oxygen species (ROS), other (non-ROS producing) mitochondrial dysfunction, and DNA damage induced by ionizing radiation, genotoxic chemotherapies, or stalled replication or transcription forks; as well as through pathways that trigger derepression of the gene locus CDKN2A, leading to increased expression of the p16INK4a protein (hereinafter, ‘p16’) [29].
Senescence plays an important role in mammalian tumor suppression by preventing damaged cells from proliferating, while allowing cells to fulfill certain necessary physiological roles, e.g. melanocytes continue to produce melanin while senescent, thus providing ongoing protection against UV damage to the skin [30]. However, senescent cells can also exhibit a proinflammatory response known as the senescence-associated secretory phenotype (‘SASP’), which in its transient form has been beneficially linked to wound healing [31] and important developmental processes in early life, but can become damaging in later life if left unchecked. Irrespective of cell type or underlying trigger for senescence, cells exhibiting a SASP secrete cytokines and chemokines such as interleukins (IL)-6 and −8, which through paracrine signaling exacerbate the growth and aggressiveness of nearby precancerous and cancerous cells [32, 33]. This somewhat paradoxical dual effect of senescence and its associated secretory phenotype, i.e. initially inhibiting but eventually promoting tumorigenesis, is consistent with the evolutionary theory of antagonistic pleiotropy. The SASP together with the chronic inflammation it induces (often referred to as ‘inflammaging’) is therefore a candidate driver of ageing [34] and a target for pharmacological intervention.
4.2. The Discovery of Senolytics
Following Hayflick’s initial discovery in 1961, progress in understanding senescence in vivo was limited until the discovery of the senescence-associated β-galactosidase (SA β-galactosidase) biomarker in 1995 [35]. As in many cases of scientific breakthrough, this discovery was one of intelligent serendipity: human skin cell cultures that had been genetically modified through transfection with plasmids expressing E. coli lacZ were being tested for β-galactosidase to confirm success. High levels of an unexpected lysosomal β-galactosidase in certain cells led researchers to take a closer look: it turned out that the cultures had been inadvertently incubated in a cell tissue incubator instead of a bacterial incubator, and the relatively high concentration of carbon dioxide in the tissue incubator had lowered the pH of the cultures to about 6.0. Following further investigation, this phenomenon was replicated by inducing senescence in primary human fibroblast cells and lowering the pH to 6.0 triggering expression (in senescent cells only) of lysosomal β-galactosidase, thus establishing the validity of SA β-galactosidase as the first senescence biomarker [J. Campisi, personal communication, February 21, 2019].
Use of SA β-galactosidase as a biomarker helped elucidate downstream pathways of senescence and revealed additional biomarkers. In general, there appear to be two senescence response pathways, one or both of which are activated depending on the type of trigger. The first pathway is p53-dependent, generally triggered through genomic stress, and results in upregulated expression of p53 and p21 (a commonly cited biomarker for senescence). The second is p16-dependent (p16 is another biomarker), generally triggered through oncogenes and other types of stress, resulting in upregulated pRB activity, leading to chromatin reorganization that prevents cellular proliferation [34]. Because of the myriad pathways that trigger senescence, there is no single biomarker present in all senescent cells, and conversely the presence of a single biomarker is not a hard indication that a cell is senescent. Therefore identification of senescent cells generally involves multiple biomarkers, of which SA β-galactosidase, p21, and p16 are prominent [36].
Senescence and the SASP have been linked to cancer and many other pathologies, including ageing [29, 37]. In 2011, in order to explore whether eliminating senescent cells (termed ‘senolysis’) would have a beneficial effect on ageing pathology, a transgenic progeroid mouse model (BubR1) was modified such that, upon administration of a synthetic drug (AP20187), cells expressing p16 would undergo apoptosis, thus providing a highly effective method of pharmacologically inducing senolysis in multiple tissue types, including adipose, skeletal muscle, and eye tissue. Lifelong administration of AP20187 significantly delayed the onset of ageing-related phenotypes, and late-life administration attenuated their progression [37**].
4.3. Senolytics in Clinical Trials
Inspired by the results from the 2011 mouse studies, biotech investor Nathaniel David, PhD, together with a team of prominent senescence researchers, cofounded Unity Biotechnology in 2012, aiming to explore the potential for targeting senescent cells as an anti-ageing therapeutic intervention. Over the next few years, researchers at Unity Biotechnology developed the senolytic UBX0101, a small molecule that inhibits MDM2/p53 protein interaction and thereby tips senescent cells into apoptosis. UBX0101’s target indication of osteoarthritis was based on observations that senescent cells accumulate in knee cartilage following injury to the anterior cruciate ligament (ACL), leading to the development of osteoarthritis [39].
In preclinical studies carried out in 2017, intra-articular injection of UBX0101 following ACL transection resulted in selective elimination of senescent cells, reduced pain, and attenuated development of osteoarthritis in transgenic, non-transgenic, and naturally-aged mice. Similarly, tissue cultures from human patients with osteoarthritis were treated with UBX0101 and showed decreased expression of senescent biomarkers and increased expression of cartilage extracellular matrix proteins [40]. In 2018, the FDA cleared Unity’s IND application for UBX0101, and by the second half of 2018, phase 1 safety studies were underway.
4.4. Future Directions for Senolytics as a Pharmacological Intervention
The Unity Biotechnology website lists a second lead candidate, UBX1967, a small molecular inhibitor of the Bcl-2 family of anti-apoptotic proteins with a target indication to treat ophthalmologic diseases [41]. Other Bcl-2 inhibitors such as Abbvie’s ABT-737 and ABT-263, originally targeted for cancer treatment, have also been identified as apoptosis-inducing senolytics [42, 43], although these may face challenges as therapeutic indications due to previous failed clinical trials for cancer. Cleara Biotech is working with a senolytic peptide called FOXO4-DRI that perturbs forkhead box protein O4 (FOXO4) interaction with p53 and tips senescent cells into apoptosis [44]. Numerous other biotech firms such as Oisin Biotechnologies, Senolytic Therapeutics, and CellAge are also focused on developing senolytics as therapeutic interventions, and new firms are emerging with increasing frequency.
Naturally occurring substances also hold potential for development as senolytics. For example, Fisetin, a naturally occurring flavonoid, displays powerful senolytic properties and is currently recruiting for clinical trial to test effects on frailty through the Mayo Clinic [45]. Work with fisetin follows previous successful phase 1 clinical studies with quercetin, another senolytic flavonoid [46]. Continued screening of known compounds may reveal further leads of interest in this area. Given the complexity of both drivers and pathways of senescence, there is potential for discovery of myriad senolytic target/lead combinations going forward.
5. Conclusion
Research in biology of ageing has made significant progress over the last 30 years in elucidating our understanding of the genetic and biochemical pathways that contribute to ageing-related damage accumulation. Not only do the processes of ageing contribute to ageing-related diseases and morbidities, but complex interrelationships exist amongst the pathways of ageing themselves. Because of this, interventions that target healthy ageing generally have multiple downstream effects that must be carefully explored and weighed against each other to ensure optimal clinical outcomes. By the same token, modulating a single ageing-related target often yields unexpected beneficial effects on indications beyond those under initial consideration.
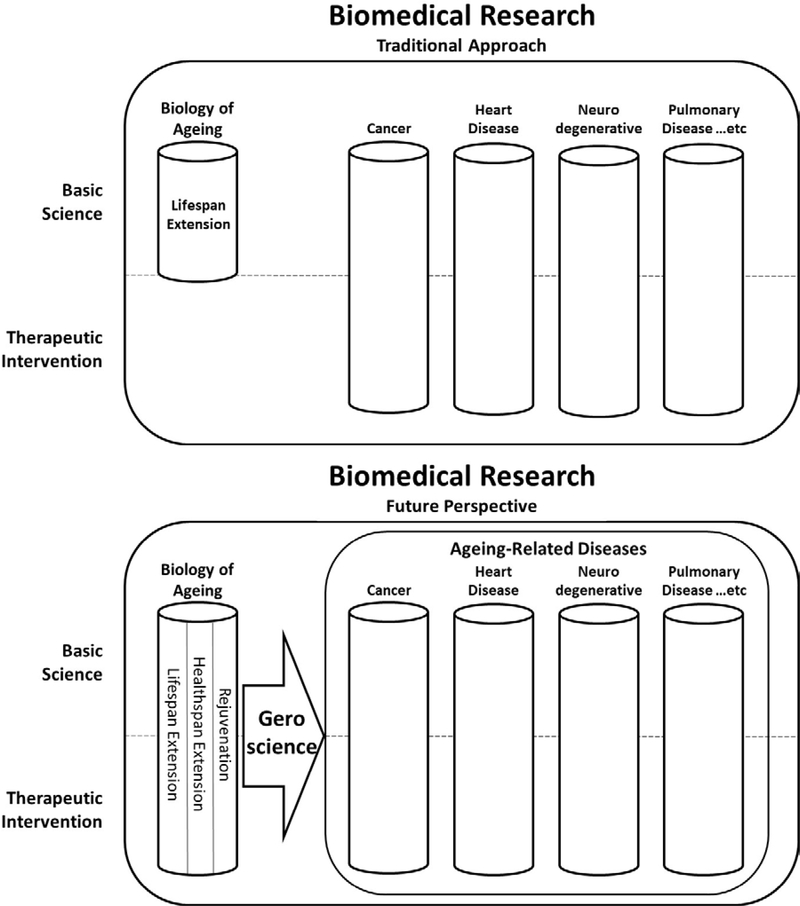
Biomedical research and patient communities stand to benefit enormously from a heightened understanding of commonality amongst ageing-related diseases and the role that ageing processes play in their pathology (Figure 3). We are just beginning to reap the fruits of 30+ years of ageing research, with the first optimized leads from validated targets of ageing entering late-stage clinical trials and a strong pipeline of additional leads in the works. This trend can be expected to continue as new players focused on biotechnology of ageing enter the market. The field of biology of ageing has truly begun to mature.
Figure 3.
Commonality Amongst Ageing-Related Diseases. Although some researchers believe the process of ageing should be classified as a disease [47] and ‘Aging’ is an acceptable medical condition for clinical trials on the UK Clinical Trials Gateway and Clinicaltrials.gov in the US, to date ageing has not been acknowledged in the International Classification of Diseases (ICD) as a disease, but rather as a symptom. However, the most recent ICD-11, released in mid-2018, has added ‘ageing-related’ as a descriptive extension, paving the way for the biomedical research industry to consider commonality amongst diseases whose highest risk factor is age. It is the hope of scientists in biology of ageing that the traditional siloed approach of indication-oriented biomedical research will give way to a more comprehensive approach to researching ageing-related diseases that makes use of discoveries in biology of ageing, with the field of geroscience bridging that gap.
6. Expert Opinion
We live at a time of unprecedented demographic change, wherein the oldest age groups are the fastest-growing, a phenomenon aptly termed the ‘silver tsunami.’ Leading edge baby boomers are now in their 70s, and with this rising tide, we are presented with a new model of how to spend one’s golden years. Many of today’s seniors are engaged in active retirement, health activism, and generally staying active. Indeed ‘active’ appears to be the operative word, and with it comes a demand for healthier ageing. We believe that the heightened self-awareness and expectations of this generation of seniors has driven and will continue to drive increased interest and investment of time, energy, and money into researching healthy ageing.
Similarly, we believe that research in ageing is poised to experience a rapid increase in translation of validated targets into pharmacological interventions that slow, halt, or even reverse the effects of ageing. Much remains to be understood about mTOR and other metabolic pathways such as IGF-1, SIR2, and AMPK, which have all been linked to effects of DR; however, the potential to target these pathways is manifest, and preclinical work is under way. Controlled senescence holds great promise for extending healthspan and is discussed in detail above; in our opinion, this is truly the low-hanging fruit in the ageing drug discovery pipeline that is quickly being explored in academia and industry. Another major area of preclinical research is in ameliorating or reversing mitochondrial dysfunction; for example CohBar’s MOTS-c analogue is currently in clinical trial targeting NASH. All these areas of focus have validated pharmacological targets in various stages of preclinical or clinical work at one or more biotechnology companies aiming to identify and optimize leads for clinical trial.
Stem cell therapies and biologics (beyond the scope of this review) will also be developed. Perhaps it will one day be commonplace to replace aged tissues and even whole organs derived from pluripotent cells. There is also great hope in the use of patient-derived cell lines and organelles in the identification of individualized therapies. This may be the first tangible benefit of stem cell technologies.
We also believe that advances in computational technology together with increasing capacity for data analysis will play a significant role in both discovering and developing ageing-related therapeutics going forward, as is also the case for other areas of drug discovery and development. In silico methodologies such as virtual drug screening, and data-intensive analytics such as pharmacogenomics and pharmacometabolomics, are all highly relevant for ageing-related research.
Meanwhile, biomedical research must move away from specific disease- and organ-based research (research silos) and instead begin to investigate why comorbidities are so common in the elderly, focusing on ageing mechanisms as a common cause of diverse diseases. The field should continue to be on the lookout for drugs such as metformin, which, although developed as an antidiabetic, actually appears to slow ageing and therefore may be useful in preventing many ageing-related conditions. Interested readers may wish to follow the progress of the proposed metformin TAME trial, which would be a novel long-range clinical study aimed at monitoring metformin’s potential to delay onset of multiple ageing-related comorbidities [48]. Perhaps other FDA-approved drugs or naturally occurring substances also affect ageing, and these should be identified and repurposed as appropriate.
For those interested in what can be done in the here and now to maximize healthy lifespan, there is ample scientific evidence supporting the benefits of a lifestyle that incorporates a healthy diet, regular exercise, adequate sleep, minimal chronic stress, and daily social interaction. Demographers have been predicting the silver tsunami for decades, but it has taken until now for the wider population to start paying attention. The tsunami is here, let’s aim for higher ground!
Article Highlights Box.
Biology of ageing is focused on elucidating drivers of cellular damage accumulation over time.
From an evolutionary perspective, traits that impart fitness in the young may be damaging in later life.
‘Geroscience’ refers to the connection between basic science research in biology of ageing and biomedical research in ageing-related chronic diseases.
Three main areas of ageing research are: lifespan/longevity, healthy lifespan (‘healthspan’), and rejuvenation.
A number of pharmacological interventions that target drivers of ageing are in clinical trial, and this trend is set to continue.
Acknowledgements:
The authors would like to acknowledge Dr Judith Campisi for sharing her expertise and personal insight in the drafting of this review.
Funding:
GJ Lithgow is supported by NIH grant UL1024917 (through the Interdisciplinary Research Consortium on Geroscience) as well as NIH grants 1R01AG029631-01A1 and R01AG029631.
Footnotes
Declaration of Interest:
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures:
Peer reviewers on this manuscript have received an honorarium from.
Contributor Information
Ariana Myers, Buck Institute for Research on Aging, 8001 Redwood Boulevard, Novato, CA 94945, USA, Job Title: Candidate, MS Drug Discovery and Development, Drexel University, Phone: +1-415-209-2000, ariana.myers@drexel.edu.
Gordon J. Lithgow, Buck Institute for Research on Aging, 8001 Redwood Boulevard, Novato, CA 94945, USA, Job Titles: Principal Investigator, Lithgow Laboratory; Chief Academic Officer; Interim Chief Science Officer, Phone: +1-415-209-2000, glithgow@buckinstitute.org.
References
- 1.Friedman DB and Johnson TE, A Mutation in the Age-1 Gene in Caenorhabditis Elegans Lengthens Life and Reduces Hermaphrodite Fertility. Genetics, 1988. 118(1): p. 75–86.* Seminal work launching Biology of Ageing as a formal research science.
- 2.Johnson TE, 25 Years after age-1: Genes, Interventions and the Revolution in Aging Research. Exp Gerontol, 2013. 48(7): p. 640–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez-Otin C, Blasco MA, Partridge L et al. , The hallmarks of aging. Cell, 2013. 153(6): p. 1194–217.** Figure 1 from this paper is frequently referenced in Biology of Ageing research.
- 4.Holliday R, Food, reproduction and longevity: is the extended lifespan of calorie-restricted animals an evolutionary adaptation? Bioessays, 1989. 10(4): p. 125–127. [DOI] [PubMed] [Google Scholar]
- 5.Kapahi P Chen D, Rogers AN, et al. , With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab, 2010. 11(6): p. 453–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kennedy BK, Berger SL, Brunet A et al. , Geroscience: linking aging to chronic disease. Cell, 2014. 159(4): p. 709–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung HJ and Kwon HJ, Target deconvolution of bioactive small molecules: the heart of chemical biology and drug discovery. Arch Pharm Res, 2015. 38(9): p. 1627–41. [DOI] [PubMed] [Google Scholar]
- 8.Newman JC, Milman S, Hashmi SK et al. , Strategies and Challenges in Clinical Trials Targeting Human Aging. J Gerontol A Biol Sci Med Sci, 2016. 71(11): p. 1424–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCay CM, Crowell MF, and Maynard LA, The effect of retarded growth upon the length of life span and upon the ultimate body size. 1935 Nutrition, 1989. 5(3): p. 155–71; discussion 172.* Historically significant paper debuting effects of dietary restriction in a controlled experiment.
- 10.McDonald RB and Ramsey JJ, Honoring Clive McCay and 75 Years of Calorie Restriction Research. J Nutr, 2010. 140(7): p. 1205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fontana L and Partridge L, Promoting health and longevity through diet: from model organisms to humans. Cell, 2015. 161(1): p. 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heitman J, Movva NR, and Hall MN, Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science, 1991. 253(5022): p. 905–9. [DOI] [PubMed] [Google Scholar]
- 13.Vellai T, Takacs-Vellai K, Zhang Y et al. , Genetics: influence of TOR kinase on lifespan in C. elegans. Nature, 2003. 426(6967): p. 620. [DOI] [PubMed] [Google Scholar]
- 14.Kapahi P, Zid BM, Harper T et al. , Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol, 2004. 14(10): p. 885–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaeberlein M Powers RW 3rd, Steffen KK, et al. , Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science, 2005. 310(5751): p. 1193–6. [DOI] [PubMed] [Google Scholar]
- 16.Mita MM, Mita A, and Rowinsky EK, The molecular target of rapamycin (mTOR) as a therapeutic target against cancer. Cancer Biol Ther, 2003. 2(4 Suppl 1): p. S169–77. [PubMed] [Google Scholar]
- 17.Vezina C, Kudelski A, and Sehgal SN, Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot (Tokyo), 1975. 28(10): p. 721–6. [DOI] [PubMed] [Google Scholar]
- 18.Martel RR, Klicius J, and Galet S, Inhibition of the immune response by rapamycin, a new antifungal antibiotic. Can J Physiol Pharmacol, 1977. 55(1): p. 48–51. [DOI] [PubMed] [Google Scholar]
- 19.Seto B, Rapamycin and mTOR: a serendipitous discovery and implications for breast cancer. Clin Transl Med, 2012. 1: p. 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrison DE Strong R, Sharp ZD, et al. , Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature, 2009. 460: p. 392.** Establishes the life-extending properties of rapamycin in mammals.
- 21.Wilkinson JE Burmeister L, Brooks SV, et al. , Rapamycin slows aging in mice. Aging Cell, 2012. 11(4): p. 675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mannick JB Del Giudice G, Lattanzi M, et al. , mTOR inhibition improves immune function in the elderly. Sci Transl Med, 2014. 6(268): p. 268ra179. [DOI] [PubMed] [Google Scholar]
- 23.Mannick JB Morris M2, Hockey HP, et al. , TORC1 inhibition enhances immune function and reduces infections in the elderly. Sci Transl Med. 2018. July 11;10(449). pii: eaaq1564. doi: 10.1126/scitranslmed.aaq1564 [DOI] [PubMed] [Google Scholar]
- 24.Pipeline. resTORbio; Available from: https://www.restorbio.com/pipeline/.
- 25.Product Development. Mount Tam Biotechnologies; Available from: http://mounttambiotech.com/productdevelopment.aspx.
- 26.Munoz-Espin D and Serrano M, Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol, 2014. 15(7): p. 482–96. [DOI] [PubMed] [Google Scholar]
- 27.Hayflick L, The Limited In Vitro Lifetime Of Human Diploid Cell Strains. Exp Cell Res, 1965. 37: p. 614–36.* Historically significant work introducing the Hayflick limit.
- 28.Hayflick L and Moorhead PS, The serial cultivation of human diploid cell strains. Exp Cell Res, 1961. 25: p. 585–621. [DOI] [PubMed] [Google Scholar]
- 29.van Deursen JM, The role of senescent cells in ageing. Nature, 2014. 509: p. 439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jan Vijg JC, Lithgow Gordon, Molecular and Cell Biology of Aging. 2014, Washington, DC: Gerontological Society of America. [Google Scholar]
- 31.Demaria M, Ohtani N, Youssef S, et al. , An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell, 2014. 31(6): p. 722–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coppé J-P Patil CK, Rodier F, et al. , Senescence-Associated Secretory Phenotypes Reveal Cell-Nonautonomous Functions of Oncogenic RAS and the p53 Tumor Suppressor. PLOS Biology, 2008. 6(12): p. e301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krtolica A Parrinello S, Lockett S, et al. , Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci U S A, 2001. 98(21): p. 12072–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campisi J, Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell, 2005. 120(4): p. 513–22. [DOI] [PubMed] [Google Scholar]
- 35.Dimri GP Lee X, Basile G, et al. , A biomarker that identifies senescent human cells in culture and in aging skin in vivo. 1995. 92(20): p. 9363–9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernardes de Jesus B and Blasco MA, Assessing cell and organ senescence biomarkers. Circ Res, 2012. 111(1): p. 97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Childs BG, Gluscevic M1, Baker DJ et al. , Senescent cells: an emerging target for diseases of ageing. Nat Rev Drug Discov, 2017. 16(10): p. 718–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baker DJ, Wijshake T, Tchkonia T et al. , Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature, 2011. 479: p. 232.** Establishes the age-delaying (healthspan-improving) properties of senolytics.
- 39.Jeon OH, David N, Campisi J et al. , Senescent cells and osteoarthritis: a painful connection. J Clin Invest, 2018. 128(4): p. 1229–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeon OH, Kim C, Laberge RM et al. , Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nature Medicine, 2017. 23: p. 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pipeline. Unity Biotechnologies; Available from: https://unitybiotechnology.com/pipeline/.
- 42.Yosef R, Pilpel N, Tokarsky-Amiel R et al. , Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat Commun, 2016. 7: p. 11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang J, Wang Y, Shao L et al. , Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nature Medicine, 2015. 22: p. 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baar M, Brandt R, Putavet D, et al. , Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging. Cell, 2017. 169(1): p. 132–147.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yousefzadeh M, Zhu Y, McGowan S, et al. , Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine, (2018). 36: p. 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Justice J, Nambiar A, Tchkonia T, et al. , Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine, (forthcoming 2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhavoronkov A and Bhullar B, Classifying aging as a disease in the context of ICD-11. Front Genet, 2015. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barzilai N, Crandall JP, Kritchevsky SB, & Espeland MA, Metformin as a Tool to Target Aging. Cell metabolism, 2016. 23(6): p. 1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]