Abstract
Purpose:
BRAF and MEK inhibitors (BRAFi, MEKi) are actively used for the treatment of metastatic melanoma in patients with BRAFV600E mutation in their tumors. However, the development of resistance to BRAFi and MEKi remains a difficult clinical challenge with limited therapeutic options available to these patients. In this study we investigated the mechanism and potential therapeutic utility of combination BRAFi and adoptive T cells therapy (ACT) in melanoma resistant to BRAFi.
Experimental design:
Investigations were performed in vitro and in vivo with various human melanoma cell lines sensitive and resistant to BRAFi as well as PDXs derived from patients. In addition, samples were evaluated from patients on a clinical trial of BRAFi in combination with ACT.
Results:
Herein we report that in human melanoma cell lines senstitive and resistant to BRAFi and in PDXs from patients who progressed on BRAFi and MEKi therapy, BRAFi caused transient up-regulation of mannose-6-phosphate receptor (M6PR). This sensitized tumor cells to CTLs via uptake of granzyme B, a main component of the cytotoxic activity of CTLs. Treatment of mice bearing resistant tumors with BRAFi enhanced the antitumor effect of patients’ TIL. A pilot clinical trial of 16 patients with metastatic melanoma who were treated with the BRAFi vemurafenib followed by therapy with TIL demonstrated significant increase of M6PR expression on tumors during vemurafenib treatment.
Conclusions:
BRAF targeted therapy sensitized resistant melanoma cells to CTLs, which opens new therapeutic opportunities for the treatment of patients with BRAF resistant disease.
INTRODUCTION
Melanoma is a skin cancer with high metastatic potential responsible for 80% of skin cancer-related deaths (1). Approximately 50% of melanoma patients have the BRAFV600E mutation in their tumors, which leads to expression of constitutively active mutant BRAF protein and induces the activation of downstream mitogen activated protein kinase (MAPK) signaling by phosphorylating MEK (2–4). Therefore, targeting of BRAF and MEK is an important therapeutic option for BRAF V600 mutated melanoma patients. BRAF inhibitors (BRAFi) vemurafenib and dabrafenib demonstrated impressive clinical responses in patients with BRAFV600E mutant melanoma (5, 6). Subsequent trials showed that the combination of BRAFi and MEKi achieved higher response rates and greater progression-free and overall survival (7–9). However, the efficacy of the treatment is limited due to development of resistance (10–12). Several studies have proposed a possible effect of BRAFi on immune responses. A significant increase in the infiltration of CD4+ and/or CD8+ T cells has been shown in metastatic melanoma patients treated with BRAFi (13, 14). BRAFi increased T cell recognition of melanoma cells without affecting the viability or function of lymphocytes (15, 16), suggesting that it might increase the effect of immunotherapy. BRAFV600E mutant SM1 melanoma-bearing mice treated with BRAFi and adoptive T cell transfer showed stronger antitumor responses and improved survival compared to either therapy alone. Expression of MHC and tumor antigen by SM1 tumor cells was not significantly altered (17).
Adoptive cell therapy (ACT) of melanoma with tumor-infiltrating lymphocytes (TIL) derived from patients’ resected tumors has demonstrated therapeutic promise (18, 19). The combination of targeted therapy and ACT would be a natural choice. In a recent pilot trial, the combination of vemurafinib and TIL ACT showed acceptable toxicity and generated objective clinical responses (20). However, the mechanism of a possible combined effect remains unclear since recognition of autologous tumor by T cells was similar between TILs grown from pre- and post-vemurafenib metastases (20). The clinically relevant question remained whether the combination of BRAFi and ACT could be beneficial in patients who developed resistance to BRAFi and MEKi and for whom clinical options are very limited.
We have previously demonstrated that transient up-regulation of cation-independent mannose 6-phosphate receptor (M6PR) (also known as insulin-like growth factor 2 receptor; IGF2R) was important for the antitumor effect of combination immune- and chemo- or radiation therapy in different mouse models of cancer (21–23). M6PR is a multifunctional membrane-associated protein involved in trafficking of soluble lysosomal proteins in the cytoplasm and binding of M6P containing ligands, such as insulin-like growth factor 2 (IGF2) (24). Importantly, it is a receptor for granzyme B (GrzB) secreted by activated cytotoxic T cells (CTL) (25). Chemotherapy and radiation therapy caused autophagy of tumor cells that resulted in re-distribution of M6PR to the surface of tumor cells and increased uptake of GrzB released by CTLs leading to expansion of tumor cell death (21–23). We asked whether BRAF targeted therapy can induce similar effects in human melanoma, and more importantly, whether this effect depends on the development of BRAF resistance by tumor cells.
MATERIAL and METHODS
Clinical Trial
The clinical trial protocol (NCT01659151) was approved by institutional review board of University of South Florida, and all subjects gave written informed consent for trial participation. The studies were conducted in accordance Declaration of Helsinki guidelines. Subjects were of age ≥ 18 years with stage III or IV metastatic melanoma that harbored an activating BRAF V600 mutation and were determined to be unresectable for intent to cure. Existing CNS metastases were required to be treated unless three or less in number, each less than 1 cm in size, and none associated with hemorrhage/edema. A focus of at least 1 cm of metastatic melanoma was harvested for TIL propagation as previously described with residual measurable disease per RECIST 1.1 criteria (26). Subjects started vemurafenib 960 mg (supplied by Genentech, Inc) by mouth twice daily one day following surgical resection with allowance for dose reduction or cessation based upon intolerance per the standard of care. TIL was expanded from tumor fragments and evaluated for reactivity as previously described, with the exception of the final two patients where rapid expansion occurred in G-REX flasks due to change in manufacturing requirements (30). Upon successful TIL propagation, subjects underwent lymphodepleting chemotherapy comprising daily inpatient cyclophosphamide 60 mg/kg x for 2 days, followed by daily outpatient fludarabine 25 mg/m2 for 5 days. Subjects then underwent ACT followed by up to 15 doses of intravenous 720,000 IU/kg of Interleukin-2 every 8 hours in the hospital under telemetry monitoring. Following clinical recovery from ACT, subjects were discharged and resumed vemurafenib for up to two years or until disease progression or intolerance.
Pre and post-treatment tumor biopsies were obtained from 9+7=16 patients receiving therapy for metastatic melanoma, either as part of a clinical trial of vemurafenib treatment prior to and after adoptive cell therapy for metastatic melanoma (n=9). Patients underwent needle core biopsies of target lesions after receiving therapy, and pre-treatment tumor tissue was retrieved from the institutional archives for comparative staining.
Human Cells and Mouse Models
Human studies were approved by The Wistar Institute IRB. Peripheral blood was collected from healthy volunteers after obtaining informed consent. Animal experiments were approved by The Wistar Institute Animal Care and Use Committee. The mice were kept under pathogen free conditions. Experiments were carried out using female NSG mice or female and male nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice, obtained from the mouse facility of the Wistar Institute and Charles River Laboratories, respectively. WM35 human melanoma and HLA-matched human tumor infiltrating lymphocytes (TIL) were generated at H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA). Human melanoma cells lines WM983B and WM983B-BR were maintained in Dulbecco’s Modified Eagles medium (DMEM) (Gibco), other human melanoma cell lines were maintained in RPMI medium supplemented with 10% fetal bovine serum (FBS, Sigma-Aldrich, St. Louis, MO) and 1 % Pen/Strep (Corning; Cat.no. 30–002-Cl) at 37 °C, 5% CO2. PLX4720-resistant cells were kept in cell culture media supplemented with 1 μM PLX4720 to keep these cells resistant against the drug. 350 μg/mL Hygromycin B (Invitrogen; Cat.no. 10687010) was kept in the culture media of WM983B-BR-pAHygCMV2, WM983B-BR-M6PR/IGF2R, WM35-pAHygCMV2, WM35-M6PR/IGF2R cells after transfection. Tumor cell lines were tested for mycoplasma contamination by using Universal Mycoplasma detection kit (ATCC) regularly. Human melanoma cells were treated with 1–10 μM PLX4720 (Selleckchem; Cat.no. S1152), 25–100 nmol/mL (nM) Trametinib (Selleckchem; Cat. no. S2673). Hypoxia (0.5% O2) was maintained using hypoxic chamber (BioSpherix).
In order to establish xenograft models, cultured WM35, WM35-BR, WM983B-BR-pAHygCMV2 and WM983B-BR-M6PR/IGF2R cells were harvested, suspended in sterile PBS, mixed in 2:1 ratio with Matrigel and injected subcutaneously into the left flank of mice (1–3 × 106 cells per mouse) (27). PLX4720 was administered daily at dose 50 mg/kg for 10 days via oral gavage. As a control, mice treated with vehicle alone (2% DMSO, 50% PEG 300, 5% Tween 80) were used. Treatments were started when tumors were 0.5–0.8 cm in diameter.
Generation of human melanoma cells overexpressing human M6PR/IGF2R
The plasmid pAHygCMV2/IGF2R encoding wild-type human M6PR/IGF2R was kindly provided by Dr. Lukas Mach from Medical University of Vienna. WM983B-BR and WM35 cell lines were stably transfected with either an empty plasmid, pAHygCMV2, or pAHygCMV2/IGF2R using Lipofectamine™ LTX reagent (Thermo Fischer Scientific; Cat.no. 15338030). Selection of transfected cells was achieved by their ability to grow in the presence of 350 μg/ml Hygromycin B (Invitrogen). Drug-resistant clones were isolated approximately after 2 weeks and tested for M6PR/IGF2R production by flow cytometry and western blotting.
Generation of WM983B-M6PR KO tumor cell line using CRISPR/Cas9
Human M6PR/IGF2R gene (gene ID number 3482) was used as target to design DNA guides. Oligoduplexes were ligated to the vector pLentiCRISPRv2 and transformed to Stbl3 bacteria. pLentiCRISPRv2 vectors containing the sgRNA guides were transfected with pMD2.G and pSPAX2 to 293T cells in order to produce lentiviral vectors. From all six guides generated, AGTCCGGGCCCGGCGCGATG gave a polyclonal population that included clones with complete knock out of M6PR. Sixteen clones were isolated by cell serial dilution and in seven of them, the gene had been deleted.
Immunohistochemistry of patients samples
Slides were stained using a Ventana Discovery XT automated system (Ventana Medical Systems, Tucson) as per manufacturer’s protocol with proprietary reagents. Antibody sources, dilutions, and incubation times were as follows: 1. CD4 rabbit anti-human #104R-18, Cell Marque, Rocklin, CA, prediluted, incubation for 8 minutes 2. CD8 rabbit anti-human #790–4460, Ventana, Tucson, AZ, prediluted, incubation for 20 minutes 3. Granzyme rabbit anti-human #760–4283, Ventana, Tucson, AZ, prediluated, incubation for 44 minutes 4. Mannose-6 phosphate receptor rabbit anti-human #ab32815, Abcam, Cambridge, MA, 1:1000 dilution, incubation 32 minutes. The Ventana UltraMap Anti-rabbit Alk Phos secondary Antibody was used for 8 minutes (CD4) and 16 minutes (CD8. Granzyme B, mannose-6 phosphate receptor). The detection system used was the Ventana ChromoMap Red kit and slides were then counterstained with hematoxylin. Stained slides were evaluated and graded by the study pathologist. For CD4 and CD8, the density of intratumoral lymphocytes was graded from 0–3 as follows: 0-absent, 1-rare lymphocytes, 2-lymphocytes scattered singly and in small aggregates, 3-dense infiltrate of lymphocytes. For mannose-6 phosphate receptor and Granzyme B, which both exhibited cytoplasmic staining, the staining was graded from 0–3 as follows: 0- no staining, 1-weak staining, 2-moderate staining, 3-strong staining.
Immunohistochemistry of tumor tissues in xenograft experiments
Tumor tissues were harvested, fixed overnight with 4% paraformaldehyde (Electron Microscopy Science; Cat.no. 15710) at 4°C, embedded in paraffin blocks and 4–5 μm thick sections of the tissue were prepared and stained with polyclonal goat M6PR/IGF2R primary antibody (R&D Systems; Cat.no. AF2447; 5 μg/mL per samples) O/N at 4°C. Biotinylated 2nd antibody in 1:200 dilution was used for 30 min at RT followed by application of ABC solution (avidin dehydroxygenase and biotinylated horseradish peroxidase) for 30 min at RT. DAB 0.05% solution was applied for 3–4 min at RT. Hematoxylin was used for counterstaining the nucleus. Images were analyzed using Nis-Elements Ar (Advanced Research) Nikon 80i Upright Microscope with 40x N.A. objective. Each slide was scanned, images were manually captured at separate X, Y locations and a multipoint ND document was created point by point. A threshold value was defined for each image (for brown M6PR staining) using the single point selection tool. Results were exported to Excel for further analysis. (Acquisition Software: Nikon NIS-Elements Br (Basic research) 4.0, Camera Name: DS-Ri1-U3 40x Objective, Numerical Aperture: 0.95, Camera Settings: Format: 1280×1024 Fine, Exposure: ME 80 ms (-+0.0 EV))
MTT Assay
CellTiter 96® Non-reactive cell proliferation assay kit (Promega; Cat.no. G4000) was used to detect viability of the cells and experiments were performed according to the manufacturer’s protocol.
Flow cytometry
M6PR expression levels were detected by flow cytometry. Cells were incubated with Aqua dead cell staining kit (Thermo Fischer Scientific; Cat.no. L34957) for 15 min at 4°C, followed by staining with Alexa Fluor 647-conjugated mouse antibody specific for human IGF2R/M6PR (BD Biosciences; Cat.no. 565105; clone no. MEM-238) or its isotype (BD Biosciences; Cat.no. 557732; clone no. MOPC-21) at 4 °C for 20 minutes. Cells were fixed using 1% paraformaldehyde for 20 minutes at 4 °C before running them on LSR14. Data was analyzed by FlowJo software (Tree Star).
Granzyme B (GrzB) uptake
Melanoma cells were were incubated with inactive granzyme B (R&D systems; Cat.no. 2906-SE-010, Stock concentration: 0.22 mg/mL, Lot: OFN0415101) at 37°C, 5% CO2 for 1 hour. Cells were then stained with Aqua dead cell staining kit (Thermo Fischer Scientific) for 15 min at 4°C fixed in fixation buffer (BD Cytofix/Cytoperm; Cat.no. 51–2090). Granzyme B antibody (Cat.no. 561142) or its isotype control (BD Bioscience; Cat.no. 555749) were used. Stained cells were run on LSR14. Data was analyzed by FlowJo software (Tree Star).
Mini rapid expansion protocol (Mini-REP)
In a T25 flask, 1.45 × 105 human TIL were stimulated with 30 ng/mL CD3 monoclonal antibody (OKT3) (eBioscience; Cat.no. 16–0037-81) in the presence of 29 × 106 irradiated (5000 rad) allogenic PBMC as feeder cells. TIL were cultured in 9.6 mL of REP Media I (comprised of RPMI 1640, 10 % heat-inactivated human AB serum (Sigma-Aldrich; Cat.no. H4522), 55 μM 2-mercaptoethanol (Gibco; Cat.no. 21985–023), 10 mM HEPES Buffer (Corning; Cat.no. 30–060-Cl)) and 10.7 mL AIM V (Gibco; Cat.no. 0870112-DK) supplemented with 6000 I.U./mL rhIL-2. On day 4, 15 mL media was replaced with fresh media (50 % AIM V and 50 % REP I) containing 3000 I.U./mL rhIL-2. On day 7, 15 mL media was removed and 15 mL of AIM V containing 3000 I.U./mL rhIL-2 was added to the culture. On day 9, TIL and media were transferred T25 to T75 flask and 20 mL of AIM V containing 3000 I.U./mL rhIL-2 was added. On Day 11, TIL and media were transferred from T75 to T150 flask and 40 mL of AIM V containing 3000 I.U./mL rhIL-2 was added. After 14 days, TIL were collected, counted and CD8+ TIL were isolated using EasySep™ Human CD8+ T Cell Enrichment Kit (Stem Cell Technologies; Cat.no. 19053) for further experiments.
CTL Assay
For Chromium (51Cr) release assays, 1 × 106 human melanoma cells were incubated with 100 μCi 51Cr (Perkin-Elmer; Cat.no. NEZ030S001MC; 1 mCi/mL) at 37°C for 60 minutes, washed 3 times with sterile PBS and plated into 96-well round-bottom plates at a cell density of 1 × 104 tumor target cells/well. Target cells were incubated with human CD8+ TILs in triplicates at the indicated effector/target (E/T) ratios in 200 μl culture medium at 37°C in a humidified CO2 incubator. After incubation, plates were centrifuged, 50 μl supernatant was harvested from each well and Cr51 release was measured using a gamma counter. The percent specific lysis was calculated as follows: 100 × [(experimental release – spontaneous release)/ (maximum release – spontaneous release)]. As controls, T cells isolated from blood of healthy donor were used.
Western Blotting
Cells were lysed in RIPA buffer (Sigma-Aldrich) in the presence of protease inhibitor cocktail (Sigma-Aldrich). Whole cell lysates were subjected to 6% SDS-PAGE and transferred to PVDF membrane. The membranes were probed with the antibodies specific for M6PR (Cell Signaling Technology; Cat.no. 14364) and HSP90 (Cell Signaling Technology; Cat.no. 4877) and secondary antibody conjugated with peroxidase (Santa Cruz; Cat.no. sc-2357).
Statistical Analysis
P values were determined by 2-tailed student’s t test (unpaired). For repeated measurements two-way ANOVA test followed by the Bonferroni-Dunn method was used. All calculations were performed on GraphPad Prism7. All results are presented as mean ± SD or ± SEM (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001). Responses and survival of subjects treated on the clinical trial were determined by RECIST 1.1 criteria and by the Kaplan-Meier method using GraphPad Prism7 respectively.
RESULTS
BRAF inhibition causes up-regulation of M6PR in human melanoma cell lines
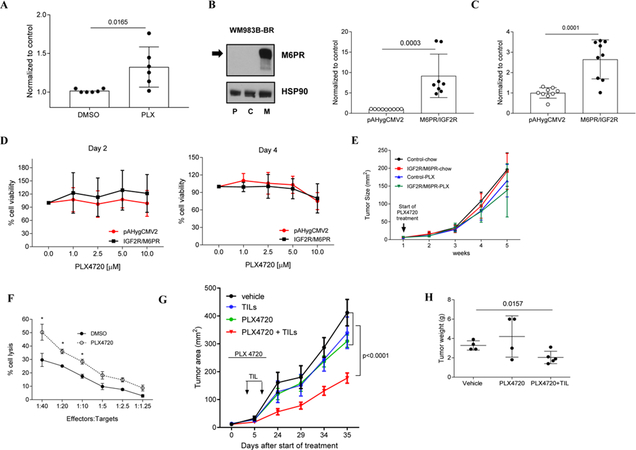
We tested the effect of the BRAFi PLX4720 on M6PR expression using BRAFV600E mutant human melanoma cell lines WM983B and WM35. Within 6 hours of treatment with 1 μM, 2.5, 5 or 10 μM PLX4720, both melanoma cell lines showed substantial dose-dependent up-regulation of M6PR on the cell surface (Fig. 1A,B). Similar up-regulation of M6PR was also detected in other melanoma cell lines (A2058, SK-Mel624, Mel624) (Fig. S1A).
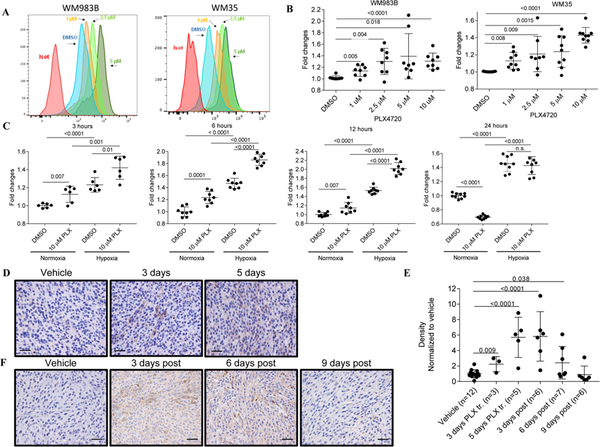
Figure 1. PLX4720 treatment induces a transient up-regulation of M6PR in PLX-sensitive melanoma cells.
A. Typical example of M6PR expression by flow cytometry in two different cell lines (WM983B and WM35) treated for 6 hr with different concentrations of PLX4270. Isot. – cells stained with isotype control. B. Expression of M6PR on the surface of WM983B and WM35 melanoma cells measured by flow cytometry. Geometric mean was calculated and results were normalized to control (DMSO treated samples). Individual results and SD are shown. P values are shown in unpaired two-tailed Student’s t test. C. WM983B cells were treated with DMSO or 10 μM PLX4720 for 3–24 hours in vitro under normoxia and hypoxia (0.5% O2). Cell surface M6PR levels were determined by flow cytometry. Geometric mean was calculated and results were normalized to control (DMSO treated samples under normoxia). Individual results and SD are shown. P values are shown in unpaired two-tailed Student’s t test. D. IHC staining of WM35 tumors with human M6PR specific antibody 3 and 5 days after start of the treatment with PLX4720. Typical example of staining is shown. Scale bar = 25 μm. E. Images from each section were analyzed using Nis-Elements Ar, sum density was calculated and results were normalized according to the values of vehicle-treated mice. Individual results and SD are shown. P values are shown in unpaired two-tailed Student’s t test. F. IHC staining of WM35 tumors with human M6PR specific antibody 3–9 days after finish of the treatment with PLX4720. Typical example of staining is shown. Scale bar = 25 μm.
Since hypoxia is an important component of the tumor microenvironment, we evaluated the effect of hypoxia on PLX4720-induced M6PR up-regulation. WM983B melanoma cells were exposed to 0.5% O2 during PLX4720 treatment. Within 3 hours after starting treatment, a significant (p<0.0001) increase in the expression of M6PR during hypoxia was detected (Fig. 1C). M6PR up-regulation upon PLX4720 treatment was more pronounced under hypoxic conditions than in normoxia and reached a maximum within 12 hours. Twenty-four hours after starting treatment no up-regulation of M6PR expression by PLX4720 was detected (Fig. 1C). Similar kinetic of M6PR up-regulation was observed in WM35 cells (Fig. S1B). To understand the effect of PLX4720 on M6PR expression in vivo, WM35 tumor cells were injected s.c. into immune deficient NOD/SCID mice. When tumors reached 0.5–0.8 cm in diameter, mice were treated with vehicle or 50 mg/kg PLX4720 by oral gavage for 3 or 5 consecutive days. A significant increase in tumor M6PR levels was detected by immunohistochemistry 3 days after start of the treatment and was further increased 2 days later (Fig. 1D, E). Expression of M6PR remained high 3 days after finish of the treatment with slight decrease by day 6. M6PR expression returned to the pretreatment level by day 9 after finishing the treatment (Fig. 1E, F).
Combination BRAF and MEK inhibitors significantly increases clinical responses and survival in metastatic melanoma patients (28, 29). We asked whether combining PLX4720 with a MEKi, trametinib, would affect the up-regulation of M6PR. As expected, 4-day in vitro treatment of melanoma cells with trametinib caused a decrease in cell viability (Fig. S2A). However, in contrast to PLX4720, trametinib did not induce M6PR expression (Fig. S2B). Treatment of tumor cells with the combination of trametinib and PLX4720 resulted in significantly higher up-regulation of M6PR than PLX4720 alone (Fig. S2B). Thus, BRAFi caused substantial up-regulation of M6PR on human melanoma cells in vitro and in vivo. In vivo this effect lasted for almost a week after cessation of the treatment.
PLX4720 treatment causes up-regulation of M6PR in PLX4720-resistant human melanoma cell lines
One of the major problems in the treatment of melanoma with BRAFi or BRAFi + MEKi is the development of resistance. We evaluated the effect of PLX4720 on M6PR expression in BRAF resistant cell lines generated by long-term exposure to increased concentrations of inhibitors that has been described previously (30). Cell lines resistant to BRAFi or to combination of BRAFi and MEKi showed increased (p=0.02) expression of M6PR as compared to untreated cell lines (Fig. S2C).
To maintain consistency with the results obtained on sensitive cell lines and to better assess the effect of BRAFi on the sensitivity of tumor cells to CTLs, we generated an additional BRAFi resistant cell line WM35-BR by exposing WM35 cells to increased concentrations of PLX4720 (Fig. S3A). Resistant cell lines (WM983B-BR and WM35-BR) were treated with 1 μM or 10 μM PLX4720 for different times, and cell surface M6PR levels were determined by flow cytometry. Despite the fact that these cells were previously exposed to BRAFi, treatment with PLX4720 caused further substantial up-regulation of MP6R on the cell surface (Fig. 2A). Similar to the effect seen in sensitive cells, we have observed increased M6PR expression in PLX4720-treated BRAF resistant cells under hypoxia (Fig. 2B). However, in contrast to PLX4720-sensitive cells, M6PR levels did not decrease to pre-treatment levels after 24 hours of treatment (Fig. 2B).
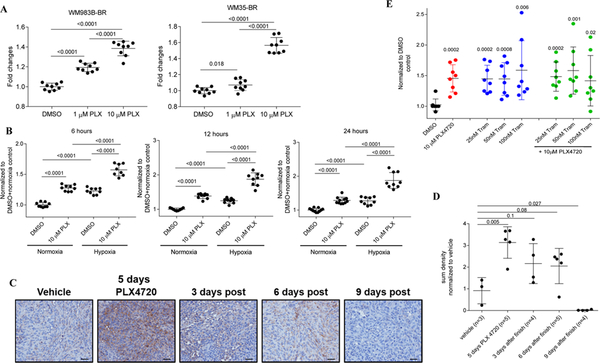
Figure 2. PLX4720 treatment induces a transient up-regulation of M6PR in PLX-resistant melanoma cells.
A. Cell surface M6PR after 1 μM and 10 μM PLX4720 treatment in WM983B-BR and WM35-BR cells, respectively. Individual results and SD are shown. P values are shown in unpaired two-tailed Student’s t test. B. WM983B-BR cells were treated with DMSO or 10 μM PLX4720 for 6–24 hours in vitro under normoxia and hypoxia. Cell surface M6PR levels were determined by flow cytometry. Geometric mean was calculated and results were normalized to control (DMSO treated samples under normoxia). Individual results and SD are shown. P values are shown in unpaired two-tailed Student’s t test. C. IHC staining of WM35-BR derived tumor sections displaying MPR levels after 5 days of PLX4720 treatment of mice. Typical example of staining is shown. Scale bar = 25 μm. D. 10 images from each section were analyzed using Nis-Elements Ar, sum density was calculated and results were normalized according to the values of vehicle (DMSO)-treated mice. Individual results and SD are shown. P values are shown in unpaired two-tailed Student’s t test. E. Cell surface M6PR on WM35-BR cells after treatment for 24 hours with DMSO, PLX4720, Trametinib or combination of PLX4720 and Trametinib treatment. Geometric mean was calculated and results were normalized to DMSO control. Individual results and SD are shown. P values are shown in unpaired two-tailed Student’s t test.
We also assessed the effect of the other BRAFi - dabrafenib (GSK2118438) on the expression of M6PR in different melanoma cell lines and observed substantial up-regulation of the receptor similar to the effect seen with PLX4720 (Fig. S3B).
WM35-BR tumor cells were injected subcutaneously to the flank of NOD/SCID mice and once the average tumor area reached around 40–50 mm2 in size, mice were treated with vehicle or 50 mg/kg PLX4720 by oral gavage for 5 days. After 5 days of PLX4720 treatment, tumors were harvested at different time points and M6PR expression in tumor tissues was detected by IHC staining. Five days of PLX4720 treatment caused a marked up-regulation of M6PR in tumor tissues, which was lower than that observed in sensitive cell lines. In contrast to sensitive lines, expression of M6PR in resistant cells decreased more rapidly after treatment was stopped (Fig. 2C, D). We then tested the effect of combined BRAFi and MEKi in PLX4720-resistant WM35-BR cells. In contrast to sensitive cells, in resistant cell lines, trametinib alone significantly up-regulated expression of M6PR and this effect was not further increased by the combination of BRAFi and MEKi (Fig. 2E). Thus, up-regulation of M6PR by BRAFi was observed not only in sensitive, but also in resistant cell lines.
M6PR up-regulation sensitizes melanoma cells to the cytotoxic effect of tumor infiltrating lymphocytes in vitro
We explored whether BRAF targeting can affect the sensitivity of melanoma cells to CTLs. HLA-A2+ human tumor infiltrating lymphocytes (TIL) that recognized HLA-A2+ matched WM35 tumor cells were obtained from a patient with metastatic melanoma and expanded by a mini rapid expansion protocol (31). CD8+ T cells were isolated and used as effector cells in a CTL assay. WM35 cells treated with DMSO or PLX4720 were used as targets. We observed that PLX4720 treatment rendered WM35 cells more sensitive to the lysis by effector cells (Fig. 3A). To assess whether M6PR up-regulation could directly enhance cell killing by CTLs, we generated WM35 and WM983B cell lines with stable over-expression of M6PR (WM35-M6PR/IGF2R, WM983B-M6PR/IGF2R) (Fig. 3B,C). Overexpression of M6PR did not affect sensitivity of melanoma cells to PLX4720 (Fig. S4A). However, incubation with TIL resulted in a significantly higher killing of M6PR over-expressing than control cells (Fig. 3D). T cells isolated from peripheral blood of healthy individual were not able to kill target cells (Fig. 3E). Granzyme B (GrzB) released by CTLs is one of the ligands of M6PR that may potentiate the cytotoxic effect of CTLs (21). We evaluated GrzB uptake by melanoma cells with overexpression of M6PR and found dramatically higher GrzB uptake in M6PR overexpressing cells than controls (Fig. 3F).
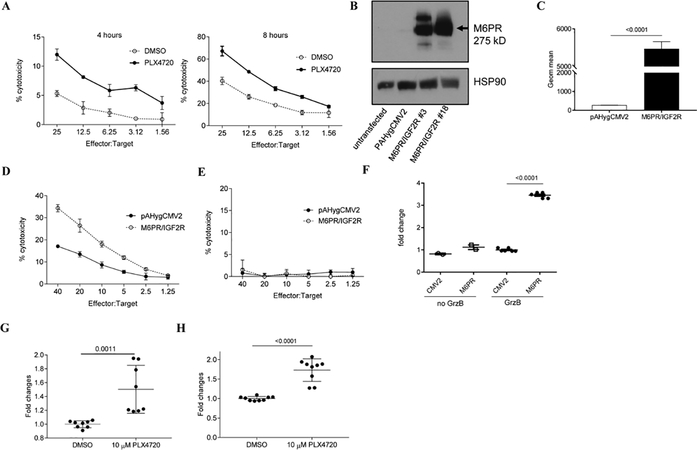
Figure 3. M6PR up-regulation on the cell surface sensitizes WM35 cells to the cytotoxic effect of TIL.
A. After pretreatment of target cells (WM35) with PLX4720 O/N, 4–8 hours Cr release assay was performed in triplicate. Cells were labeled with 51Cr and cultured with effector cells (HLA-A2+ TIL) at the indicated ratios. Appropriate maximum and minimum release controls were determined in each experiment. Representative results of 3 different experiments are shown. B. Analysis of total protein from control (pAHygCMV2) or M6PR over-expressing (M6PR/IGF2R) WM35 cells. Lysates were probed with M6PR and HSP90 specific antibodies. C. Cell surface levels of M6PR was determined by flow cytometry in control (WM35-pAHygCMV2) and M6PR-overexpressing cells (WM35-IGF2R/M6PR). Bars represent standard deviation (SD). Statistical analysis was done using unpaired two-tailed Student’s t test. D, E. 6 hours 51Cr experiment was performed in triplicates. As target cells, 51Cr labeled WM35-pAHygCMV2 (control) and WM35- IGF2R/M6PR were used and cultured with HLA-matching TIL (D) or healthy donor derived CD8+ T cells (E). F. Cells were incubated with inactive GrzB for 1 hr and intracellular GrzB level was measured by flow cytometry. Mean and SD of individual experiments are shown (n=6). P values were calculated in two-sided Student’s t-test. G, H. WM35 cells were treated with DMSO or PLX4720 O/N and cell surface levels of M6PR was detected by flow cytometry (G). H. GrzB uptake by WM35 cells were detected by intracellular GrzB staining using GrzB specific mouse anti-human antibody. Geometric mean was calculated and all results were normalized to control (DMSO-treated cells). Individual results and SD are shown. P values are shown in unpaired two-tailed Student’s t test.
In order to understand whether BRAF targeting affects GrzB uptake by tumor cells WM35 cells were treated overnight with PLX4720 and M6PR up-regulation was confirmed by flow cytometry (Fig. 3G). Cells were then incubated with inactive recombinant GrzB for an hour and intracellular GrzB was assessed by flow cytometry. PLX4720 treatment caused significant up-regulation of GrzB uptake by tumor cells (Fig. 3H).
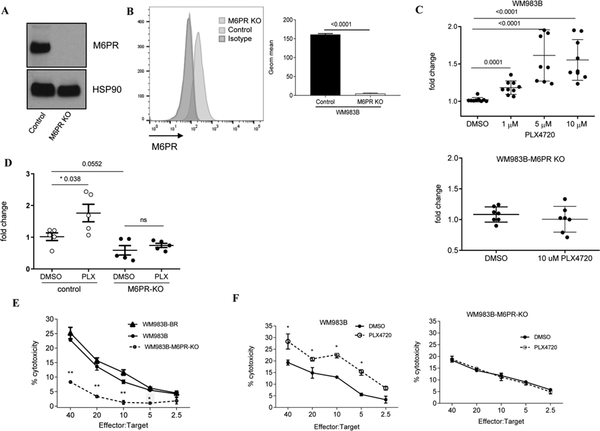
To investigate if M6PR has a direct role in the increased uptake of GrzB induced by PLX4720, we generated a WM983B-M6PR-KO cell line lacking M6PR expression, using Crispr/Cas9 technology. Deletion of M6PR was confirmed by western blotting (Fig. 4A) and flow cytometry (Fig. 4B). WM983B and WM983B-M6PR-KO cells were cultured with DMSO or different concentrations of PLX4720. As expected, PLX4720 induced substantial up-regulation of M6PR in WM983B cells but not in WM983B-M6PR-KO cells (Fig. 4C). Deletion of M6PR did not affect the sensitivity of WM983B tumor cells to PLX4720 treatment (Fig. S4B). WM983B and WM983B-M6PR-KO cells were treated overnight with DMSO or PLX4720 and then incubated with inactive GrzB for 1 hour at 37°C and intracellular GrzB levels were measured by flow cytometry. In the absence of M6PR, PLX4720- inducible up-regulation of GrzB uptake was abrogated (Fig. 4D) suggesting that PLX4720-induced GrzB uptake depends on the up-regulation of M6PR. To test the sensitivity of WM983B cells to CTLs, we expanded TILs isolated from a HLA-A1+ patient with metastatic melanoma. These TILs recognized HLA-A1+ WM983B cells. As targets, we used WM983B and WM983B-M6PR-KO cells. Deletion of M6PR in tumor cells markedly reduced their killing by TILs as compared with WT cells. BRAFi sensitive and resistant WM983B cells were recognized by CTLs equally well (Fig. 4E). Treatment with PLX4720 significantly (p<0.05) increased killing of WM983B tumor cells by TILs, whereas this effect was not observed in WM983B-M6PR-KO cells (Fig. 4F). We asked whether manipulation with expression of M6PR could affect expression of molecules associated with antigen presentation and regulation of immune responses by tumor cells. We evaluated expression of MHC class I, MHC class II, PDL1, and FasL on tumor cells with overexpression or deletion of M6PR. No significant changes were observed in any of those molecules (Fig. S5). Taken together, these results indicate that BRAF inhibition sensitized tumor cells to CTLs via up-regulation of M6PR in both sensitive and resistant melanoma cells.
Figure 4. Effect of M6PR deletion on TIL mediated killing of melanoma cells.
A, B. Expression of M6PR in cell lysates of control (WM983B) and M6PR-deleted (WM983B-M6PR KO) cells tested by western blot (A) and on the surface of the cells by flow cytometry (B). Representative data with triplicate for both groups are shown. P values are shown in unpaired two-tailed Student’s t test. C. M6PR expression on the cell surface after O/N treatment with DMSO or PLX4720 on WM983B and WM983B-M6PR KO cells. Individual replicates, mean and SD are shown. P values were calculated in unpaired two-sided Student’s t test. D. Intracellular GrzB levels were detected by flow cytometry in DMSO and PLX4720 treated cells. Individual results and SD are shown. P values are shown in unpaired two-tailed Student’s t test. E. 51Cr-release cytotoxicity assay was performed in triplicates with TIL and indicated target cells. F. 51Cr-release assay performed with TIL and indicated target cells treated ON with PLX-4720. Typical example of three independent experiments is shown. Mean, SD and p values (* p<0.05 in unpaired Student’s t-test) are shown.
M6PR up-regulation sensitizes PLX4720-resistant melanoma cells to the cytotoxic effect of TIL
We asked whether PLX4720 treatment could increase the uptake of GrzB by BRAFi resistant cells. WM35-BR cells were treated with DMSO and 10 μM PLX4720 overnight, and after confirming up-regulation of M6PR on the cell surface, pretreated cells were incubated with inactive GrzB at 37°C for one hour and intracellular GrzB levels assessed by flow cytometry. PLX4720 treated cells had significantly higher amount of GrzB than DMSO treated cells (Fig. 5A). Overexpression of M6PR in WM983B-BR cells (WM983B-BR-M6PR/IGF2R) (Fig. 5B) markedly increased GrzB uptake (Fig. 5C). Over-expression of M6PR did not improve the survival of WM983B-BR cells in response to treatment with PLX4720 in vitro (Fig. 5D). WM983B-BR control and M6PR-overexpressing cells were subcutaneously injected into opposite flanks of immune-deficient NSG mice, and when tumors became palpable, mice were treated with PLX4720. No difference in tumor growth was seen (Fig. 5E) supporting the conclusion that M6PR overexpression by itself does not reverse resistance of melanoma cells to BRAF inhibition.
Figure 5. The anti-tumor effect of combined therapy.
A. Intracellular GrzB level in WM35-BR cells after O/N treatment with DMSO or PLX4720. Individual results and SD are shown. P values are shown in unpaired two-tailed Student’s t test. B. Left panel - expression of M6PR in cell lysates (parental (P; WM983B-BR), control (C; WM983B-BR-pAHygCMV2) and M6PR over-expressing (M; WM983B-BR- M6PR/IGF2R)). Right panel – M6PR expression on cell surface. C. Grz B uptake by M6PR over-expressing WM983B-BR cells were determined by intracellular Grz B staining. Cumulative data of 3 separate experiments are shown. Geometric mean was calculated and all results were normalized to DMSO-treated control. Individual results and SD are shown. P value is shown in unpaired two-tailed Student’s t test. D. MTT assay results displaying the percentage of live control and M6PR over-expressing WM983B-BR cells after 2 and 4 days of PLX4720 treatment, respectively. Cumulative data of 2 separate experiments are shown. E. Tumor growth kinetics in NSG mice fed with either control diet or a special diet supplemented with 200 mg PLX4720/kg. Each group has 5 mice. Mean and SD are shown. F. 6 hours 51Cr release assay performed in triplicates. As target, 51Cr labeled DMSO (control) or PLX4720 treated WM35-BR cells were cultured with HLA-matching TIL (effector cells) at the indicated ratios. Appropriate maximum and minimum release controls were set up in each experiment. Typical example of 4 different experiments is shown. G. Tumor growth kinetics in WM35-BR bearing NOD/SCID mice treated with PLX4720 and TIL. Arrows indicate TILs injection. PLX4720 was started 5 days before TIL injection and stopped after second TIL injection. Each group has 8 mice. Mean and SEM are shown. P – values were calculated in two-way ANOVA test with Bonferroni-Dunn analysis. H. Tumor weight on day 30-post TIL injection. Individual results and SD are shown. P values are shown in unpaired two-tailed Student’s t test.
We next tested whether PLX-induced M6PR up-regulation on the cell surface of resistant cells sensitized them to HLA-matched TIL. Overnight treatment of WM35-BR with PLX4720 increased sensitivity of tumor cells to TIL in cytotoxicity assay (Fig. 5F). We asked whether BRAFi could sensitize resistant tumors to TILs in vivo. WM35-BR cells were injected subcutaneously to the flanks of immune deficient NOD/SCID mice and when tumors became palpable, mice were split to 4 groups. Mice treated with vehicle alone, mice treated with 50 mg/kg PLX4720 for 10 days, mice treated with CD8+ T-cell enriched TIL i.v. twice at a 4 day interval and mice treated with combination of PLX4720 and TILs. TIL injections alone or PLX4720 did not affect tumor growth. However, when PLX4720 treatment was combined with TIL transfer, tumor growth was significantly decreased (Fig. 5G) and the tumor weight of this group was significantly lower than the vehicle-treated or PLX-treated groups (Fig. 5H), suggesting that PLX4720 - potentiated the anti-tumor effect of TIL in vivo in resistant tumor cells.
The effect of BRAF inhibitor vemurafenib on M6PR expression in melanoma patients
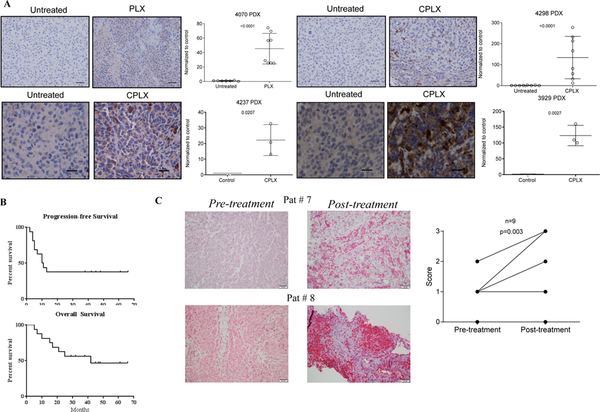
To test the effect of therapy on M6PR expression in clinical samples, we established patient derived xenografts (PDX) from four patients who were either treatment naïve (4237, 3929), or progressed on BRAFi (4070) or combination of BRAFi and MEKi (4298). Mice with PDX were left untreated or treated for 3 weeks with single agent BRAFi (PLX-PLX4720) or combination with MEKi (CPLX-PLX4720 and PD0325901). In all four cases, treatment with inhibitors caused marked up-regulation of M6PR in tumors (Fig. 6A).
Figure 6. M6PR expression in patients treated with vemurafenib plus ACT with TIL.
A. PDX were established from four patients who either treatment naïve (4237, 3929), or progress on BRAFi (4070) or combination of BRAFi and MEKi (4298). Mice with PDX were left untreated for 4 weeks (untreated), or treated for 3 weeks with corresponding single agent BRAFi (PLX-PLX4720) or combination with MEKi (CPLX – PLX4720 and PD0325901). Tissues were stained for M6PR and intensity of staining was assessed in 8 fields. Typical example of staining (scale bar = 25μm) and statistical analysis are shown. Intensity was normalized to untreated samples. Mean and SD for 8 fields are shown. P values were calculated in unpaired Student’s t-test. B. Kaplan-Meier progression-free survival and overall survival curves in 16 patients treated with BRAFi and TIL ACT. C. Typical example of M6PR staining in two patients pre- and post-treatment and cumulative results of M6PR staining in 9 patients before and after treatment. H-score results are shown. P value is shown in paired two-tailed Student’s t test.
A clinical trial of vemurafenib and TIL has been performed in patients with metastatic melanoma. Previously completed clinical trial of ACT with TIL was associated with a 26% objective response rate based upon intention to treat (26). However, it had 32% patient attrition rate largely due to disease progression prior to ACT. Therefore, the goals of combining vemurafenib with TIL ACT were to reduce patient attrition and to improve clinical responses. Clinical trial (NCT01659151) was conducted at H. Lee Moffitt Cancer Center in 2014–2017. Seventeen subjects with BRAF V600-mutated tumors were accrued with subject characteristics and TIL phenotype listed in Table S1. All subjects started vemurafenib the day after tumor harvest, and one subject dropped out prior to ACT due to inadequate TIL growth, representing an attrition rate of 6%. The null hypothesis for this endpoint was 32% (based upon the historical track record at H. Lee Moffitt Cancer Center). Thus, this trial demonstrated a marked improvement in the attrition rate of patients after tumor harvest who were due for TIL therapy.
Vemurafenib was held during the ACT regimen and was resumed upon clinical recovery for up to 2 years. There were no treatment-related deaths. Toxicity was expected and included the typical vemurafenib and adoptive cell therapy-related toxicities of bone marrow suppression, neutropenic fever, chronic fatigue neuropathy, and skin toxicity (Table S2). There was no long-term toxicity that was definitely related to therapy aside from the above. Six of the 16 accrued patients (38%) manifested an objective response at the pre-specified endpoint of 12 months. The null hypothesis for this endpoint was < 30% (based upon historical track record). The median progression-free survival was 10.5 months, and the median overall survival was 42 months (Fig. 6B). The 6 objective responders achieved an overall survival that ranged from 38 to 66 months. One responding subject who developed symptomatic disease not meeting criteria for progression underwent surgical resection and is without evidence of disease after 48 months of follow up without additional treatment. One subject who achieved a complete response died from a cause independent from melanoma after 42 months.
To assess the effect of the treatment on the expression of M6PR in tumors from patients on this trial we evaluated pre- and on-treatment biopsies. Biopsies from nine patients treated with vemurafenib followed by ACT were available for evaluation. On-treatment biopsies were obtained from 10–321 days after initiation of vemurafenib treatment (median 77 days). Expression of M6PR was noted in both tumor cells and lymphocytes (Fig. 6C). M6PR tumor scores increased in 8 samples from 7 patients and were stable in 2 samples from 2 patients (Figure 6C) while on treatment (p=0.003). There were no significant changes in either CD4 or CD8 lymphocytes within or surrounding the tumor after initiation of vemurafenib treatment. Thus, BRAF inhibitor up-regulated M6PR in tumors from cancer patients and in combination with BRAF inhibition and ACT demonstrated promising clinical results.
DISCUSSION
In this study, we demonstrated that BRAFi sensitized human melanoma cells to killing by CTLs. Because of widespread resistance to BRAFi, new combination modalities are necessary, and one possible approach would be to combine BRAFi with ACT. We tested the hypothesis that BRAFi can cause up-regulation of M6PR and thus sensitize tumor cells to TILs. Koya et al. previously showed that the combination of vemurafenib with ACT showed an enhanced antitumor effect in immunocompetent mice bearing BRAFV600E mutant SM1 melanoma cells (17). Vemurafenib treatment did not cause any increase in the expansion or tumor infiltration of adoptively transferred T cells. However, the antitumor activity of antigen-specific T cells was significantly increased after vemurafenib treatment (17), suggesting that understanding the mechanism behind the improvement in the anti-tumor effect of combination therapy might lead to more effective therapy for metastatic melanoma. We have previously demonstrated in models of lung cancer and lymphoma that several chemotherapeutic drugs (paclitaxel, doxorubicin, cisplatin) as well as radiation therapy could potentiate the anti-tumor effect of immunotherapy via up-regulation of M6PR on the tumor cell surface (21–23). Since M6PR can bind GrzB, this may explain enhanced tumor cell killing in a perforin-independent manner (21). In the current study, we tested the effect of BRAFi (PLX4720) on induction of M6PR in different human melanoma cell lines. PLX4720 and vemurafenib has been shown to promote the anti-tumor effects of T cells. PLX4720 treatment decreased tumor CCL2 expression in BRAFV600E mouse melanoma transplants. PLX4720 did not directly increase tumor immunogenicity, but caused a robust increase in the CD8+T/FoxP3+CD4+ T cell ratio and in NK cells (32). Using TILs isolated from cancer patients and HLA matched melanoma cell lines with overexpressed or deleted M6PR, we determined that up-regulation of M6PR was directly responsible for BRAFi-induced increased sensitivity of tumor cells to CTLs. We have previously demonstrated that M6PR up-regulation was mediated by autophagy induced by various stress signals (22). It is possible that a similar mechanism was involved in the impact of BRAFi. In the current work we did not study the specific mechanism of M6PR upregulation, but focused on the impact of this effect on the treatment of BRAFi resistant cells. We observed that BRAFi caused upregulation of M6PR in both sensitive and resistant melanoma cells. The magnitude of up-regulation was similar in vitro, whereas the BRAFi effect in sensitive cells was stronger. This is consistent with a higher basal level of the receptor being expressed in resistant cells. Although cell surface M6PR returned to the pre-treatment level in sensitive cells after 24 hours of treatment, in resistant cells MPR levels were still significantly higher in comparison to DMSO treated cells after 24 hours. Considering that BRAFi resistance is induced by exposing cells to sustained and increased doses of inhibitor, continuous stress may result in higher levels of the receptor. Consistent with stress-induced up-regulation of the receptor were also the results of stronger up-regulation of M6PR by BRAFi in hypoxia.
Expression of M6PR did not affect the sensitivity of melanoma cells to BRAFi since neither up-regulation nor deletion of the receptor affected the viability of cells exposed to BRAFi. However, augmentation of M6PR levels was a major mechanism regulating increased sensitivity of tumor cells to CTLs, since deletion of the receptor abrogated killing of tumor cells by TIL.
Several clinical trials have demonstrated the clinical efficacy of the BRAFi and MEKi combination in metastatic melanoma patients (28, 29). Furthermore, dabrafenib and trametinib combination therapy was shown to increase the expression of melanoma antigen, T cell cytotoxicity markers and CD8+ T cell infiltration in biopsies from metastatic melanoma patients in comparison to vemurafenib treatment alone (14), suggesting that the combination of BRAFi and MEKi might augment the antitumor effect of immunotherapy. In our study, we observed that although trametinib alone did not induce M6PR in sensitive cell lines, in combination with PLX4720, up-regulation of M6PR was stronger than PLX4720 alone. In contrast, combining trametinib with PLX4720 did not enhance M6PR up-regulation in BRAFi-resistant cells. These results further support the hypothesis that the effect of BRAFi on M6PR expression is not associated with canonical signaling via MAPK.
The results of a clinical trial demonstrated that treatment with BRAFi caused marked up-regulation of M6PR in tumors. Our data in PDX showed that up-regulation of M6PR can be observed in tumors after failure of BRAFi or with the combination of BRAFi and MEKi therapy suggesting that in these patients addition of ACT might be beneficial. This study demonstrated that targeting of BRAF in BRAFV600E mutant melanoma sensitizes tumor cells to killing by CTLs at least in part via up-regulation of M6PR. Combining ACT with BRAFi treatment in patients who progressed on BRAFi and MEKi therapy may be therapeutically useful.
Supplementary Material
Translational relevance.
Although treatment of melanoma patients demonstrating the BRAFV600E mutation in their tumor with BRAFi and MEKi results in remarkable initial responses, it also results in rapid development of resistance. Only limited therapeutic options exists for these patients. In this study, we identified a combination strategy of BRAFi and ACT with tumor infiltrating lymphocytes that utilizes novel mechanisms associated with up-regulation of IGFR2 and results in elimination of BRAFi resistant tumor cells. This approach can be readily translated to patients with metastatic melanoma.
Acknowledgements
This study was supported by Melanoma SPORE grant (P50CA168536) to DG, AS, JW, Research grant from Genentech Inc., the Donald A. Adam Comprehensive Melanoma Research Center at Moffitt Cancer Center, and the Swim Across America Foundation to AS, NCI-K23CA178083 to AS, Leo and Anne Albert American Cancer Society Foundation Research Scholar Grant (RSG-16–117-01-LIB) to SP-T, Melanoma SPORE grant (P50CA174523), NIH grants 5P01CA114046, 1U54CA224070, the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, and department of defense grant WX1XWH-16–1-0119 (CA150619) to M.H.
Flow cytometry, imaging, and animal cores at Wistar Institute Cancer Center, tissue, flow cytometry and the cell therapies cores at Moffitt Cancer Center. Vemurafenib for clinical trial was provided by Genentech Inc, and IL-2 for TIL generation was provided by Prometheus Laboratories Inc.
Footnotes
Authors declare no conflict of interest exists
References
- 1.Merhavi-Shoham E, Itzhaki O, Markel G, Schachter J, Besser MJ. Adoptive Cell Therapy for Metastatic Melanoma. Cancer journal (Sudbury, Mass. 2017;23:48–53. [DOI] [PubMed] [Google Scholar]
- 2.Ascierto PA, Kirkwood JM, Grob JJ, Simeone E, Grimaldi AM, Maio M, et al. The role of BRAF V600 mutation in melanoma. J Transl Med. 2012;10:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim A, Cohen MS. The discovery of vemurafenib for the treatment of BRAF-mutated metastatic melanoma. Expert opinion on drug discovery. 2016;11:907–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wan PT, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–67. [DOI] [PubMed] [Google Scholar]
- 5.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366:707–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schadendorf D, Long GV, Stroiakovski D, Karaszewska B, Hauschild A, Levchenko E, et al. Three-year pooled analysis of factors associated with clinical outcomes across dabrafenib and trametinib combination therapy phase 3 randomised trials. Eur J Cancer. 2017;82:45–55. [DOI] [PubMed] [Google Scholar]
- 8.Long GV, Grob JJ, Nathan P, Ribas A, Robert C, Schadendorf D, et al. Factors predictive of response, disease progression, and overall survival after dabrafenib and trametinib combination treatment: a pooled analysis of individual patient data from randomised trials. The lancet oncology. 2016;17:1743–54. [DOI] [PubMed] [Google Scholar]
- 9.Long GV, Flaherty KT, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol. 2017;28:1631–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartsough E, Shao Y, Aplin AE. Resistance to RAF inhibitors revisited. J Invest Dermatol. 2014;134:319–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paraiso KH, Xiang Y, Rebecca VW, Abel EV, Chen YA, Munko AC, et al. PTEN loss confers BRAF inhibitor resistance to melanoma cells through the suppression of BIM expression. Cancer Res. 2011;71:2750–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilmott JS, Long GV, Howle JR, Haydu LE, Sharma RN, Thompson JF, et al. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin Cancer Res. 2012;18:1386–94. [DOI] [PubMed] [Google Scholar]
- 14.Frederick DT, Piris A, Cogdill AP, Cooper ZA, Lezcano C, Ferrone CR, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res. 2013;19:1225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Comin-Anduix B, Chodon T, Sazegar H, Matsunaga D, Mock S, Jalil J, et al. The oncogenic BRAF kinase inhibitor PLX4032/RG7204 does not affect the viability or function of human lymphocytes across a wide range of concentrations. Clin Cancer Res. 2010;16:6040–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boni A, Cogdill AP, Dang P, Udayakumar D, Njauw CN, Sloss CM, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 2010;70:5213–9. [DOI] [PubMed] [Google Scholar]
- 17.Koya RC, Mok S, Otte N, Blacketor KJ, Comin-Anduix B, Tumeh PC, et al. BRAF inhibitor vemurafenib improves the antitumor activity of adoptive cell immunotherapy. Cancer Res. 2012;72:3928–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yee C Adoptive T cell therapy: points to consider. Curr Opin Immunol. 2018;51:197–203. [DOI] [PubMed] [Google Scholar]
- 19.Baruch EN, Berg AL, Besser MJ, Schachter J, Markel G. Adoptive T cell therapy: An overview of obstacles and opportunities. Cancer. 2017;123:2154–62. [DOI] [PubMed] [Google Scholar]
- 20.Deniger DC, Kwong ML, Pasetto A, Dudley ME, Wunderlich JR, Langhan MM, et al. A Pilot Trial of the Combination of Vemurafenib with Adoptive Cell Therapy in Patients with Metastatic Melanoma. Clin Cancer Res. 2017;23:351–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramakrishnan R, Assudani D, Nagaraj S, Hunter T, Cho HI, Antonia S, et al. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest. 2010;120:1111–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramakrishnan R, Huang C, Cho HI, Lloyd M, Johnson J, Ren X, et al. Autophagy induced by conventional chemotherapy mediates tumor cell sensitivity to immunotherapy. Cancer Res. 2012;72:5483–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim S, Ramakrishnan R, Lavilla-Alonso S, Chinnaiyan P, Rao N, Fowler E, et al. Radiation-induced autophagy potentiates immunotherapy of cancer via up-regulation of mannose 6-phosphate receptor on tumor cells in mice. Cancer Immunol Immunother. 2014;63:1009–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Probst OC, Karayel E, Schida N, Nimmerfall E, Hehenberger E, Puxbaum V, et al. The mannose 6-phosphate-binding sites of M6P/IGF2R determine its capacity to suppress matrix invasion by squamous cell carcinoma cells. Biochem J. 2013;451:91–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motyka B, Korbutt G, Pinkoski MJ, Heibein JA, Caputo A, Hobman M, et al. Mannose 6-phosphate/insulin-like growth factor II receptor is a death receptor for granzyme B during cytotoxic T cell-induced apoptosis. Cell. 2000;103:491–500. [DOI] [PubMed] [Google Scholar]
- 26.Pilon-Thomas S, Kuhn L, Ellwanger S, Janssen W, Royster E, Marzban S, et al. Efficacy of adoptive cell transfer of tumor-infiltrating lymphocytes after lymphopenia induction for metastatic melanoma. J Immunother. 2012;35:615–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krepler C, Sproesser K, Brafford P, Beqiri M, Garman B, Xiao M, et al. A Comprehensive Patient-Derived Xenograft Collection Representing the Heterogeneity of Melanoma. Cell reports. 2017;21:1953–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eroglu Z, Ribas A. Combination therapy with BRAF and MEK inhibitors for melanoma: latest evidence and place in therapy. Therapeutic advances in medical oncology. 2016;8:48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367:1694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shannan B, Watters A, Chen Q, Mollin S, Dorr M, Meggers E, et al. PIM kinases as therapeutic targets against advanced melanoma. Oncotarget. 2016;7:54897–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall M, Liu H, Malafa M, Centeno B, Hodul PJ, Pimiento J, et al. Expansion of tumor-infiltrating lymphocytes (TIL) from human pancreatic tumors. J Immunother Cancer. 2016;4:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knight DA, Ngiow SF, Li M, Parmenter T, Mok S, Cass A, et al. Host immunity contributes to the anti-melanoma activity of BRAF inhibitors. J Clin Invest. 2013;123:1371–81. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.