Abstract
Aims/Introduction
The purpose of the present study was to investigate the possible effect of oral magnesium sulfate (MgSO 4) in the reduction of atherosclerosis plaques through inhibition of lectin‐like low‐density lipoprotein receptor‐1 (LOX‐1) gene expression in diabetic vessels.
Materials and methods
A total of 50 rats were divided into five groups, including non‐diabetic control, Mg‐treated non‐diabetic control, chronic diabetic, Mg‐treated chronic diabetic and insulin‐treated chronic diabetic. The induction of diabetes was carried out by streptozotocin. The Mg‐treated chronic diabetic and Mg‐treated non‐diabetic control groups were treated with 10 g/L of MgSO 4 added to their drinking water. The insulin‐treated chronic diabetic group received 2.5 U/kg of insulin twice per day. The fasting blood glucose level and bodyweight were determined weekly. Blood pressure measurement and the intraperitoneal glucose tolerance test were carried out after 16 weeks, and the plasma levels of Mg, lipid profile and oxidized low‐density lipoprotein cholesterol (oxLDL) were determined. The mesenteric bed was isolated and perfused according to the McGregor method. The aorta was isolated for LOX‐1 genes and proteins expression, and pathological investigation.
Results
MgSO 4 administration improved blood pressure, sensitivity to phenylephrine, intraperitoneal glucose tolerance test, lipid profile and plasma ox‐LDL level, and also lowered the blood glucose level to the normal range, and decreased LOX‐1 gene and protein expressions. Insulin decreased blood pressure, sensitivity to phenylephrine, blood glucose, lipid profiles and plasma oxLDL level, but it did not decrease LOX‐1 gene and protein expressions.
Conclusions
The present findings suggested that MgSO 4 improves blood pressure and vessel structure through decreasing oxLDL, and LOX‐1 gene and protein expressions; however, insulin did not repair vessel structure, and LOX‐1 gene and protein expressions.
Keywords: Diabetes, LOX‐1, Magnesium
Introduction
Diabetes microvascular sickness is a leading cause of blindness, renal failure and nerve injury in the clinic setting1. In diabetes, the elevated plasma cholesterol and low‐density lipoprotein cholesterol (LDL) levels are usually responsible for development of atherosclerotic vascular disease, which is the major risk factor in myocardial infarction, stroke and limb amputation2.
Atherosclerosis is one of the causes of mortality in diabetes patients. The first step in the development of plaques is related to subendothelial deposition of LDL, which is subsequently modified to oxidized LDL (oxLDL). This process is influenced by hypertension and diabetes, which increase oxidative stress in the vascular wall3, 4. It has been posited that reactive oxygen species are required for transformation of LDL to oxLDL5. Innumerable studies have proposed that intracellular reactive oxygen species serve as a common intracellular downstream messenger of the different stimulus‐specific pathways that result in nuclear factor‐κB activation, and it plays an important role for the induction of apoptosis by glucose5, 6. As a lectin‐like 52‐kD receptor, lectin‐like low‐density lipoprotein receptor‐1 (LOX ‐1) works on oxLDLs, and it is present primarily in endothelial cells7, being upregulated in the hypertensive, dyslipidemic and diabetic animals’ arteries. In humans and diabetic animal models with atherosclerotic plaques, the LOX‐1 gene expression increases, which is the reason for elevated blood pressure and endothelial dysfunction8. High glucose changes oxidative stress through NADPH oxidase activation, and this contributes to LOX‐1 upregulation and endothelial nitric oxide synthase downregulation in endothelial cells8. Some researchers have shown that magnesium (Mg) deficiency is closely correlated with atherosclerosis and vascular problems in diabetes patients9. A low level of plasma Mg accelerates atherosclerosis by rising LDL concentrations and their oxidative modifications.
Furthermore, in our previous studies, it was shown that oral magnesium sulfate (MgSO4) administration improves blood glucose level and endothelial function, as well as being useful in preventing atherosclerosis in diabetic vessels10, 11, 12, 13. Accordingly, it seems that MgSO4 administration has an acceptable effect on prevention of atherosclerosis development in diabetic vessels; however, the mechanism is not completely clear. The present study was designed to determine the significance of oral MgSO4 administration atherosclerosis reduction through inhibition of LOX‐1 gene expiration in streptozotocin‐induced diabetic rat vessels.
Methods
Animals
Animals were handled in accordance with acceptable guidelines (Canadian Council on Animal Care 1993). The research protocol was approved by the Ethical Committee for Animal Care of Hormozgan University of Medical Science (HUMS.REC.1394.169).
Male Wistar rats, weighing 180–250 g, were kept in standard conditions of temperature (23 ± 2°C) and dark–light cycle (12:12‐h), and food and water were easily accessible. The animals were categorized into five groups (10 rats in each group); namely, the non‐diabetic control (NDC), Mg‐treated non‐diabetic control (Mg‐NDC), chronic diabetic (CD), Mg‐treated chronic diabetic (Mg‐CD) and insulin‐treated chronic diabetic (Ins‐CD).
The diabetes was induced by streptozotocin (60 mg/kg, i.p.) injection. Within 10 days of diabetes induction, the fasting blood glucose level was measured and diabetes was confirmed with blood sugar concentrations exceeding 250 mg/dL. The animals for which the diabetic parameters were observed for a period of 4 months were considered as CD. The Mg‐CD and Mg‐NDC groups received 10 g/L of MgSO4 in their drinking water13 for 16 weeks. The animals’ water consumption was measured daily. The Mg‐treated CD and Mg‐NDC groups apparently had considerably lower water intake in comparison with the CD group (40 ± 2, 41 ± 2 and 207 ± 3.2 mL/24 h for rats that were Mg‐treated CD, Mg‐NDC and CD, respectively). Hence, the last dose of MgSO4 was 0.4 g/24 h that animals drank each day. As for the Ins‐CD group, they were treated with 2.5 U/kg of insulin (at 1/3 regular and 2/3 NPH) to maintain their blood sugar at approximately 136 ± 6 mg/dL on the 10th day after induction of diabetes through intraperitoneal injection for 4 months. All rats were screened for blood sugar level by a glucometer (Ascensia ELITE XL, Leverkusen, Germany), and they were subjected to a weekly record of their bodyweight using a digital scale.
Intraperitoneal Glucose Tolerance Test
In order to determine the intraperitoneal glucose tolerance test (IPGTT), all the animals were deprived of food for 15 h overnight, receiving 1.5 g/kg bodyweight glucose by intraperitoneal injection. The blood sample was drawn from the tail vein at 0, 10, 20, 30, 60, 90 and 120 min after glucose administration14, 15.
Blood Pressure Measurement and Biochemical Assay
The animals were anesthetized with ketamine HCl (50 mg/kg, i.p.). The left femoral artery was cannulated to record blood pressure continuously for a period of 30 min using both a pressure transducer (MLT0380; AD Instruments, Sydney, NSW, Australia) and Power Lab Recording System (4SP; AD Instruments). Blood samples were collected from the neck vascular trunk to measure the plasma levels of Mg, calcium, triglycerides, total cholesterol and LDL cholesterol levels by micro plate reader (Biotek, Winooski, VT, USA) and appropriate kits (Pars Azmone, Tehran, Iran). The very low‐density lipoprotein (VLDL) was calculated by triglycerides / 513.
The plasma oxLDL level was measured by a rat oxLDL ELISA kit (Abcam, Cambridge, UK). The aorta was isolated for LOX‐1 gene expression, and its protein was measured by real‐time polymerase chain reaction (PCR) and immunohistochemistry methods, respectively.
After 16 weeks, the animals in the NDC, CD, Ins‐CD and Mg‐CD groups received an intraperitoneal injection of ketamine HCl 50 mg/kg for anesthesia to prepare the mesenteric vascular beds by the McGregor method16. In this stage, the abdominal wall was opened, the superior mesenteric artery was exposed and cannulated, then it was gently flushed with modified Krebs–Henseleit solution (comprising [in mmol/L] NaCl: 118, KCl: 4.7, CaCl2; 2.5, MgSO4; 1.2, glucose; 2, NaHCO3; 2.5, NaHPO4; 1.2) while it was simultaneously bubbled with a mixture of 95% O2 and 5% CO2 (final pH 7.4) and warmed to 37°C. The mesentery was separated from the intestine, and it was placed in a water‐jacketed perfusion chamber that was maintained warm at 37°C. The preparation was accomplished at 1 mL/min with modified Krebs–Henseleit solution introduced by a peristaltic pump (Meredos GmbH, Baoding, China). Tissues were continuously kept wet with modified Krebs–Henseleit solution to prevent tissue drying. The pressure used for perfusion was screened by a T‐tube, which was inserted between the pump and the inflow cannula. This was adjoined to a pressure transducer (by MLT0380 AD Instruments). Power Lab System (16SP; AD Instruments) was used for recording. Tissues were used for examination after equilibration of 30 min. Then, the vascular bed was constricted by Krebs–Henseleit solution that contained phenylephrine, an α1‐adrenoceptor agonist, from 0.0001 to 0.1 mol/L and perfusion pressure was recorded.
Histopathological Procedures
The aorta was isolated and fixed in 10% formalin solution, then embedded in paraffin for histopathological staining. Hematoxylin–eosin staining was carried out to examine the tissue injury. To consider the vessel damage, the presence of foam cells, collagen thickness and endothelium damage were evaluated. Based on the damage intensity, the samples were rated from 1 to 4, while a score of zero was assigned to normal tissue.
LOX‐1 Gene Expression
Tissue preparation was carried out at the end of 16 weeks of treatment. The aorta of rats were separated and washed in very cold isotonic saline, and subsequently, 50–100 mg of tissue was put in 1 mL RNAse Later (Qiagen, Hilden, Germany), and it was stored at −80°C before ribonucleic acid (RNA) isolation.
Real‐time PCR
The quantitation of messenger RNA (mRNA) level in the aorta was carried out through real‐time PCR. Total RNA from aorta tissue was extracted using Trizol kit (SIGMA, Taufkirchen, Germany) using the manufacturer's instructions. The quality of the RNA samples was determined by electrophoresis through denaturing agarose gels stained with ethidium bromide; 5 μL of total RNA was used for complementary DNAs production using a Prime Script™ RT Reagent Kit (Cat. No. RR037A, Takara, Japan), according to the manufacturer's instructions. To evaluate the mRNA expression level, quantitative real‐time PCR was carried out using SYBR‐green (Takara, Tokyo, Japan) with a Step One real‐time PCR machine (Rotor‐Gene 6000; Corbett, Sydney, NSW, Australia). One microliter of total complementary DNA was added to 10 mL of 2× SYBR Green PCR mix with ROX, treated water, and 10 pmol/mL of each forward and reverse primers for the measured genes. The primers sequences are given in Table 1, with an initial denaturation temperature at 95°C. The average expression of β‐actin was used as an internal reference gene to normalize input complementary DNA. The comparative CT method was used to compute the relative expression value.
Table 1.
Primers for quantitative real‐time polymerase chain reaction analysis of gene expression
| Primer | Antisense primer | Sense primer |
|---|---|---|
| LOX‐1 | ACTCTTCAGGTCCTTGTCCACA | GTTGGGATTTTTCCGATGTAAT |
| β‐Actin | CACACCCGCCACCAGTTCG | ACCCATTCCCACCATCACACC |
LOX‐1, lectin‐like low‐density lipoprotein receptor‐1.
Immunohistochemistry
Five‐micrometer sections of the fixed tissue were prepared for immunohistochemical staining, then processed through xylene and a graded alcohol series, and treated with 3% hydrogen peroxide to eliminate the endogenous peroxidase activity before immunostaining and being blocked with 5% normal goat serum. Slides were then treated with mouse anti‐rat LOX‐1 antibody at 10 μg/mL (by Santa Cruz Biotechnology, Santa Cruz, CA, USA).
After an overnight incubation, the secondary antibody of horse anti‐mouse immunoglobulin G‐biotin (by Vector Labs, Burlingame, CA, USA) was added at a 1:200 dilution, then streptavidin–horseradish peroxidase (Jackson Labs, West Grove, PA, USA) was given at 1:1,000. Slides were prepared with AEC Reagent (by Zymed Laboratories, San Francisco, CA,USA), and hematoxylin was used for counterstaining (by Sigma Co, St. Louis, MO, USA). Finally, photographs of the cells were taken under a bright field.
Statistical Analysis
Data were expressed as mean ± standard error of the mean. Degrees of difference among groups were evaluated by one‐way and two‐way anova with the Tukey's post‐hoc test.
The relative changes of gene expression were calculated by the 2−ΔΔCt formula, where ΔCt = Ct (LOX−1)−Ct (β‐actin), and Ct demonstrates the threshold cycle number. In addition, Spearman and Mann–Whitney U‐tests were used to investigate the expression pattern of the target gene. Statistical analysis was carried out using SPSS 21 (SPSS Schweig AG, Zurich, Switzerland), while P < 0.05 was selected as statistically significant.
Results
As baseline data, no significant changes between the groups for blood glucose, IPGTT, weight and plasma level of Mg were observed before the intervention.
Changes in Plasma Glucose
The changes in feeding level of glucose in plasma were measured in all of the groups (Figure 1a). Diabetes induction led to an increase in blood glucose level (to 394.37 ± 18.3 mg/dL), and the blood sugar level continued to rise (590.25 ± 7.32 mg/dL) during the 16 weeks of following diabetes generation. The 16 weeks of MgSO4 or insulin treatment (from day 10) led to a decrease in the plasma glucose levels in the Mg‐CD and Ins‐CD groups to 127.5 ± 0.81 and 86.5 ± 12.47 mg/dL, respectively.
Figure 1.
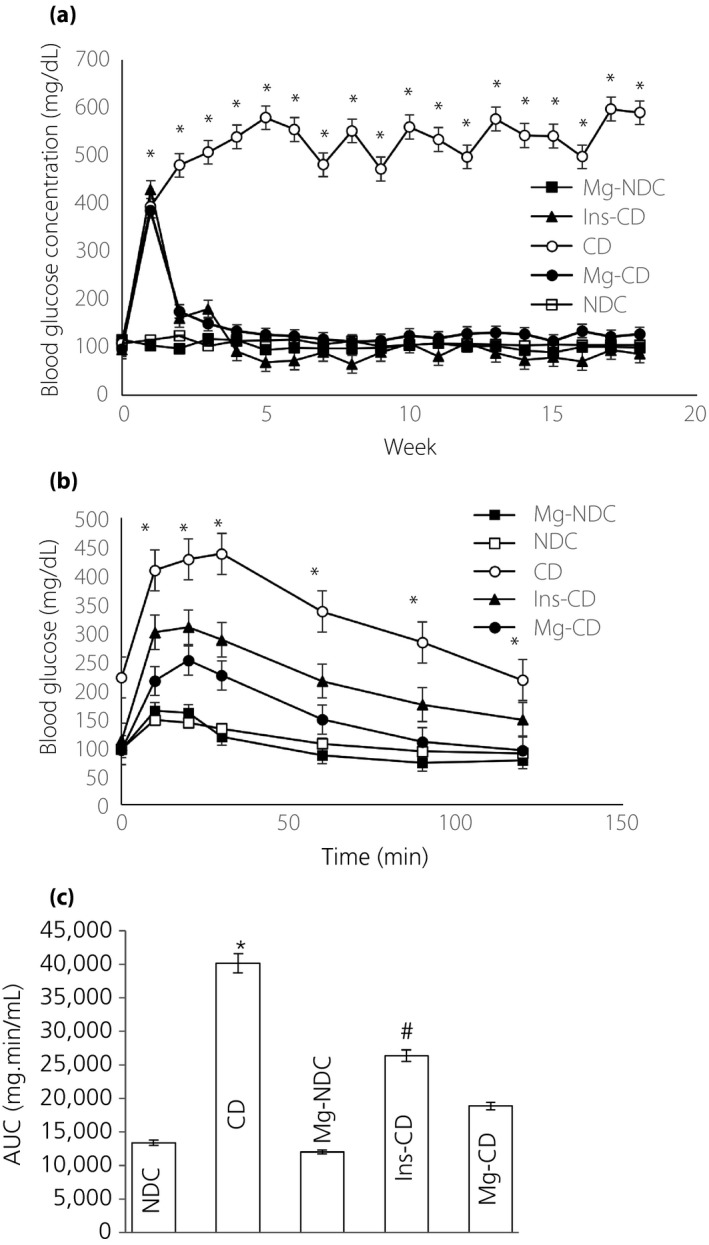
Comparison of (a) fed blood glucose, (b) intraperitoneal glucose tolerance test (IPGTT) and (c) the area under the glycemic curve (AUC) in non‐diabetic control (NDC), chronic diabetic (CD), Mg‐treated non‐diabetic control (Mg‐NDC), insulin‐treated chronic diabetic (Ins‐CD) and Mg‐treated chronic diabetic (Mg‐CD) groups (10 rats in each group, data are expressed as mean ± standard error of the mean). *Significant difference between chronic diabetic and other groups (P < 0.0001). #Significant difference between Ins‐CD and other groups (P < 0.001).
Effects of MgSO4 on IPGTT
Before starting MgSO4 and streptozotocin administration, the IPGTT designs and the area under the curve for all of the groups were quite similar, with no significant difference between the groups (data was not shown).
However, by the 16th week, severe glucose intolerance was shown by the CD group (Figure 1b), being significantly improved in the Mg‐CD group to an extent that was considered effective (area under the curve: NDC, CD and Mg‐CD were 13,370 ± 408.41, 40,085 ± 1,369.55 and 18,827 ± 587.69 mg/min·mL, respectively, at P < 0.0001; Figure 1c). Alterations in bodyweight and plasma biochemical levels in the CD group animals showed that the total bodyweight was decreased insignificantly when compared with NDC animals, whereas an increase in bodyweight after the administration of Mg2+ and insulin was observed compared with CD animals (Figure 2). Also, in order to find the duration and concentration of orally‐administered Mg in plasma, alterations in plasma calcium (Ca) and magnesium (Mg) levels were recorded in all of the groups (Table 2). The plasma Mg level in the CD group (0.46 ± 0.08) significantly (P < 0.01) decreased in comparison with the NDC group (2.17 ± 0.03) 16 weeks after diabetes induction. Administration of Mg2+ and insulin for 16 weeks (from day 10) caused plasma Mg concentrations in the Ins‐CD and Mg‐CD groups to increase (Mg‐CD 3.56 ± 0.26, Ins‐CD 4.99 ± 0.9; Table 2). The plasma Ca level in Mg‐CD, Ins‐CD and Mg‐NDC groups decreased significantly compared with CD animals. Fluctuations in plasma triglycerides, the total cholesterol, oxLDL, LDL and VLDL concentrations were recorded in all of the groups (Table 2). LDL, VLDL, oxLDL, and cumulative cholesterol and triglyceride levels were increased significantly (P < 0.001) for the CD rats 16 weeks after the induction of diabetes. Administration of MgSO4 for 16 weeks (starting from day 10) resulted in plasma oxLDL, LDL, VLDL, total cholesterol and triglyceride concentrations to drop to normal levels.
Figure 2.
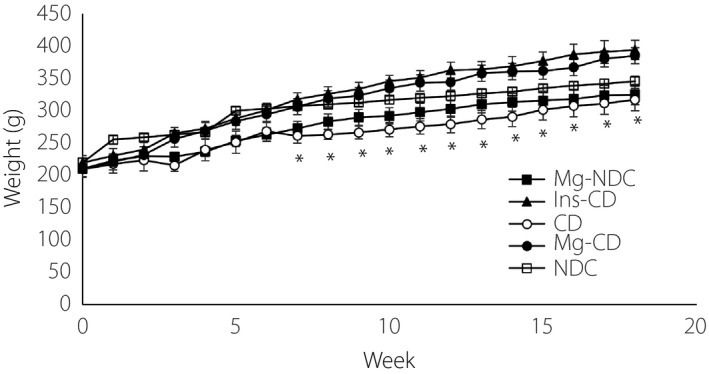
Comparison of bodyweight in non‐diabetic control (NDC), chronic diabetic (CD), Mg‐treated non‐diabetic control (Mg‐NDC), insulin‐treated chronic diabetic (Ins‐CD) and Mg‐treated chronic diabetic (Mg‐CD) groups (10 rats in each group, data are expressed as mean ± standard error of the mean). *Significant difference between CD and other groups (P < 0.001).
Table 2.
Plasma triglyceride, cholesterol, low‐density lipoprotein cholesterol, oxidized low‐density lipoprotein cholesterol, very low‐density lipoprotein cholesterol, calcium and magnesium concentrations in non‐diabetic control, chronic diabetic, magnesium‐treated non‐diabetic control, insulin‐treated chronic diabetic and magnesium‐treated chronic diabetic groups
| Cholesterol | LDL‐c | oxLDL | TG | VLDL | Mg | Ca | |
|---|---|---|---|---|---|---|---|
| NDC | 53.43 ± 1.7 | 6.49 ± 1.2 | 8.6 ± 0.1 | 51.62 ± 4.6a | 9.46 ± 1.2 | 2.17 ± 0.03 | 9.87 ± 0.4 |
| CD | 68.65 ± 5.5b | 14.57 ± 2b | 10.6 ± 0.4b | 93.12 ± 10.6b | 14.72 ± 1.6b | 0.46 ± 0.08b | 10.7 ± 0.3b |
| Mg‐NDC | 46.42 ± 2.7 | 9.16 ± 1.5 | 9.1 ± 0.3 | 32.34 ± 3.6 | 6.46 ± 0.7 | 3.04 ± 0.5 | 7.79 ± 0.7 |
| Ins‐CD | 50.24 ± 2.8 | 3.74 ± 0.04 | 7.2 ± 1.4 | 71.33 ± 9.7 | 14.26 ± 1.9 | 4.99 ± 0.9 | 7.46 ± 0.4 |
| Mg‐CD | 51.74 ± 5.8 | 7.92 ± 2.1 | 8.8 ± 0.6 | 30.9 ± 4.2 | 6.18 ± 0.8 | 3.56 ± 0.2 | 7.56 ± 0.3 |
Data were expressed as mean ± standard error of the mean, 10 rats in each group.
Significant difference in non‐diabetic control (NDC) and other groups (P < 0.001).
Significant difference between chronic diabetic (CD) and other groups (P < 0.001). Ca, calcium; Ins‐CD, insulin‐treated chronic diabetic; LDL‐c, low‐density lipoprotein cholesterol; Mg, magnesium; Mg‐CD, Mg‐treated chronic diabetic; Mg‐NDC, magnesium‐treated non‐diabetic control; oxLDL, oxidized low‐density lipoprotein cholesterol; VLDL, very low‐density lipoprotein cholesterol.
Mesenteric Bed Response, Blood Pressure, Ca/Mg Ratio and Changes in Atherosclerosis Parameters
The Ca/Mg ratio in the CD group elevated significantly (P < 0.001) 16 weeks after the induction of diabetes compared with the other groups (NDC, Mg‐CD, CD, Mg‐NDC and Ins‐CD were 4.64 ± 0.2, 2.22 ± 0.14, 21.17 ± 3.2, 2.81 ± 0.5 and 1.64 ± 0.2, Figure 3a). Mean arterial pressures in CD animals were significantly elevated compared with the NDC group. Mg2+ administration for 16 weeks returned blood pressure to normal levels (Figure 3b).
Figure 3.
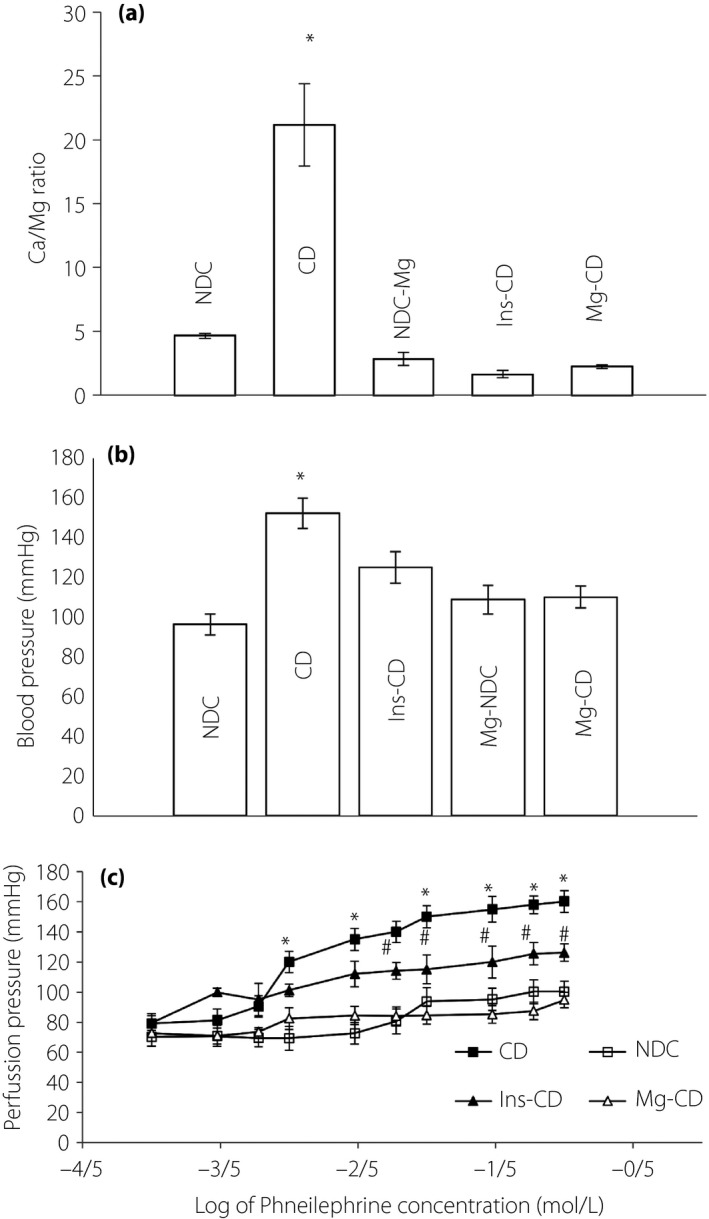
(a) Calcium/magnesium (Ca/Mg) ratio, (b) mean arterial blood pressure and (c) dose–response curve of phenylephrine in the mesenteric vascular bed with intact endothelium in non‐diabetic control (NDC), chronic diabetic (CD), Mg‐treated non‐diabetic control (Mg‐NDC), insulin‐treated chronic diabetic (Ins‐CD) and Mg‐treated chronic diabetic (Mg‐CD) groups (10 rats in each group, data were expressed as mean ± standard error of the mean). *Significant difference between CD and other groups (P < 0.001). #Significant difference between Ins‐CD and Mg‐CD group (P < 0.001).
Baseline perfusion pressure for the CD rats after 16 weeks was reported to be significantly (P < 0.001) higher than other groups, but there was no significant difference between NDC rats and the Mg‐CD rats (Figure 3c). After 30 min of equilibration, a curve was plotted for phenylephrine, a α1 adrenoceptor agonist, showing cumulative concentration response (from 0.0001 to 0.1 mol/L) in unchanged mesenteric beds. Phenylephrine was also introduced into the medium and while recording the perfusion pressure during 15 min for each concentration (Figure 3c). The concentration‐dependent vasoconstriction induced by phenylephrine in mesenteric beds led to an increase in pressure of perfusion. Maximum vasoconstriction was recorded at phenylephrine concentration of 0.01 mol/L. Phenylephrine contractile responses at concentrations of >0.006 mol/L increased significantly in the mesenteric beds of CD rats in comparison with the NDC and Mg‐CD groups, with no significant differences between the NDC and Mg‐CD groups. There was, however, a significant difference witnessed between Mg‐CD animals, and CD and Ins‐CD groups.
Changes in Expression of the LOX‐1 Gene
On average, the CT obtained from real‐time PCR in all of the five groups of experiments, as well as the role MgSO4 and insulin play in expression of LOX‐1, are shown in Figure 4a. Transcription of LOX‐1 relatively decreased to approximately 1.5‐fold (P < 0.01), as compared with the CD group due to Mg2+ treatment. However, insulin administration in diabetic rats did not reduce LOX‐1 gene expression in comparison with the CD group. Changes in the LOX‐1 protein level immunohistochemistry were carried out for all of the groups for LOX‐1 protein level detection, and the results are shown in Figure 4b,c. According to our findings, the LOX‐1 protein level increased in the CD group, and Mg2+ therapy in diabetic rats decreased LOX‐1 protein expression, but this effect was not observed by insulin administration in diabetic rats. Changes in the aorta structure section of the aorta showed that some atherosclerosis parameters significantly changed in the CD group, and MgSO4 administration could improve aortic stricture. However, this effect was not observed in the Ins‐CD animals (Figure 5a,b).
Figure 4.
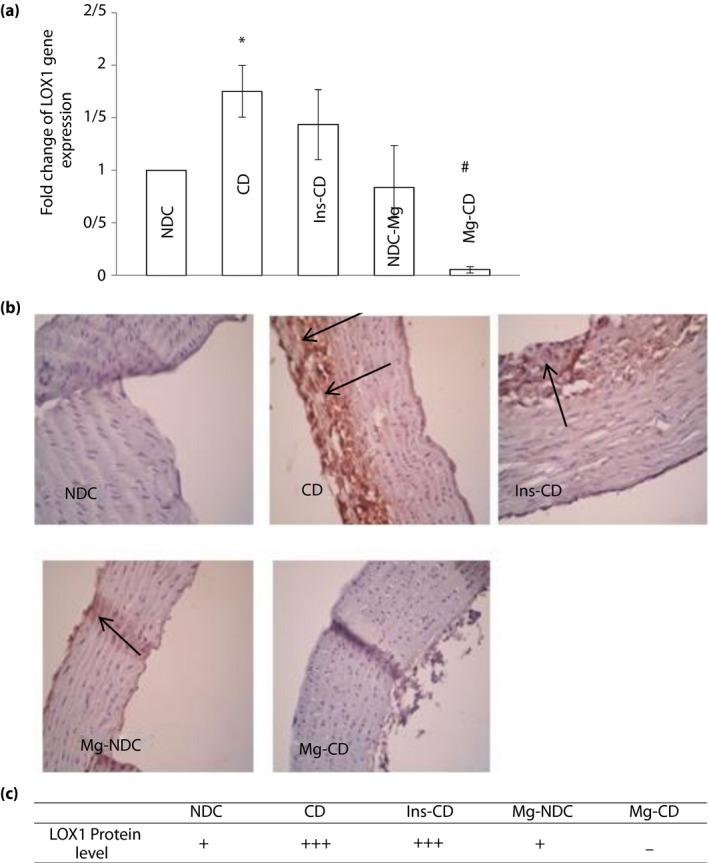
Comparison of (a) lectin‐like low‐density lipoprotein receptor‐1 (LOX‐1) gene expression and (b,c) immunohistochemistry for LOX‐ 1 in non‐diabetic control (NDC), chronic diabetic (CD), Mg‐treated non‐diabetic control (Mg‐NDC), insulin‐treated chronic diabetic (Ins‐CD) and Mg‐treated chronic diabetic (Mg‐CD) groups (10 rats in each group, data are expressed as mean ± standard error of the mean and protein level of LOX‐ 1 is shown by black arrows). *Significant difference between CD and other groups (P < 0.01). #Significant difference between the Mg‐CD and Ins‐CD and Mg‐NDC groups (P < 0.01).
Figure 5.
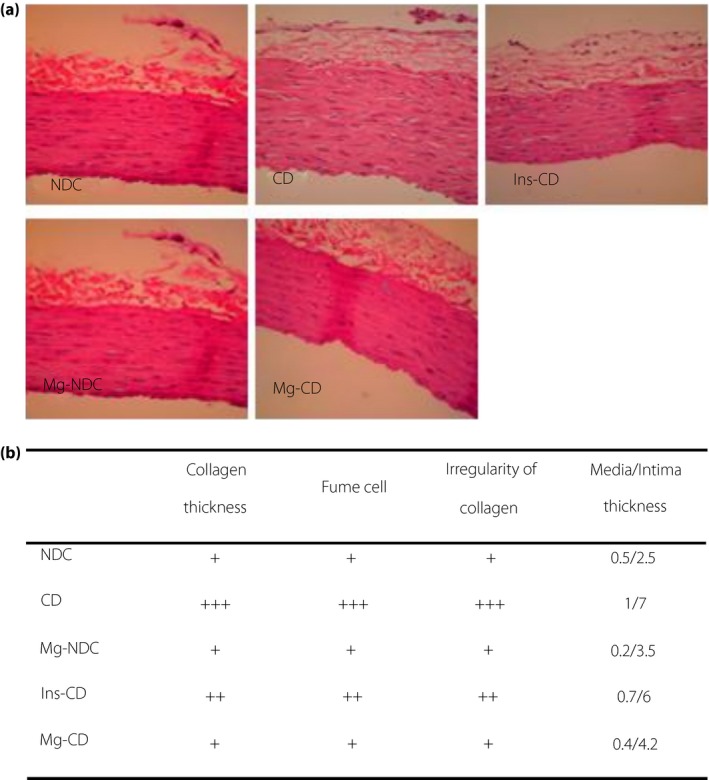
(a) Section of aorta and (b) changes in atherosclerosis parameters in non‐diabetic control (NDC), chronic diabetic (CD), Mg‐treated non‐diabetic control (Mg‐NDC), insulin‐ treated chronic diabetic (Ins‐CD) and Mg‐treated chronic diabetic (Mg‐CD) groups (10 rats in each group).
Discussion
The results from our previous studies argue for the hypothesis that Mg2+ can play a role in the management of diabetes, and thus, the prevention of atherosclerosis in streptozotocin‐induced diabetic rats. Mg2+ is also useful in the treatment of hyperlipidemia in diabetic cases12, 13, but its mechanism remains unclear. We have tried to show the potential role of LOX‐1 inhibition in decreasing the atherosclerosis of diabetic animals’ vessels after administration of MgSO4. In the current study, we attempted to investigate the possible role of inhibition of LOX‐1 gene expression by 16 weeks of MgSO4 administration to prevent atherosclerosis in diabetic animals’ vessels.
The present findings showed that MgSO4 administration in diabetic rats’ drinking water for 16 weeks could increase the plasma Mg level, while it decreases blood glucose concentration and improves the glucose tolerance test. IPGTT results from the current study suggest that MgSO4 might be able to repair pancreas β‐cells and enhance the secretion of insulin or elevate glucose tramsporter type 4 translocation in the cell membrane, which in turn lowers the blood glucose level. However, our previous findings14 showed that Mg2+ has the ability to decrease blood glucose levels by increased glucose tramsporter type 4 mRNA expression, which acts separately from insulin secretion.
According to our previous studies11, 12, 13, the lack of magnesium in the body has been introduced in some studies as a possible cause of diabetes vascular disease, such as hypertension and atherosclerosis. The rate of hypertension in diabetes patients is higher than that of non‐diabetic individuals.
It has long been documented that significant changes in the reactivity of blood vessels to vasoconstriction neurotransmitters are the leading cause of diabetic vessels complications17. In contrast, many studies showed that LOX‐1 gene expression is upregulated in atherosclerotic vessels18, 19, 20, and it plays a key part in the pathogenesis of atherosclerosis. Furthermore, endothelial dysfunction induced by oxLDL through the LOX‐1 receptor and endothelial dysfunction precedes the obviously structural vascular changes and development of atherosclerosis20. Therefore, in the present study, a response to phenylephrine was deemed an alteration in vascular reactivity to vasoconstrictor agents. Also, alteration in the Ca/Mg ratio, and an increase in plasma oxLDL level and LOX‐1 mRNA gene expression elevation were considered markers for atherosclerosis. These results show that Mg2+ therapy lasting for 4 months can reduce the Ca/Mg ratio and plasma oxLDL level, and change the vascular reactivity to vasoconstrictor agents and lower the vessel atherosclerosis criteria. This can be related to observe mean arterial blood pressure, collagen thickness, intima/media thickness and the lumen/media ratio in the aorta in the Mg‐CD group, that are lower than in CD animals. Some investigators have reported that part of Mg2+‐induced vasorelaxation in normal and diabetic vessels is mediated by nitric oxide10, 21. Also, inhibition of expression of LOX‐1 on endothelium cells by MgSO4 might result in an increase of nitric oxide production, as well as a decrease in blood pressure. In contrast, some studies showed that atherosclerotic is interlinked with LDL cholesterol, and Mg is an essential cofactor for many enzymes, namely those involved in lipid metabolism2, 13. The results of the present study showed that MgSO4 has a beneficial effect on lipid profiles, and its action is more sufficient than insulin. Administration of MgSO4 and insulin could also decrease plasma oxLDL levels, but unlike Mg2+, insulin could not reduce LOX‐1 gene expression. Additionally, according to our pathology findings, insulin could not protect the blood vessels against atherosclerosis. The present data also showed that insulin administration for 16 weeks could not alter the sensitivity of mesenteric beds to phenylephrine.
This finding has resulted in the claim that insulin might contribute to increased blood pressure in Ins‐CD animals. The present findings showed that MgSO4 administration in diabetic rats could decrease both plasma oxLDL levels and LOX‐1 gene expression, and it also limited atherosclerosis formation in the aorta, and decreased blood pressure and sensitivity of mesenteric beds to phenylephrine. Some studies have proclaimed that Mg2+ might be able to prevent atherosclerotic disease by decreasing intracellular calcium concentration22, 23, while confirming the negative correlation between low red blood cell magnesium levels and the severity of microangiopathy.
Other studies have shown that Mg2+ is effective in preventing atherosclerosis, and it plays an important role in this field because chronic Mg2+ intake could decrease collagen and ADP‐induced platelet agreeability in type 2 diabetes patients24. However, the findings of the present study showed that Mg2+ administration for 16 weeks could prevent atherosclerosis by decreasing oxLDL and its receptor. It is reported that oxLDL is taken up by macrophages through LOX‐1 receptors, and oxLDL plays a main role in the creation and evolution of atherosclerosis, and leads to endothelial dysfunctions and expression of molecules’ adhesion in the vessel25. All in all, hypertension is the most significant risk factor for atherosclerosis.
In contrast, endothelial cells activation by oxLDL through LOX‐1 might be a key element in causing hypertension in diabetes. The present findings showed that Mg therapy in diabetic rats could decrease hypertension and atherosclerosis through inhibition of oxLDL, LOX‐1 gene expression and sensitivity of vessels to phenylephrine, and Mg was more effective than insulin. The reason for this can be interpreted as insulin being necessary for Mg uptake to the insulin target cells. As our previous studies26, 27, 28 showed that long‐term insulin therapy increases insulin resistance as a result of decreased glucose tramsporter type 4 translocation to the cell membrane, therefore plasma Mg level in Ins‐CD animals was higher than other groups, but insulin resistance altered the Mg entry, while intracellular magnesium concentrations are required for insulin function. Finally, according the IPGTT data, some degree of resistance to insulin was observed in the Ins‐CD group, which might increase the expression of the LOX1.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
Hormozgan University of Medical Sciences supported the present study. The grant number is 94141. The authors also thank Dr Nima Pouladian for editing the manuscript.
J Diabetes Investig 2019; 10: 650–658
References
- 1. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001; 414: 813–820. [DOI] [PubMed] [Google Scholar]
- 2. Jenkins AJ, Lyons TJ, Zheng D, et al Serum lipoproteins in the diabetes control and complications trial/epidemiology of diabetes intervention and complications cohort associations with gender and glycemia. Diabetes Care 2003; 26: 810–818. [DOI] [PubMed] [Google Scholar]
- 3. Witztum JL, Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest 1991; 88: 1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Parthasarathy S, Steinberg D, Witztum J. The role of oxidized low‐density lipoproteins in the pathogenesis of atherosclerosis. Annu Rev Med 1992; 43: 219–225. [DOI] [PubMed] [Google Scholar]
- 5. Nishimura S, Akagi M, Yoshida K, et al Oxidized low‐ density lipoprotein (ox‐LDL) binding to lectin‐like ox‐LDL receptor‐1 (LOX‐1) in cultured bovine articular chondrocytes increases production of intracellular reactive oxygen species (ROS) resulting in the activation of NF‐κB. Osteoarthritis Cartilage 2004; 12: 568–576. [DOI] [PubMed] [Google Scholar]
- 6. Cominacini L, Pasini AF, Garbin U, et al Oxidized low density lipoprotein (ox‐LDL) binding to ox‐LDL receptor‐1 in endothelial cells induces the activation of NF‐κB through an increased production of intracellular reactive oxygen species. J Biol Chem 2000; 275: 12633–12638. [DOI] [PubMed] [Google Scholar]
- 7. Mehta JL, Chen J, Hermonat PL, et al Lectin‐like, oxidized low‐density lipoprotein receptor‐1 (LOX‐1): a critical player in the development of atherosclerosis and related disorders. Cardiovasc Res 2006; 69: 36–45. [DOI] [PubMed] [Google Scholar]
- 8. Taye A, Saad AH, Kumar AH, et al Effect of apocynin on NADPH oxidase‐mediated oxidative stress‐LOX‐1‐eNOS pathway in human endothelial cells exposed to high glucose. Eur J Pharmacol 2010; 627: 42–48. [DOI] [PubMed] [Google Scholar]
- 9. Liao F, Folsom AR, Brancati FL. Is low magnesium concentration a risk factor for coronary heart disease? The Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J 1998; 136: 480–490. [DOI] [PubMed] [Google Scholar]
- 10. Soltani N, Keshavarz M, Sohanaki H, et al Relaxatory effect of magnesium on mesenteric vascular beds differs from normal and streptozotocin induced diabetic rats. Eur J Pharmacol 2005; 508: 177–181. [DOI] [PubMed] [Google Scholar]
- 11. Soltani N, Keshavarz M, Sohanaki H, et al Oral magnesium administration prevents vascular complications in STZ‐diabetic rats. Life Sci 2005; 76: 1455–1464. [DOI] [PubMed] [Google Scholar]
- 12. Soltani N, Keshavarz M, Minaii B, et al Effects of administration of oral magnesium on plasma glucose and pathological changes in the aorta and pancreas of diabetic rats. Clin Exp Pharmacol Physiol 2005; 32: 604–610. [DOI] [PubMed] [Google Scholar]
- 13. Soltani N, Keshavarz M, Dehpour AR. Effect of oral magnesium sulfate administration on blood pressure and lipid profile in streptozocin diabetic rat. Eur J Pharmacol 2007; 560: 201–205. [DOI] [PubMed] [Google Scholar]
- 14. Solaimani H, Soltani N, Malekzadeh K, et al Modulation of GLUT4 expression by oral administration of Mg2+ to control sugar levels in STZ‐induced diabetic rats. Can J Physiol Pharmacol 2014; 92: 438–444. [DOI] [PubMed] [Google Scholar]
- 15. Soltani N, Qiu H, Aleksic M, et al GABA exerts protective and regenerative effects on islet beta cells and reverses diabetes. Proc Natl Acad Sci USA 2011; 108: 11692–11697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McGregor D. The effect of sympathetic nerve stimulation on vasoconstrictor responses in perfused mesenteric blood vessels of the rat. J Physiol 1965; 177: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Özcelikay AT, Tay A, Guner S, et al Reversal effects of L‐arginine treatment on blood pressure and vascular responsiveness of streptozotocin‐ diabetic rats. Pharmacol Res 2000; 41: 201–209. [DOI] [PubMed] [Google Scholar]
- 18. Wang L, Yu Y, Zhang L, et al Taurine rescues vascular endothelial dysfunction in streptozocin‐induced diabetic rats: correlated with downregulation of LOX‐1 and ICAM‐1 expression on aortas. Eur J Pharmacol 2008; 597: 75–80. [DOI] [PubMed] [Google Scholar]
- 19. Chen M, Masaki T, Sawamura T. LOX‐1, the receptor for oxidized low‐density lipoprotein identified from endothelial cells: implications in endothelial dysfunction and atherosclerosis. Pharmacol Ther 2002; 95: 89–100. [DOI] [PubMed] [Google Scholar]
- 20. Hamakawa Y, Omori N, Ouchida M, et al Severity dependent up‐regulations of LOX‐1 and MCP‐1 in early sclerotic changes of common carotid arteries in spontaneously hypertensive rats. Neurol Res 2004; 26: 767–773. [DOI] [PubMed] [Google Scholar]
- 21. Pearson PJ, Evora PR, Seccombe JF, et al Hypomagnesemia inhibits nitric oxide release from coronary endothelium: protective role of magnesium infusion after cardiac operations. Ann Thorac Surg 1998; 65: 967–972. [DOI] [PubMed] [Google Scholar]
- 22. Seelig MS, Heggtveit HA. Magnesium interrelationships in ischemic heart disease: a review. Am J Clin Nutr 1974; 27: 59–79. [DOI] [PubMed] [Google Scholar]
- 23. Mather H, Levin G, Nisbet J. Hypomagnesemia and ischemic heart disease in diabetes. Diabetes Care 1982; 5: 452–453. [Google Scholar]
- 24. Vanroelen WF, Van Gaal LF, Van Rooy PE, et al Serum and erythrocyte magnesium levels in type I and type II diabetics. Acta Diabetol 1985; 22: 185–190. [DOI] [PubMed] [Google Scholar]
- 25. Mitra S, Goyal T, Mehta JL. Oxidized LDL, LOX‐1 and atherosclerosis. Cardiovasc Drugs Ther 2011; 25: 419. [DOI] [PubMed] [Google Scholar]
- 26. Khosravi F, Kharazmi F, Kamran M, et al The role of PPAR‐γ and NFKB genes expression in muscle to improve hyperglycemia in STZ‐induced diabetic rat following magnesium sulfate administration. Int J Physiol Pathophysiol Pharmacol 2018; 10: 124–131. [PMC free article] [PubMed] [Google Scholar]
- 27. Sohrabipour S, Sharifi MR, Sharifi M, et al Effect of magnesium sulfate administration to improve insulin resistance in type 2 diabetes animal model: using the hyperinsulinemic‐euglycemic clamp technique. Fundam Clin Pharmacol 2018; 32: 603–616. [DOI] [PubMed] [Google Scholar]
- 28. Sohrabipour S, Sharifi MR, Talebi A, et al GABA dramatically improves glucose tolerance in streptozotocin‐induced diabetic rats fed with high‐fat diet. Eur J Pharmacol 2018; 826: 75–84. [DOI] [PubMed] [Google Scholar]


