Abstract
Aims/Introduction
Flash and continuous glucose monitoring systems are becoming prevalent in clinical practice. We directly compared a flash glucose monitoring system (FreeStyle Libre Pro [FSL‐Pro]) with a continuous glucose monitoring system (iPro2) in patients with diabetes mellitus.
Materials and Methods
Glucose concentrations were simultaneously measured using the FSL‐Pro, iPro2 and self‐monitoring blood glucose in 10 patients with diabetes mellitus, and agreement among them was assessed.
Results
Parkes error grid analysis showed that the 92.9 and 7.1% of glucose values measured using the FSL‐Pro fell into areas A and B, respectively, and that 96.3, 2.8 and 0.9% of those determined using iPro2 fell into areas A, B and C, respectively. The median absolute relative differences compared with self‐monitoring blood glucose were 8.1% (3.9–12.7%) and 5.0% (2.6–9.1%) for the FSL‐Pro and iPro2, respectively. Analysis of 5,555 paired values showed a close correlation between FSL‐Pro and iPro2 glucose values (ρ = 0.96, P < 0.01). Notably, 65.3% of all glucose values were lower for the FSL‐Pro than the iPro2. Median glucose values also decreased by 3.3% for the FSL‐Pro compared with the iPro2 (177.0 [133.0–228.0] vs 183.0 [145.0–230.0] mg/dL, P < 0.01). The difference in glucose values between the two systems was more pronounced in hypoglycemia. The median absolute relative difference between FSL‐Pro and iPro2 during hypoglycemia was much larger than that during euglycemia and hyperglycemia.
Conclusions
Both the FSL‐Pro and iPro2 systems are clinically acceptable, but glucose values tended to be lower when measured using the FSL‐Pro than the iPro2. Agreement was not close between these systems during hypoglycemia.
Keywords: Nocturnal hypoglycemia, Postprandial hyperglycemia, Self‐management
Introduction
Regular self‐monitoring of blood glucose (SMBG) is required to improve blood glucose control in patients with types 1 and 2 diabetes mellitus requiring insulin therapy1, 2. In contrast, evidence of the effectiveness of self‐monitoring is controversial for patients with type 2 diabetes mellitus managed without insulin3, 4. Although an integral part of treatment, SMBG is clinically limited, as the overall daily blood glucose profile is not captured, and postprandial hyperglycemia or nocturnal hypoglycemia is often underestimated5. Furthermore, SMBG is accompanied by the burden and pain associated with multiple measurements of capillary blood glucose using fingersticks.
Continuous glucose monitoring systems (CGMS) measure interstitial fluid glucose concentrations (ISFG) throughout the day and provide information that is unattainable by SMBG6. Several devices have been developed to measure ISFG7. The iPro2 is a professional CGMS that enables the collection of ISFG profiles for up to 7 days of wear per sensor. The iPro2 requires calibrations with SMBG four times daily to calibrate ISFG. The FreeStyle Libre Pro (FSL‐Pro) is a professional flash glucose monitoring system (FGMS) that was approved in Japan after the iPro2. This system can monitor ISFG for 14 consecutive days of wear per sensor. Unlike the iPro2 CGMS, the FSL‐Pro is factory‐calibrated for ISFG, meaning that patients do not need to measure capillary glucose concentrations for calibration. The application of the iPro2 and FSL‐Pro systems in clinical practice has increased, and they help to understand postprandial hyperglycemia and nocturnal hypoglycemia, which provides benefits to many patients with diabetes mellitus.
Here, ISFG was simultaneously measured in patients with diabetes mellitus requiring insulin therapy using the FSL‐Pro and iPro2 and agreement between them was assessed. To the best of our knowledge, these systems have not yet been compared in hospitalized patients.
Methods
The present study enrolled patients with diabetes mellitus requiring insulin therapy (men n = 7; women n = 3; age 59.5 ± 17.6 years; Table 1) who were hospitalized to achieve optimal glucose control. Two patients with type 1 diabetes mellitus and eight with type 2 diabetes mellitus received multiple‐dose insulin injection therapy. They were also treated with biguanides (n = 4), sodium–glucose co‐transporter 2 inhibitor (n = 3), dipeptidyl peptidase‐4 inhibitor (n = 2), glinides (n = 1), α‐glucosidase inhibitor (n = 1) and liraglutide (n = 1). The ethics committees at Mito Medical Center, Tsukuba University Hospital and Mito Kyodo General Hospital approved this study, and written informed consent was obtained from all patients to participate.
Table 1.
Patients’ characteristics at baseline
| Parameter | |
|---|---|
| Men/women | 7/3 |
| Age (years) | 59.5 ± 17.6 |
| BMI | 25.3 ± 4.0 |
| T1DM/T2DM | 2/8 |
| BG (mg/dL) | 410.3 ± 122.0 |
| HbA1c (%) | 13.7 ± 2.5 |
| Insulin (U/day) | 35.8 ± 22.3 |
| Biguanides | 4 |
| SGLT2i | 3 |
| DPP4i | 2 |
| Glinides | 1 |
| α‐GI | 1 |
| Liraglutide | 1 |
α‐GI, α‐glucosidase inhibitor; BG, blood glucose; BMI, body mass index; DPP4i, dipeptidyl peptidase‐4 inhibitor; HbA1c, hemoglobin A1c; SGLT2i, sodium–glucose co‐transporter 2 inhibitor; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
Laboratory studies proceeded on admission. Venous blood glucose (BG) values were determined using the glucose oxidation method. The enrolled patients underwent simultaneous 24‐h CGM using the iPro2 (Medtronic Inc., Northridge, PA, USA) and FSL‐Pro FGMS (Abbott Diabetes Care Inc., Alameda, CA, USA) systems. A total of 10 patients measured ISFG using both systems for 7 days. The FSL‐Pro and iPro2 Enlite sensors were applied to the upper arm on one side. The FSL‐Pro and iPro2 Enlite sensors record ISFG every 15 and 5 min, respectively. SMBG was carried out four times daily (before meals and at bedtime) to calibrate ISFG determined using the iPro2. The iPro2 and FSL‐Pro recordings were aligned to obtain paired glucose measures, and glucose values obtained from the FSL‐Pro were compared with the closest of those obtained using the iPro2. The lag time was maintained at <2 min between iPro2 and FSL‐Pro glucose values. Unpaired iPro2 glucose values were discarded. Likewise, glucose values from the two systems were also compared with the closest capillary BG value determined by SMBG.
The consistency of glucose values provided by FSL‐Pro or iPro2 with SMBG in the clinically meaningful areas from A to E was evaluated using Parkes error grid analysis8, 9. Because boundaries are stricter for the type 1 diabetes mellitus than the type 2 diabetes mellitus version of the Parkes error grid, the stricter type 1 diabetes mellitus version should therefore be the analytical method of choice for accurate assessments9. Thus, the present study applied the type 1 diabetes mellitus version for the combined population of type 1 and type 2 diabetes mellitus. Absolute differences (AD) and absolute relative differences (ARD) against the SMBG value for the FSL‐Pro and iPro2 were determined as follows:
The median AD and median ARD were calculated for each patient.
Agreement between FSL‐Pro and iPro2 glucose values was checked based on linear correlations. Differences in glucose values between the two systems were calculated and plotted in accordance with glucose values measured using the iPro2. The AD and ARD between FSL‐Pro and iPro2 were also determined based on glucose values provided by iPro2 for a head‐to‐head comparison:
The median AD and median ARD were calculated for each patient.
Continuous variables with normal distribution are presented as means (standard deviation), and non‐normal variables are expressed as medians (interquartile range). Pairs of groups were compared using the Wilcoxon signed‐rank test. The Kruskal–Wallis test was applied for multiple comparisons, and statistically significant results of multiple comparisons were individually compared using Mann–Whitney U‐tests with Bonferroni correction. Linear correlations were checked using the Spearman rank correlation coefficient. A value of P < 0.05 was considered significant. The Bonferroni correction yielded a threshold of significance for uncorrected P‐values of 0.017 instead of 0.05. Data were statistically analyzed using SPSS version 23 (SPSS Inc., Chicago, IL, USA).
Results
A total of 10 of the enrolled patients with diabetes mellitus (type 1, n = 2; type 2, n = 8) were hospitalized to achieve optimal plasma glucose control. Values for BG and hemoglobin A1c were 410.3 ± 122.0 mg/dL and 13.7 ± 2.5%, respectively (Table 1). All patients required multiple‐dose insulin injection therapy, and the mean insulin dose was 35.8 ± 22.3 units per day.
The accuracy of the FSL‐Pro and iPro2 was initially compared with SMBG. Parkes error grid analyses showed that 92.9 and 7.1% of glucose values generated by the FSL‐Pro fell within areas A and B, respectively, and that 96.3, 2.8 and 0.9% of those measured using the iPro2 fell within areas A, B and C, respectively (Figure 1a,b).
Figure 1.
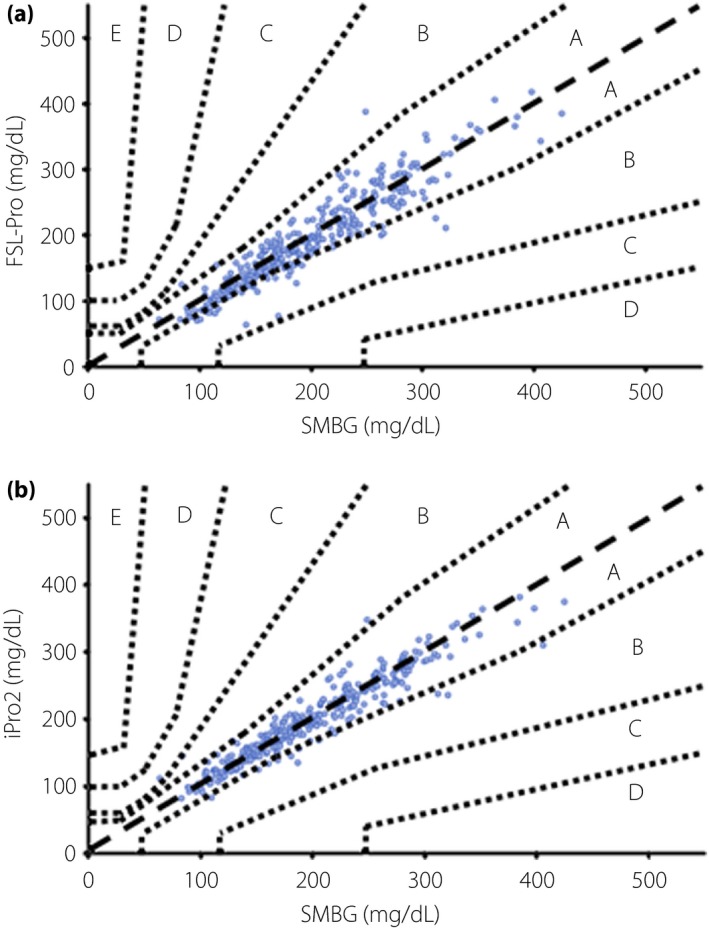
Parkes error grid analyses. Clinical validity of (a) the FreeStyle Libre Pro (FSL‐Pro) and (b) iPro2 assessed by Parkes error grid analyses. SMBG, self‐monitoring glucose monitoring.
Glucose values generated by the FSL‐Pro and iPro2 were compared with the closest capillary BG values determined using SMBG (Table 2). The overall glucose values were significantly decreased by 2.6% for the FSL‐Pro compared with the SMBG. In contrast, overall glucose values did not significantly differ between the iPro2 and SMBG. Although the glucose values were decreased by 4.1% for the FSL‐Pro compared with SMBG during euglycemia (SMBG 70–180 mg/dL), those determined using the iPro2 were increased by 2.1% compared with SMBG. Glucose values were comparable among these three systems during hyperglycemia (>180 mg/dL). Because just two glucose values were determined using SMBG as <70 mg/dL, statistical analyses were not possible during hypoglycemia <70 mg/dL.
Table 2.
Comparison of glucose values generated using FreeStyle Libre Pro and iPro2 with closest values generated using self‐monitoring blood glucose
| n | SMBG (mg/dL) | FSL‐Pro (mg/dL) | iPro2 (mg/dL) | |
|---|---|---|---|---|
| Overall | 323 | 189.0 (152.0–248.0) | 184.0 (144.0–249.0)* , ** | 195.0 (151.0–247.0) |
| Hypoglycemia | 2 | 63.5 | 67.5 | 105.5 |
| Euglycemia | 141 | 145.0 (123.5–165.5) | 139.0 (115.0–158.5)* , ** | 148.0 (125.0–167.0)*** |
| Hyperglycemia | 180 | 238.0 (207.3–278.8) | 239.5 (201.0–279.0) | 238.0 (206.0–272.0) |
Glycemia is classified based on the glucose values determined by self‐monitoring blood glucose (SMBG). Data are expressed as medians (interquartile range). *P < 0.01 versus SMBG. **P < 0.01 versus iPro2. ***P < 0.01 versus SMBG. Values were compared using Wilcoxon signed‐rank test with Bonferroni correction. FSL‐Pro, FreeStyle Libre Pro.
The overall median ARD versus SMBG for the FSL‐Pro and iPro2 were 8.1% (3.9–12.7%) and 5.0% (2.6–9.1%), respectively (Table 3). Although the median ARD significantly differed overall between the FSL‐Pro and the iPro2, the accuracy of both systems compared with SMBG was acceptable. The overall median AD against SMBG was significantly higher for the FSL‐Pro than the iPro2 (Table 3). The median ARD and AD were also assessed in terms of degrees of glycemia. Table 3 shows that the median ARD and AD were significantly higher for the FSL‐Pro than the iPro2 during euglycemia and hyperglycemia. Because just two glucose values were determined by SMBG as being <70 mg/dL, statistical analyses were not possible during hypoglycemia. In addition, whereas values determined by SMBG indicated hypoglycemia, those determined using the iPro2 were normal, which would lead to higher ARD and AD for the iPro2 than the FSL‐Pro during hypoglycemia (ARD 66.1 vs 6.3; AD 42.0 vs 4.0; Table 3).
Table 3.
Median median absolute relative differences and median absolute differences between FreeStyle Libre Pro and self‐monitoring blood glucose, and between iPro2 and self‐monitoring blood glucose
| FSL‐Pro | iPro2 | |
|---|---|---|
| Median ARD (%) | ||
| Overall | 8.1 (3.9–12.7)* | 5.0 (2.6–9.1) |
| Hypoglycemia | 6.3 | 66.1 |
| Euglycemia | 8.9 (4.1–14.0)* | 5.6 (2.8–10.3) |
| Hyperglycemia | 7.8 (3.6–12.4)* | 4.4 (2.5–8.1) |
| Median AD (mg/dL) | ||
| Overall | 15.0 (7.0–25.0)* | 10.0 (5.0–17.0) |
| Hypoglycemia | 4.0 | 42.0 |
| Euglycemia | 13.0 (6.0–19.0)* | 8.0 (4.0–14.0) |
| Hyperglycemia | 18.0 (9.0–30.0)* | 12.0 (6.0–19.8) |
Data are expressed as medians (interquartile range). *P < 0.01 versus iPro2. Wilcoxon signed‐rank test was used for a comparison. AD, absolute differences; ARD, median absolute relative differences; FSL‐Pro, FreeStyle Libre Pro.
We then compared glucose values determined using the FSL‐Pro with the closest of those generated using the iPro2 and found a close correlation between them (ρ = 0.96, P < 0.01) in a pooled analysis of 5,555 paired values from the 10 patients (Figure 2a). Notably, 65.3% of overall glucose values were lower for the FSL‐Pro than the iPro2. The difference between FSL‐Pro and iPro2 was more pronounced when lower glucose values were determined by the iPro2 (Figure 2b). The difference in the two systems weakly, but significantly, correlated positively with glucose values determined using the iPro2 (ρ = 0.21, P < 0.01).
Figure 2.
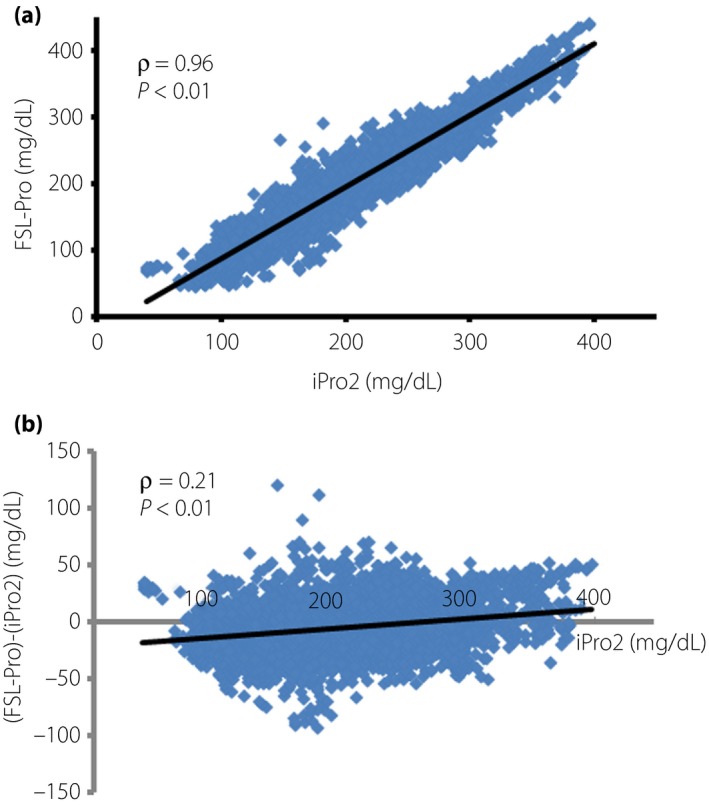
Agreement between the FreeStyle Libre Pro (FSL‐Pro) and iPro2. (a) FSL‐Pro versus iPro2. (b) Differences in glucose values between systems plotted according to glucose values generated using iPro2. (a,b) Linear correlations were confirmed using the Spearman rank correlation coefficient.
The iPro2 generated glucose values of 0.3, 48.0 and 51.7% in hypoglycemia, euglycemia and hyperglycemia (iPro2 <70, 70–180 and >180 mg/dL, respectively; Table 4). Overall median glucose values were significantly decreased by 3.3% when measured using the FSL‐Pro compared with the iPro2 (177.0 [133.0–228.0] vs 183.0 [145.0–230.0] mg/dL, P < 0.01; Table 4). In detail, glucose values were significantly decreased by 8.3 and 1.3% according to the FSL‐Pro during euglycemia and hyperglycemia, respectively, whereas they were significantly increased by 58.5% according to the FSL‐Pro compared with the iPro2 during hypoglycemia.
Table 4.
Comparison of glucose values between FreeStyle Libre Pro and iPro2
| n | FSL‐Pro (mg/dL) | iPro2 (mg/dL) | |
|---|---|---|---|
| Overall | 5,555 | 177.0 (133.0–228.0)* | 183.0 (145.0–230.0) |
| Hypoglycemia | 16 | 74.5 (60.8–76.8)* | 47.0 (40.5–65.0) |
| Euglycemia | 2,669 | 132.0 (108.0–154.5)* | 144.0 (120.0–162.0) |
| Hyperglycemia | 2,870 | 225.0 (196.0–266.0)* | 228.0 (202.0–265.0) |
Glycemia is classified based on the glucose values determined by the iPro2. Data are expressed as medians (interquartile range). *P < 0.01 versus iPro2. Wilcoxon signed‐rank test was used for a comparison. FSL‐Pro, FreeStyle Libre Pro.
The overall median ARD between the two systems was 6.5% (3.6–11.5%, P < 0.01). The median ARD between glycemic values obtained using the two systems differed the most and least under conditions of hypoglycemia and hyperglycemia, respectively (Figure 3a). The median ARD in hypoglycemia was much higher than that of euglycemia or hyperglycemia (57.1% [17.1–76.7%] vs 6.7% [4.1–12.9%] vs 6.2% [3.0–10.5%]). The overall median AD between the two systems was 12.0 (6.0–22.0 mg/dL, P < 0.01). Consistent with the difference between two systems (Figure 2b; Table 4), the median AD was also higher in hypoglycemia than in euglycemia or hyperglycemia (27.0 [15.0–32.0] vs 9.0 [6.0–18.0] vs 14.0 [7.0–24.0] mg/dL; Figure 3b).
Figure 3.
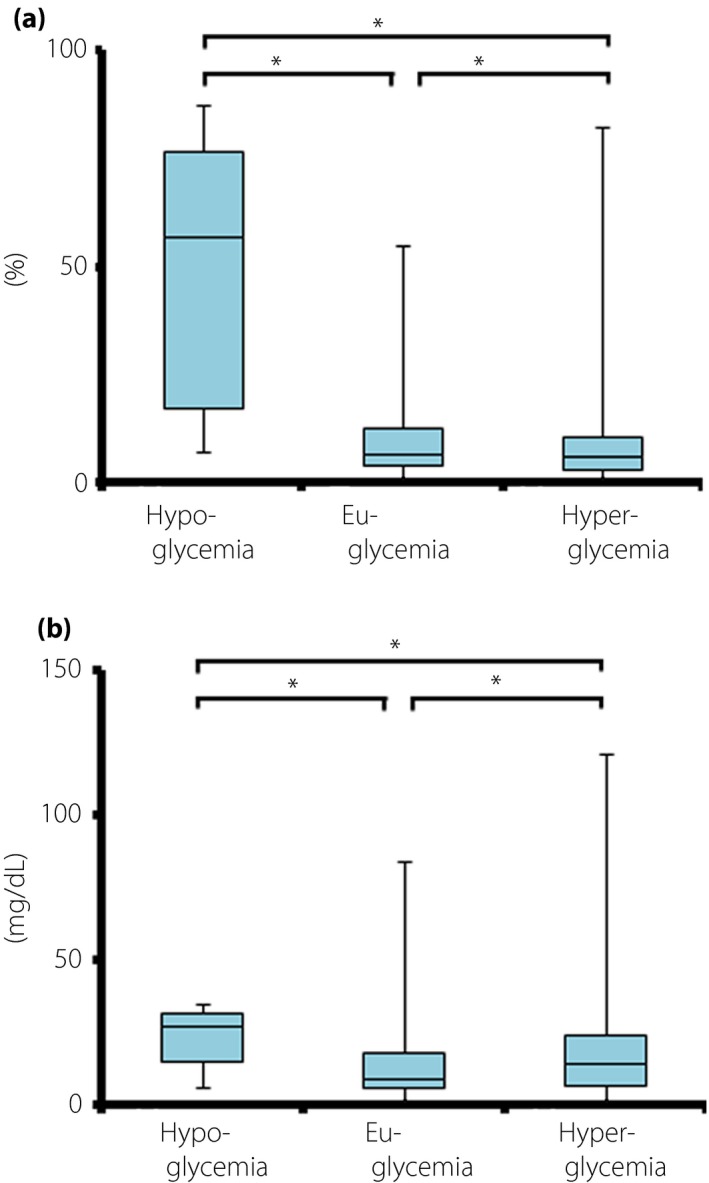
Median (a) absolute relative differences (ARD) and (b) absolute differences (AD) between the FreeStyle Libre Pro and iPro2 in terms of glycemic values. *P < 0.01. Kruskal–Wallis test for multiple comparisons followed by Mann–Whitney U tests with Bonferroni correction.
Discussion
Patients who are unaware of having nocturnal hypoglycemia, have significant glucose variability or have discrepancies between hemoglobin A1c and SMBG should use CGMS6, 10, as it improves hemoglobin A1c without increasing the risk of hypoglycemia compared with SMBG alone11, 12. New methods of CGMS have recently been developed7, and the iPro2 CGMS is widely used in Japan. The FSL‐Pro FGMS was recently approved and will become more popular in clinical practice, replacing iPro2 CGMS. Herein, we assessed FSL‐Pro FGMS and iPro2 CGMS in a head‐to‐head comparison, and found that both were clinically acceptable. We also identified a close correlation between FSL‐Pro and iPro2 glucose values, although a difference in glucose values between the two systems was pronounced in hypoglycemia. Agreement between the two systems determined by ARD was the worst and best during hypoglycemia and hyperglycemia, respectively.
Parkes error grid analysis showed that 92.9 and 7.1% of all glucose values determined by the FSL‐Pro fell into areas A and B, respectively, which was consistent with the outcomes reported by Bailey et al.13. In addition, 96.3, 2.8 and 0.9% of glucose values determined by the iPro2 also fell into areas A, B and C, respectively, suggesting that both systems are clinically acceptable.
While the glucose values were significantly decreased for FSL‐Pro compared with SMBG, the iPro2 generated significantly increased glucose values compared with SMBG during euglycemia (Table 2). In contrast, glucose values generated using the FSL‐Pro and iPro2 were comparable with those generated by SMBG during hyperglycemia. As a result, overall glucose values were significantly more decreased for the FSL‐Pro than SMBG. Overall glucose values did not significantly differ between the iPro2 and SMBG. The accuracy of these two systems was compared with that of SMBG as median ARD (Table 3). Although the median ARD was significantly higher for the FSL‐Pro than the iPro2, both systems were accurate with regard to SMBG7, except in the hypoglycemic range, which was barely confirmed by SMBG in the present study. A comparison of the FSL with capillary BG found a mean ARD (MARD) of 8.3% in patients with type 1 and type 2 diabetes mellitus14, which was similar to the present outcomes. The results from the Parkes error grid analysis and the median ARD showed that both systems can contribute to the appropriate self‐management of diabetes in clinical practice. We used capillary BG provided by SMBG rather than venous BG to assess ARD because of the practical limitations of frequent blood collection. Furthermore, SMBG provided more reference points and represented real‐life accuracy during daily patient use. One report has described that SMBG can serve as the primary comparator for FGMS performance evaluation13. Many studies have used the MARD to determine the accuracy of FGMS and CGMS13, 14, 15, 16, 17, 18. However, we applied median ARD instead of MARD, because the distribution of the glucose values measured in the present study was not normal. The average reported ARD is 0.8% × MARD7.
We then found a close correlation in overall glucose values between the FSL‐Pro and iPro2. The median overall glucose value was decreased by 3.3% when measured by the FSL‐Pro compared with the iPro2 (Table 4). Notably, 65.3% of overall glucose values were lower for the FSL‐Pro than the iPro2. Glucose values were significantly more deceased for the FSL‐Pro compared with the iPro2 during euglycemia and hyperglycemia, and significantly more increased for the FSL‐Pro than the iPro2 during hypoglycemia. The difference in glucose values between the two systems was more pronounced at lower glucose values determined using the iPro2 (Figure 2b; Table 4). The median AD between the two systems was significantly higher in hypoglycemia than in euglycemia and hyperglycemia in the present study, which raises concerns about direct comparisons of absolute glucose values provided by these two systems during hypoglycemia. Although few comparisons of FSL‐Pro and iPro2 have been reported, one study found significantly lower glucose values generated by the FSL than the iPro2 during oral glucose loading tests of outpatients with type 1 and type 2 diabetes mellitus14.
Glucose values provided by CGMS might be inaccurate during hypoglycemia, and the MARD reportedly varies in terms of glucose levels19, 20. The median ARD between FSL‐Pro and iPro2 in hypoglycemia was much higher than that in either euglycemia or hyperglycemia in the present study, indicating that the two systems did not closely agree during hypoglycemia. In contrast, the median ARD was optimal during hyperglycemia. The present study findings are in line with the recently published accuracy of FSL CGMS14, 15, 16, 17. The MARD between FSL and Dexcom G4 Platinum (Dexcom Inc., San Diego, CA, USA) CGMS was higher in hypoglycemia than in either euglycemia or hyperglycemia15, 17. The MARD of several devices, including the FSL, compared with venous or capillary BG are also higher in hypoglycemia than in euglycemia or hyperglycemia14, 16, 17. Therefore, care is required if CGMS or FGMS is applied to patients who are frequently unaware of being hypoglycemic, or who have nocturnal hypoglycemia. Low glucose values measured by these systems should routinely be confirmed by SBMG. Furthermore, even if these systems do not indicate hypoglycemia, SMBG should proceed if symptoms of hypoglycemia arise.
The present study was limited by the small number of patients and the lack of a gold standard against which to measure plasma glucose values. Furthermore, because SMBG determined that just two glucose values were <70 mg/dL, the accuracy of the two systems could not be compared with capillary BG during hypoglycemia. Nevertheless, values determined using the FSL‐Pro and iPro2 systems agreed in hospitalized patients.
In conclusion, both the FSL‐Pro and iPro2 systems are clinically acceptable, but glucose values tended to be lower when generated using the FSL‐Pro than the iPro2. In addition, values generated by these two systems did not closely agree during hypoglycemia. These data should be helpful when considering therapeutic regimens based on glucose values provided by the FSL‐Pro and iPro2 systems.
Disclosure
The authors declare no conflict of interest.
J Diabetes Investig 2019; 10: 851–856
References
- 1. Schutt M, Kern W, Krause U, et al Is the frequency of self‐monitoring of blood glucose related to long‐term metabolic control? Multicenter analysis including 24,500 patients from 191 centers in Germany and Austria. Exp Clin Endocrinol Diabetes 2006; 114: 384–388. [DOI] [PubMed] [Google Scholar]
- 2. Murata GH, Shah JH, Hoffman RM, et al Intensified blood glucose monitoring improves glycemic control in stable, insulin‐treated veterans with type 2 diabetes: the Diabetes Outcomes in Veterans Study (DOVES). Diabetes Care 2003; 26: 1759–1763. [DOI] [PubMed] [Google Scholar]
- 3. McIntosh B, Yu C, Lal A, et al Efficacy of self‐monitoring of blood glucose in patients with type 2 diabetes mellitus managed without insulin: a systematic review and meta‐analysis. Open Med 2010; 4: e102–e113. [PMC free article] [PubMed] [Google Scholar]
- 4. Szymborska‐Kajanek A, Psurek A, Hese R, et al Self‐monitoring of blood glucose in treatment of type 2 diabetes. Diabetes Res Clin Pract 2009; 86(suppl 1): S49–S52. [DOI] [PubMed] [Google Scholar]
- 5. Boland E, Monsod T, Delucia M, et al Limitations of conventional methods of self‐monitoring of blood glucose: lessons learned from 3 days of continuous glucose sensing in pediatric patients with type 1 diabetes. Diabetes Care 2001; 24: 1858–1862. [DOI] [PubMed] [Google Scholar]
- 6. Blevins TC. Professional continuous glucose monitoring in clinical practice 2010. J Diabetes Sci Technol 2010; 4: 440–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rodbard D. Continuous glucose monitoring: a review of successes, challenges, and opportunities. Diabetes Technol Ther 2016; 18(suppl 2): S3–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Parkes JL, Slatin SL, Pardo S, et al A new consensus error grid to evaluate the clinical significance of inaccuracies in the measurement of blood glucose. Diabetes Care 2000; 23: 1143–1148. [DOI] [PubMed] [Google Scholar]
- 9. Pfutzner A, Klonoff DC, Pardo S, et al Technical aspects of the Parkes error grid. J Diabetes Sci Technol 2013; 7: 1275–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choudhary P, Ramasamy S, Green L, et al Real‐time continuous glucose monitoring significantly reduces severe hypoglycemia in hypoglycemia‐unaware patients with type 1 diabetes. Diabetes Care 2013; 36: 4160–4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tamborlane WV, Beck RW, Bode BW, et al Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008; 359: 1464–1476. [DOI] [PubMed] [Google Scholar]
- 12. Tanenberg R, Bode B, Lane W, et al Use of the Continuous Glucose Monitoring System to guide therapy in patients with insulin‐treated diabetes: a randomized controlled trial. Mayo Clin Proc 2004; 79: 1521–1526. [DOI] [PubMed] [Google Scholar]
- 13. Bailey T, Bode BW, Christiansen MP, et al The performance and usability of a factory‐calibrated flash glucose monitoring system. Diabetes Technol Ther 2015; 17: 787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fokkert MJ, van Dijk PR, Edens MA, et al Performance of the FreeStyle Libre Flash glucose monitoring system in patients with type 1 and 2 diabetes mellitus. BMJ Open Diabetes Res Care 2017; 5: e000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bonora B, Maran A, Ciciliot S, et al Head‐to‐head comparison between flash and continuous glucose monitoring systems in outpatients with type 1 diabetes. J Endocrinol Invest 2016; 39: 1391–1399. [DOI] [PubMed] [Google Scholar]
- 16. Aberer F, Hajnsek M, Rumpler M, et al Evaluation of subcutaneous glucose monitoring systems under routine environmental conditions in patients with type 1 diabetes. Diabetes Obes Metab 2017; 19: 1051–1055. [DOI] [PubMed] [Google Scholar]
- 17. Boscari F, Galasso S, Facchinetti A, et al FreeStyle Libre and Dexcom G4 Platinum sensors: accuracy comparisons during two weeks of home use and use during experimentally induced glucose excursions. Nutr Metab Cardiovasc Dis 2018; 28: 180–186. [DOI] [PubMed] [Google Scholar]
- 18. Sekido K, Sekido T, Kaneko A, et al Careful readings for a flash glucose monitoring system in nondiabetic Japanese subjects: individual differences and discrepancy in glucose concentration after glucose loading [Rapid Communication]. Endocr J 2017; 64: 827–832. [DOI] [PubMed] [Google Scholar]
- 19. Rodbard D. Characterizing accuracy and precision of glucose sensors and meters. J Diabetes Sci Technol 2014; 8: 980–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Klonoff DC. Continuous glucose monitoring: roadmap for 21st century diabetes therapy. Diabetes Care 2005; 28: 1231–1239. [DOI] [PubMed] [Google Scholar]


