Abstract
Aims/Introduction
Blockade or reversal the progression of diabetic nephropathy is a clinical challenge. The aim of the present study was to examine whether recombinant human glucagon‐like peptide‐1 (rhGLP‐1) has an effect on alleviating urinary protein and urinary albumin levels in diabetic rats.
Materials and Methods
Streptozotocin‐induced diabetes rats were treated with rhGLP‐1 insulin and saline. Using immunostaining, hematoxylin–eosin, electron microscopy and periodic acid–Schiff staining to study the pathology of diabetic nephropathy, and we carried out quantitative reverse transcription polymerase chain reaction, western blot and immunohistochemistry to identify the differentially expressed proteins. The mechanism was studied through advanced glycation end‐products‐induced tubular epithelial cells.
Results
rhGLP‐1 inhibits protein kinase C (PKC)‐β, but increases protein kinase A (PKA), which reduces oxidative stress in glomeruli and in cultured glomerular microvascular endothelial cells. In tubules, rhGLP‐1 increased the expression of two key proteins related to re‐absorption – megalin and cubilin – which was accompanied by downregulation of PKC‐β and upregulation of PKA. On human proximal tubular epithelial cells, rhGLP‐1 enhanced the absorption of albumin, and this was blocked by a PKC activator or PKA inhibitor.
Conclusions
These findings suggest that rhGLP‐1 can reverse diabetic nephropathy by protecting both glomeruli and tubules by inhibiting PKC and activating PKA.
Keywords: Diabetic nephropathy, Protein kinase, Recombinant human glucagon‐like peptide‐1
Introduction
Diabetic nephropathy (DN) is the leading cause of end‐stage renal disease worldwide1. The development of DN involves complex pathological mechanisms, including interaction among inflammatory, metabolic, oxidative stress and hemodynamic factors2. The exact reason for DN currently remains unknown, but accumulated evidence has shown that hyperglycemia‐induced renal hyperfiltration and renal injury play an important role in DN. Furthermore, protein kinase C (PKC)‐induced cytokines, advanced glycation end‐product (AGE)‐induced oxidative stress, and different inflammatory, chemokines and apoptotic signals might also be involved in the pathology of DN3.
At present, tight glycemic and blood pressure control are used for the prevention of DN, but the efficacy is unsatisfactory4. Finding a new method of treatment for DN is urgent and important. Glucagon‐like peptide‐1 (GLP‐1) is one of the two primary incretin hormones produced mostly by intestinal L cells that can stimulate insulin secretion and inhibit glucagon secretion dependent on glucose5. GLP‐1 can be broken down by dipeptidyl peptidase‐4 within 2 min in vivo. Some GLP1 analogs with a longer half‐life were recently developed, such as GLP‐1 receptor agonists6 or GLP‐1 analogs and dipeptidyl peptidase‐4 inhibitors5, which are clinically applied for the treatment of type 2 diabetes.
GLP‐1‐induced insulin secretion is mediated by the high‐affinity GLP‐1 receptor (GLP‐1R) in pancreatic β‐cells5. GLP‐1R agonists ameliorate DN in both type 1 and type 2 DN animal models by activating GLP‐1R on glomerular endothelial and infiltrating inflammatory cells6, 7. GLP‐1R agonists can reduce AGE receptor expression, and act as anti‐inflammatory agents against AGEs through activation of the cyclic adenosine monophosphate (cAMP) pathway on mesangial cells8. GLP‐1‐based drugs were also reported to prevent glomerular macrophage infiltration in glomeruli, and reduce oxidative stress and inflammation in tubular cells in streptozotocin (STZ)‐induced diabetic animals9. GLP‐1 receptor agonists, liraglutide, semaglutide, lixisenatide and extended‐release exenatide, were reported to prevent the inflammatory response in diabetic kidneys, they have shown cardiovascular safety and have the potential to be chosen for the treatment of DN10, 11.
In addition, exendin‐4 – a GLP‐1 analog – can also attenuate renal tubular injury in a STZ‐induced diabetes model12. These data suggest that GLP‐1 protects the kidney in multiple pathways. In the present study, we investigated the actions and underlying mechanism of recombinant human GLP‐1 (rhGLP‐1) on the glomerular filtration barrier and on renal tubular protein reabsorption by using STZ‐induced Wistar rats with DN and in cultured cells.
Methods
rhGLP‐1
For the present study, rhGLP‐1 (7–36; beinaglutide) was purchased from Shanghai Benemae Pharmaceutical Corporation (Shanghai, China). rhGLP‐1 is an analog of native GLP‐1, and its half‐life in blood is approximately 2 h13.
Animals
All animal experiments followed the national guidelines and the relevant national laws on the protection of animals. Rats were housed under specific pathogen‐free conditions in strict accordance with the guidelines of the care and use of laboratory animals established by the Beijing Association of Laboratory Animal Science. All rat experiments were carried out in accordance with the protocols and guidelines approved by the Animal Ethics Committee of the China‐Japan Friendship Hospital (permit No: 130401). Eight‐week‐old male/female Wistar rats weighing 300 ± 10 g were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd (Beijing, China). A diabetic model was induced by administration of 12 g/L STZ (fresh diluent with 0.1 mol/L citrate buffer at pH 4.5) intraperitoneally (30 mg/kg) twice with a 1‐week interval. Blood was taken from the caudal vein, and blood glucose of >16.7 mmol/L for three consecutive days after the second STZ injection is considered the standard of a diabetic model. All the diabetic rats were fed with conventional chow for an additional 8 weeks13. Then, 24 diabetic rats were divided into the three groups according to a random number‐generating table (n = 8) as follows. For the rhGLP‐1 group, rhGLP‐1 (dissolved in 0.9% saline, 1.5 pmol/kg/min) was administered as a continuous intravenous infusion for 12 weeks by osmotic pumps (type 2004; Alzet; Alza Corp, Palo Alto, CA, USA). The pump was placed in the abdominal cavity and replaced with a new pump at 4‐weeks. For the vehicle group, diabetic rats were treated with isotonic 0.9% NaCl. For the insulin group, diabetic rats were treated with glargine insulin (2.5 U/day; LANTUS®, Paris, France) subcutaneously. Eight normal Wistar rats of comparable age and weight were used as controls (normal group).
Detection of 24‐h Urinary Protein and 24‐h Urinary Albumin
At 0, 2, 4, 6, 8, 10 and 12 weeks after treatment, all rats were placed in metabolic cages (Suzhou Fengshi Laboratory Animal Equipment Co, LTD, Suzhou, China) for 24 h, and excretion of urine was measured individually. Urine samples were collected for analysis of albumin and total protein. The AssayMax Rat Albumin Elisa Kit (Assaypro, St Charles, MO, USA) was used to measure urinary albumin. The urinary proteins were measured by a BCA Protein Assay Kit (Beyotime Institute of Biotechnology, Nanjing, China).
Measurement of Serum C‐peptide
Rat blood was collected from the femoral vein under anesthesia into BD Vacutainer® Rapid Serum Tubes, left for 30 min at room temperature, then centrifuged and serum was collected. The concentration of C‐peptide was measured with rat C‐peptide ELISA kit (ALPCO Diagnostics, Salem, NH, USA).
Histology and Immunohistochemistry
Rat pancreatic tissue was fixed in 4% formaldehyde. Sections were stained with rabbit anti‐rat insulin antibody (1:100; Sigma, Shanghai, China) and horseradish peroxidase‐conjugated secondary antibody.
Freshly dissected kidneys were fixed overnight in 4% formaldehyde, and periodic acid–Schiff staining was carried out according to standard procedures. Semi‐quantitative scoring of glomerular sclerosis in periodic acid–Schiff‐stained slides was carried out13, 14 using a masked protocol of >25 tissue sections for each group. Section immunostaining was carried out using primary antibody against PKC‐β2 (Abcam, Shanghai, China, ab32026), protein kinase A (PKA; Abcam, ab75991), nitric oxide (NO) synthase (iNOS; Abcam, ab15323), superoxide dismutase 1 (SOD1; Abcam, ab13498), megalin (Abcam, ab184676), cubilin (Abcam, ab191073), GLP‐1R (Abcam, ab218532), insulin (Abcam, ab7842), podocin (Abcam, ab50339), nephrin (Abcam, ab58968) and collagen IV (ThermoFisher, Shanghai, China, PA1‐36001). Antigen signals were detected with horseradish peroxidase‐conjugated secondary antibody and visualized by 3,3′‐diaminobenzidine or immunofluorescence.
Electron Microscopy
Kidney cortex was fixed in 2.5% glutaraldehyde for 1 h at room temperature, and glomerular basement membrane (GBM) thickness was determined as previously described15. Briefly, GBM thicknesses were measured with a ruler in the Adobe PDF file at >50 sites, and the results are presented as mean ± standard error of the mean.
Isolation of Glomeruli and Tubules
After 12‐week treatment, the rats were euthanized by CO2, the renal cortex was separated and homogenized, the lysis was passed through a 100‐mesh metal sieve, then using a 300‐mesh metal sieve to filtrate the lysis, the glomeruli and tubules were saved for the experiment.
Real‐Rime Reverse Transcription Polymerase Chain Reaction
First‐strand complementary deoxyribonucleic acid was synthesized from 2 μg of total ribonucleic acid (RNA) in a 40‐μL reaction system using avian myeloblastosis virus reverse transcriptase (Invitrogen, Shanghai, China) and oligo‐dT primers (Invitrogen). Real‐time polymerase chain reaction (PCR) was carried out by a SYBR green PCR reagent kit (Toyobo, Osaka, Japan), then run by Applied Biosystems 7500 (Applied Biosystems, Foster City, CA, USA). The change in messenger RNA levels was determined by the formula 2−ΔΔCt. The PCR primers used in the present study are listed in Table 1.
Table 1.
Primer sequences for polymervase chain reaction amplification of rat genes
| Genes | Primer sequences | Product length (bp) |
|---|---|---|
| PKC‐β | sense: 5′‐GAGACAAGCGAGACACCTCC‐3′ | 192 |
| antisense: 5′‐CAGCCTTACACACAGGCTCA‐3′ | ||
| PKA | sense: 5′‐TAAACCGGTTCACAAGGCGTG‐3′ | 326 |
| antisense: 5′‐GTTACCAACGCATCTTCCAAC‐3′ | ||
| Megalin | sense:: 5′‐AGGACACTTCGCACTGCGCC‐3′ | 215 |
| antisense: 5′‐CCACTGCGGGATGCAACGGT‐3′ | ||
| Cubilin | sense: 5′‐CCCGTGTCGCCAGTGTGTGT‐3′ | 121 |
| antisense: 5′‐TCAGCCCGGAAGCCCCTGTT‐3′ | ||
| β‐actin | sense: 5′‐GACATCCGTAAAGACCTCTATGC‐3′ | 173 |
| antisense: 5′‐ATAGAGCCACCAATCCACACAG‐3′ | ||
| CD2AP | sense: 5′‐ACGAACTGGAACTGACCGTG‐3′ | 168 |
| antisense: 5′‐CTGATTCCTCCTGAGCGTCG‐3′ | ||
| Nephrin | sense: 5′‐GGCTGACATCTGGGATGACC‐3′ | 294 |
| antisense: 5′‐AGAGCTGGAATGACAGTGATGG‐3′ | ||
| NEPH1 | sense: 5′‐GGATTCAAACATGGTCCTGAGTA‐3′ | 216 |
| antisense: 5′‐CCGATGAAGCACTCCACCTT‐3′ | ||
| Podocin | sense: 5′‐TGCTACTACCGCATGGAAAATG‐3′ | 178 |
| antisense: 5′‐CTGCATCTAAGGCAACCTTTACA‐3′ | ||
| α‐Actinin 4 | sense: 5′‐CCAGGAGGATGACTGGGAC‐3′ | 106 |
| antisense: 5′‐GCCAGCCTTCCGAAGATGA‐3′ | ||
| iNOS | sense: 5′‐CCTTGCACTGCCAAGAATTTG‐3′ | 95 |
| antisense: 5′‐CATTGCGTCACTGGATAGTAGTT‐3′ | ||
| SOD1 | sense: 5′‐AACCAGTTGTGTTGTCAGGAC‐3′ | 139 |
| antisense: 5′‐CCACCATGTTTCTTAGAGTGAGG‐3′ | ||
| GLP‐1R | sense: 5′‐ACGGTGTCCCTCTCAGAGAC‐3′ | 117 |
| antisense: 5′‐ATCAAAGGTCCGGTTGCAGAA‐3′ |
GLP‐1R, glucagon‐like peptide‐1 receptor; iNOS, nitric oxide synthase; PKA, protein kinase A; PKC‐β, protein kinase Cβ; SOD, SOD1, superoxide dismutase 1.
Western blot
The total protein of 100 μg was fractionated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, and then transferred to polyvinylidene fluoride membranes. β‐Actin (A5316), CD2AP (PA5‐51894), NEPH1 (ABS1511) and PKC‐β2 (P3203) purchased from Sigma; GLP‐1R (ab186051), α‐actinin4 (ab108198), nephrin (ab58968), podocin (ab50339), PKA (ab75991), megalin (ab184676), iNOS (ab15323) and SOD1 (ab13498) purchased from Abcam; and xc ubilin (sc‐20609) purchased from Santa Cruz, Santa Cruz, CA, USA) were used in western blotting. Horseradish peroxidase‐conjugated rabbit anti‐mouse, goat anti‐rabbit or donkey anti‐goat immunoglobulin G (Sigma) was used at a 1:30,000 dilution. The antigens were visualized using the ECL System purchased from Millipore (Bedford, MA, USA). For kidney tissue, eight samples in each group were pooled. For cultured cells, the experiment was carried out independently three times. Images from one experiment are shown in the figures. The gray scale was analyzed with Image J (National Institutes of Health, Bethesda, MD, USA), and the data are presented as a ratio of target protein : actin (mean ± standard error of the mean).
Culture of Rat Glomerular Endothelial Cells
Rat glomerular microvascular endothelial cells (RGECs) were cultured in M199 (Gibco, Grand Island, NY, USA) medium that was supplemented with 100 μg/mL endothelial cell growth factor, 20% fetal calf serum (Hyclone; Fisher Scientific, South Longan, UT, USA), 2 mmol/L l‐glutamine, 100 U/mL penicillin and streptomycin.
Effects of rhGLP‐1 on AGE‐Induced Reactive Oxygen Species and NO Production
RGECs were cultured in 96‐well plates for 24 h, followed by a pre‐incubation with PKC‐β specific inhibitor LY‐333531 (10 nmol/L; Axon Ligands, Groningen, the Netherlands), PKC agonist phorbol‐myristate‐acetate (PMA; 10 μmol/L; Sigma), PKA inhibitor H‐89 (10 μmol/L; Sigma) or PKA agonist 8‐bromo‐adenosine 3′,5′‐cyclic monophosphate (8‐Br‐cAMP; 100 μmol/L; Sigma) for 30 min. Then RGECs were treated with AGEs (200 μg/mL; Abcam), AGEs (200 μg/mL) + insulin (1 IU/mL, Lantus Solostar, Sanofi, Beijing, China) or AGEs (200 μg/mL) + rhGLP‐1 (1.0 mg/mL; Shanghai Benemae Pharmaceutical Corporation) for another 24 h according to a previously used method detect of reactive oxygen species (ROS) production16.
RGECs were seeded in 24‐well plates and pre‐incubated with PKC inhibitor LY‐333531, PKC agonist PMA, PKA inhibitor H‐89 or PKA agonist 8‐Br‐cAMP for 30 min, and then treated with AGEs (200 μg/mL), AGEs (200 μg/mL) + insulin or AGEs (200 μg/mL) + rhGLP‐1 for 24 h. Detection of NO production followed a previous study16.
Culture of Human Proximal Tubular Epithelial Cells
For maintain the cells, we used Dulbecco's modified Eagle's medium/F12 medium (Gibco) that contains 10% fetal bovine serum and 100 U/mL penicillin and streptomycin to culture human proximal tubular epithelial cells (HK‐2). HK‐2 cells were treated with medium containing AGEs (200 μg/mL), AGEs (200 μg/mL) + insulin, AGEs (200 μg/mL) + rhGLP‐1, rhGLP‐1 + (200 μg/mL) + LY33531 (10 nmol/L), rhGLP‐1 + AGEs (200 μg/mL) + 8‐Br‐cAMP (100 μmol/L), rhGLP‐1 + AGEs (200 μg/mL) + PKC agonist PMA (20 nmol/L) or rhGLP‐1 + AGEs (200 μg/mL) + PKA inhibitor H‐89 (100 μmol/L) for 24 h. Albumin absorption was evaluated as described previously15.
PKC/PKA Activity Assays
PKC/PKA activity were measured as shown previously16.
Measurement of Total Diacylglycerol Contents
The total renal DAG contents were measured as shown previously17. The DAG ELISA kit (Product No. ABIN5524780) was purchased from antibodies‐online Inc. (Atlanta, GA, USA).
Statistical Analysis
Data are presented as mean ± standard error of the mean. One‐way anova (Tukey's) and two‐way anova (Bonferroni post‐tests) analysis were used. A value of P < 0.05, P < 0.01 or P < 0.001 was indicated by one (*) and (#), two (**) and (##) or three asterisks (***) and (###), respectively.
Results
rhGLP‐1 Alleviates DN in Rats
After treatment for 12 weeks, the blood glucose in the rhGLP‐1 group was slightly lower than that in the vehicle group (Figure 1a); however, treatment with insulin reduced blood glucose greatly (Figure 1a). Urinary protein as an indicator of diabetic nephropathy was increased in the vehicle group over time compared with the normal group (Figure 1b). After treatment by GLP‐1 for 6 weeks, urinary protein was lower in the rhGLP‐1 group compared with the vehicle and insulin groups (Figure 1b). Furthermore, we observed a diminution in 24‐h urinary albumin excretion compared with the vehicle and insulin groups after treatment with rhGLP‐1 for 6 weeks (Figure 1c).
Figure 1.
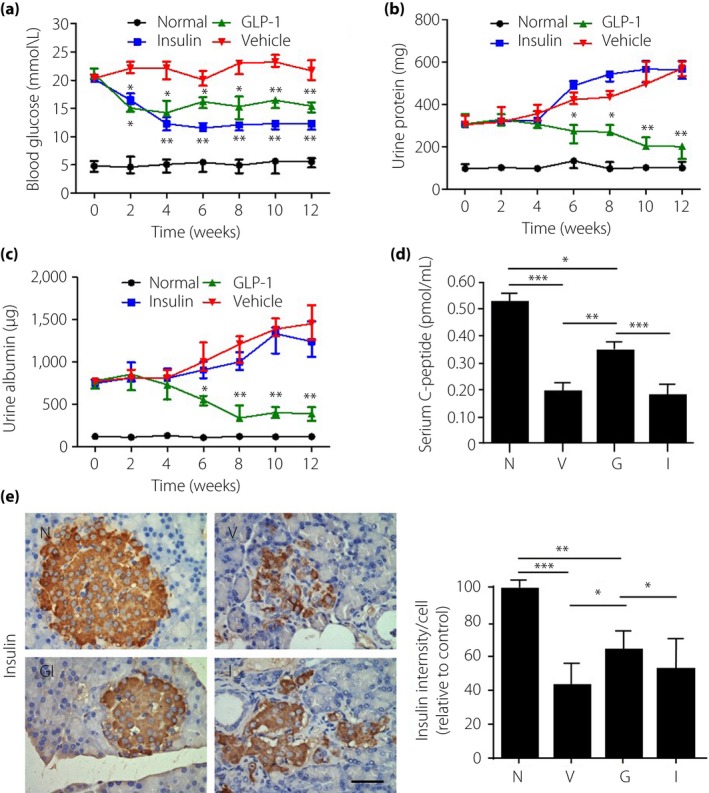
The efficacy of recombinant human glucagon‐like peptide‐1 (GLP‐1) in diabetic nephropathy. (a) Blood glucose, (b) urinary protein and (c) urinary albumin were measured at different time‐points after treatment for up to 3 months. (d) Serum C‐peptide levels were measured by enzyme‐linked immunosorbent assay at the end of the experiment. (e) Insulin level in islets were detected by immunohistochemical staining in pancreatic tissue. Scale bar, 20 μm. Data are presented as mean ± standard error the mean (*P < 0.05, **P < 0.01 and ***P < 0.001; one‐way anova test). G, diabetic rats treated with recombinant human glucagon‐like peptide‐1 (n = 8; 5 male, 3 female); I, diabetic rats treated with insulin (n = 8; 3 male, 5 female); N, normal rats (n = 8l; 4 male, 4 female); V, diabetic rats treated with saline (n = 8; 4 male, 4 female).
Analysis of serum C‐peptide concentration showed that rhGLP‐1 increased serum C‐peptide in the diabetic rats, but insulin treatment had no effect on serum C‐peptide concentration (Figure 1d). By immunohistochemical staining and quantitative analysis, we found that insulin decreased in the diabetic pancreas compared with the normal group, and rhGLP‐1 can increase the insulin production in the islet (Figure 1e). All the aforementioned results suggest that rhGLP‐1 alleviates diabetic nephropathy in rats.
rhGLP‐1 Upregulates GLP‐1R Expression in Kidneys of Diabetic Rats
GLP‐1 is a potent vasodilator, and is associated with improvement of endothelial function in animal models and in type 2 diabetes patients18, 19. GLP‐1 acts through the GLP‐1 receptor, a G‐coupled‐protein receptor that is abundantly present in the gastrointestinal tract, and has also been detected at lower levels in vascular smooth muscle and endothelial cells20, 21, 22. To explore whether GLP‐1 alleviates DN through GLP‐1R, we evaluated the expression of GLP‐1R. The results showed that GLP‐1R was reduced in both glomeruli and renal tubules in the vehicle group and insulin group, but it can be increased by rhGLP‐1 (Figure S1a). Furthermore, this result was confirmed in isolated glomeruli (Figure S1b) and tubules (Figure S1c) by western blotting.
rhGLP‐1 Improves the Glomerular Lesion
Podocyte injury plays a key role in the development of albuminuria in DN23. To explore the mechanism of GLP‐1 underlying the prevention of proteinuria, we examined sclerosis in glomeruli, and the structure of the glomerular filtration barrier using periodic acid–Schiff staining and electron microscopy. Histological examination of the kidney revealed the increased glomerular sclerosis in the vehicle group compared with the normal group (Figure 2a). Glomerular sclerosis could be improved by rhGLP‐1, but not by insulin (Figure 2a). By electron microscopy, we found that the thickness of GBM was normal and podocyte foot processes were well arranged in normal rats (Figure 2b). Chronic hyperglycemia caused a marked increase in GBM thickness and severe foot process effacement in vehicle‐treated diabetic rats (Figure 2b), consistent with the dramatic albuminuria and proteinuria observed. Treatment with rhGLP‐1 maintained the normal thickness of the GBM and normal podocyte structure (Figure 2b). However, insulin treatment showed a negligible effect on reducing GBM thickness and foot process effacement (Figure 2b).
Figure 2.
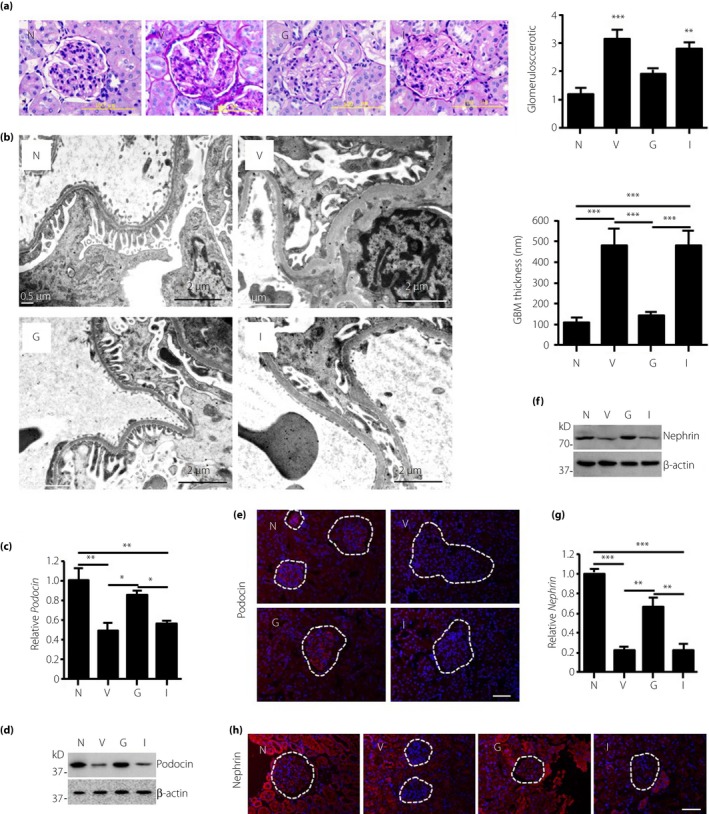
Recombinant human glucagon‐like peptide‐1 protects the glomerular filtration barrier in diabetic rats. After treatment for 12 weeks, the animals in all groups were euthanized and the kidneys were harvested for structural evaluation of the glomerular filtration barrier. (a) Glomerular morphology was carried out by periodic acid–Schiff staining. Glomerulosclerosis scores were semiquantified in the normal group (N), vehicle group (V), recombinant human glucagon‐like peptide‐1 group (G), and insulin group (I). ***P < 0.001 (vs V). (b) Transmission electron microscopy for the structure of the glomerular basement membrane (GBM) and foot processes of podocytes. Scale bars, 2 μm. The GBM thicknesses were measured with a ruler in Adobe PDF files at >50 sites, and the results are presented as mean ± standard error of the mean. (c) Real‐time polymerase chain reaction, (d) western blot and (e) immunofluorescence imaging was used to measure the expression of podocin in the glomeruli. (f) Western blot, (g) real‐time polymerase chain reaction and (h) immunofluorescence imaging was used to measure the expression of nephrin in the glomeruli. Data are presented as mean ± standard error of the mean, n = 8 (*P < 0.05, **P < 0.01 and ***P < 0.001; one‐way anova test).
The slit diaphragm is the key structure that controls protein filtration through the glomerular filtration barrier24. We therefore quantified the expression of key proteins within the slit diaphragm using real‐time reverse transcription PCR, IF and western Blot. As shown in Figure 2c–h and Figure S2a–c, compared with the normal controls, the messenger RNA and protein levels of α‐actinin4, CD2AP, nephrin, NEPH1 and podocin were all dramatically reduced in the vehicle group, and rhGLP‐1 treatment partially restored their expression. However, there were no significant differences between the insulin and vehicle groups both in messenger RNA expression and protein level (Figure 2c–h, Figure S2a–c). Overall, the present results showed that rhGLP‐1 exerts a protective effect on the glomerular filtration barrier and glomerular sclerosis in rats with diabetic nephropathy.
Effects of rhGLP‐1 on the Expression of Megalin and Cubilin in Renal Tubules
Megalin and cubilin are two important membrane proteins on tubular epithelial cells, and are associated with the reabsorption of urinary protein. We thus evaluated in isolated tubular tissue the expression of megalin and cubilin by reverse transcription PCR (Figure S3a), and western blotting and quantification analysis (Figure S3b). The results showed that megalin and cubilin decreased in the rats with diabetic nephropathy, and expression was restored by rhGLP‐1, but not by insulin (Figure S3a, b). This finding was further confirmed by immunohistochemical staining on kidney tissue from four groups (Figure S3c). These results suggested that rhGLP‐1 positively affected megalin and cubilin expression.
rhGLP‐1 Inhibits PKC‐β and Activates PKA in Both Glomeruli and in Tubules
It is well known that hyperglycemia induces the activation of PKC, leading to renal injury19. By immunohistochemistry staining, we found that PKC‐β was increased in the vehicle group and insulin group in both the glomerular and tubular regions as compared with the normal group (Figure 3a); however, rhGLP‐1 treatment reduced PKC‐β levels in the glomerular and tubular regions. These results were confirmed in isolated glomeruli (Figure 3b,c) and tubule tissue (Figure 3d) by real‐time PCR and western blotting analysis. Furthermore, the activity of PKC‐β was increased in the glomeruli and tubules in the vehicle group compared with the normal group, but the activity of PKC‐β was decreased in the rhGLP‐1 group compared with the vehicle or insulin group (Figure 3e).
Figure 3.
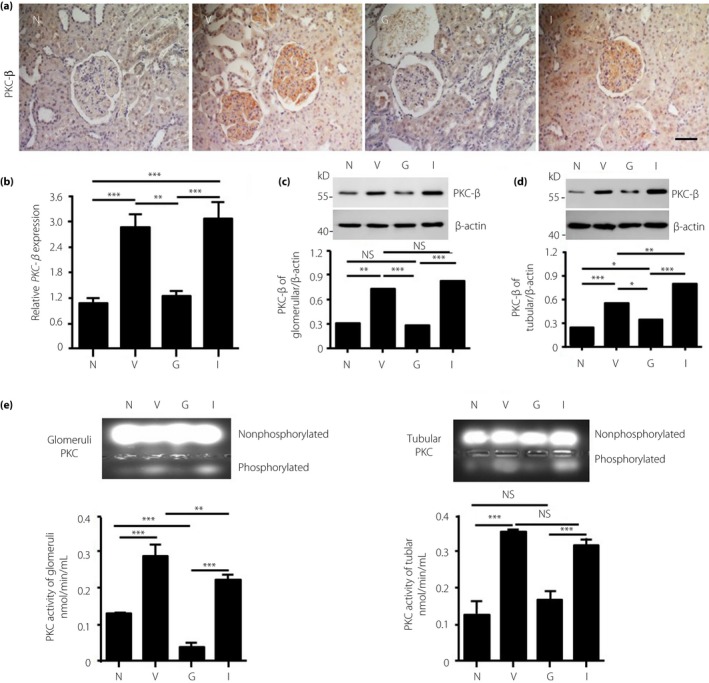
The effect of recombinant human glucagon‐like peptide‐1 treatment on the expression of protein kinase C (PKC) ‐β. (a–b) Eight weeks after treatment, animals were euthanized and the PKC‐β expression in kidneys was evaluated by immunohistochemistry and reverse transcription polymerase chain reaction. (c,d) The expression level of PKC‐β was also measured in isolated glomerular tissue and tubular tissue by western blotting (n = 8 each group, one‐way anova test). In addition, (e) PKC activity was evaluated by measuring the amount of phosphorylated PKC in isolated glomerular tissue (left) and tubular tissue (right). Data are presented as means ± standard error of the mean (*P < 0.05, **P < 0.01 and ***P < 0.001, NS, not significant, by one‐way anova test). G, recombinant human glucagon‐like peptide‐1 group; I, insulin group; N, normal group; V, vehicle group.
Activation of the GLP‐1 receptor can trigger the generation of the second messenger cAMP, followed by activation of PKA on endothelial cells25. Thus, we evaluated the PKA levels 8 weeks after rhGLP‐1 treatment. Compared with normal rats, the expression of PKA was markedly reduced in renal glomeruli and tubules of diabetic rats treated with either vehicle or insulin (Figure S4a); whereas rhGLP‐1 treatment upregulated PKA expression in the glomeruli (Figure S4b and c) and tubules (Figure S4d). In addition, PKA activity was decreased in the glomerular and tubular regions in vehicle‐treated rats compared with normal rats, and there is an increased activity of PKA in the rhGLP‐1 group, but not in insulin group (Figure S4e). Collectively, these results indicated that rhGLP‐1 regulates PKC and PKA in both glomeruli and tubules.
rhGLP‐1 Reduces Oxidative Stress in Glomeruli Through PKC and PKA
Oxidative stress is implicated in the pathogenesis of diabetic nephropathy. By immunohistochemical staining, we observed increased expression of iNOS and decreased SOD1 in the glomeruli of vehicle‐treated rats compared with normal rats (Figure 4a). Downregulated iNOS and upregulated SOD1 were observed in the rhGLP‐1 group, as compared with the vehicle group. Insulin did not influence the expression of iNOS and SOD1 significantly in glomeruli (Figure 4a). This finding was confirmed by reverse transcription PCR (Figure 4b) and western blotting (Figure 4c). Because the DAG–PKC pathway was suggested to be responsible for glomerular dysfunction in diabetic rats26, we also examined whether rhGLP‐1 could prevent an increase of DAG content in DN rats; it was possible to reduce the DAG content in the kidneys of diabetic rats after treatment with GLP‐1, but insulin has no effect on the decrease of the DAG content in DN rats (Figure 4d). We also quantified the collagen IV level in the kidney and found that the rhGLP‐1 can inhibit DN‐induced collagen IV upregulation (Figure 4e).
Figure 4.
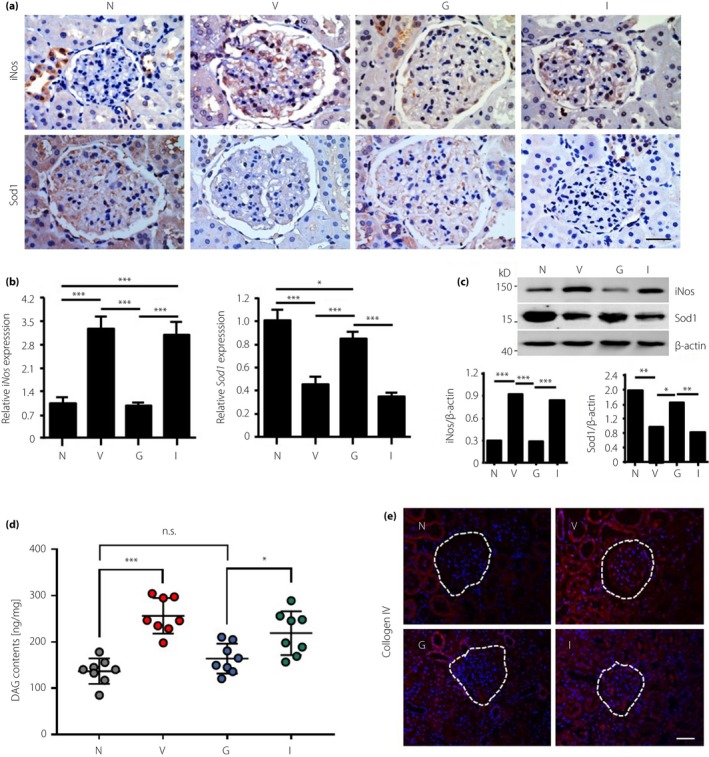
Recombinant human glucagon‐like peptide‐1 inhibits oxidative stress and diacylglycerol (DAG) in the glomeruli of diabetic nephropathy rats. (a) The expression of nitric oxide synthase (iNOS) and superoxide dismutase 1 (SOD1) was measured in kidneys by immunohistochemistry. Scale bar, 20 μm. In addition, the expression levels of iNOS and SOD1 were also measured in isolated glomeruli by (b) reverse transcription polymerase chain reaction and (c) western blotting. (d) DAG contents were measured by enzyme‐linked immunosorbent assay in the isolated glomerular tissue. (e) Immunofluorescence images of collagen IV in the diabetic nephropathy rat's kidney. G, recombinant human glucagon‐like peptide‐group; I, insulin group; N, normal group; V, vehicle group.
Microvascular endothelial cells are key components of the glomerular filtration barrier. Thus, we studied the possible roles of rhGLP‐1 in oxidative stress using cultured RGECs. RGECs were cultured with AGEs, perhaps to stipulate that elevated AGE is present in the diabetic milieu and is believed to contribute to DN development. By western blotting and real‐time PCR (Figure 5a,b), we found that iNOS was upregulated in RGECs by AGEs, and downregulated by rhGLP1, but SOD1 was the opposite to iNOS. Furthermore, the PKC activator (PMA) and PKA inhibitor (H‐89) weakened this effect of rhGLP‐1 (Figure 5a). These results showed that oxidative stress was reduced by rhGLP‐1, maybe by inhibiting PKC and activating PKA.
Figure 5.
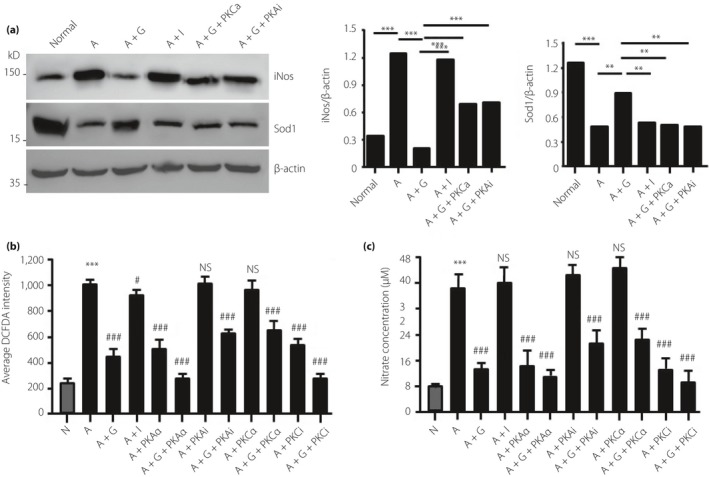
Recombinant human glucagon‐like peptide‐1 inhibits oxidative stress in rat glomerular endothelial cells (RGECs). (a) The effects of protein kinase C (PKC) and protein kinase A (PKA) on the expression of nitric oxide synthase (iNOS) were analyzed in by western blotting. RGEC was incubated with advanced glycation end‐products (200 μg/mL) to mimic diabetic injury, and the activator, inhibitor of PKC or PKA, was used to analyze the effect of the two pathways. **P < 0.01, ***P < 0.001. (b,c) Production of reactive oxygen species and nitric oxide was measured in cultured RGECs under different conditions. Data are presented as mean ± standard error of the mean (***P < 0.001 vs normal; #P < 0.05, ###P < 0.001 vs advanced glycation end‐products group; one‐way anova test; NS, not significant). A, advanced glycation end‐products; G, recombinant human glucagon‐like peptide‐1; I, insulin; iNOS, nitric oxide synthase; N, normal; PKAa, protein kinase A activator; PKCa, protein kinase C activator; PKAi, protein kinase A inhibitor; PKCi, protein kinase C inhibitor; SOD1, superoxide dismutase 1.
Next, we measured the effect of rhGLP‐1 on AGE‐induced ROS and NO production. The ROS (Figure 5b) and NO production (Figure 5c) in RGECs was significantly enhanced by AGEs; but inhibited by rhGLP1, PKA activator or PKC inhibitor. These results show that inhibition of PKC and activation of PKA are involved in the reduction in oxidative stress by rhGLP‐1.
rhGLP‐1 Enhances the Absorption of Albumin in HK‐2 Cells
In cultured HK‐2 cells, the expression of megalin and cubilin was evaluated at the protein level (Figure 6a). The results showed that megalin and cubilin were downregulated by AGEs, but restored by rhGLP‐1. The PKC inhibitor and PKA activator also increased megalin and cubilin expression, but PKC activator and PKA inhibitor can abolish the enhancement effect by rhGLP‐1 (Figure 6a). These results showed that rhGLP‐1 could inhibit PKC and activate PKA in tubules, and then increase the expression of megalin and cubilin.
Figure 6.
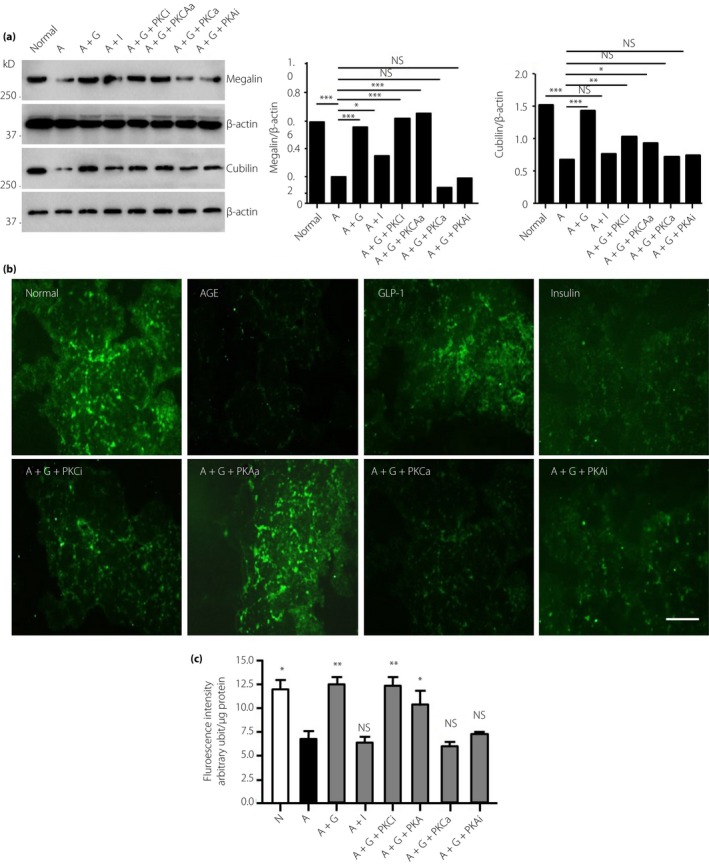
Recombinant human glucagon‐like peptide‐1 promotes the absorption of albumin in advanced glycation end‐product (AGE) ‐induced tubular epithelial cells through protein kinase C (PKC) ‐β and protein kinase A (PKA). (a) HK‐2 cells were cultured with AGEs to mimic diabetic nephropathy. The effects of PKC and PKA on the expression of megalin and cubilin were detected by western blotting (*P < 0.05, **P < 0.01, ***P < 0.001). (b) PKC and PKA regulate the absorption of fluorescein‐labeled albumin by HK‐2 cells observed under microscopy. (c) Quantitative fluorescence intensity was measured with a fluorescence plate reader and cell numbers were normalized to total protein. Data are presented as means ± standard error of the mean (*P < 0.05, **P < 0.01, ***P < 0.001; NS, not significant vs advanced glycation end‐products group; one‐way anova test). A, advanced glycation end‐products; G, recombinant human glucagon‐like peptide‐1; GLP‐1, glucagon‐like peptide‐1; I, insulin; N, normal; PKAa, protein kinase A activator; PKAi, protein kinase A inhibitor; PKCa, protein kinase C activator; PKCi, protein kinase C inhibitor.
Megalin and cubilin are two important receptors for re‐absorption. Next, we analyzed the effect of rhGLP‐1 on albumin absorption. Cultured HK‐2 cells can absorb fluorescein‐labeled human albumin from culture medium; and after incubation with AGEs, the HK‐2 cells showed a decreased intracellular fluorescence intensity compared with the normal group. In the rhGLP‐1 + AGEs group, the fluorescence intensity was stronger than in the AGEs group (Figure 6b,c), and the PKC inhibitor and PKA activator exerted an action similar to that of rhGLP‐1. The action of rhGLP‐1 on albumin absorption could be weakened by the PKC activator and PKA inhibitor (Figure 6c). This result showed that megalin and cubilin could be upregulated through PKC inhibition and PKA activation, which might contribute to the action of rhGLP‐1.
Discussion
DN is a long‐term complication of diabetes mellitus, and effective blockade of its progression remains a clinical challenge. Analysis of the Action to Control Cardiovascular Risk in Type 2 Diabetes study showed that intensive blood glucose control did not reduce composite advanced microvascular outcomes, including renal complications27.
Microalbuminuria is a major risk factor in the progressive decline observed in renal function in DN25, and microalbuminuria is thought to be the first step in an inevitable progression to proteinuria and renal failure. Thus, a reduction in albuminuria is a key marker of the efficacy of renoprotective therapy in type 2 diabetes28. The most profound effect of rhGLP‐1 therapy is the prevention of albuminuria in diabetic rats, without significant changes in blood glucose.
The lesions in the glomerular filtration barrier constitute one of the main causes of proteinuria. Toyoda et al.29 described severe disruption and detachment of podocyte foot processes in patients with type 1 diabetes that lead to denuded areas of GBM30. The occurrences of proteinuria were related to injury of the glomerular filtration barrier and tubular reabsorption of protein. In DN, one of the major contributors to proteinuria is attenuated function of tubular protein reabsorption31. In the proximal tubules, the reabsorption of albumin is primarily through megalin and cubilin32.
Oxidative stress mediated by PKC activation and PKA inhibition is a key factor in diabetic nephropathy. Some studies showed that PKC‐β‐null mice with STZ‐induced diabetes showed improvements in renal abnormalities, including albuminuria, renal hypertrophy and mesangial expansion33, 34, 35, 36. Here, we found that the PKC‐β level was statistically higher, and that PKA was lower in both renal glomeruli and in tubules in the DN group compared with the normal group.
GLP‐1 might protect against microvascular outcomes. Recently, Julia et al.37 reported that GLP‐1 (7‐37Mut8) can significantly reduce tubule interstitial renal damage, but it cannot improve glucose metabolism. GLP‐1 can also activate the AMPK and prevent the nicotinamide adenine dinucleotide phosphate oxidase activation to stimulate ROS production, and then it inhibits the PKC activation in mouse cardiomyocytes38. The conventional PKCs (α, βI, βII and γ) require Ca2+, DAG and a phospholipid, such as phosphatidylserine, for activation. We quantified the DAG content in the kidney, and we found it was highly expressed in the DN rats and it can be inhibited when treated with rhGLP‐1, which means rhGLP‐1 decreases PKC expression in the kidneys of DN rats through DAG. Phospholipase C is a class of membrane‐associated enzymes that cleave phospholipids, such as PIP2 (phosphatidylinositol bisphosphate), to IP3 and DAG. GLP‐1R is the GLP‐1 receptor, it belongs to G protein‐coupled receptor, so it might through the G protein‐mediated signaling pathway that phospholipase C is activated, then PIP2 can be digested to IP3 and DAG, and the high content of DAG can induce PKC activation in DN.
Disclosure
The authors declare no conflict of interest.
Supporting information
Figure S1 ¦ The glucagon‐like peptide‐1 receptor levels were increased in the glomeruli and tubules after treatment with recombinant human glucagon‐like peptide‐1.
Figure S2 ¦ Recombinant human glucagon‐like peptide‐1 protects the glomerular filtration barrier in diabetic rats.
Figure S3 ¦ Recombinant human glucagon‐like peptide‐1 regulates the expression of megalin and cubilin in the diabetic nephropathy kidney.
Figure S4 ¦ Enhance of protein kinase A expression when treated by recombinant human glucagon‐like peptide‐1 in the diabetic nephropathy rat kidney.
Acknowledgments
We thank the all the members of the Institute of Clinical Medical Sciences, China–Japan Friendship Hospital, and the Electron Microscopy Core at the Chinese Academy of Medical Sciences & Peking Union Medical College.This study was supported by the National Basic Research Program of China (2012CB966402) and National Natural Science Foundation of China (No. 81370873 and No. 81370918).
J Diabetes Investig 2019; 10: 613–625
References
- 1. Hsieh MC, Tien KJ, Perng DS, et al Diabetic nephropathy and risk factors for peripheral artery disease in Chinese with type 2 diabetes mellitus. Metabolism 2009; 58: 504–509. [DOI] [PubMed] [Google Scholar]
- 2. Stanton RC, King GL. A complex interplay of factors causes diabetic nephropathy. Metabolism 2011; 60: 591–593. [DOI] [PubMed] [Google Scholar]
- 3. Bhattacharjee N, Barma S, Konwar N, et al Mechanistic insight of diabetic nephropathy and its pharmacotherapeutic targets: an update. Eur J Pharmacol 2016; 791: 8–24. [DOI] [PubMed] [Google Scholar]
- 4. Pofi R, Di Mario F, Gigante A, et al Diabetic nephropathy: focus on current and future therapeutic strategies. Curr Drug Metab 2016; 17: 497–502. [DOI] [PubMed] [Google Scholar]
- 5. Lee YS, Jun HS. Anti‐diabetic actions of glucagon‐like peptide‐1 on pancreatic beta‐cells. Metabolism 2014; 63: 9–19. [DOI] [PubMed] [Google Scholar]
- 6. Kodera R, Shikata K, Kataoka HU, et al Glucagon‐like peptide‐1 receptor agonist ameliorates renal injury through its anti‐inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia 2011; 54: 965–978. [DOI] [PubMed] [Google Scholar]
- 7. Park CW, Kim HW, Ko SH, et al Long‐term treatment of glucagon‐like peptide 1 analog exendin‐4 ameliorates diabetic nephropathy through improving metabolic anomalies in db/db mice. J Am Soc Nephrol 2007; 18: 1227–1238. [DOI] [PubMed] [Google Scholar]
- 8. Ishibashi Y, Nishino Y, Matsui T, et al Glucagon‐like peptide‐1 suppresses advanced glycation end product‐induced monocyte chemoattractant protein‐1 expression in mesangial cells by reducing advanced glysacation end product receptor level. Metabolism 2011; 60: 1271–1277. [DOI] [PubMed] [Google Scholar]
- 9. Hendarto H, Inoguchi T, Maeda Y, et al GLP‐1 analog liraglutide protects against oxidative stress and albuminuria in streptozotocin‐induced diabetic rats via protein kinase A‐mediated inhibition of renal NAD(P)H oxidases. Metabolism 2012; 61: 1422–1434. [DOI] [PubMed] [Google Scholar]
- 10. Einbinder Y, Ohana M, Benchetrit S, et al Glucagon‐like‐peptide‐1 and vitamin D: anti‐inflammatory response in diabetic kidney disease in db/db mice and in cultured endothelial cells. Diabetes Metab Res Rev 2016; 32: 805–815. [DOI] [PubMed] [Google Scholar]
- 11. Bethel MA, Patel RA, Merrill P, et al Cardiovascular outcomes with glucagon‐like peptide‐1 receptor agonists in patients with type 2 diabetes: a meta‐analysis. Lancet Diabetes Endocrinol 2018; 6: 105–113. [DOI] [PubMed] [Google Scholar]
- 12. Sancar‐Bas S, Gezginci‐Oktayoglu S, Bolkent S. Exendin‐4 attenuates renal tubular injury by decreasing oxidative stress and inflammation in streptozotocin‐induced diabetic mice. Growth Factors 2015; 33: 419–429. [DOI] [PubMed] [Google Scholar]
- 13. Ke JT, Li M, Xu SQ, et al Gliquidone decreases urinary protein by promoting tubular reabsorption in diabetic Goto‐Kakizaki rats. J Endocrinol 2014; 220: 129–141. [DOI] [PubMed] [Google Scholar]
- 14. Taneda S, Pippin JW, Sage EH, et al Amelioration of diabetic nephropathy in SPARC‐null mice. J Am Soc Nephrol 2003; 14: 968–980. [DOI] [PubMed] [Google Scholar]
- 15. Zhang Z, Sun L, Wang Y, et al Renoprotective role of the vitamin D receptor in diabetic nephropathy. Kidney Int 2008a; 73: 163–171. [DOI] [PubMed] [Google Scholar]
- 16. Wang H, Jiang YW, Zhang WJ, et al Differential activations of PKC/PKA related to microvasculopathy in diabetic GK rats. Am J Physiol Endocrinol Metab 2012; 302: 173–182. [DOI] [PubMed] [Google Scholar]
- 17. Isshiki K, Haneda M, Koya D, et al Thiazolidinedione compounds ameliorate glomerular dysfunction independent of their insulin‐sensitizing action in diabetic rats. Diabetes 2000; 49: 1022–1032. [DOI] [PubMed] [Google Scholar]
- 18. Das AK, Pickett TM, Tungekar MF. Glomerular basement membrane thickness‐ a comparison of two methods of measurement in patients with unexplained haematuria. Nephrol Dial Transplant 1996; 11: 1256–1260. [PubMed] [Google Scholar]
- 19. Sukumaran SK, Prasadarao NV. Regulation of protein kinase C in Escherichia coli K1 invasion of human brain microvascular endothelial cells. J Biol Chem 2002; 277: 12253–12262. [DOI] [PubMed] [Google Scholar]
- 20. Golpon HA, Puechner A, Welte T, et al Vasorelaxant effect of glucagon‐like peptide‐(7‐36) amide and amylin on the pulmonary circulation of the rat. Regul Pept 2001; 102: 81–86. [DOI] [PubMed] [Google Scholar]
- 21. Aronis KN, Chamberland JP, Mantzoros CS. GLP‐1 promotes angiogenesis in human endothelial cells in a dose‐dependent manner, through the Akt. Src and PKC pathways. Metabolism 2013; 62: 1279–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ban K, Noyan‐Ashraf MH, Hoefer J, et al Cardioprotective and vasodilatory actions of glucagon‐like peptide 1 receptor are mediated through both glucagon‐like peptide 1 receptor‐dependent and ‐independent pathways. Circulation 2008; 117: 2340–2350. [DOI] [PubMed] [Google Scholar]
- 23. Bullock BP, Heller RS, Habener JF. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon‐like peptide‐1 receptor. Endocrinology 1996; 137: 2968–2978. [DOI] [PubMed] [Google Scholar]
- 24. Drucker DJ. The biology of incretin hormones. Cell Metab 2006; 3: 153–165. [DOI] [PubMed] [Google Scholar]
- 25. Kawachi H, Miyauchi N, Suzuki K, et al Role of podocyte slit diaphragm as a filtration barrier. Nephrology 2006; 11: 274–281. [DOI] [PubMed] [Google Scholar]
- 26. Koya D, King GL. Protein kinase C activation and the development of diabetic complications. Diabetes 1998; 47: 859–866. [DOI] [PubMed] [Google Scholar]
- 27. Nakamura T, Ushiyama C, Osada S, et al Pioglitazone reduces urinary podocyte excretion in type 2 diabetes patients with microalbuminuria. Metabolism 2001; 50: 1193–1196. [DOI] [PubMed] [Google Scholar]
- 28. Ismail‐Beigi F, Craven T, Banerji MA, et al Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010; 376: 419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Toyoda M, Najafian B, Kim Y, et al Podocyte detachment and reduced glomerular capillary endothelial fenestration in human type 1 diabetic nephropathy. Diabetes 2007; 56: 2155–2160. [DOI] [PubMed] [Google Scholar]
- 30. Kriz W, Gretz N, Lemley KV. Progression of glomerular diseases: is the podocyte the culprit? Kidney Int 1998; 54: 687–697. [DOI] [PubMed] [Google Scholar]
- 31. Russo LM, Sandoval RM, Campos SB, et al Impaired tubular uptake explains albuminuria in early diabetic nephropathy. J Am Soc Nephrol 2009; 20: 489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nielsen R, Christensen EI, Birn H. Megalin and cubilin in proximal tubule protein reabsorption: from experimental models to human disease. Kidney Int 2016; 89: 58–67. [DOI] [PubMed] [Google Scholar]
- 33. Ohshiro Y, Ma RC, Yasuda Y, et al Reduction of diabetes‐induced oxidative stress, fibrotic cytokine expression, and renal dysfunction in protein kinase C beta‐null mice. Diabetes 2006; 55: 3112–3120. [DOI] [PubMed] [Google Scholar]
- 34. Chung SS, Ho EC, Lam KS, et al Contribution of polyol pathway to diabetes‐induced oxidative stress. J Am Soc Nephrol 2003; 14: S233–S236. [DOI] [PubMed] [Google Scholar]
- 35. Lee HB, Yu MR, Yang Y, et al Reactive oxygen species‐regulated signaling pathways in diabetic nephropathy. J Am Soc Nephrol 2003; 14: S241–S245. [DOI] [PubMed] [Google Scholar]
- 36. Forbes JM, Cooper ME, Oldfield MD, et al Role of advanced glycation end products in diabetic nephropathy. J Am Soc Nephrol 2003; 14: S254–S258. [DOI] [PubMed] [Google Scholar]
- 37. Moellmann J, Klinkhammer BM, Onstein J, et al Glucagon‐like peptide‐1 and its cleavage products are renoprotective in murine diabetic nephropathy. Diabetes 2018; 67: 2410–2419. [DOI] [PubMed] [Google Scholar]
- 38. Balteau M, Van Steenbergen A, Timmermans AD, et al AMPK activation by glucagon‐like peptide‐1 prevents NADPH oxidase activation induced by hyperglycemia in adult cardiomyocytes. Am J Physiol Heart Circ Physiol 2014; 307: H1120–H1133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 ¦ The glucagon‐like peptide‐1 receptor levels were increased in the glomeruli and tubules after treatment with recombinant human glucagon‐like peptide‐1.
Figure S2 ¦ Recombinant human glucagon‐like peptide‐1 protects the glomerular filtration barrier in diabetic rats.
Figure S3 ¦ Recombinant human glucagon‐like peptide‐1 regulates the expression of megalin and cubilin in the diabetic nephropathy kidney.
Figure S4 ¦ Enhance of protein kinase A expression when treated by recombinant human glucagon‐like peptide‐1 in the diabetic nephropathy rat kidney.


