Abstract
Aims/Introduction
The objective of the present study was to elucidate the effect of switching to teneligliptin from other dipeptidyl peptidase‐4 (DPP‐4) inhibitors on glucose control and renoprotection in type 2 diabetes mellitus patients with diabetic kidney disease.
Materials and Methods
The present study was a single‐arm, open‐label, observational study. A total of 23 patients, who had urinary albumin/creatinine ratios (UACR) ≥30 mg/gCr in their first urine in the early morning, and received other DPP‐4 inhibitors and renin‐angiotensin system inhibitors, switched to teneligliptin 20 mg/day. After switching to teneligliptin for 24 weeks, we evaluated changes in glycated hemoglobin (HbA1c), fasting plasma glucose levels, plasma DPP‐4 activity and UACR.
Results
HbA1c, fasting plasma glucose and UACR values showed no significant change after 24 weeks compared with baseline. However, plasma DPP‐4 activity was significantly reduced after 24 weeks (0.57 ± 0.26 nmol/min/mL, P = 0.012, vs baseline), compared with baseline (1.49 ± 1.73 nmol/min/mL), and there was a positive relationship between the change rate of plasma DPP‐4 activity (Δ%DPP‐4) for 24 weeks and the levels of plasma DPP‐4 activity (r = −0.5997, P = 0.0025) and fasting plasma glucose (r = −0.4235, P = 0.0440) at baseline. Additionally, the Δ%DPP‐4 for 24 weeks was significantly correlated to the change rate of UACR (r = 0.556, P = 0.0059). However, there was no relationship between Δ%DPP‐4 and ΔHbA1c (amount of HbA1c change).
Conclusions
Switching to teneligliptin from other DPP‐4 inhibitors for 24 weeks reduces plasma DPP‐4 activity, which is associated with a reduction in albuminuria, independent of the change in glucose levels, in type 2 diabetes mellitus patients with diabetic kidney disease.
Keywords: Diabetic kidney disease, Plasma DPP‐4 activity, Teneligliptin
Introduction
Diabetic kidney disease (DKD), which is a diabetic vascular complication, is recognized as a major leading cause of end‐stage renal disease. Glucose control is fundamentally important for the prevention of DKD, as well as the control of blood pressure (BP) using renin–angiotensin system (RAS) inhibitors1. However, hypoglycemia should be avoided, because hypoglycemia is closely related to increased mortality, which is associated with an increased incidence of cardiovascular disease2, 3. Treatment with dipeptidyl peptidase‐4 (DPP‐4) inhibitors, which are incretin‐related antidiabetic agents, is widely accepted in clinical practice because of their low risk of hypoglycemia and their beneficial effect on glucose control4. In addition to their glucose‐lowering effect, previous data from clinical studies showed that DPP‐4 inhibitors have renoprotective effects, which are mainly a reduction in albuminuria, independent of the glucose‐lowering effect5, 6, 7, 8, 9, 10, 11, 12. Multiple DPP‐4 inhibitors are clinically available, and each DPP‐4 inhibitor has different features, such as chemical structure, inhibitory activity towards DPP‐4 and distribution in tissues, including the kidney. However, there are few reports regarding the difference in the renoprotective effect among DPP‐4 inhibitors. Teneligliptin has strong and long DPP‐4 inhibitory effects, and no dose adjustment may be required, even if the patient has renal function decline13, 14, because 34% of the administered dose of teneligliptin is excreted unchanged through the renal route, whereas 66% is metabolized and eliminated through the hepatic and renal routes13. In addition, the distribution of teneligliptin to the kidney is high because of its lipophilicity, possibly showing a renoprotective effect. However, there are no sufficient clinical data regarding the renoprotective effect of teneligliptin in type 2 diabetes mellitus patients with DKD. Therefore, the aim of the present study was to investigate the effects of teneligliptin on glycemic control and albuminuria compared with the effects of other DPP‐4 inhibitors, in particular, by focusing on the relationship with the change of plasma DPP‐4 activity in type 2 diabetes mellitus patients with DKD.
Methods
Participants
A total of 40 participants with type 2 diabetes mellitus (24 men and 16 women) were selected for the present study from outpatients who visited the Department of Endocrinology and Metabolism at Kanazawa Medical University Hospital. The entry criteria included the following: (i) age ≥20 years; (ii) type 2 diabetes mellitus with a glycated hemoglobin (HbA1c) ≥6.0%; (iii) urinary albumin/creatinine (Cr) ratio (UACR) ≥30 mg/gCr in spot urine for screening of DKD; (iv) treatment with diet, exercise therapy and DPP‐4 inhibitor, excluding teneligliptin; and (v) treatment with RAS inhibitors. The exclusion criteria were as follows: (i) type 1 diabetes; (ii) severe diabetic metabolic complications, such as ketoacidosis; (iii) severe liver dysfunction; (iv) pregnant or breast‐feeding women and those who might be pregnant; and (v) any patient whom the investigator judged to be inappropriate for this study. Patients were given detailed explanations of the study protocol, and informed consent was obtained from each patient. The study protocol was approved by the ethics committee of Kanazawa Medical University. This trial was registered with the University Hospital Medical Information Network (UMIN000015922).
Study protocol
The present study is a single‐arm, open‐label, observational study. At the start of the study, patients were switched from other DPP‐4 inhibitors to teneligliptin 20 mg/day. Participants were assessed for several parameters at baseline and 24‐weeks after switching to teneligliptin. No changes were made to the type and dose of the glucose‐lowering agents if the participants showed hypoglycemia. RAS inhibitors, including angiotensin‐converting enzyme inhibitors or/and angiotensin II receptor blockers, were also not changed during the study period. These agents were prescribed for at least 3 months before the study.
After carrying out a screening of UACR ≥30 mg/gCr in spot urine, DKD was finally diagnosed by a UACR ≥30 mg/gCr in the first urine in the early morning. We evaluated data including the change in HbA1c, plasma DPP‐4 activity, UACR, BP and body mass index (BMI) during the treatment with teneligliptin.
Measurements
Blood samples were collected in the morning after an overnight fast. The first urine in the early morning sample was collected at the home of participants, and the urine was carried in a cooler box to the hospital. Levels of HbA1c, plasma fasting glucose, serum total cholesterol, high‐density lipoprotein‐cholesterol and triglyceride, serum and urinary Cr, urinary albumin, serum cystatin C, urinary liver‐type fatty acid binding protein, serum aspartate transaminase, and alanine aminotransferase were measured, and the estimated glomerular filtration rate was calculated, as previously described11. Plasma DPP‐4 activity was measured by an enzymatic method, using the substrate H‐glycyl‐L‐proline‐amino‐4‐methyl coumarin hydrogen bromide (BACHEM, Inc., Bubendorf, Switzerland) and a standard amino‐4‐methyl coumarin (Sigma‐Aldrich Corp., St. Louis, MO, USA) by LSI Medience Co. (Tokyo, Japan).
Statistical analysis
Statistical analyses were carried out using SAS 9.4 (SAS Institute, Cary, North Carolina, USA) by KUREHA SPECIAL LABORATORY CO., LTD. (Tokyo, Japan). All values are summarized as the mean and standard deviation. Data at baseline and 24‐weeks after switching to teneligliptin were assessed by a paired t‐test or Mann–Whitney U‐test. The correlation of two variables was analyzed by a single linear regression analysis as a Pearson correlation coefficient. Statistical significance was defined as P < 0.05.
Results
Characteristics of the participants are shown in Table 1. Initially, 40 participants who showed albuminuria of >30 mg/gCr in spot urine were enrolled. However, 11 participants were excluded, because their albuminuria was <30 mg/gCr in the early morning first urine. Additionally, six participants were excluded. Two participants were excluded before starting treatment with teneligliptin due to the participants’ wishes, and one participant was also excluded because of a UACR <30 mg/gCr in the spot urine, which was a mistake during the screening before starting this study. After treatment with teneligliptin, one participant declined to take teneligliptin due to an epileptic seizure, but this was not associated with teneligliptin, and one participant was excluded by doctor's judgement due to poor adherence to the treatment. Furthermore, because an antihypertensive agent was added during the study period, one participant was excluded. Therefore, we evaluated 23 participants for analysis in the present study. Baseline clinical characteristics, as well as concomitant background therapies, are shown in Table 1. All participants received treatment with another DPP‐4 inhibitor, including linagliptin (5 mg/day, n = 7), sitagliptin (100 mg/day, n = 2; 50 mg/day, n = 3; 25 mg/day, n = 1), vildagliptin (100 mg/day, n = 5), anagliptin (200 mg/day, n = 3) or alogliptin (25 mg/day, n = 1; 12.5 mg/day, n = 1), in addition to other antidiabetic agents at baseline. Seven participants received insulin therapy. Additionally, all participants received RAS inhibitors including angiotensin‐converting enzyme inhibitors or/and angiotensin II receptor blockers, and three participants received spironolactone.
Table 1.
Characteristics of participants
| n | 23 | Antihypertensive agents (n) | 23 |
|---|---|---|---|
| Male:female | 13:10 | RAS inhibitors (n) | 23 |
| Age (years) | 68.7 ± 7.1 | ARBs (n) | 21 |
| BMI (kg/m2) | 27.3 ± 4.4 | ACEIs (n) | 5 |
| Duration of diabetes (years) | 20.0 ± 8.1 | ARBs + ACEIs (n) | 3 |
| Spironolactone (n) | 3 | ||
| Antidiabetic agents (n) | 23 | Ca‐Blockers (n) | 10 |
| Sulfonylurea (n) | 10 | β‐Blockers (n) | 1 |
| Glinide (n) | 1 | α‐Blockers (n) | 1 |
| Metformin (n) | 20 | Diuretics (n) | 5 |
| α‐Glucosidase inhibitor (n) | 2 | ||
| Insulin (n) | 7 | Antidyslipidemia agents (n) | 17 |
| Statins (n) | 14 | ||
| DPP‐4 inhibitors (n) | 23 | Fibrates (n) | 1 |
| Linagliptin (n) | 7 | Ezetimibe (n) | 2 |
| Sitagliptin (n) | 6 | ||
| Vildagliptin (n) | 5 | Anti‐uric acid agents (n) | 4 |
| Anagliptin (n) | 3 | Allopurinol | 2 |
| Alogliptin (n) | 2 | Febuxostat | 2 |
ACEIs, angiotensin‐converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; BMI, body mass index; DPP‐4, dipeptidyl peptidase‐4; RAS, renin–angiotensin system.
The levels of HbA1c, FPG and other data, including UACR, renal and liver function tests, lipid data, BMI, and BP, were not significantly different after switching to teneligliptin from other DPP‐4 inhibitors (Table 2). However, plasma DPP‐4 activity tended to decrease from baseline (1.49 ± 1.73 nmol/min/mL) at 8 weeks after switching to teneligliptin (0.78 ± 0.44 nmol/min/mL, P = 0.139, vs baseline); and after 24 weeks, it was significantly decreased (0.57 ± 0.26 nmol/min/mL, P = 0.012, vs baseline; Table 2). The change rate of plasma DPP‐4 activity (Δ%DPP‐4) for 24 weeks was positively correlated with both the plasma DPP‐4 activity (r = −0.5997, P = 0.0025) and FPG levels (r = −0.4235, P = 0.0440) at baseline, which was evaluated by a Pearson's correlation coefficient analysis (Table 3). However, there was no correlation between plasma DPP‐4 activity at baseline and the levels of FPG at baseline (r = 0.2957, P = 0.1706). The levels of HbA1c, BMI and UACR at baseline also showed no correlation to Δ%DPP‐4 (Table 3). The relationship between Δ%DPP‐4 and the change rate of UACR (Δ%UACR) from the Pearson's correlation coefficient analysis showed a positive correlation (r = 0.556, P = 0.0059; Table 3; Figure 1a). The change amount of HbA1c (r = 0.2024, P = 0.3543), FPG (r = 0.3960, P = 0.0614), UACR (r = 0.2997, P = 0.1647), BMI (r = 0.2943, P = 0.1729), systolic BP (r = 0.1940, P = 0.3750) and diastolic BP (r = 0.0439, P = 0.1729) was not correlated to Δ%DPP‐4 at 24 weeks after switching to teneligliptin from other DPP‐4 inhibitors (Table 3). There was also no significant correlation between the Δ%UACR and both plasma DPP‐4 activity (r = 0.2862, P = 0.1855) and FPG (r = 0.2760, P = 0.2024) at baseline. Furthermore, there was no significant relationship between Δ%DPP‐4 and ΔHbA1c (r = 0.2024, P = 0.3543; Figure 1b), and between the amount of HbA1c change (ΔHbA1c) and Δ%UACR (r = 0.1909, P = 0.3830) at 24 weeks (Figure 1c). Thus, the Δ%DPP‐4 after treatment with teneligliptin contributes to the Δ%UACR, which is independent of the change in glucose control, BMI or BP at 24 weeks, and plasma DPP‐4 activity and FPG at baseline.
Table 2.
Changes in laboratory data
| Baseline (week 0) | Week 24 | P‐value | |
|---|---|---|---|
| HbA1c (%) | 7.82 ± 0.7 | 7.77 ± 0.96 | 0.765* |
| FPG (mg/dL) | 164.7 ± 71.6 | 169.3 ± 71.6 | 0.746** |
| Plasma DPP‐4 activity (nmol/min/mL) | 1.49 ± 1.73 | 0.57 ± 0.26 | 0.010** |
| UACR (mg/gCr) | 309.1 ± 407.1 | 329.2 ± 432.7 | 0.616** |
| Log‐transformed UACR | 2.23 ± 0.48 | 2.17 ± 0.63 | 0.419* |
| Cr (mg/dL) | 1.0 ± 0.2 | 1.02 ± 0.23 | 0.153* |
| eGFRcrea (mL/min/1.73 m2) | 54.1 ± 11.5 | 52.0 ± 12.4 | 0.250** |
| Cystatin C (mg/L) | 1.17 ± 0.23 | 1.21 ± 0.25 | 0.263* |
| eGFRcys (mL/min/1.73 m2) | 60.4 ± 17.5 | 58.5 ± 17.6 | 0.496** |
| Urinary L‐FABP/Cr (μg/gCr) | 11.4 ± 15.2 | 15.4 ± 22.0 | 0.468** |
| Log‐transformed urinary L‐FABP | 0.80 ± 0.44 | 0.81 ± 0.60 | 0.921* |
| Uric acid (mg/dL) | 5.6 ± 0.9 | 5.7 ± 1.1 | 0.638** |
| AST (IU/L) | 22.0 ± 6.8 | 22.3 ± 6.3 | 0.933** |
| ALT (IU/L) | 24.9 ± 14.2 | 24.3 ± 10.5 | 0.836** |
| T‐Cho (mg/dL) | 175.3 ± 30.5 | 173.3 ± 32.6 | 0.903* |
| TG (mg/dL) | 131.7 ± 55.9 | 150.9 ± 94.4 | 0.213** |
| HDL‐C (mg/dL) | 50.6 ± 13.1 | 47.7 ± 10.8 | 0.265** |
| BMI (kg/m2) | 27.4 ± 4.5 | 27.2 ± 4.3 | 0.626** |
| Systolic BP (mmHg) | 135.2 ± 12.7 | 134.6 ± 13.3 | 0.657* |
| Diastolic BP (mmHg) | 72.9 ± 8.5 | 73.9 ± 7.5 | 0.637* |
Data are mean ± standard deviation. *P‐value, paired t‐test; **P‐value, Mann–Whitney U‐test. ALT, alanine aminotransferase; AST, aspartate transaminase; BMI, body mass index; BP, blood pressure; Cr, creatinine; DPP‐4, dipeptidyl peptidase‐4; eGFR, estimated glomerular filtration rate; eGFRcrea, serum creatinine‐based estimated glomerular filtration rate; eGFRcys, serum cystatin C‐based estimated glomerular filtration rate; FPG, fasting plasma glucose; HDL‐C, high density lipoprotein‐cholesterol; L‐FABP, liver‐type fatty acid binding protein; T‐Cho, total cholesterol; TG, triglyceride; UACR, urinary albumin/creatinine ratio.
Table 3.
Relationship between changes in plasma dipeptidyl peptidase‐4 activity and various characteristics
| n | r | P‐value | ||
|---|---|---|---|---|
| Δ%DPP‐4 | Week 0 | |||
| HbA1c (%) | 23 | −0.0760 | 0.7303 | |
| FPG (mg/dL) | 23 | −0.4235 | 0.0440 | |
| Plasma DPP‐4 activity (nmol/min/mL) | 23 | −0.5997 | 0.0025 | |
| UACR (mg/gCr) | 23 | −0.3038 | 0.1588 | |
| BMI (kg/m2) | 23 | −0.3296 | 0.1342 | |
| Changes between week 24 and baseline | ||||
| ΔHbA1c (%) | 23 | 0.2024 | 0.3543 | |
| ΔFPG (mg/dL) | 23 | 0.3960 | 0.0614 | |
| Δ%UACR | 23 | 0.5560 | 0.0059 | |
| ΔBMI (kg/m2) | 23 | 0.2943 | 0.1729 | |
| ΔSystolic BP (mmHg) | 23 | 0.1940 | 0.3750 | |
| ΔDiastolic BP (mmHg) | 23 | 0.0439 | 0.1729 | |
Pearson's correlation coefficient. Δ%DPP‐4, change rate in level of plasma dipeptidyl peptidase‐4 activity at 24‐weeks after switching to teneligliptin; BMI, body mass index; BP, blood pressure; DPP‐4, dipeptidyl peptidase‐4; FPG, fasting plasma glucose; UACR, urinary albumin/creatinine ratio.
Figure 1.
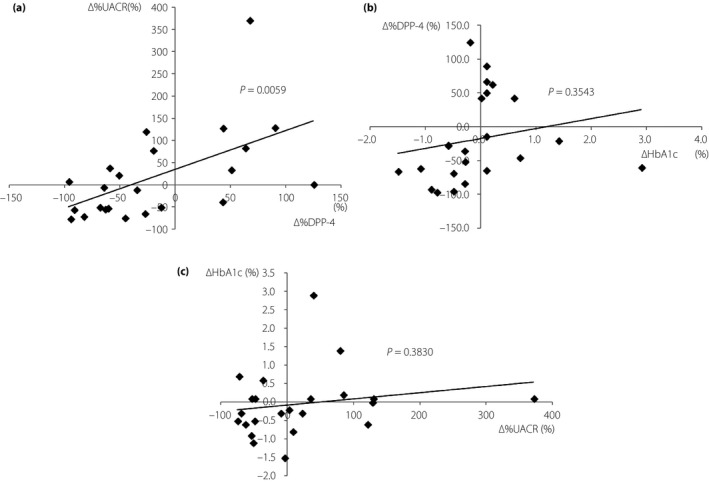
(a) The relationship between the change rate in urinary albumin/creatinine ratio (Δ%UACR) and plasma dipeptidyl peptidase‐4 activity (Δ%DPP‐4) at 24 weeks after switching to teneligliptin from other DPP‐4 inhibitors showed a positive correlation. (b) The relationship between Δ%DPP‐4 and the amount of glycated hemoglobin change (ΔHbA1c) at 24 weeks did not show a significant correlation. (c) The relationship between ΔHbA1c and Δ%UACR at 24 weeks did not show a significant correlation. Pearson's correlation coefficient analysis.
Discussion
The present study showed that the levels of HbA1c, FPG and other data, including UACR and lipids, showed no significant change by switching to teneligliptin from other DPP‐4 inhibitors for 24 weeks. However, the switch to teneligliptin for 24 weeks showed a significant decrease in plasma DPP‐4 activity. The decrease in plasma DPP‐4 activity by switching to teneligliptin did not contribute to an improvement in glucose control; however, we found a positive correlation between the change rate of plasma DPP‐4 activity and the change rate of UACR.
In a diabetic state, DPP‐4 activity might be enhanced15, and it is related to the pathogenesis of DKD16, 17. Previous clinical studies showed that DPP‐4 inhibitors have renoprotective effects, which are mainly a reduction in albuminuria, independent of the glucose‐lowering effect5, 6, 7, 8, 9, 10, 11, 12. In addition, Shah et al.18 reported, in a retrospective analysis, that teneligliptin (20mg/day) for 24 weeks significantly improved glycemic control, proteinuria evaluated by urine dipstick analysis and estimated glomerular filtration rate in type 2 diabetes mellitus patients. What are the molecular mechanisms of the renoprotective effect of DPP‐4 inhibitors in the diabetic kidney? Previous reports in animal studies showed that DPP‐4 inhibitors exerted their renoprotective effect through anti‐inflammation19, 20, 21, anti‐oxidative stress22, 23, 24 and anti‐fibrosis21, 23, 24, 25 activities, through glucose‐dependent or glucagon like peptide‐1 (GLP‐1) pathways via increased substrates for DPP‐4, such as GLP‐1 or stromal cell‐derived factor‐1α or substrates including a GLP‐1‐independent pathway. Additionally, teneligliptin can directly scavenge reactive oxygen species because of its structural features26. However, in the present study, the amount of albuminuria showed no significant change by switching to teneligliptin in the patients treated with RAS inhibitors, and we did not evaluate changes in the substrates for DPP‐4, including GLP‐1 and stromal cell‐derived factor‐1α, inflammation, and oxidative stress.
Multiple DPP‐4 inhibitors are available in the current clinical setting. Each DPP‐4 inhibitor has differences in chemical structure, the binding mode to DPP‐4 and their physical properties, including lipophilicity; therefore, DPP‐4 inhibitors might show differences in DPP‐4 inhibitory action on glucose control and renoprotection. However, the differences in the renoprotective effect for DKD among DPP‐4 inhibitors are unclear. In the present study, our data clearly showed that teneligliptin might have stronger inhibitory action against DPP‐4 than other DPP‐4 inhibitors, because the plasma DPP‐4 activity was significantly decreased after switching to teneligliptin. Teneligliptin has a unique structure, and binds to the S1, S2 and S2 extensive subsite of the DPP‐4 enzyme, leading to enhanced potency and selectivity, and it is also a class 3 DPP‐4 inhibitor27. Additionally, binding of teneligliptin to the S2 extensive site, apart from the S1 and S2 sites, imparts stronger inhibitory action on the DPP‐4 enzyme28. Furthermore, teneligliptin was reported to have the J‐shaped anchor‐lock domain, strong covalent bonds with DPP‐4 and more extensive S2 extensive binding, showing its higher inhibitory activity13. Plasma DPP‐4 activity mainly measures a soluble type of DPP‐4 activity; therefore, teneligliptin showed stronger inhibition of a soluble type of DPP‐4 compared with other DPP‐4 inhibitors, in the present study. However, glucose control, which was evaluated by HbA1c and FPG levels, was not changed after switching to teneligliptin, although the plasma DPP‐4 activity was reduced by teneligliptin. DPP‐4 inhibitors show a glucose‐lowering effect through increased levels of incretins, including GLP‐1, and their glucose‐lowering effect is dependent on pancreatic β‐cell function. All DPP‐4 inhibitors fundamentally have enough activity for a glucose‐lowering effect, and they show a similar effect on the reduction of HbA1c levels. Therefore, in the present study, although we could not assess changes in the levels of incretins, switching to teneligliptin from other DPP‐4 inhibitors did not lead to a greater glucose‐lowering effect. However, interestingly, we found that the change rate of plasma DPP‐4 activity is positively correlated to the change rate of UACR at 24 weeks. DPP‐4 exists in endothelial cells, tubular cells, mesangial cells and podocytes in the kidney, as a membrane‐bound type29, as well as in the blood as a soluble type. Additional studies are required to investigate the pharmacological differences between soluble and membrane‐bound DPP‐4 and to compare DPP‐4 inhibitors regarding their ability to inhibit different forms of DPP‐4. Teneligliptin has a higher lipophilicity and greater distribution to the kidney than the other DPP‐4 inhibitors, sitagliptin, alogliptin, saxagliptin, anagliptin and vildagliptin30. In addition, previously, De Nigris et al.31 showed that teneligliptin reduces DPP‐4 messenger ribonucleic acid and protein levels under high‐glucose conditions in cultured human vascular endothelial cells. They also showed that teneligliptin indirectly inhibits the shedding of DPP‐4 by downregulating metalloproteinase 1 (MMP1), MMP2 and MMP14 gene expression through tumor necrosis factor‐α under hyperglycemic conditions. A decrease in these metalloproteinases reduces the release of the soluble form of DPP‐4 by human umbilical vein endothelial cells. Thus, teneligliptin might contribute to the renoprotection that was evaluated by the reduction of albuminuria in type 2 diabetes mellitus patients, associating with the suppression of both membrane bound in endothelial cells and release of soluble DPP‐4 from endothelial cells. Linagliptin also has a high lipophilicity and distribution to the kidney30, 32, and a previous animal study showed the renoprotection through possibly suppressing membrane‐bound DPP‐4 in renal endothelial cells25. Additionally, several previous clinical studies have shown the renoprotective effects of linagliptin in type 2 diabetes mellitus patients7, 33. In the present study, a reduction of plasma DPP‐4 activity and a positive relationship between Δ%DPP‐4 and Δ%UACR in the patients who were switched to teneligliptin from linagliptin was not observed (Table S1 and Figure S1); however, the patients who were switched to teneligliptin from other DPP‐4 inhibitors, including sitagliptin, vildagliptin, alogliptin and anagliptin, showed a significant reduction of plasma DPP‐4 activity, and a positive relationship between Δ%DPP‐4 and Δ%UACR (Table S1 and Figure S1). In addition, DPP‐4 inhibitors have the difference of excretion route, such as the reabsorption rate of excreted DPP‐4 inhibitor from glomeruli in tubular cells, and the hepatic or biliary route. Both teneligliptin and linagliptin have a high lipophilicity and distribution to the kidney, and teneligliptin is excreted through both the renal and hepatic route, whereas linagliptin is excreted through the biliary route. Thus, we suggest that the features of DPP‐4 inhibitor, such as lipophilicity and distribution to the kidney, might be more important for suppressing membrane‐bound DPP‐4 in the kidney, rather than the excretion route of drugs. However, further studies might be necessary to clarify the relationship between the distribution of DPP‐4 inhibitors to the kidney and the renoprotective effect by suppressing DPP‐4. In addition, the change rate of plasma DPP‐4 activity was correlated to higher plasma DPP‐4 activity and FPG levels at baseline, but there was no relationship between plasma DPP‐4 activity and FPG at baseline. Furthermore, plasma DPP‐4 activity and FPG levels at baseline showed no relationship with Δ%UACR. There was also no correlation between Δ%DPP‐4 and change in BMI and BP. Therefore, the Δ%DPP‐4 after switching to teneligliptin strongly contributes to the Δ%UACR, independently of the change in glucose control, BMI and BP, plasma DPP‐4 activity, and FPG at baseline. By contrast, Sagara et al.34 previously showed that teneligliptin switching from sitagliptin can improve endothelial function, and reduce renal and vascular oxidative stress in patients with type 2 diabetes mellitus and CKD compared with those taking sitagliptin. The effect of teneligliptin was independent of improving glucose control, and the amount of albuminuria showed no significant difference between teneligliptin and sitagliptin treatment34. However, because they did not assess plasma DPP‐4 activity, the relationship between the change in DPP‐4 activity and UACR is unclear.
There were several limitations to the present study. This was a single‐arm, open‐label, non‐controlled study that occurred over a short time‐period, and the number of participants was small. We could not assess changes in the levels of the enzymatic substrates of DPP‐4, such as GLP‐1, stromal cell‐derived factor‐1α, oxidative stress and inflammation, after teneligliptin treatment. The detailed mechanism by which teneligliptin decreases the UACR through a reduction in DPP‐4 activity, in a glucose‐lowering independent manner, is unclear; therefore, further study is necessary to elucidate these points.
In conclusion, in the present study, switching to teneligliptin from other DPP‐4 inhibitors for 24 weeks leads to a decrease in plasma DPP‐4 activity, which is closely related to a reduction in the UACR, independently of the glucose‐lowering effect, in type 2 diabetes mellitus patients with DKD.
Disclosure
Boehringer Ingelheim, Mitsubishi Tanabe Pharma, Kyowa Hakko Kirin, Taisho Toyama Pharmaceutical Co. and Ono Pharmaceutical Co. contributed to establishing the Division of Anticipatory Molecular Food Science and Technology. The authors declare no conflict of interest.
Supporting information
Figure S1 (a) The relationship between the change rate in urinary albumin/creatinine ratio (Δ%UACR) and plasma dipeptidyl peptidase‐4 activity (Δ%DPP‐4) at 24 weeks after switching to teneligliptin from linagliptin showed no significantly correlation (n = 7). (b) The relationship between the change rate in Δ%UACR and Δ%DPP‐4 at 24‐weeks after switching to teneligliptin from other DPP‐4 inhibitors, such as sitagliptin, vildagliptin, alogliptin and anagliptin, showed a positive correlation (n = 16). Pearson's correlation coefficient analysis.
Table S1 Changes in plasma dipeptidyl peptidase‐4 activity.
Acknowledgments
We thank Yuka Kuroshima and Erii Hayashi, who are clinical research coordinators; and Ai Watanabe, Takako Nagai, Shin‐ichi Tsuda, Makoto Nishizawa and all the staff at the Department of Endocrinology and Metabolism of Kanazawa Medical University Hospital for their great assistance in this study.
This work was financially supported by Mitsubishi Tanabe Pharma.
J Diabetes Investig 2019; 10: 706–713
Clinical Trial Registry
University Hospital Medical Information Network
UMIN000015922
References
- 1. Kitada M, Kanasaki K, Koya D. Clinical therapeutic strategies for early stage of diabetic kidney disease. World J Diabetes 2014; 5: 342–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Action to Control Cardiovascular Risk in Diabetes Study Group , Gerstein HC, Miller ME, et al Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358: 2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goto A, Arah OA, Goto M, et al Severe hypoglycaemia and cardiovascular disease: systematic review and meta‐analysis with bias analysis. BMJ 2013; 347: f4533. [DOI] [PubMed] [Google Scholar]
- 4. Howse PM, Chibrikova LN, Twells LK, et al Safety and efficacy of incretin‐based therapies in patients with type 2 diabetes mellitus and CKD: a systematic review and meta‐analysis. Am J Kidney Dis 2016; 68: 733–742. [DOI] [PubMed] [Google Scholar]
- 5. Mori H, Okada Y, Arao T, et al Sitagliptin improves albuminuria in patients with type 2 diabetes mellitus. J Diabetes Investig 2014; 5: 313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hattori S. Sitagliptin reduces albuminuria in patients with type 2 diabetes. Endocr J 2011; 58: 69–73. [DOI] [PubMed] [Google Scholar]
- 7. Groop PH, Cooper ME, Perkovic V, et al Linagliptin lowers albuminuria on top of recommended standard treatment in patients with type 2 diabetes and renal dysfunction. Diabetes Care 2013; 36: 3460–3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fujita H, Taniai H, Murayama H, et al DPP‐4 inhibition with alogliptin on top of angiotensin II type 1 receptor blockade ameliorates albuminuria via up‐regulation of SDF‐1alpha in type 2 diabetic patients with incipient nephropathy. Endocr J 2014; 61: 159–166. [DOI] [PubMed] [Google Scholar]
- 9. Tani S, Nagao K, Hirayama A. Association between urinary albumin excretion and low‐density lipoprotein heterogeneity following treatment of type 2 diabetes patients with the dipeptidyl peptidase‐4 inhibitor, vildagliptin: a pilot study. Am J Cardiovasc Drugs 2013; 13: 443–450. [DOI] [PubMed] [Google Scholar]
- 10. Mosenzon O, Leibowitz G, Bhatt DL, et al Effect of saxagliptin on renal outcomes in the SAVOR‐TIMI 53 Trial. Diabetes Care 2017; 40: 69–76. [DOI] [PubMed] [Google Scholar]
- 11. Kitada M, Tsuda SI, Konishi K, et al Anagliptin ameliorates albuminuria and urinary liver‐type fatty acid‐binding protein excretion in patients with type 2 diabetes with nephropathy in a glucose‐lowering‐independent manner. BMJ Open Diabetes Res Care 2017; 5: e000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scirica BM, Bhatt DL, Braunwald E, et al Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369: 1317–1326. [DOI] [PubMed] [Google Scholar]
- 13. Sharma SK, Panneerselvam A, Singh KP, et al Teneligliptin in management of type 2 diabetes mellitus. Diabetes Metab Syndr Obes 2016; 9: 251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abubaker M, Mishra P, Swami OC. Teneligliptin in management of diabetic kidney disease: a review of place in therapy. J Clin Diagn 2017; 11: OE05–OE09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mannucci E, Pala L, Ciani S, et al Hyperglycaemia increases dipeptidyl peptidase IV activity in diabetes mellitus. Diabetologia 2005; 48: 1168–1172. [DOI] [PubMed] [Google Scholar]
- 16. Sharkovska Y, Reichetzeder C, Alter M, et al Blood pressure and glucose independent renoprotective effects of dipeptidyl peptidase‐4 inhibition in a mouse model of type‐2 diabetic nephropathy. J Hypertens 2014; 32: 2211–2223. discussion 23. [DOI] [PubMed] [Google Scholar]
- 17. Makino Y, Fujita Y, Haneda M. Dipeptidyl peptidase‐4 inhibitors in progressive kidney disease. Curr Opin Nephrol Hypertens 2015; 24: 67–73. [DOI] [PubMed] [Google Scholar]
- 18. Shah K. Teneligliptin in early diabetic kidney disease: an observation in Asian Indian patients with type 2 diabetes mellitus in real‐life scenario. J Clin Diagn Res 2017; 11: OC22–OC25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kodera R, Shikata K, Takatsuka T, et al Dipeptidyl peptidase‐4 inhibitor ameliorates early renal injury through its anti‐inflammatory action in a rat model of type 1 diabetes. Biochem Biophys Res Comm 2014; 443: 828–833. [DOI] [PubMed] [Google Scholar]
- 20. Tanaka Y, Kume S, Chin‐Kanasaki M, et al Renoprotective effect of DPP‐4 inhibitors against free fatty acid‐bound albumin‐induced renal proximal tubular cell injury. Biochem Biophys Res Comm 2016; 470: 539–545. [DOI] [PubMed] [Google Scholar]
- 21. Gangadharan Komala M, Gross S, Zaky A, et al Saxagliptin reduces renal tubulointerstitial inflammation, hypertrophy and fibrosis in diabetes. Nephrology 2016; 21: 423–431. [DOI] [PubMed] [Google Scholar]
- 22. Mega C, de Lemos ET, Vala H, et al Diabetic nephropathy amelioration by a low‐dose sitagliptin in an animal model of type 2 diabetes (Zucker diabetic fatty rat). Experiment Diabetes Res 2011; 2011: 162092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Takashima S, Fujita H, Fujishima H, et al Stromal cell‐derived factor‐1 is upregulated by dipeptidyl peptidase‐4 inhibition and has protective roles in progressive diabetic nephropathy. Kidney Int 2016; 90: 783–796. [DOI] [PubMed] [Google Scholar]
- 24. Vavrinec P, Henning RH, Landheer SW, et al Vildagliptin restores renal myogenic function and attenuates renal sclerosis independently of effects on blood glucose or proteinuria in zucker diabetic fatty rat. Curr Vasc Pharmacol 2014; 12: 836–844. [DOI] [PubMed] [Google Scholar]
- 25. Kanasaki K, Shi S, Kanasaki M, et al Linagliptin‐mediated DPP‐4 inhibition ameliorates kidney fibrosis in streptozotocin‐induced diabetic mice by inhibiting endothelial‐to‐mesenchymal transition in a therapeutic regimen. Diabetes 2014; 63: 2120–2131. [DOI] [PubMed] [Google Scholar]
- 26. Kimura S, Inoguchi T, Yamasaki T, et al A novel DPP‐4 inhibitor teneligliptin scavenges hydroxyl radicals: in vitro study evaluated by electron spin resonance spectroscopy and in vivo study using DPP‐4 deficient rats. Metabolism 2016; 65: 138–145. [DOI] [PubMed] [Google Scholar]
- 27. Nabeno M, Akahoshi F, Kishida H, et al A comparative study of the binding modes of recently launched dipeptidyl peptidase IV inhibitors in the active site. Biochem Biophys Res Comm 2013; 434: 191–196. [DOI] [PubMed] [Google Scholar]
- 28. Kishimoto M. Teneligliptin: a DPP‐4 inhibitor for the treatment of type 2 diabetes. Diabetes Metab Syndr Obes 2013; 6: 187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hasan AA, Hocher B. Role of soluble and membrane‐bound dipeptidyl peptidase‐4 in diabetic nephropathy. J Mol Endocrinol 2017; 59: R1–R10. [DOI] [PubMed] [Google Scholar]
- 30. Nakamaru Y, Akahoshi F, Iijima H, et al Tissue distribution of teneligliptin in rats and comparisons with data reported for other dipeptidyl peptidase‐4 inhibitors. Biopharm Drug Dispos 2016; 37: 142–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. De Nigris V, Prattichizzo F, Mancuso E, et al Teneligliptin enhances the beneficial effects of GLP‐1 in endothelial cells exposed to hyperglycemic conditions. Oncotarget 2018; 9: 8898–8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fuchs H, Binder R, Greischel A. Tissue distribution of the novel DPP‐4 inhibitor BI 1356 is dominated by saturable binding to its target in rats. Biopharm Drug Dispos 2009; 30: 229–240. [DOI] [PubMed] [Google Scholar]
- 33. Cooper ME, Perkovic V, McGill JB, et al Kidney disease end points in a pooled analysis of individual patient‐level data from a large clinical trials program of the dipeptidyl peptidase 4 inhibitor linagliptin in type 2 diabetes. Am J Kidney Dis 2015; 66: 441–449. [DOI] [PubMed] [Google Scholar]
- 34. Sagara M, Suzuki K, Aoki C, et al Impact of teneligliptin on oxidative stress and endothelial function in type 2 diabetes patients with chronic kidney disease: a case‐control study. Cardiovasc Diabetol 2016; 15: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 (a) The relationship between the change rate in urinary albumin/creatinine ratio (Δ%UACR) and plasma dipeptidyl peptidase‐4 activity (Δ%DPP‐4) at 24 weeks after switching to teneligliptin from linagliptin showed no significantly correlation (n = 7). (b) The relationship between the change rate in Δ%UACR and Δ%DPP‐4 at 24‐weeks after switching to teneligliptin from other DPP‐4 inhibitors, such as sitagliptin, vildagliptin, alogliptin and anagliptin, showed a positive correlation (n = 16). Pearson's correlation coefficient analysis.
Table S1 Changes in plasma dipeptidyl peptidase‐4 activity.


