Abstract
Aims/Introduction
Risk factors of type 2 diabetes mellitus in Japanese women with recent gestational diabetes mellitus are unknown. The objective of the present study was to investigate the clinical and genetic characteristics associated with postpartum abnormal glucose tolerance in Japanese women with gestational diabetes mellitus.
Materials and Methods
A total of 213 Japanese women with recent gestational diabetes mellitus who underwent a postpartum 2‐h oral glucose tolerance test were investigated. The association between antepartum clinical characteristics and postpartum abnormal glucose tolerance (diabetes or prediabetes based on the Japan Diabetes Society criteria) was examined. Frequencies of 45 known type 2 diabetes mellitus‐associated genetic variants were also compared between women with and without postpartum abnormal glucose tolerance.
Results
A total of 59 women showed postpartum abnormal glucose tolerance (prediabetes, n = 51; diabetes, n = 8). Plasma glucose levels at 1 or 2 h, the insulinogenic index and the insulin secretion‐sensitivity index‐2 of the antepartum oral glucose tolerance test were independent of postpartum abnormal glucose tolerance risk factors (P = 0.006, P = 0.00002, P = 0.01 and P = 0.006, respectively). Four genetic variants (rs266729 [ADIPOQ], rs6017317 [HNF 4A], rs5215 [KCNJ 11] and rs7177055 [HMG 20A]) showed a nominally significant association with postpartum abnormal glucose tolerance (P < 0.05, respectively). Among these, three were related to insulin secretion. Postpartum abnormal glucose tolerance risk significantly increased with increasing risk‐allele number (P = 0.0005; odds ratio 1.91).
Conclusions
Clinical features and genetic variants related to impaired insulin secretion are risk factors of postpartum abnormal glucose tolerance in Japanese women with recent gestational diabetes mellitus.
Keywords: Gestational diabetes, Glucose tolerance test, Single‐nucleotide polymorphism
Introduction
Women with a history of gestational diabetes mellitus (GDM) are at a high risk of developing type 2 diabetes mellitus in the future; therefore, postpartum diabetes screening is highly recommended for the improvement of health outcomes1, 2, 3. However, the rate of postpartum follow up for women with GDM is low; therefore, it is desirable to identify those at the greatest risk by determining the risk factors3. The clinical risk factors associated with postpartum abnormal glucose tolerance (pAGT) include a family history of diabetes, obesity, early GDM diagnosis, fasting hyperglycemia, insulin requirement during pregnancy and antepartum β‐cell dysfunction4, 5, 6, 7, 8, 9, 10, 11, 12, 13. Additionally, recent studies have shown that certain genetic variants are associated with the development of future type 2 diabetes mellitus in women with a history of GDM9, 14, 15. It is shown that cyclin‐dependent kinase inhibitor 2A/2B (CDKN2A/2B), hematopoietically expressed homeobox (HHEX), CDK5 regulatory subunit associated protein 1‐like 1 (CDKAL1), transcription factor 7‐like 2 (TCF7L2), and fat mass and obesity‐associated protein (FTO) genes could be candidates for risk variants of pAGT9, 14, 15. Given that the susceptibility to glucose intolerance varies among different racial or ethnic groups16, 17, the clinical risk factors and genetic variants of pAGT might differ between Caucasian and Japanese women. However, few reports on the risk factors of pAGT are available with regard to Japanese women with GDM10, 11, 12, and, to the best of our knowledge, there are no data on the genetic variants associated with pAGT in this population subgroup.
These analyses are further complicated by a lack of international agreement regarding the diagnostic criteria of GDM. The diagnosis of GDM in most of the studies on maternal characteristics associated with postpartum glucose intolerance was based on the Carpenter–Coustan (i.e., a 100‐g, 3‐h oral glucose tolerance test [OGTT])]18 or the World Health Organization (WHO) 1999 (i.e., a 75‐g, 2‐h OGTT) criteria19. In 2010, the International Association of Diabetes in Pregnancy Study Group (IADPSG) proposed new criteria for the diagnosis of GDM20. Since 2013, the WHO has also advised the use of the IADPSG criteria for the diagnosis of GDM21; however, the risk factors of pAGT in GDM according to the IADPSG criteria (i.e., WHO 2013 criteria) are yet unknown, because the number of healthcare providers adopting the new criteria remains limited.
With this background, we retrospectively investigated the postpartum glucose tolerance status in Japanese women with recent GDM using the IADPSG criteria (i.e., WHO 2013 criteria). Furthermore, we analyzed antenatal clinical and genetic characteristics in Japanese women with recently developed GDM and those having pAGT. We also examined the association between previously reported type 2 diabetes mellitus‐ or GDM‐susceptibility genes and the development of pAGT in Japanese women with recently developed GDM.
Methods
Participants
We retrospectively investigated a cohort of 213 women with a recent history of GDM who underwent postpartum diabetes screening at Keio University Hospital or the National Center for Child Health and Development between April 2011 and December 2016. During the study period, GDM was diagnosed according to the IADPSG criteria proposed in 201020. Women with multifetal pregnancies and women whose neonates showed congenital anomalies were excluded from the present study. Women with overt diabetes in pregnancy and pre‐pregnancy diabetes were also excluded. The research was carried out in accordance with the Declaration of Helsinki, and informed consent was obtained from patients where appropriate. This study was approved by the Keio University School of Medicine Ethics Committee (Nos. 20100154 and 20110321) and the institutional review board of the National Research Institute for Child Health and Development (No. 406).
Postpartum glucose tolerance status
Each woman with GDM was scheduled to undergo a postpartum diabetes screening using the 75‐g OGTT ~6–12 weeks after delivery as part of the routine care recommendation by the Japan Society of Obstetrics and Gynecology22. Postpartum diabetes screening was rearranged if the mother failed to attend the scheduled appointment. According to the results of the postpartum OGTT, women with recently developed GDM were characterized into three categories based on the Japan Diabetes Society criteria: diabetic, fasting plasma glucose (PG) ≥126 mg/dL (7.0 mmol/L) and/or 2‐h PG ≥200 mg/dL (11.1 mmol/L); normal, fasting PG ≤110 mg/dL (6.1 mmol/L) and 2‐h PG ≤140 mg/dL (7.8 mmol/L); or prediabetes, neither normal nor diabetic23. In the present study, pAGT included women classified as either the diabetic or prediabetes type. Postpartum normal glucose tolerance (NGT) was defined as postpartum normal OGTT results.
Antepartum clinical and metabolic characteristics
Maternal characteristics in the index pregnancy were collected from patient hospital records as follows: maternal age, pregravid body mass index (BMI), a first‐degree family history of diabetes, gestational age at the time of diagnosis of GDM and insulin requirement in pregnancy. Participants were categorized by pregravid BMI: underweight, BMI <18.5; normal weight, BMI from 18.5–24.9; and overweight, BMI ≥25.022. Glycemic and insulin profiles of the OGTT, as well as levels of glycated hemoglobin at the time of diagnosis of GDM, were also reviewed. Additionally, metabolic features (i.e., insulin sensitivity, insulin secretion and β‐cell function) were assessed using antepartum OGTT results. Insulin sensitivity was estimated according to the whole‐body insulin sensitivity index derived from the OGTT (ISOGTT) and the homeostasis model assessment for insulin resistance. The ISOGTT was calculated using the following formula: 10,000/square root {PG0 × Ins0 × (PG0 + PG60 × 2 + PG120)/2 × (Ins0 + Ins60 × 2 + Ins120)/2}, where PGy (mg/dL) and Insy (mU/L) represent PG and insulin values, respectively, at time y min during the OGTT24. Homeostasis model assessment for insulin resistance was calculated as follows: Ins0 × PG0/40525. Insulin secretion was assessed according to the insulinogenic index (IGI: {Ins30 − Ins0}/{PG30 − PG0}) and the ratio of the total area under the insulin curve to the total area under the glucose curve (AUCins/glu) during the OGTT26. To evaluate β‐cell function, we calculated the OGTT‐derived disposition index using the Insulin Secretion‐Sensitivity Index‐2 (ISSI‐2; the AUCins/glu multiplied by ISOGTT)27.
Single‐nucleotide polymorphism selection and genotyping
Single‐nucleotide polymorphism (SNP) selection from previously reported type 2 diabetes mellitus‐ or GDM‐susceptibility genes and genotyping were carried out as previously described28. We selected the SNPs based on the criterion of minor allele frequency (MAF) >30% in the Japanese population, because this selection could provide adequate statistical power to detect SNPs with genotype relative risk ≥1.6 in our study cohort. Finally, we confirmed the association between 45 SNPs from 36 genes and the development of pAGT. All the polymorphisms analyzed in the present study were in Hardy–Weinberg equilibrium.
During the study period, maternal peripheral blood samples were collected soon after delivery, and genomic deoxyribonucleic acid was extracted using the QIAsymphony DNA mini kit (96) (Qiagen, Valencia, CA, USA) for exploratory research when informed consent was obtained. As all of the 213 women agreed to the present study, genotyping was carried out using the high‐throughput genotyping MassARRAY platform (Sequenom Inc., San Diego, CA, USA) after postpartum OGTT. Primers, including those used for amplification and extension, were designed using Assay Design Suite (Sequenom Inc.; https://seqpws1.sequenom.com/AssayDesignerSuite.html; Table S1). Negative controls, run at least in quadruplicate, were placed on all 384 plates as quality controls. The SNP genotyping success rate was >94%, and the concordance rate for genotyping was >99.8% in the present study.
Statistical analysis
Data are presented as the median (range), mean ± standard deviation or the number of cases (percentage). Continuous data were compared between groups using Student's t‐test or logistic regression analysis. Categorical variables were analyzed by the χ2‐test or Fisher's exact test.
For metabolic measurements, multivariate regression analysis was used to identify independent risk factors for pAGT. Additionally, predictive values of clinical characteristics for the risk of pAGT were obtained using multiple logistic regression analysis and receiver operating characteristics (ROC) analysis. For genetic variants, we analyzed the association between 45 SNPs (36 genes) and the risk of pAGT among 213 women. Per‐allele odds ratios (ORs) and their 95% confidence intervals (CIs) for the association between SNPs and pAGT were evaluated using logistic regression analysis adjusted for maternal age, pregravid BMI, family history of type 2 diabetes mellitus and antepartum metabolic features that were significantly associated with pAGT (i.e., 2‐h PG). The possibility of multiple testing burden was avoided by Bonferroni correction, and an adjusted P < 0.05 was considered significant. Therefore, we examined the combined effects of multiple genetic variants on pAGT in Japanese women with recent GDM and the cumulative effects of risk alleles at pAGT‐associated SNPs having a lower P‐value. Statistical analyses, the calculation of linkage disequilibrium among SNPs, and construction of a forest plot of per‐allele ORs were carried out using R (version 3.3.1; https://cran.r-project.org/bin/macosx/).
Results
Antepartum clinical and metabolic characteristics in women with a recent history of GDM
Women in the present study cohort underwent postpartum OGTT at a median of 24.9 weeks (range 6.0–53.7 weeks). At the time of GDM diagnosis, 142 women had a single abnormal OGTT value, 51 had two abnormal values and 20 had three abnormal values. Maternal age at delivery and pregravid BMI in this study cohort were 37 years (range 23–51 years) and 21.6 (range 16.2–38.8 tears), respectively. Of all the women, 128 (60%) were nulliparous.
During the follow‐up period, 59 women (28%) developed pAGT: eight had diabetes and 51 had prediabetes as classified according to the Japan Diabetes Society criteria. These included 33 of 142 (23.2%) women with a single abnormal antepartum OGTT value, 18 of 51 (35.3%) with two abnormal values and eight of 20 (40.0%) with three abnormal values.
There were no significant differences in pregravid BMI, overweight status, GDM diagnosed before 20 weeks of pregnancy, and insulin requirement during pregnancy between the pAGT and NGT groups (Table 1). Women with pAGT showed older maternal age and a higher rate of family history of diabetes as compared with those in the NGT group. With regard to antepartum OGTT profile, women with pAGT showed significantly higher levels of antepartum 1‐h PG and 2‐h PG, as compared with those in the NGT group (P < 0.001). Among antepartum metabolic features, IGI in the pAGT group was significantly lower than that in the NGT group (P < 0.01). There was a significant difference in antepartum ISSI‐2 between the pAGT and NGT groups (P < 0.001). After adjustment for maternal age and family history of diabetes using the logistic regression model, 1‐h PG, 2‐h PG, IGI and ISSI‐2 derived from the antenatal OGTT remained independent risk factors of pAGT (P = 0.006, P = 0.00002, P = 0.01 and P = 0.006, respectively). The AUC was used to evaluate the predictive power of these antepartum factors (Table 2). Among clinical characteristics, the 2‐h PG showed the largest area under the ROC curve (AUC 0.72).
Table 1.
Comparison of antepartum clinical features between women with postpartum abnormal glucose tolerance and normal glucose tolerance
| Abnormal glucose tolerance (n = 59) | Normal glucose tolerance (n = 154) | |
|---|---|---|
| Maternal age at delivery (years) | 39 (27–51) | 37 (23–46)* |
| Pregravid BMI (kg/m2) | 21.4 (17.0–33.9) | 21.6 (16.2–38.8) |
| Pregravid overweight | 18 (31) | 27 (17) |
| Nulliparous | 41 (69) | 87 (56) |
| Family history of diabetes | 19 (32) | 22 (14)** |
| GW at diagnosis of GDM (weeks) | 21 (9–33) | 16 (7–34) |
| GDM diagnosed before 20 weeks of pregnancy | 28 (47) | 84 (55) |
| Insulin use in pregnancy | 29 (49) | 54 (35) |
| Plasma glucose of the antepartum OGTT (mg/dL) | ||
| 0 min | 88 (76–109) | 92 (68–116)* |
| 30 min | 157 (107–211) | 153 (79–208) |
| 60 min | 182 (110–242) | 165 (88–272)*** |
| 120 min | 164 (112–230) | 147 (88–256)*** |
| Insulinogenic index | 0.56 (0.17–1.71) | 0.69 (0.02–7.53)** |
| ISOGTT | 2.69 (0.74–6.91) | 2.53 (0.54–7.27) |
| HOMA‐IR | 1.29 (0.39–6.57) | 1.49 (0.38–6.84) |
| ISSI‐2 | 0.80 (0.41–1.62) | 0.94 (0.36–2.51)*** |
| HbA1c at diagnosis of GDM (%) | 5.4 (4.8–6.1) | 5.3 (4.3–6.1) |
Data are median (range) or n (%). *P < 0.05, **P < 0.01, ***P < 0.001. BMI, body mass index; GDM, gestational diabetes mellitus; GW, gestational week; HbA1c, glycated hemoglobin; HOMA‐IR, homeostasis model assessment for insulin resistance; ISOGTT, insulin sensitivity index from the oral glucose tolerance test; ISSI‐2, Insulin Secretion‐Sensitivity Index‐2; OGTT, oral glucose tolerance test; overweight, body mass index ≥25 kg/m2.
Table 2.
Predictive values of antepartum clinical characteristics for the risk of postpartum abnormal glucose tolerance
| AUC | 95% CI | Cut‐off | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|---|
| 1‐h PG | 0.66 | 0.58–0.74 | 175 | 66.1 | 58.4 | 37.9 | 81.8 |
| 2‐h PG | 0.72 | 0.64–0.80 | 156 | 74.6 | 61.7 | 42.7 | 86.4 |
| Insulinogenic Index | 0.62 | 0.54–0.70 | 0.66 | 65.5 | 52.6 | 34.5 | 80.0 |
| ISSI‐2 | 0.65 | 0.57–0.73 | 0.88 | 70.7 | 56.6 | 38.3 | 83.5 |
1‐h PG, plasma glucose at 1 h in the antepartum oral glucose‐tolerance test; 2‐h PG, plasma glucose at 2 h in the antepartum oral glucose‐tolerance test; AUC, area under the receiver operating characteristics curve; CI, confidence interval; IGI, Insulinogenic Index; ISSI‐2, Insulin Secretion‐Sensitivity Index‐2; NPV, negative predictive value; PPV, positive predictive value.
Genetic variants associated with pAGT in women with a recent history of GDM
We compared the risk‐allele frequencies of 45 SNPs between the pAGT and NGT groups. The individual SNP results are shown in Figure 1 and Table 3. After adjustment for maternal age at delivery, pregravid BMI, family history of type 2 diabetes mellitus and antepartum 2‐h PG, four out of the 45 SNPs showed a nominally significant association with the development of pAGT (rs266729 [P = 0.029, OR 2.02, 95% CI 1.08–3.78], rs6017317 [P = 0.031, OR 1.77, 95% CI 1.05–2.99], rs5215 [P = 0.032, OR 1.82, 95% CI 1.05–3.14] and rs7177055 [P = 0.043, OR 1.82, 95% CI 1.02–3.26]). Therefore, we carried out a combined analysis of the four identified risk alleles, for which each individual could harbor between zero and eight possible risk alleles. With the increasing number of risk alleles, the risk of pAGT increased (P = 0.00005, OR 1.91, 95% CI 1.40–2.61; Figure 2a). In particular, women with seven or more risk alleles showed a 7.67‐fold increased risk of pAGT (P = 0.0085, 95% CI 1.68–34.9), as compared with those having three or fewer risk alleles (Figure 2b). The linkage disequilibrium values among four SNPs calculated using r 2 were <0.02 in both the pAGT and NGT groups. Additionally, we carried out the association analysis between the number of identified risk alleles and postpartum metabolic features using linear regression analysis. The 1 h‐PG, 2 h‐PG and ISSI‐2 from the postpartum OGTT were significantly associated with increasing the number of risk alleles (P = 0.00027, P = 0.008 and P = 0.0087, respectively; Table S2).
Figure 1.
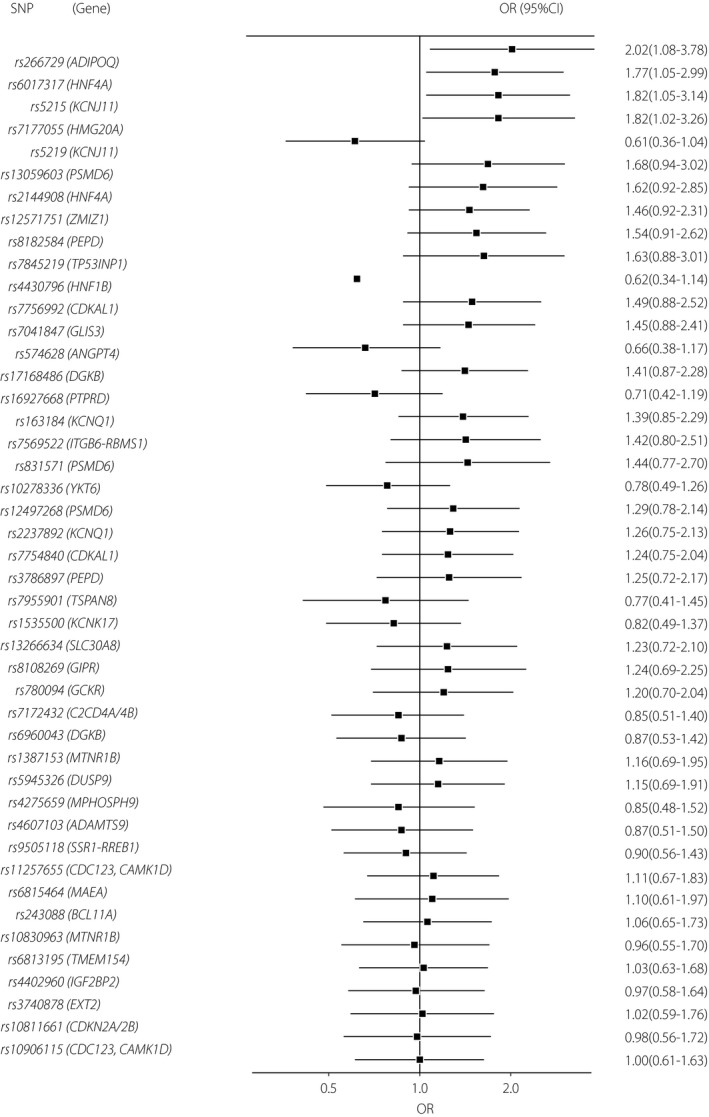
Forest plot of per‐allele odds ratio of 45 single‐nucleotide polymorphisms assessed in the present study. CI, confidence interval; OR, odds ratio; SNP, single‐nucleotide polymorphism.
Table 3.
Association analysis results between single nucleotide polymorphisms and the risk of gestational diabetes mellitus
| SNP | Chr | Nearby gene | Insulin sensitivity | Insulin secretion | Risk allele | Japanese major allele | Japanese minor allele | RAF of Japanese pAGT | The present study | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| P‐value | OR (95% CI) | ||||||||||
| 1 | rs266729 | 3 | ADIPOQ | Yes | C | C | G | 0.69 | 0.029 | 2.02 (1.08–3.78) | |
| 2 | rs6017317 | 20 | HNF4A | Yes | G | G | T | 0.55 | 0.031 | 1.77 (1.05–2.99) | |
| 3 | rs5215 | 11 | KCNJ11 | Yes | T | T | C | 0.34 | 0.032 | 1.82 (1.05–3.14) | |
| 4 | rs7177055 | 15 | HMG20A | Yes | A | G | A | 0.41 | 0.043 | 1.82 (1.02–3.26) | |
| 5 | rs5219 | 11 | KCNJ11 | Yes | T | C | T | 0.35 | 0.07 | 0.61 (0.36–1.04) | |
| 6 | rs13059603 | 3 | PSMD6(THOC7) | Yes | Yes | A | A | G | 0.68 | 0.08 | 1.68 (0.94–3.02) |
| 7 | rs2144908 | 20 | HNF4A | Yes | A | G | A | 0.42 | 0.09 | 1.62 (0.92–2.85) | |
| 8 | rs12571751 | 10 | ZMIZ1 | G | A | G | 0.42 | 0.11 | 1.46 (0.92–2.31) | ||
| 9 | rs8182584 | 19 | PEPD | Yes | T | T | G | 0.64 | 0.11 | 1.54 (0.91–2.62) | |
| 10 | rs7845219 | 8 | TP53INP1 | Yes | T | C | T | 0.27 | 0.12 | 1.63 (0.88–3.01) | |
| 11 | rs4430796 | 17 | HNF1B | Yes | G | A | G | 0.36 | 0.13 | 0.62 (0.34–1.14) | |
| 12 | rs7756992 | 6 | CDKAL1 | Yes | G | A | G | 0.46 | 0.14 | 1.49 (0.88–2.52) | |
| 13 | rs7041847 | 9 | GLIS3 | Yes | A | G | A | 0.47 | 0.15 | 1.45 (0.88–2.41) | |
| 14 | rs574628 | 20 | ANGPT4 | Yes | G | G | A | 0.63 | 0.16 | 0.66 (0.38–1.17) | |
| 15 | rs17168486 | 7 | DGKB | Yes | T | C | T | 0.41 | 0.17 | 1.41 (0.87–2.28) | |
| 16 | rs16927668 | 9 | PTPRD | Yes | T | T | C | 0.57 | 0.19 | 0.71 (0.42–1.19) | |
| 17 | rs163184 | 11 | KCNQ1 | Yes | G | T | G | 0.42 | 0.19 | 1.39 (0.85–2.29) | |
| 18 | rs7569522 | 2 | ITGB6‐RBMS1 | Yes | A | G | A | 0.38 | 0.23 | 1.42 (0.80–2.51) | |
| 19 | rs831571 | 3 | PSMD6(PRICKLE2) | Yes | Yes | C | C | T | 0.69 | 0.26 | 1.44 (0.77–2.70) |
| 20 | rs10278336 | 7 | YKT6 | A | A | G | 0.57 | 0.31 | 0.78 (0.49–1.26) | ||
| 21 | rs12497268 | 3 | PSMD6(PRICKLE2) | Yes | G | G | C | 0.61 | 0.32 | 1.29 (0.78–2.14) | |
| 22 | rs2237892 | 11 | KCNQ1 | Yes | C | C | T | 0.64 | 0.38 | 1.26 (0.75–2.13) | |
| 23 | rs7754840 | 6 | CDKAL1 | Yes | C | G | C | 0.41 | 0.40 | 1.24 (0.75–2.04) | |
| 24 | rs3786897 | 19 | PEPD | Yes | A | A | G | 0.55 | 0.42 | 1.25 (0.72–2.17) | |
| 25 | rs7955901 | 12 | TSPAN8, LGR5 | Yes | C | C | T | 0.70 | 0.42 | 0.77 (0.41–1.45) | |
| 26 | rs1535500 | 6 | KCNK17 | T | G | T | 0.40 | 0.43 | 0.82 (0.49–1.37) | ||
| 27 | rs13266634 | 8 | SLC30A8 | Yes | C | C | T | 0.56 | 0.44 | 1.23 (0.72–2.10) | |
| 28 | rs8108269 | 19 | GIPR | Yes | G | G | T | 0.61 | 0.47 | 1.24 (0.69–2.25) | |
| 29 | rs780094 | 2 | GCKR | Yes | G | A | G | 0.44 | 0.50 | 1.20 (0.70–2.04) | |
| 30 | rs7172432 | 15 | C2CD4A/4B | Yes | A | A | G | 0.55 | 0.52 | 0.85 (0.51–1.40) | |
| 31 | rs6960043 | 7 | DGKB | Yes | C | C | T | 0.50 | 0.58 | 0.87 (0.53–1.42) | |
| 32 | rs1387153 | 11 | MTNR1B | Yes | T | C | T | 0.48 | 0.58 | 1.16 (0.69–1.95) | |
| 33 | rs5945326 | X | DUSP9 | Yes | A | A | G | 0.62 | 0.60 | 1.15 (0.69–1.91) | |
| 34 | rs4275659 | 12 | MPHOSPH9(ABCB9) | C | C | T | 0.67 | 0.60 | 0.85 (0.48–1.52) | ||
| 35 | rs4607103 | 3 | ADAMTS9 | Yes | C | C | T | 0.59 | 0.62 | 0.87 (0.51–1.50) | |
| 36 | rs9505118 | 6 | SSR1‐RREB1 | Yes | A | A | G | 0.59 | 0.65 | 0.90 (0.56–1.43) | |
| 37 | rs11257655 | 10 | CDC123, CAMK1D | Yes | T | C | T | 0.41 | 0.69 | 1.11 (0.67–1.83) | |
| 38 | rs6815464 | 4 | MAEA | Yes | C | C | G | 0.64 | 0.76 | 1.10 (0.61–1.97) | |
| 39 | rs243088 | 2 | BCL11A | Yes | T | A | T | 0.27 | 0.82 | 1.06 (0.65–1.73) | |
| 40 | rs10830963 | 11 | MTNR1B | Yes | G | C | G | 0.47 | 0.90 | 0.96 (0.55–1.70) | |
| 41 | rs6813195 | 4 | TMEM154 | Yes | C | T | C | 0.46 | 0.91 | 1.03 (0.63–1.68) | |
| 42 | rs4402960 | 3 | IGF2BP2 | Yes | T | G | T | 0.30 | 0.92 | 0.97 (0.58–1.64) | |
| 43 | rs3740878 | 11 | EXT2 | Yes | A | A | G | 0.64 | 0.95 | 1.02 (0.59–1.76) | |
| 44 | rs10811661 | 9 | CDKN2A/2B | Yes | T | T | C | 0.52 | 0.96 | 0.98 (0.56–1.72) | |
| 45 | rs10906115 | 10 | CDC123, CAMK1D | Yes | A | A | G | 0.51 | 0.99 | 1.00 (0.61–1.63) | |
P‐values before Bonferroni correction are shown. Chr, chromosome; CI, confidence interval; OR, odds ratio; pAGT, postpartum abnormal glucose tolerance; RAF, risk allele frequency; SNP, single‐nucleotide polymorphism.
Figure 2.
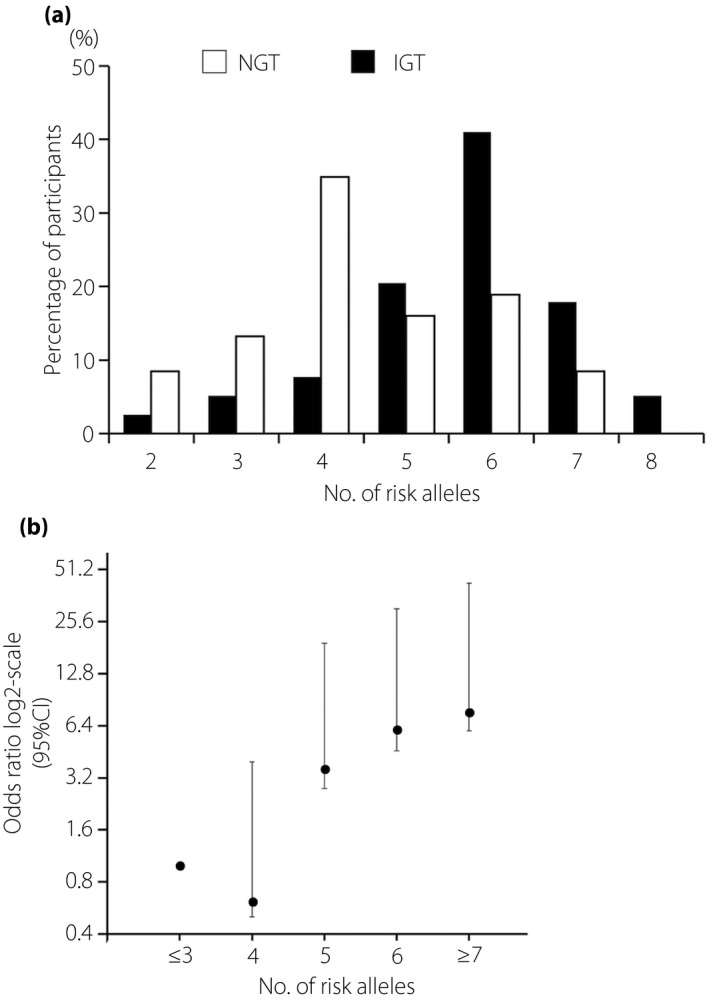
Risk alleles of four genetic variants and the development of postpartum abnormal glucose tolerance (pAGT). (a) Distribution of risk alleles of four genetic variants in postpartum abnormal glucose tolerance and normal glucose tolerance (NGT). Black bars: abnormal glucose tolerance (n = 59); white bars: NGT (n = 154). (b) Odds ratio for the risk of postpartum abnormal glucose tolerance according to the number of risk alleles carried. 95% CI, 95% confidence interval; IGT, impaired glucose tolerance.
Discussion
As women with GDM are at a high risk of progression to prediabetes or type 2 diabetes mellitus, risk factors associated with the development of this condition have received special attention8. The majority of studies have focused on the clinical features of GDM mainly as defined by the Carpenter–Coustan18 or WHO 1999 criteria19. In contrast to genetic studies on the development of GDM, reports on genetic risk variants associated with glucose intolerance after pregnancies with GDM are very limited. In particular, there is a paucity of information on the risk of pAGT in Japanese women with a history of GDM. To the best of our knowledge, this is the first report highlighting both clinical and genetic characteristics associated with pAGT in Japanese women with recently developed GDM, as defined by the IADPSG criteria (i.e., WHO 2013 criteria).
The rate of pAGT (i.e., diabetes and prediabetes, as defined by Japan Diabetes Society criteria) was 28% in the present study cohort. In comparison, O'Reilly et al.29 reported that 19% of women with GDM defined by the IADPSG criteria showed abnormal glucose tolerance (impaired fasting glucose, impaired glucose intolerance, both or type 2 diabetes mellitus using the American Diabetes Association criteria) in the tests carried out up to 6 months postpartum. Retnakaran et al.1 showed that ~25% of women with gestational glucose intolerance (i.e., a single abnormal OGTT value and/or GDM by the Carpenter–Coustan criteria) developed prediabetes or diabetes as defined by the WHO 1999 criteria within the first year postpartum. Notably, gestational glucose intolerance defined in the study of Retnakaran et al.1 was comparable with GDM according to the IADPSG criteria. These findings suggested that women with recently developed GDM, as defined by the IADPSG criteria, are at risk of pAGT, although the IADPSG diagnostic threshold was based on the risk for adverse perinatal outcomes associated with hyperglycemia. Those with a single abnormal value in the OGTT are thought to have less severe dysglycemia as compared with women with two or three abnormal values. However, the present results highlighted that even women with a single abnormal value have a ~25% risk of glucose intolerance after delivery, which should be recognized by clinicians.
Risk factors of pAGT might be dependent on the diagnostic criteria of GDM and the postpartum follow‐up period. Ethnicity also influences the risk of persistent pAGT30. Compared with Caucasian and Hispanic women, Asian women have a relatively low BMI, but show an increased propensity to develop prediabetes or diabetes. Therefore, Asian women might have impaired β‐cell compensation as compared with women of other ethnicities when a similar extent of insulin resistance exists16, 17. In the present study, metabolic parameters (1 h‐PG, 2 h‐PG, IGI and ISSI‐2) of antenatal OGTT were associated with pAGT. These parameters are thought to be related to β‐cell function. Therefore, the present results suggested that women with lower levels of antepartum β‐cell function were at a higher risk of postpartum glucose intolerance.
The present study showed that the antepartum 2‐h PG exhibited the largest AUC. In clinical practice, antepartum predictors for pAGT are useful because of a potential increase in GDM prevalence according to the IADPSG criteria. Until now, only a few reports have been published with regard to predictors of pAGT in women with GDM, as defined by the IADPSG criteria. Capula et al.7 reported that 2‐h PG in the antepartum OGTT was correlated with the development of prediabetes and type 2 diabetes mellitus within 1 year after delivery in cases of GDM, as defined by the IADPSG criteria. Benhalima et al.13 showed that ethnicity and glycated hemoglobin at the time of OGTT during pregnancy were significant predictors of postpartum glucose intolerance in women with IADPSG criteria‐based GDM. However, to the best of our knowledge, no reports on predictors of pAGT in Japanese GDM based on the IADPSG criteria are available. Therefore, further investigations are warranted to identify antenatal predictors of pAGT in Japanese women with GDM, as defined by the IADPSG criteria (i.e., WHO 2013 criteria).
The present study demonstrated that four SNPs showed a nominally significant association with the development of pAGT in women with recently developed IADPSG‐defined GDM: rs266729 (ADIPOQ), rs6017317 (HNF4A), rs5215 (KCNJ11) and rs7177055 (HMG20A). Notably, women with an increasing number of risk alleles had a significantly higher risk of pAGT. Several authors have shown that CDKN2A/2B, HHEX, CDKAL1, TCF7L2 and FTO carry genetic risk variants associated with pAGT in women with GDM, as defined by the Carpenter–Coustan or WHO 1999 criteria9, 14, 15. Our observation suggests that multiple genetic factors might contribute to the development of pAGT in Japanese women with GDM based on the IADPSG criteria.
Of the four genetic variants (four genes) identified in the present study, several studies showed that a single polymorphism, rs266729 (ADIPOQ), was associated with decreased levels of serum adiponectin in GDM, as well as type 2 diabetes mellitus31. Our previous investigation showed that rs266729 (ADIPOQ) was associated with Japanese GDM28. Furthermore, hepatocyte nuclear factor 4 alpha (HNF4A) is known as the gene responsible for maturity onset diabetes in the young population32. Although the mechanisms underlying the development of impaired insulin secretion remain unknown, an early hypersecretion of insulin in utero and in the neonatal periods, and pancreatic β‐cell exhaustion later in life are suspected contributing factors, along with possible variations in gene expression over time33. KCNJ11, encoding a member of the potassium channel gene family, contributes to insulin secretion34. Previous reports showed that rs5215 is associated with the development of type 2 diabetes mellitus35. High mobility group protein 20A (HMG20A) was related to the development of type 2 diabetes mellitus in European individuals36, and it might contribute to insulin secretion37. It is important that three variants (rs6017317 [HNF4A], rs5215 [KCNJ11] and rs7177055 [HMG20A]) identified in the present study were found to be insulin‐secretion candidate genes. The genetic features found in this study are consistent with the fact that glucose intolerance in East Asian individuals, including Japanese individuals, is characterized as impaired insulin secretion (i.e., β‐cell dysfunction)38. Based on these findings, impaired insulin secretion might play an important role in the pathophysiology of pAGT in Japanese women with GDM.
There were several limitations to the present retrospective study. First, 156 women (73%) underwent postpartum diabetic screening between 13‐weeks and 1‐year postpartum. Therefore, the clinical and genetic characteristics found in the present study were derived from women showing glucose intolerance within 1‐year postpartum after delivery. Second, postpartum diabetes screening is not mandatory for all GDM women in our institutions. As the rate of postpartum diabetes screening was 48% in our experience, the study cohort in this investigation might have selection bias. However, antepartum metabolic features were comparable between GDM women with and without postpartum diabetes screening during the study period (data not shown). Thus, we speculate that women receiving postpartum follow up appeared similar to those without the test with regard to the degree of glucose intolerance during pregnancy. Third, we focused on only SNPs with MAF >30%, as the present study cohort constituted a relatively small panel. For example, the FTO gene, a risk variant related to type 2 diabetes mellitus in Japanese men as well as European people, was not included in this analysis, because the MAF was ≤30% in Japanese people. Therefore, we might have missed SNPs with MAF ≤30% that are associated with pAGT in Japanese women with recently developed GDM. It is also important to replicate and evaluate the current findings in another independent case–control set. Finally, we did not examine the genetic risk score, because the genetic variants detected showed a nominally significant association with pAGT in the present study. As reported by Kwak et al.9, the use of a genetic risk score might improve prediction over clinical risk factors. Further research using a larger cohort is required to determine genetic characteristics associated with pAGT in Japanese women with GDM.
In summary, we investigated the clinical and genetic features associated with pAGT in Japanese women with recently developed GDM, as defined by the IADPSG criteria (i.e., WHO 2013 criteria) for the first time. The clinical risk factors included 1‐h PG, 2‐h PG, IGI and ISSI‐2 derived from the antenatal OGTT. Among genetic characteristics, four SNPs in four genes (ADIPOQ, HNF4A, KCNJ11 and HMG20A) showed a nominally significant association with the development of pAGT. In particular, three variants were related to insulin secretion. Our results suggested that antepartum clinical and genetic characteristics related to impaired insulin secretion appeared to function as risk factors of pAGT in Japanese women with recently developed GDM. Further investigations are required to determine the predictors of pAGT after pregnancies with GDM.
Disclosure
The authors declare no conflict of interest.
Supporting information
Table S1 ¦ Primers designed by Assay Design Suite in the present study.
Table S2 ¦ Linear regression analysis between four single‐nucleotide polymorphisms and postpartum metabolic features.
Acknowledgments
This study was supported by the Japan Agency for Medical Research and Development (AMED, 16gm0510011h0205, 16ek0109067h0003, 16gk0110013s0801, 16gk0110018s0601), KAKENHI (26293365 and 26462500), grants from the NCCHD of Japan (26‐13), and a CREST Program of JST (Epigenomic analysis of the human placenta and endometrium constituting the fetal‐maternal interface). The authors are grateful to all medical staff at Keio University Hospital for excellent patient care. We also appreciate all medical staff in the perinatal unit of the National Center for Child Health and Development.
J Diabetes Investig 2019; 10: 817–826
Contributor Information
Kei Miyakoshi, Email: kei.z7@keio.jp.
Kenichiro Hata, Email: hata-k@ncchd.go.jp.
References
- 1. Retnakaran R, Qi Y, Sermer M, et al Glucose intolerance in pregnancy and future risk of pre‐diabetes or diabetes. Diabetes Care 2008; 31: 2026–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Retnakaran R, Qi Y, Sermer M, et al Beta‐cell function declines within the first year postpartum in women with recent glucose intolerance in pregnancy. Diabetes Care 2010; 33: 1798–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tovar A, Chasan‐Taber L, Eggleston E, et al Postpartum screening for diabetes among women with a history of gestational diabetes mellitus. Prev Chronic Dis 2011; 8: A124. [PMC free article] [PubMed] [Google Scholar]
- 4. Ekelund M, Shaat N, Almgren P, et al Prediction of postpartum diabetes in women with gestational diabetes mellitus. Diabetologia 2010; 53: 452–457. [DOI] [PubMed] [Google Scholar]
- 5. Baptiste‐Roberts K, Barone BB, Gary TL, et al Risk factors for type 2 diabetes among women with gestational diabetes: a systematic review. Am J Med 2009; 122: 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pallardo F, Herranz L, Garcia‐Ingelmo T, et al Early postpartum metabolic assessment in women with prior gestational diabetes. Diabetes Care 1999; 22: 1053–1058. [DOI] [PubMed] [Google Scholar]
- 7. Capula C, Chiefari E, Vero A, et al Prevalence and predictors of postpartum glucose intolerance in Italian women with gestational diabetes mellitus. Diabetes Res Clin Pract 2014; 105: 223–230. [DOI] [PubMed] [Google Scholar]
- 8. Leuridan L, Wens J, Devlieger R, et al Glucose intolerance in early postpartum in women with gestational diabetes: who is at increased risk? Prim Care Diabetes 2015; 9: 244–252. [DOI] [PubMed] [Google Scholar]
- 9. Kwak SH, Choi SH, Jung HS, et al Clinical and genetic risk factors for type 2 diabetes at early or late postpartum after gestational diabetes mellitus. J Clin Endocrinol Metab 2013; 98: E744–E752. [DOI] [PubMed] [Google Scholar]
- 10. Kugishima Y, Yasui I, Yamashita H, et al Risk factors associated with abnormal glucose tolerance in the early postpartum period among Japanese women with gestational diabetes. Int J Gynaecol Obstet 2015; 129: 42–45. [DOI] [PubMed] [Google Scholar]
- 11. Saisho Y, Miyakoshi K, Tanaka M, et al Antepartum oral disposition index as a predictor of glucose intolerance postpartum. Diabetes Care 2012; 35: e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kojima N, Tanimura K, Deguchi M, et al Risk factors for postpartum glucose intolerance in women with gestational diabetes mellitus. Gynecol Endocrinol 2016; 32: 803–806. [DOI] [PubMed] [Google Scholar]
- 13. Benhalima K, Jegers K, Devlieger R, et al Glucose intolerance after a recent history of gestational diabetes based on the 2013 WHO criteria. PLoS ONE 2016; 11: e0157272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kwak SH, Choi SH, Kim K, et al Prediction of type 2 diabetes in women with a history of gestational diabetes using a genetic risk score. Diabetologia 2013; 56: 2556–2563. [DOI] [PubMed] [Google Scholar]
- 15. Ekelund M, Shaat N, Almgren P, et al Genetic prediction of postpartum diabetes in women with gestational diabetes mellitus. Diabetes Res Clin Pract 2012; 97: 394–398. [DOI] [PubMed] [Google Scholar]
- 16. Mørkrid K, Jenum AK, Sletner L, et al Failure to increase insulin secretory capacity during pregnancy‐induced insulin resistance is associated with ethnicity and gestational diabetes. Eur J Endocrinol 2012; 167: 579–588. [DOI] [PubMed] [Google Scholar]
- 17. Tutino GE, Tam WH, Yang X, et al Diabetes and pregnancy: perspectives from Asia. Diabet Med 2014; 31: 302–318. [DOI] [PubMed] [Google Scholar]
- 18. Coustan DR, Carpenter MW. The diagnosis of gestational diabetes. Diabetes Care 1998; 21: B5–B38. [PubMed] [Google Scholar]
- 19. Alberti KG, Zimmet PZ. Definition, diagnosis, and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998; 15: 539–553. [DOI] [PubMed] [Google Scholar]
- 20. International Association of Diabetes and Pregnancy Study Groups Consensus Panel . International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010; 33: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. World Health Organization . Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy. Geneva: WHO, 2013. [PubMed] [Google Scholar]
- 22. Minakami H, Maeda T, Fujii T, et al Guidelines for obstetrical practice in Japan: Japan Society of Obstetrics and Gynecology (JSOG) and Japan Association of Obstetricians and Gynecologists (JAOG) 2014 edition. J Obstet Gynaecol Res 2014; 40: 1469–1499. [DOI] [PubMed] [Google Scholar]
- 23. Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus , Seino Y, Nanjo K, et al Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig 2010; 1: 212–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999; 22: 1462–1470. [DOI] [PubMed] [Google Scholar]
- 25. Matthews DR, Hosker JP, Rudenski AS, et al Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 26. Kosaka K, Hagura R, Kuzuya T, et al Insulin secretory response of diabetics during the period of improvement of glucose tolerance to normal range. Diabetologia 1974; 10: 775–782. [DOI] [PubMed] [Google Scholar]
- 27. Retnakaran R, Qi Y, Goran MI, et al Evaluation of proposed oral disposition index measures in relation to the actual disposition index. Diabet Med 2009; 26: 1198–1203. [DOI] [PubMed] [Google Scholar]
- 28. Kasuga Y, Hata K, Tajima A, et al Association of common polymorphisms with gestational diabetes mellitus in Japanese women: a case‐control study. Endocr J 2017; 64: 463–475. [DOI] [PubMed] [Google Scholar]
- 29. O'Reilly MW, Avalos G, Dennedy MC, et al Atlantic DIP: high prevalence of abnormal glucose tolerance postpartum is reduced by breast‐feeding in women with prior gestational diabetes mellitus. Eur J Endocrinol 2011; 165: 953–959. [DOI] [PubMed] [Google Scholar]
- 30. Sinha B, Brydon P, Taylor RS, et al Maternal antenatal parameters as predictors of persistent postnatal glucose intolerance: a comparative study between Afro‐Caribbeans, Asians, and Caucasians. Diabet Med 2003; 20: 382–386. [DOI] [PubMed] [Google Scholar]
- 31. Hivert MF, Manning AK, McAteer JB, et al Common variants in the adiponectin gene (ADIPOQ) associated with plasma adiponectin levels, type 2 diabetes, and diabetes‐related quantitative traits: the Framingham Offspring Study. Diabetes 2008; 57: 3353–3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ellard S, Bellanné‐Chantelot C, Hattersley AT, et al Best practice guidelines for the molecular genetic diagnosis of maturity‐onset diabetes of the young. Diabetologia 2008; 51: 546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pearson ER, Boj SF, Steele AM, et al Macrosomia and hyperinsulinaemic hypoglycaemia in patients with heterozygous mutations in the HNF4A gene. PLoS Med 2007; 4: e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Haghvirdizadeh P, Mohamed Z, Abdullah NA, et al KCNJ11: genetic polymorphisms and risk of diabetes mellitus. J Diabetes Res 2015; 2015: 908152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Phani NM, Guddattu V, Bellampalli R, et al Population specific impact of genetic variants in KCNJ11 gene to type 2 diabetes: a case‐control and meta‐analysis study. PLoS ONE 2014; 9: e107021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morris AP, Voight BF, Teslovich TM, et al Large‐scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet 2012; 44: 981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Locke JM, Hysenaj G, Wood AR, et al Targeted allelic expression profiling in human islets identifies cis‐regulatory effects for multiple variants identified by type 2 diabetes genome‐wide association studies. Diabetes 2015; 64: 1484–1491. [DOI] [PubMed] [Google Scholar]
- 38. Yabe D, Seino Y. Type 2 diabetes via beta‐cell dysfunction in east Asian people. Lancet Diabetes Endocrinol 2016; 4: 2–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 ¦ Primers designed by Assay Design Suite in the present study.
Table S2 ¦ Linear regression analysis between four single‐nucleotide polymorphisms and postpartum metabolic features.


