Abstract
Aims/Introduction
Recent studies advocate that omega‐3 polyunsaturated fatty acids (ω‐3 PUFAs) have direct anti‐oxidative and anti‐inflammatory effects in the vasculature; however, the role of ω‐3 PUFAs in Schwann cells remains undetermined.
Materials and methods
Immortalized mouse Schwann (IMS32) cells were incubated with the ω‐3 PUFAs docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA). The messenger ribonucleic acid levels of several anti‐oxidant enzymes (heme oxygenase‐1 [Ho‐1], nicotinamide adenine dinucleotide [phosphate] H quinone oxidoreductase 1, catalase, superoxide dismutase and glutathione peroxidase) were identified using real‐time reverse transcription polymerase chain reaction. Ho‐1 and nicotinamide adenine dinucleotide [phosphate] H quinone oxidoreductase 1 protein levels were evaluated using Western blotting. Nuclear factor (erythroid‐derived 2)‐related factor 2 (Nrf2) of the nuclear fraction was also quantified using western blotting. Catalase activity and glutathione content were determined by colorimetric assay kits. Nrf2 promoter‐luciferase activity was evaluated by a dual luciferase assay system.
Results
Treatment with tert‐butyl hydroperoxide decreased cell viability dose‐dependently. DHA or EPA pretreatment significantly alleviated tert‐butyl hydroperoxide‐induced cytotoxicity. DHA or EPA increased the messenger ribonucleic acid levels of Ho‐1, nicotinamide adenine dinucleotide (phosphate) H quinone oxidoreductase 1 and catalase dose‐dependently. Ho‐1 protein level, catalase activity, Nrf2 promoter‐luciferase activity and intracellular glutathione content were significantly increased by DHA and EPA.
Conclusions
These findings show that DHA and EPA can induce Ho‐1 and catalase through Nrf2, thus protecting Schwann cells against oxidative stress. ω‐3 PUFAs appear to exert their neuroprotective effect by increasing defense mechanisms against oxidative stress in diabetic neuropathies.
Keywords: Diabetic neuropathy, Omega‐3 polyunsaturated fatty acids, Oxidative stress
Introduction
Diabetic peripheral neuropathies, which are the most prevalent chronic complications that affect patients with diabetes, decrease quality of life and increase morbidity1. The metabolic mechanisms responsible for diabetic complications as a result of hyperglycemia include oxidative stress upregulation2, protein kinase C abnormalities3, non‐enzymatic glycation endproduct increase4 and polyol pathway hyperactivity5. Oxidative stress is specifically considered the final common pathway of cellular injury in hyperglycemic conditions6.
Reportedly, omega‐3 polyunsaturated fatty acids (ω‐3 PUFAs), such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) occurring in fish oil, decreased cardiovascular events in clinical studies of Eskimos7. Recent large‐scale studies also reported that ω‐3 PUFAs reduce the risk of cardiovascular disease8, 9. Several studies have shown that ω‐3 PUFAs have anti‐inflammatory10, 11 and anti‐oxidant effects12. DHA upregulates anti‐oxidant enzymes in human umbilical vein endothelial cells (HUVECs)13, and both DHA and EPA can prevent H2O2‐induced cytotoxicity in 3T3‐L1 adipocytes14. Furthermore, there is evidence that DHA and EPA can decrease oxidative stress in patients with type 2 diabetes15. Despite interest in the anti‐oxidative actions of ω‐3 PUFAs, their effects on neural cells, such as Schwann cells, have not been elucidated.
Nuclear factor (erythroid‐derived 2)‐related factor 2 (Nrf2) exerts significant cytoprotective effects on oxidative stress through the Nrf2–anti‐oxidant response element (ARE) pathway16, 17. Nrf2 pathway activation initiates the transcriptional regulation of ARE‐dependent expression of diverse anti‐oxidants and phase II detoxification enzymes, including heme oxygenase‐1 (Ho‐1) and nicotinamide adenine dinucleotide (phosphate) H quinone oxidoreductase 1 (Nqo1)18. Furthermore, the overexpression of Nrf2 reportedly inhibits apoptosis that is induced by high glucose in Schwann cells, and the transplantation of Nrf2‐overexpressing Schwann cells can recover nerve functions in diabetic animals19. Therefore, Nrf2 has become a therapeutic target in diabetic peripheral neuropathy.
Schwann cells are glial cells of the peripheral nervous system, and support neurons and maintain the structural and functional integrity of nerves. In diabetes, Schwann cells themselves undergo hyperglycemic insults20 and the supporting function is disrupted, which results in peripheral nerve dysfunctions21, 22. The disruption of Schwann cell mitochondrial function in connection with glial support can cause primary neuronal degeneration, indicating that Schwann cell dysfunction has direct effects on neuronal function23. Thus, Schwann cells and its cell line, such as immortalized mouse Schwann (IMS32) cells, are extensively applied for in vitro models of diabetic neuropathy20, 24.
We investigated whether ω‐3 PUFAs might induce the expression of the anti‐oxidant enzymes through the Nrf2 pathway and suppress the oxidative stress‐induced Schwann cell death.
Methods
The present study was an in vitro study and ethics approval was unnecessary.
Reagents
Bovine serum albumin (BSA) was obtained from BBI Solutions (Cardiff, UK). DHA, EPA and catalase assay kits were purchased from Cayman (Ann Arbor, MI, USA). Low‐glucose Dulbecco's modified Eagle's medium, tert‐butyl hydroperoxide (tBHP) and MTT were purchased from Sigma‐Aldrich (St. Louis, MO, USA). Fetal bovine serum was purchased from Gibco (Poisley, UK). Anti‐Ho‐1 antibody was purchased from Assay Design (Ann Arbor, MI, USA). Anti‐Nqo1 antibody was obtained from Abcam (Cambridge, UK). Anti‐Nrf2 antibody, anti‐β‐actin and anti‐lamin A/C antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The glutathione assay kit was purchased from OxisResearch (Foster City, CA, USA). CellROX Deep Red reagent was purchased from Invitrogen (Carlsbad, CA). Other reagents and chemicals were obtained from standard suppliers.
Cell culture
Immortalized mouse Schwann (IMS32) cells were willingly provided by Professor Kazuhiko Watabe (Kyorin University, Tokyo, Japan). IMS32 cells were cultured in low‐glucose Dulbecco's modified Eagle's medium containing 5% fetal bovine serum at 37°C in 5% CO2/95% air.
Fatty acid preparation
DHA and EPA were separately prepared as complexes with BSA. DHA and EPA (75 μmol/L) were dissolved in ethanol and gradually solubilized in 2.6 mmol/L fatty acid‐free BSA solution. BSA‐conjugated fatty acids were lysed in Dulbecco's modified Eagle's medium at the final desired concentrations.
MTT assay
IMS32 cells were cultured in 96‐well plates. To identify the effects of DHA and EPA on tBHP‐induced cytotoxicity, the cells were exposed to DHA (2.5–25 μmol/L) or EPA (2.5–25 μmol/L) for 16 h, followed by incubation with 50 μmol/L tBHP for 6 h. Cell viability was measured by conventional MTT assay25. Cells were treated with 0.5 mg/mL MTT in the medium for 3 h. The medium was then discarded, the formazan product was solubilized by dimethyl sulfoxide and the absorbance at 570 nm was determined using a microplate reader. Values are presented as percentages of cell survival. Absorbance of the control cells was set at 100%.
Quantitative real‐time reverse transcription polymerase chain reaction
Total RNA was prepared using the Nucleospin RNA kit (Macherey‐Nagel GmbH & Co., KG Düren, Germany). Single‐stranded complementary deoxyribonucleic acid was prepared from 0.5 μg total ribonucleic acid (RNA) using the PrimeScript RT reagent kit (Takara Bio, Shiga, Japan). Quantitative analyses of heme oxygenase‐1 (Ho‐1), nicotinamide adenine dinucleotide [phosphate] H quinone oxidoreductase 1 (Nqo1), catalase (Cat), superoxide dismutase (Sod) and glutathione peroxidase (Gpx) messenger RNAs (mRNAs) were carried out using real‐time reverse transcription polymerase chain reaction with the Takara Dice thermal cycler (Takara Bio, Shiga, Japan). The primer pair sequences were as follows: mouse Ho‐1, 5′‐CCTCACTGGCAGGAAATCATC‐3′ and 5′‐CCTCGTGGAGACGCTTTACATA‐3′; mouse Nqo1, 5′‐TATCCTTCCGAGTCATCTCTAGCA‐3′ and 5′‐TCTGCAGCTTCCAGCTTCTT‐3′; mouse Cat, 5′‐GAACGAGGAGGAGAGGAAAC‐3′ and 5′‐TGAAATTCTTGACCGCTTTC‐3′; mouse Sod, 5′‐CAATGGTGGGGGACATATTA‐3′ and 5′‐TTGATAGCCTCCAGCAACTC‐3′; mouse Gpx, 5′‐ACATTCCCAGTCATTCTACC‐3′ and 5′‐TTCAAGCAGGCAGATACG‐3′; and mouse glyceraldehyde 3‐phosphate dehydrogenase (Gapdh), 5′‐AACTTTGGCATTGTGGAAGG‐3′ and 5′‐GGATGCAGGGATGATGTTCT‐3′. Relative quantities were calculated by the 2(−ΔΔCt) method26.
Western blotting
Whole‐cell lysates from IMS32 cells were extracted in radioimmunoprecipitation assay buffer containing protease inhibitors (Sigma‐Aldrich). Nuclear and cytoplasmic proteins were extracted using NE‐PER™ Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific, Rockford, IL, USA). An equal amount of the proteins was loaded, separated by sodium dodecyl sulfate‐polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane (Millipore Company, Bedford, MA, USA). The membranes were incubated with anti‐Ho‐1, anti‐Nqo1, anti‐Nrf2, anti‐β‐actin or anti‐lamin A/C, followed by horseradish peroxidase‐conjugated second antibody (GE Healthcare, Buckinghamshire, UK) and detected using ECL Prime (GE Healthcare). To quantify the relative Ho‐1, Nqo1, Nrf2, β‐actin and lamin A/C protein levels, stained band intensity was determined using ImageJ software (developed at the National Institutes of Health, Bethesda, MD, USA).
Assessment of cat activity
Cat activity was determined using a catalase assay kit (Cayman), according to the instructions.
Intracellular glutathione and glutathione disulfide
Glutathione (GSH) and glutathione disulfide (GSSG) content were determined using a Bioxytech GSH/GSSG‐412 colorimetric assay kit (OxisResearch), according to the instructions.
Measurement of reactive oxygen species
Reactive oxygen species were determined using CellROX Deep Red reagent (Invitrogen) according to the manufacturer's instructions.
Cell transfection and luciferase assay
The ARE‐luciferase reporter plasmid (pNL[NlucP/ARE/Hygro]) was purchased from Promega (Madison, WI, USA). Cells were cotransfected with 0.2 μg of luciferase expression plasmid and 0.05 μg of pGL 4.53 (luc2/PGK) plasmid (Promega) using Lipofectamine 3000 (Invitrogen, Carlsbad, CA) for 24 h to normalize the transfection efficiency. Cells were then exposed to DHA or EPA for 12 h, and Firefly and Renilla luciferase activities were measured by the Dual Luciferase Reporter assay system (Promega).
Statistical analysis
Data are presented as the mean ± standard error for the indicated number of experiments. Statistical analyses were carried out by one‐way analysis of variance (anova), and the Tukey–Kramer correction for multiple comparisons. The statistical significance of differences between the control (BSA only) and DHA or EPA‐treated cells was analyzed using one‐way anova followed by Dunnett's multiple‐comparison test. P‐values <0.05 were considered to show statistical significance.
Results
Preventive effects of DHA and EPA on oxidative stress‐induced cytotoxicity
As ω‐3 PUFAs are known to exert anti‐oxidant effects in HUVECs13 and adipocytes14, we evaluated whether ω‐3 PUFAs also have anti‐oxidative effects in IMS32 cells. tBHP was used to induce oxidative stress. Treatment with 5–50 μmol/L tBHP for 6 h elicited cell toxicity in a dose‐dependent manner (Figure 1a). The cytoprotective effects of DHA and EPA against tBHP were investigated in IMS32 cells. DHA or EPA pretreatment for 16 h significantly prevented tBHP‐induced cytotoxicity in a dose‐dependent manner (Figure 1b,c; black bars). Neither DHA nor EPA alone altered cell viability compared with the BSA control (Figure 1b,c; white bars).
Figure 1.
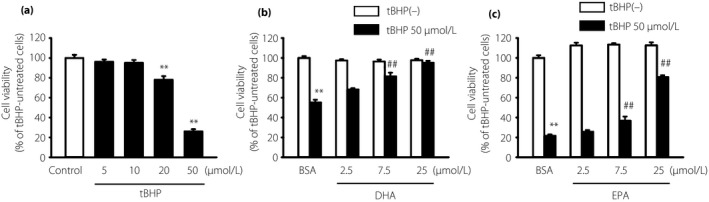
Pre‐emptive effects of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) on the oxidative stress‐induced cytotoxicity in immortalized mouse Schwann (IMS32) cells. (a) IMS32 cells were cultured with the indicated concentrations of tert‐butyl hydroperoxide (tBHP) for 6 h. IMS32 cells were pretreated with the indicated (b) DHA or (c) EPA concentration for 16 h, followed by stimulation with 50 μmol/L tBHP for 6 h. Cell viability was determined by the MTT assay. Each value represents the mean ± standard error of six experiments. **P < 0.01 compared with the tBHP‐untreated bovine serum albumin (BSA); ## P < 0.01 compared with the 50 μmol/L tBHP BSA control.
Effects of DHA and EPA on anti‐oxidant enzyme expression
We measured the mRNA levels of various anti‐oxidant enzymes to explore the protective mechanisms of DHA and EPA against oxidative stress. Both DHA and EPA increased the mRNA levels of Ho‐1, Nqo1 and Cat in a dose‐dependent manner (Figure 2a–c,f–h); however, DHA and EPA treatment did not have any effect on the mRNA levels of Sod and Gpx (Figure 2d,e,i,j). The mRNA levels of Ho‐1 increased maximally between 3 and 6 h after treatment with 7.5 μmol/L DHA (Figure 3a) or 25 μmol/L EPA (Figure 3b), whereas the mRNA levels of Nqo1 and Cat showed maximal increases at 12 h (Figure 3c–f). Furthermore, DHA or EPA treatment for 12 h significantly enhanced Ho‐1 protein levels (Figure 4a,b,d,e). Similarly, treatment with DHA for 12–24 h significantly enhanced the Nqo1 protein level (Figure 4a,c).
Figure 2.
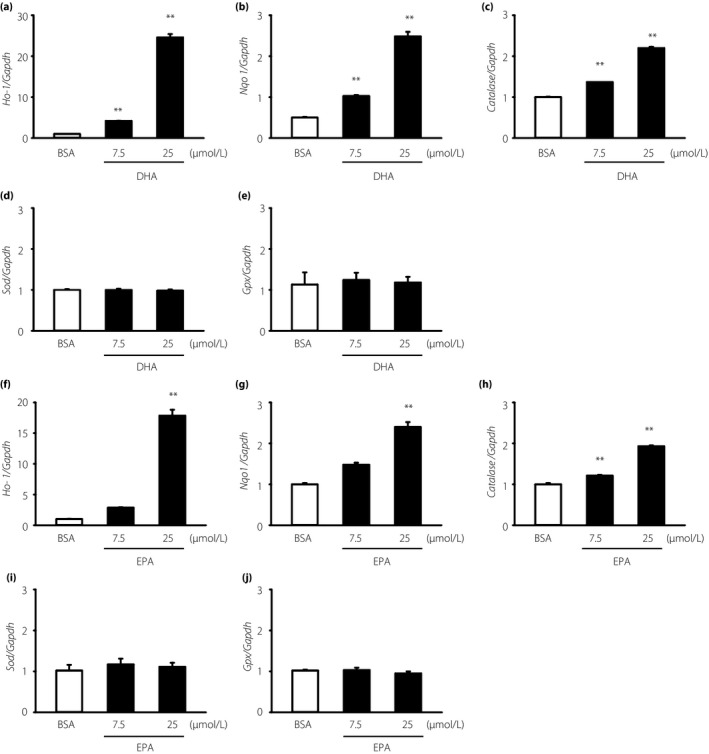
Dose‐dependent effects of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) on the messenger ribonucleic acid levels of Ho‐1, Nqo1 and Catalase in immortalized mouse Schwann (IMS32) cells. IMS32 cells were cultured with the indicated (a–e) DHA or (f–j) EPA concentration for 6 h. Total ribonucleic acid was purified and quantified by real‐time reverse transcription polymerase chain reaction. Each value represents the mean ± standard error of three experiments. **P < 0.01 compared with the bovine serum albumin control. BSA, bovine serum albumin; Gaphd, glyceraldehyde 3‐phosphate dehydrogenase; Gpx, glutathione peroxidase; Ho‐1, heme oxygenase‐1; Nqo1, nicotinamide adenine dinucleotide (phosphate) H quinone oxidoreductase 1; Sod, superoxide dismutase.
Figure 3.
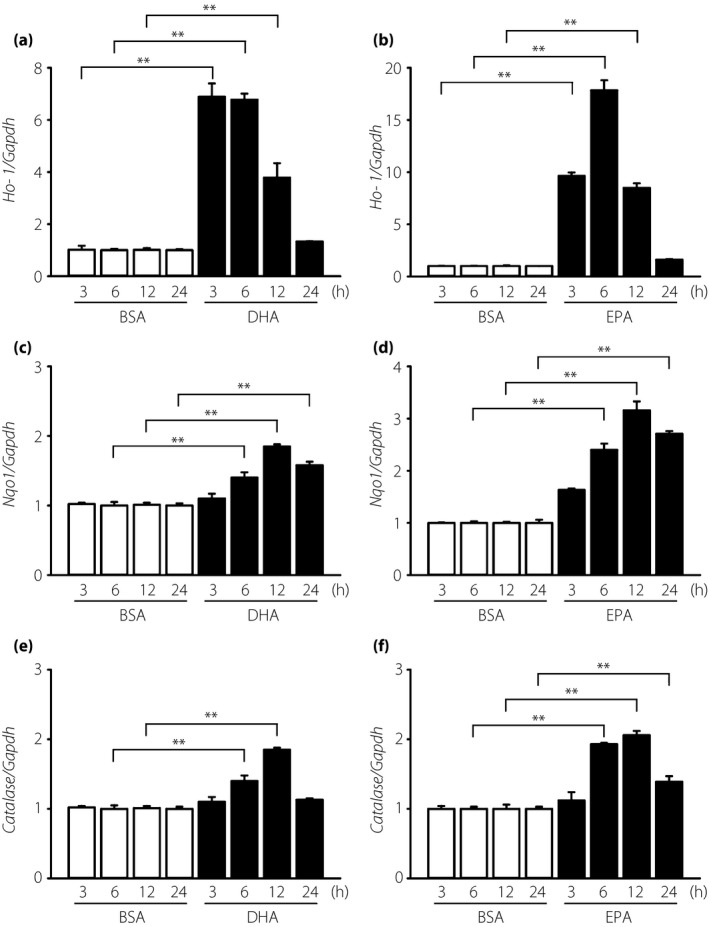
Time‐dependent effects of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) on the messenger ribonucleic acid levels of heme oxygenase‐1 (Ho‐1), nicotinamide adenine dinucleotide (phosphate) H quinone oxidoreductase 1 (Nqo1) and Catalase in immortalized mouse Schwann (IMS32) cells. IMS32 cells were cultured with (a,c,e) 7.5 μmol/L DHA or (b,d,f) 25 μmol/L EPA for the indicated times. Total ribonucleic acid was purified and quantified by real‐time reverse transcription polymerase chain reaction. Each value represents the mean ± standard error of three experiments. **P < 0.01 compared with each bovine serum albumin control. BSA, bovine serum albumin; Gaphd, glyceraldehyde 3‐phosphate dehydrogenase.
Figure 4.
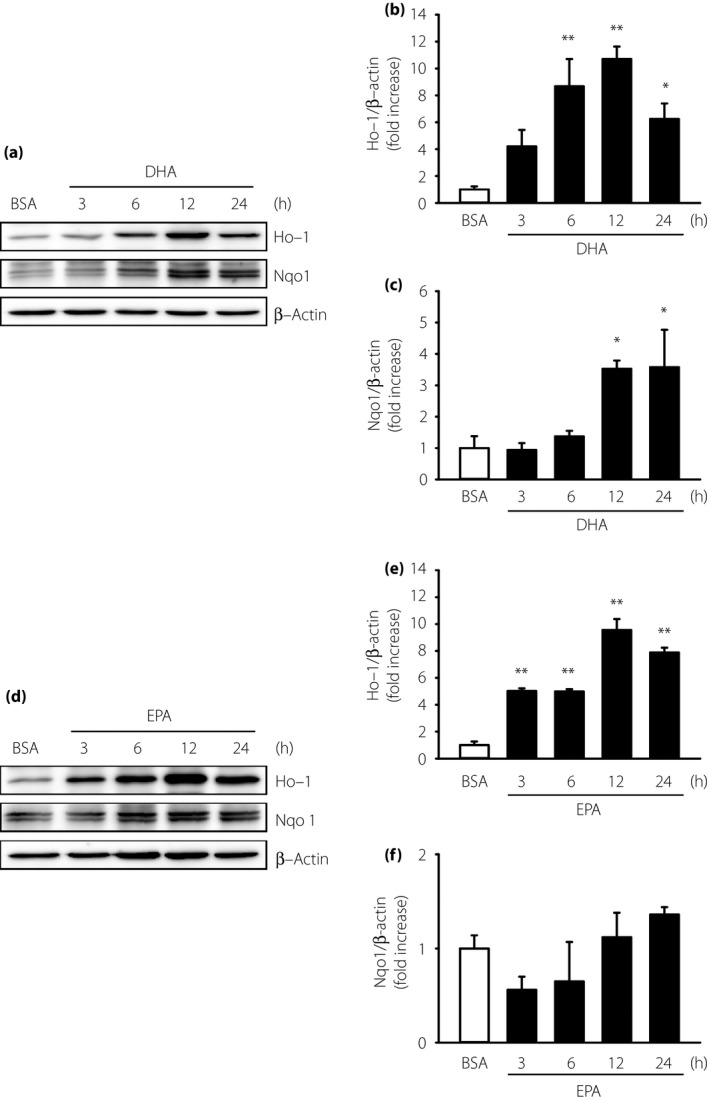
Effects of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) on the protein expression of heme oxygenase‐1 (Ho‐1), nicotinamide adenine dinucleotide (phosphate) H quinone oxidoreductase 1 (Nqo1) in immortalized mouse Schwann (IMS32) cells. IMS32 cells were cultured with (a–c) 7.5 μmol/L DHA or (d–f) 25 μmol/L EPA for the indicated times. Total cell lysates were used for western blotting analyses. Each value represents the mean ± standard error of three experiments. *P < 0.05, **P < 0.01 compared with each bovine serum albumin (BSA) control (one‐way anova and Dunnett's test).
Effects of DHA and EPA on catalase activity
Because we previously reported that either DHA or EPA could increase the mRNA levels of Cat, we then measured the catalase activity in IMS32 cells. DHA or EPA treatment for 12 h significantly enhanced the catalase activity by 1.7‐ and 1.6‐fold, respectively, compared with the controls (Figure 5).
Figure 5.
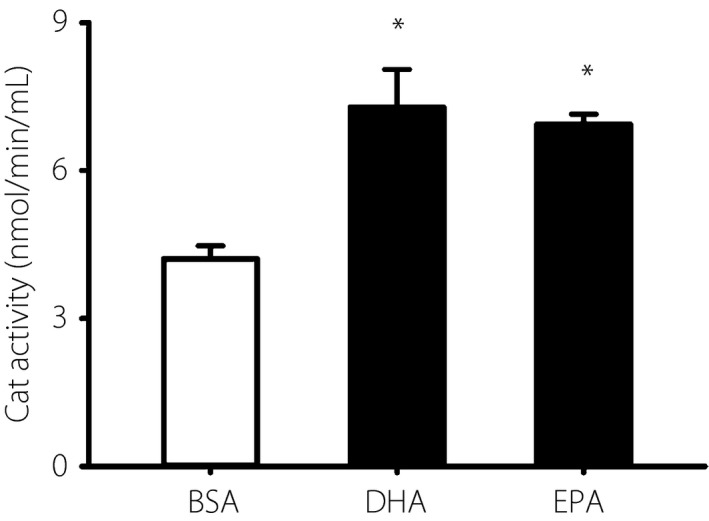
Effects of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) on catalase (Cat) activity in immortalized mouse Schwann (IMS32) cells. IMS32 cells were cultured with 7.5 μmol/L DHA or 25 μmol/L EPA for 12 h. The catalase activity was analyzed with a catalase assay kit. Each value represents the mean ± standard error of four experiments. *P < 0.05 compared with each bovine serum albumin (BSA) control.
Effects of DHA and EPA on redox homeostasis
As both DHA and EPA could induce anti‐oxidant enzymes in IMS32 cells, we measured the GSH and GSSG content to determine whether the protective effects of DHA and EPA were involved in GSH metabolism. Figure 6a–c shows the intracellular GSH and GSSG content, and the GSH/GSSG ratio, respectively. DHA or EPA treatment for 12 h significantly enhanced the intracellular GSH content by 1.5‐ and 2.7‐fold, respectively, compared with the controls (Figure 6a); however, the GSSG content remained unchanged after either DHA or EPA treatment (Figure 6b). The GSH/GSSG ratio was enhanced after either DHA or EPA treatment compared with the controls (Figure 6c). Treatment with 50 μmol/L tBHP for 90 min significantly increased reactive oxygen species compared with the BSA control. The increase in reactive oxygen species by 50 μmol/L tBHP was suppressed by pretreatment with 7.5 μmol/L DHA or 25 μmol/L EPA for 16 h (Figure 6d).
Figure 6.
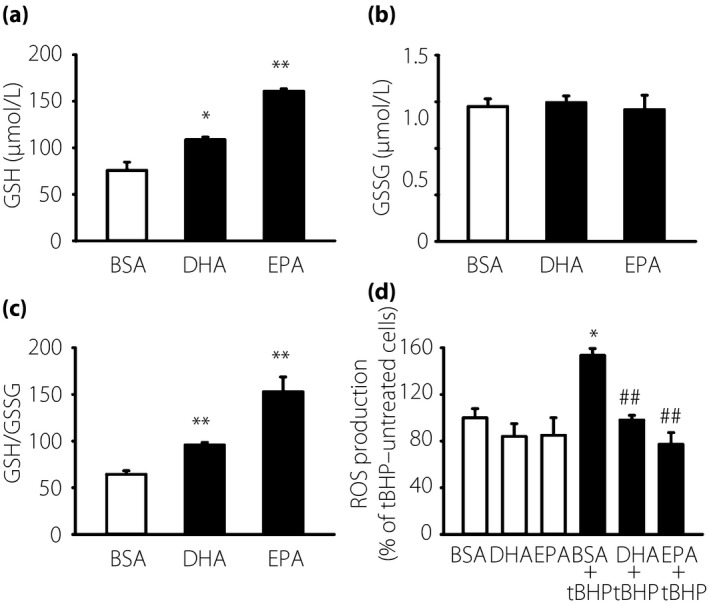
Anti‐oxidant effects of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) in immortalized mouse Schwann (IMS32) cells. IMS32 cells were cultured with 7.5 μmol/L DHA or 25 μmol/L EPA for 12 h. (a) Glutathione (GSH) content, (b) glutathione disulfide (GSSG) contents and (c) the GSH/GSSG ratio were determined with a GSH assay kit. Each value represents the mean ± standard error of three experiments. *P < 0.05, **P < 0.01 compared with each bovine serum albumin (BSA) control. (d) Measurement of reactive oxygen species (ROS) was determined using a fluorescence plate reader (Techan, Germany) at excitation/emission of 630/670 nm. Each value represents the mean ± standard error of four experiments. *P < 0.05 compared with each BSA control; ## P < 0.01 compared with the 50 μmol/L tert‐butyl hydroperoxide (tBHP) BSA control.
Effects of DHA and EPA on Nrf2 activation
Nrf2 is a key transcription factor responsible for the upregulation of anti‐oxidant enzymes, including Ho‐1, Nqo1 and Cat. To investigate whether Nrf2 is activated by DHA or EPA, we measured the ARE‐luciferase activity and Nrf2 translocation into nuclei in IMS32 cells. Luciferase activities induced by DHA and EPA were evaluated by the ARE‐luciferase expression plasmid in IMS32 cells. DHA and EPA significantly increased the ARE‐luciferase activities by 2.8‐ and 3.1‐fold, respectively, compared with the controls (Figure 7a). Nrf2 translocation into the nuclei was confirmed by western blotting of the nuclear and cytosolic fractions. DHA or EPA treatment for 3 and 6 h significantly increased Nrf2 in the nuclear fraction (Figure 7b,d). Conversely, DHA or EPA treatment for 3 and 6 h significantly decreased Nrf2 in the cytosolic fraction (Figure 7c,e). These data suggest that DHA or EPA can both increase Nrf2 nuclear translocation and that Nrf2 binds to ARE.
Figure 7.
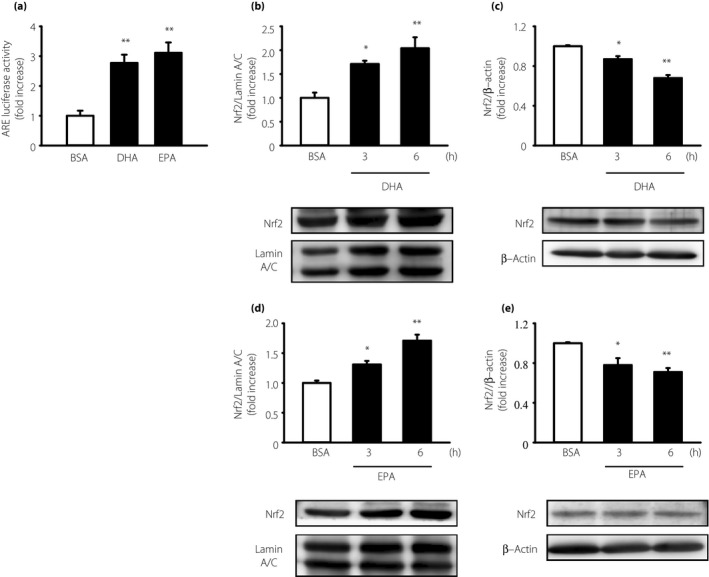
Activation of nuclear factor (erythroid‐derived 2)‐related factor 2 (Nrf2) by docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) in immortalized mouse Schwann (IMS32) cells. (a) IMS32 cells were cotransfected with a reporter plasmid (pNL[NlucP/ARE/Hygro]) and a control plasmid (pGL4.53[luc2/PGK]). After transfection, IMS32 cells were cultured with 7.5 μmol/L DHA or 25 μmol/L EPA for 12 h. Each value represents the mean ± standard error of three experiments. **P < 0.01 compared with the bovine serum albumin (BSA) control. IMS32 cells were cultured with (b,c) 7.5 μmol/L DHA or (d,e) 25 μmol/L EPA for the indicated times. Nuclear and cytosolic proteins were used for western blotting analyses. Each value represents the mean ± standard error of three experiments. *P < 0.05, **P < 0.01 compared with each BSA control. ARE, anti‐oxidant response element.
Discussion
In the present work, we showed that ω‐3 PUFAs exert anti‐oxidant effects on IMS32 cells. ω‐3 PUFAs stimulate Nrf2 translocation from the cytoplasm into the nucleus, where it binds to ARE and initiates the expression of anti‐oxidant enzymes, including Ho‐1, Nqo1 and Cat, in IMS32 cells. Furthermore, we identified the preventive effects of ω‐3 PUFAs against the oxidative stress‐induced cell death. The present findings are consistent with recent studies suggesting that the anti‐oxidant effects are induced by ω‐3 PUFAs in HUVECs13 and 3T3‐L1 adipocytes14. ω‐3 PUFAs suppress inflammatory responses by inhibiting the arachidonate cascade in neutrophils27, and the stimulation of the G protein‐coupled receptor 120 by DHA exerts anti‐inflammatory effects in macrophages28. Recent studies report the anti‐oxidant effects of 4‐hydroxy‐2‐hexanal (4‐HHE), resolvin E1 and protectin D1, which are intracellular metabolites of ω‐3 PUFAs13, 29, 30, 31, 32, 33. We also examined the effects of 4‐HHE on the mRNA levels of anti‐oxidant enzymes and found that 4‐HHE increased the mRNA levels of Ho‐1, Nqo‐1 and Cat (data not shown). Future studies should investigate whether ω‐3 PUFAs are involved in the arachidonate cascade, the G protein‐coupled receptor 120 pathway or 4‐HHE.
DHA and EPA can induce the expression of the same anti‐oxidant enzymes, including Ho‐1, Nqo1 and Cat; however, DHA concentrations that induce the expression of these enzymes differ from those of EPA (Figure 2). DHA increases the mRNA levels of Ho‐1, Nqo1 and Cat at 7.5 μmol/L. In contrast, EPA increases only the mRNA levels of Cat at 7.5 μmol/L, whereas 25 μmol/L EPA is required to increase that of Ho‐1 and Nqo1. It has been suggested that DHA induces anti‐oxidant enzymes more potently than EPA. The differences between DHA and EPA might result from differences in their metabolism14. Studies13, 14 have reported that DHA and EPA increase the mRNA levels of Ho‐1 and Nqo1 in HUVECs and 3T3‐L1 cells, respectively; however, the increase in the mRNA levels of Cat was confirmed in both cell types. The present results showed that both DHA and EPA can increase the mRNA levels of Cat, Ho‐1 and Nqo1 in neural cells. We suggest that differences in the induction of anti‐oxidant enzyme expression might depend on the cell or tissue type being examined.
Both DHA and EPA can induce the expression of Ho‐1 mRNA within 3 h, peaking at 6 h. Furthermore, the expression of Nqo1 mRNA was induced at 6 h and peaked at 12 h (Figure 3). In our results, mRNA time lapse and DHA or EPA protein expression was different. As there are several processes for transcription or translation, the time for transcription or translation is known to vary34, and we speculate that these processes differ between DHA and EPA. The time required for ω‐3 PUFAs to be metabolized into 4‐HHE might have reportedly caused this time lag14. The different induction times might be responsible for the difference in timing of anti‐oxidant effects after Nrf2/ARE pathway activation.
The present results showed that the levels of GSH, an anti‐oxidant, were increased by both DHA and EPA (Figure 6). Glutathione was synthesized by glutamate cysteine ligase modifier subunit (GCLM) and glutathione reductase (GR). Lee et al.35 reported that DHA prevents cell death induced by paraquat in dopaminergic SN4741 cells. These protective effects against paraquat‐induced oxidative stress suggest that DHA increases the mRNA levels of GR and GCLM through Nrf2, and enhances the accumulation of intracellular GSH35. We found the same results for GCLM protein (data not shown). Therefore, DHA and EPA increase GSH levels, possibly enhancing their preventive effects against oxidative stress in Schwann cells.
We showed that DHA and EPA increased ARE‐luciferase activity by 2.8‐ and 3.1‐fold, respectively, compared with controls (Figure 7). Ishikado et al.13 reported that DHA increased ARE‐luciferase activity by 50‐fold in HUVECs; the extent of the increase was much higher than that seen in the present results. The difference in the extent of the ARE‐luciferase activity induction might be due to low plasmid transfection efficiency in neural cells. In the present study, DHA and EPA both showed a weak induction of ARE‐luciferase activity in IMS32 cells; however, we confirmed that both DHA and EPA can induce Nrf2 translocation, increase the mRNA levels of Ho‐1, Nqo1 and Cat, and stimulate Ho‐1 protein levels. Collectively, DHA and EPA exert anti‐oxidant effects through the Nrf2/ARE pathway in IMS32 cells.
Nerve conduction velocity and nerve blood flow can be restored by DHA administration in diabetic rats36, 37. DHA entered the sciatic nerve in an in vivo study36. Additionally, Na+, K+‐ATPase activity of red blood cells was elevated by DHA36. Recently, ω‐3 PUFAs supplementation for 12 months was shown to increase the corneal nerve fiber length by 29% in patients with type 1 diabetes38. We speculate that the induction of anti‐oxidant enzymes in neural cells contribute, at least partly, to improving diabetic neuropathy observed in previous studies.
Previously, DHA and EPA were used at high concentrations (75–100 μmol/L)13, 14, 28. The maximum concentrations of a single oral dose of 2 g ethyl ω‐3 fatty acid capsules (comprising EPA and DHA) are as follows: 58.1 μg/mL EPA (192 μmol/L) and 115.0 μg/mL DHA (350 μmol/L). In the present study, 25 μmol/L EPA or 7.5 μmol/L DHA protected neural cells from oxidative stress. The 25 μmol/L EPA and 7.5 μmol/L DHA doses used in the present study were approximately 1/8 and 1/45 of the maximum concentrations of a single oral dose of EPA and DHA capsules, respectively. Therefore, DHA or EPA concentrations used in the present study might be able to exert sufficient anti‐oxidant effects in peripheral nerves.
Diabetic neuropathy progresses through multiple mechanisms, including oxidative stress upregulation39, 40, protein kinase C abnormalities41, increased non‐enzymatic glycation end‐products42 and polyol pathway activation43. Of these, oxidative stress is recognized as the most significant common pathway of these pathogenic mechanisms6.
Hyperglycemia‐enhanced oxidative stress is exacerbated by a concomitant reduction in endogenous anti‐oxidant defenses39. Genetic variations and polymorphisms in endogenous anti‐oxidant enzymes are associated with an increased diabetic neuropathy44, 45. Thus, the enhancement of endogenous anti‐oxidant enzymes in nerve tissues might help prevent diabetic neuropathy.
In the present study, we showed that both DHA and EPA can prevent cell death by inducing numerous anti‐oxidants in Schwann cells. The present findings suggest that the enhancement of anti‐oxidative defenses facilitated by ω‐3 PUFAs might have clinical values in diabetic neuropathy treatment.
Disclosure
YT and AK both received scholarship grants from Shonan. KS received a clinical research grant from Sumitomo Dainippon Pharma. TH and MK both received scholarship grants from Kyowa Hakko Kirin, MSD, Mitsubishi Tanabe Pharma and Novartis Pharma. YK received scholarship grants from Kyowa Hakko Kirin, MSD and Mitsubishi Tanabe Pharma. HK received honoraria for lectures from Eli Lilly, Ono, Novartis Pharma, Astellas, MSD, Sanofi and Mitsubishi Tanabe; and scholarship grants from Kyowa Hakko Kirin, MSD and Mitsubishi Tanabe Pharma. JN received honoraria for lectures from Kyowa Hakko Kirin, Ono, Pfizer, Eli Lilly, Sanofi, MSD, Taisho Toyama, Mitsubishi Tanabe, Astellas and Sanofi; clinical research grants from Fukuda Colin, Sumitomo Dainippon Pharma, Daiichi Sankyo and Kissei Pharmaceutical; and scholarship grants from Astellas, AstraZeneca, Johnson & Johnson, Daiichi Sankyo, Eli Lilly, Kyowa Hakko Kirin, Taisho Toyama Pharmaceutical, MSD, Mitsubishi Tanabe Pharma, Sanofi, Takeda, Japan Tobacco, Novo Nordisk Pharma, Ono, Novartis Pharma, Sanwa Kagaku Kenkyusho, Kowa Pharmaceutical, Sumitomo Dainippon Pharma and Nippon Boehringer Ingelheim. KK received scholarship grants from Eli Lilly, Takeda and Shonan.
Acknowledgments
This research was supported in part by a Grant‐in‐Aid for Scientific Research (16K09768) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT). We thank an undergraduate student, Ms Mio Mano (Laboratory of Medicine, Aichi Gakuin University School of Pharmacy).
J Diabetes Investig 2019; 10: 602–612
References
- 1. Vinik AI, Park TS, Stansberry KB, et al Diabetic neuropathies. Diabetologia 2000; 43: 957–973. [DOI] [PubMed] [Google Scholar]
- 2. Tesfamariam B. Free radicals in diabetic endothelial cell dysfunction. Free Radic Biol Med 1994; 16: 383–391. [DOI] [PubMed] [Google Scholar]
- 3. Koya D, King GL. Protein kinase C activation and the development of diabetic complications. Diabetes 1998; 47: 859–866. [DOI] [PubMed] [Google Scholar]
- 4. Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med 1988; 318: 1315–1321. [DOI] [PubMed] [Google Scholar]
- 5. Kasuya Y, Ito M, Nakamura J, et al An aldose redutase inhibitor prevents the intimal thickening in coronary arteries of galactose‐fed beagle dogs. Diabetologia 1999; 42: 1404–1409. [DOI] [PubMed] [Google Scholar]
- 6. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001; 414: 813–820. [DOI] [PubMed] [Google Scholar]
- 7. Kromhout D, Bosschieter EB, de Lezenne Coulander C. The inverse relation between fish consumption and 20‐year mortality from coronary heart disease. N Engl J Med 1985; 312: 1205–1209. [DOI] [PubMed] [Google Scholar]
- 8. Yokoyama M, Origasa H, Matsuzaki M, et al Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open‐label, blinded endpoint analysis. Lancet 2007; 369: 1090–1098. [DOI] [PubMed] [Google Scholar]
- 9. Saito Y, Yokoyama M, Origasa H, et al Effects of EPA on coronary artery disease in hypercholesterolemic patients with multiple risk factors: sub‐analysis of primary prevention cases from the Japan EPA Lipid Intervention Study (JELIS). Atherosclerosis 2008; 200: 135–140. [DOI] [PubMed] [Google Scholar]
- 10. De Caterina R, Cybulsky MI, Clinton SK, et al The omega‐3 fatty acid docosahexaenoate reduces cytokine‐induced expression of proatherogenic and proinflammatory proteins in human endothelial cells. Arterioscler Thromb 1994; 14: 1829–1836. [DOI] [PubMed] [Google Scholar]
- 11. Weber C, Erl W, Pietsch A, et al Docosahexaenoic acid selectively attenuates induction of vascular cell adhesion molecule‐1 and subsequent monocytic cell adhesion to human endothelial cells stimulated by tumor necrosis factor‐alpha. Arterioscler Thromb Vasc Biol 1995; 15: 622–628. [DOI] [PubMed] [Google Scholar]
- 12. Casós K, Zaragozá MC, Zarkovic N, et al A fish‐oil‐rich diet reduces vascular oxidative stress in apoE(‐/‐) mice. Free Radic Res 2010; 44: 821–829. [DOI] [PubMed] [Google Scholar]
- 13. Ishikado A, Morino K, Nishio Y, et al 4‐Hydroxy hexenal derived from docosahexaenoic acid protects endothelial cells via Nrf2 activation. PLoS One 2013; 8: e69415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kusunoki C, Yang L, Yoshizaki T, et al Omega‐3 polyunsaturated fatty acid has an anti‐oxidant effect via the Nrf‐2/HO‐1 pathway in 3T3‐L1 adipocytes. Biochem Biophys Res Commun 2013; 430: 225–230. [DOI] [PubMed] [Google Scholar]
- 15. Mori TA, Woodman RJ, Burke V, et al Effect of eicosapentaenoic acid and docosahexaenoic acid on oxidative stress and inflammatory markers in treated‐hypertensive type 2 diabetic subjects. Free Radic Biol Med 2003; 35: 772–781. [DOI] [PubMed] [Google Scholar]
- 16. Johnson JA, Johnson DA, Kraft AD, et al The Nrf2‐ARE pathway: an indicator and modulator of oxidative stress in neurodegeneration. Ann N Y Acad Sci 2008; 1147: 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kraft AD, Johnson DA, Johnson JA. Nuclear factor E2‐related factor 2‐dependent antioxidant response element activation by tert‐butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J Neurosci 2004; 24: 1101–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ma Q. Role of Nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol 2013; 53: 401–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tang W, Chen X, Liu H, et al Expression of Nrf2 promotes schwann cell‐mediated sciatic nerve recovery in diabetic peripheral neuropathy. Cell Physiol Biochem 2018; 46: 1879–1894. [DOI] [PubMed] [Google Scholar]
- 20. Cinci L, Corti F, Di Cesare Mannelli L, et al Oxidative, metabolic, and apoptotic responses of Schwann cells to high glucose levels. J Biochem Mol Toxicol 2015; 29: 274–279. [DOI] [PubMed] [Google Scholar]
- 21. Gonçalves NP, Vægter CB, Andersen H, et al Schwann cell interactions with axons and microvessels in diabetic neuropathy. Nat Rev Neurol 2017; 13: 135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kato K, Feldman E, Nakamura J. Schwann cell development and pathology: pathogenesis of diabetic neuropathy from the pont of view of schwann cell abnormalities. Springer 2014:135–146.
- 23. Viader A, Golden JP, Baloh RH, et al Schwann cell mitochondrial metabolism supports long‐term axonal survival and peripheral nerve function. J Neurosci 2011; 31: 10128–10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sango K, Yanagisawa H, Takaku S, et al Immortalized adult rodent Schwann cells as in vitro models to study diabetic neuropathy. Exp Diabetes Res 2011; 2011: 374943–374952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983; 65: 55–63. [DOI] [PubMed] [Google Scholar]
- 26. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C(T)) Method. Methods 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 27. Sperling RI, Benincaso AI, Knoell CT, et al Dietary omega‐3 polyunsaturated fatty acids inhibit phosphoinositide formation and chemotaxis in neutrophils. J Clin Invest 1993; 91: 651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oh DY, Talukdar S, Bae EJ, et al GPR120 is an omega‐3 fatty acid receptor mediating potent anti‐inflammatory and insulin‐sensitizing effects. Cell 2010; 142: 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bannenberg G, Serhan CN. Specialized pro‐resolving lipid mediators in the inflammatory response: an update. Biochim Biophys Acta 2010; 1801: 1260–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arita M, Bianchini F, Aliberti J, et al Stereochemical assignment, antiinflammatory properties, and receptor for the omega‐3 lipid mediator resolvin E1. J Exp Med 2005; 201: 713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schwab JM, Chiang N, Arita M, et al Resolvin E1 and protectin D1 activate inflammation‐resolution programmes. Nature 2007; 447: 869–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang MJ, Spite M. Resolvins: anti‐inflammatory and proresolving mediators derived from omega‐3 polyunsaturated fatty acids. Annu Rev Nutr 2012; 32: 203–227. [DOI] [PubMed] [Google Scholar]
- 33. Obrosov A, Coppey LJ, Shevalye H, et al Effect of fish oil Vs Resolvin D1, E1, methyl esters of Resolvins D1 or D2 on diabetic peripheral neuropathy. J Neurol Neurophysiol 2017; 8: 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kozak M. Regulation of translation in eukaryotic systems. Annu Rev Cell Dev Biol 1992; 8: 197–225. [DOI] [PubMed] [Google Scholar]
- 35. Lee HJ, Han J, Jang Y, et al Docosahexaenoic acid prevents paraquat‐induced reactive oxygen species production in dopaminergic neurons via enhancement of glutathione homeostasis. Biochem Biophys Res Commun 2015; 457: 95–100. [DOI] [PubMed] [Google Scholar]
- 36. Coste TC, Gerbi A, Vague P, et al Neuroprotective effect of docosahexaenoic acid‐enriched phospholipids in experimental diabetic neuropathy. Diabetes 2003; 52: 2578–2585. [DOI] [PubMed] [Google Scholar]
- 37. Pitel S, Raccah D, Gerbi A, et al At low doses, a gamma‐linolenic acid‐lipoic acid conjugate is more effective than docosahexaenoic acid‐enriched phospholipids in preventing neuropathy in diabetic rats. J Nutr 2007; 137: 368–372. [DOI] [PubMed] [Google Scholar]
- 38. Lewis EJH, Perkins BA, Lovblom LE, et al Effect of omega‐3 supplementation on neuropathy in type 1 diabetes: a 12‐month pilot trial. Neurology 2017; 88: 2294–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vincent AM, Russell JW, Low P, et al Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev 2004; 25: 612–628. [DOI] [PubMed] [Google Scholar]
- 40. Vincent AM, Kato K, McLean LL, et al Sensory neurons and schwann cells respond to oxidative stress by increasing antioxidant defense mechanisms. Antioxid Redox Signal 2009; 11: 425–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cameron NE, Cotter MA, Jack AM. Protein kinase C effects on nerve function, perfusion, Na(+), K(+)‐ATPase activity, and glutathione content in diabetic rats. Diabetologia 1999; 42: 1120–1130. [DOI] [PubMed] [Google Scholar]
- 42. Brownlee M, Cerami A, Vlassara H. Advanced products of nonenzymatic glycosylation and the pathogenesis of diabetic vascular disease. Diabetes Metab Rev 1988; 4: 437–451. [DOI] [PubMed] [Google Scholar]
- 43. Yagihashi S, Yamagishi SI, Wada Ri R, et al Neuropathy in diabetic mice overexpressing human aldose reductase and effects of aldose reductase inhibitor. Brain 2001; 124: 2448–2458. [DOI] [PubMed] [Google Scholar]
- 44. Babizhayev MA, Strokov IA, Nosikov VV, et al The role of oxidative stress in diabetic neuropathy: generation of free radical species in the glycation reaction and gene polymorphisms encoding antioxidant enzymes to genetic susceptibility to diabetic neuropathy in population of type I diabetic patients. Cell Biochem Biophys 2015; 71: 1425–1443. [DOI] [PubMed] [Google Scholar]
- 45. Buraczynska M, Buraczynska K, Dragan M, et al Pro198Leu polymorphism in the glutathione peroxidase 1 gene contributes to diabetic peripheral neuropathy in type 2 diabetes patients. Neuromolecular Med 2017; 19: 147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]


