Abstract
Aims/Introduction
We compared treatment satisfaction in type 2 diabetes patients taking daily and weekly glucagon‐like peptide‐1 receptor agonists.
Materials and Methods
The study was a 12‐week, multicenter, open‐label, prospective, randomized, parallel‐group comparison trial. The participants were Japanese patients with type 2 diabetes being administered with the glucagon‐like peptide‐1 receptor agonist, liraglutide, daily for >3 months. Patients were randomly assigned to either continue taking liraglutide once daily (Lira group) or switch to dulaglutide once weekly (Dula group). The primary outcome was the change in the Diabetes Treatment Satisfaction Questionnaire score from baseline to week 12 in the two groups. The secondary outcomes comprised changes in the Diabetes Therapy‐Related Quality of Life score, body mass and glycemic control.
Results
A total of 33 participants were initially enrolled in the trial, and 31 participants completed the protocol. The change in the Diabetes Treatment Satisfaction Questionnaire score in the Dula group was significantly greater than that in the Lira group (+0.1 ± 4.7 in the Lira group vs +4.9 ± 5.2 in the Dula group; P = 0.013). The change in Diabetes Therapy‐Related Quality of Life score in the Dula group was significantly greater than that in the Lira group (−3.7 ± 6.9 vs +8.9 ± 15.1; P = 0.007). There were no significant differences between groups in the changes in body mass, plasma glucose or glycated hemoglobin.
Conclusions
Weekly administration of dulaglutide was superior to liraglutide with regard to treatment satisfaction in patients with type 2 diabetes, in the absence of any negative effect on glycemic control.
Keywords: Dulaglutide, Treatment satisfaction, Type 2 diabetes
Introduction
The primary goal of diabetes treatment is to prolong the lifespan of patients and to maintain their quality of life (QOL) by preventing diabetes‐related complications. To achieve this goal, patients need to maintain good glycemic and metabolic control, while also being satisfied with the treatment. Patients who are dissatisfied with their treatment are less likely to maintain their treatment1, and non‐compliance with treatments for type 2 diabetes can result in poor glycemic control2.
Glucagon‐like peptide‐1 receptor agonists (GLP‐1 RAs) are administered by injection, and improve glycemic control, blood pressure and lipid profile, while reducing body mass in type 2 diabetes patients3. Several previous studies have shown that patients who are receiving GLP‐1 RAs show better compliance with therapy than those receiving insulin glargine4, 5.
Dulaglutide is a long‐acting GLP‐1 RA that is administered once weekly, and a dose of 0.75 mg was recently approved for the treatment of type 2 diabetes in Japan6. When its efficacy was tested at this dose in Japan, 0.9 mg liraglutide was equivalent for the reduction of glycated hemoglobin (HbA1c) over 26 weeks, with acceptable safety and tolerance7. Although dulaglutide is administered by injection, its use in injection‐naïve patients in clinical practice is likely to be better accepted than GLP‐1 RAs that are administered daily, because the required frequency of injection is less (once a week)8. Furthermore, the needle is pre‐attached by the manufacturer, for the convenience of the patients. Thus, the use of dulaglutide makes the induction of injection therapy easier for patients with type 2 diabetes, and the number of users is rapidly growing in Japan. However, it has not been established whether patient satisfaction and QOL are improved by switching to dulaglutide from daily GLP‐1 RA administration if the patients are already used to daily injections.
To address this issue, we initiated a multicenter randomized control trial to compare satisfaction and QOL in patients who had been treated with liraglutide daily for >3 months and were then either switched to weekly dulaglutide therapy or remained on daily liraglutide therapy. To evaluate treatment satisfaction, we used two methods: the Diabetes Treatment Satisfaction Questionnaire (DTSQ), which is one of the most common questionnaires used worldwide, and the Diabetes Therapy‐Related Quality of Life (DTR‐QOL) questionnaire, which has been recently developed and its reliability and validity confirmed in the previous studies9, 10, 11.
Methods
Protocol
The study was a 12‐week, multicenter, open‐label, prospective, randomized, parallel‐group comparison trial that was carried out at six medical institutions (Hokkaido University Hospital, Tomakomai City Hospital, Kushiro Red Cross Hospital, Manda Memorial Hospital, Kurihara Clinic and Aoki Clinic). Participants were Japanese patients with type 2 diabetes being treated at these centers. After obtaining informed consent from the patients, they were randomly assigned to either continue taking liraglutide (Victoza®; Novo Nordisk Pharma, Copenhagen, Denmark) once daily before breakfast (Lira group) or switch to weekly administration of dulaglutide (Trulicity®; Eli Lilly, Indianapolis, IN, USA and Sumitomo Dainippon Pharma Co., Ltd, Osaka, Japan; Dula group). Randomization of patients and allocation to each treatment group were carried out using a central computer‐based randomization system. Patients were stratified by screening age (<65 or ≥65 years), HbA1c (<7.5 or ≥7.5%) and body mass index (<25 or ≥25 kg/m2).
Study population
Japanese patients with type 2 diabetes were recruited into the trial between December 2016 and April 2017. Signed informed consent was obtained from all the participants. The inclusion criteria were as follows: patients with type 2 diabetes receiving 0.9 mg liraglutide for >3 months, in addition to diet and exercise therapy; age ≥20 years; and HbA1c ≥6.0 and <9.0%. The exclusion criteria were as follows: type 1 diabetes, diabetic ketosis or coma, pregnancy or lactation, current steroid therapy, severe liver or renal dysfunction, hypersensitivity to dulaglutide, current weekly GLP‐1 RA therapy, serious infection or trauma and scheduled surgery during the study.
Patients in the Lira group continued 0.9 mg liraglutide throughout the study, whereas those in the Dula group discontinued liraglutide and started 0.75 mg dulaglutide. For patients who were also receiving insulin injection therapy, insulin doses were adjusted at every clinic visit by the attending physician, based on self‐measured fasting blood glucose (target 100–120 mg/dL). During the study period, diet and exercise therapy were continued. Adverse effects, such as hypoglycemia or gastrointestinal symptoms, were monitored during the trial. Hypoglycemia was defined as blood glucose <70 mg/dL or the presence of symptoms of hypoglycemia.
The primary outcome was the change in the DTSQ score from baseline to week 12, which was compared between the two groups. The DTSQ is a self‐administered questionnaire that assesses patient‐reported outcomes9. The DTSQ includes eight items, and responses are scored on a 7‐point scale, from +6 to 0. The scores of six items of the DTSQ (current treatment, convenience, flexibility, understanding, recommend and continue) were added together to give the overall treatment satisfaction score (range +36–0), with higher scores denoting greater treatment satisfaction. In addition, the perceived frequencies of hyperglycemia and hypoglycemia are assessed in the DTSQ, rated on a scale of +6 (“Most of the time”) to 0 (“Never”). Patients completed a Japanese version of the DTSQ at baseline and in week 1210.
The secondary outcomes comprised changes in DTR‐QOL score, body mass, plasma glucose and HbA1c, from baseline to week 12 as well. The DTR‐QOL was established by Ishii et al.11 to evaluate patient QOL in 1995. The DTR‐QOL includes 29 items, and the responses are scored on a 7‐point scale, from +7 to +1. The assessment contains four domains: domain 1, burden on social activities and daily activities; domain 2, anxiety and dissatisfaction with treatment; domain 3, hypoglycemia; and domain 4, satisfaction with treatment. Total DTR‐QOL score and subscale scores were calculated using the following equation and were converted to a score of 0 (representing the poorest QOL) to 100 (representing perfect QOL), as described previously11.
Statistical analysis
Based on a previous study, which investigated the improvement in DTSQ score after a reduction in the injection frequency12, power calculations determined that a sample size of 14 individuals per group was required to have at least 80% power to detect a difference between treatments. P < 0.05 was considered statistically significant. All tests were two‐sided. Assuming a dropout rate of 10%, the sample size was set at 16 patients per group. The results are expressed as mean ± standard deviation. Differences between the two groups were analyzed for statistical significance using the unpaired t‐test or Mann–Whitney U‐test. As the primary outcome of DTSQ total score was shown to be normally distributed in the Shapiro–Wilk test, an unpaired t‐test was used to compare changes in DTSQ total score. Correlation coefficients were calculated using Spearman's rank‐order correlation. We analyzed using JMP 13 (SAS Institute Inc., Cary, NC, USA).
Ethics statement
This research protocol was approved by the institutional review board of Hokkaido University Hospital Clinical Research and Medical Innovation Center (701‐7636), and conformed to the provisions of the Declaration of Helsinki (as revised in Fortaleza, Brazil, in October 2013). The research was registered at the University Hospital Medical Information Network Center under the identifier UMIN 000024552.
Results
Patient enrollment and baseline characteristics
A total of 33 participants (15 men and 18 women) were randomly assigned to the Lira group or the Dula group, and 31 participants completed the protocol (Lira group: 15 participants; Dula group: 16 participants). Treatment was discontinued in two participants (one per group) because they were lost to follow up (Figure 1). There were no statistically significant differences between the baseline characteristics of the participants in both groups (Table 1). Baseline DTSQ and DTR‐QOL scores were also comparable between the two groups.
Figure 1.
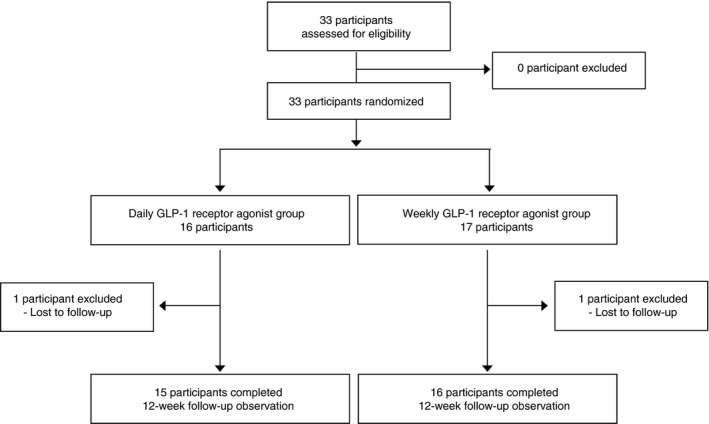
Study protocol. Patients were allocated to one of two groups and either continued taking daily the glucagon‐like peptide‐1 (GLP‐1) receptor agonist, liraglutide, or switched from taking liraglutide daily to taking the GLP‐1 receptor agonist, dulaglutide, weekly.
Table 1.
Baseline characteristics of the participants
| Total | Lira group | Dula group | P‐value | |
|---|---|---|---|---|
| n | 33 | 16 | 17 | |
| Age (years) | 62.0 ± 11.8 | 61.2 ± 12.8 | 62.7 ± 11.2 | 0.72 |
| Sex (female/male) | 18/15 | 8/8 | 10/7 | 0.73 |
| Work on weekdays (yes/no) | 17/16 | 8/8 | 9/8 | 1.00 |
| Bodyweight (kg) | 70.9 ± 15.2 | 70.7 ± 11.9 | 71.2 ± 18.1 | 0.93 |
| Duration of diabetes (years) | 18.6 ± 10.1 | 18.5 ± 9.8 | 18.6 ± 10.6 | 0.86 |
| Plasma glucose (mg/dL) | 164.8 ± 58.4 | 156.3 ± 54.0 | 172.8 ± 62.9 | 0.40 |
| HbA1c (%) | 7.6 ± 0.7 | 7.4 ± 0.5 | 7.7 ± 0.8 | 0.14 |
| No. concomitant medicine | 2.4 ± 1.0 | 2.3 ± 1.2 | 2.4 ± 0.9 | 0.82 |
| Oral hypoglycemic agents | ||||
| Metformin (%) | 78.8 | 68.8 | 88.2 | 0.22 |
| Sulfonylurea (%) | 33.3 | 18.8 | 47.1 | 0.14 |
| Glinide (%) | 15.2 | 18.8 | 11.8 | 0.66 |
| Thiazolidinediones (%) | 6.1 | 12.5 | 0.0 | 0.23 |
| α‐Glucosidase inhibitor (%) | 21.2 | 25.0 | 17.6 | 0.69 |
| SGLT2 inhibitor (%) | 27.3 | 37.5 | 17.6 | 0.26 |
| Insulin therapy (%) | 54.5 | 50.0 | 58.8 | 0.73 |
| ALT (U/L) | 27.2 ± 15.9 | 23.1 ± 12.1 | 31.2 ± 18.2 | 0.36 |
| Cr (mg/dL) | 0.88 ± 0.28 | 0.95 ± 0.34 | 0.82 ± 0.21 | 0.18 |
| Week 0 DTSQ score | ||||
| Treatment satisfaction | 24.7 ± 6.8 | 26.0 ± 7.9 | 23.5 ± 5.6 | 0.29 |
| Frequency of hyperglycemia and hypoglycemia | 5.1 ± 2.2 | 5.2 ± 1.9 | 4.9 ± 2.5 | 0.75 |
| Week 0 DTR‐QOL score | ||||
| Total score | 62.4 ± 17.6 | 65.9 ± 18.0 | 59.2 ± 17.0 | 0.28 |
| Subscale score | ||||
| Domain 1 | 67.4 ± 21.6 | 69.8 ± 22.2 | 65.2 ± 21.4 | 0.55 |
| Domain 2 | 53.4 ± 23.6 | 59.8 ± 23.5 | 47.4 ± 22.6 | 0.13 |
| Domain 3 | 72.1 ± 29.3 | 75.0 ± 30.7 | 69.4 ± 28.6 | 0.53 |
| Domain 4 | 55.6 ± 16.8 | 56.5 ± 15.4 | 54.7 ± 18.4 | 0.42 |
Data are mean ± standard deviation. The P‐value for the comparison of the liraglutide‐ and dulaglutide‐treated groups is stated. ALT, alanine aminotransferase; AST, aspartate aminotransferase; DTR‐QOL, Diabetes Therapy‐Related Quality of Life; DTSQ, Diabetes Treatment Satisfaction Questionnaire; HbA1c, glycated hemoglobin; SGLT2, sodium glucose cotransporter 2.
Changes in DTSQ and DTR‐QOL scores
Patient‐reported outcome measures were assessed in all 31 participants (15 men and 16 women). As shown in Table 2, the change in the overall DTSQ score in the Dula group was significantly greater than the change in the Lira group at week 12 (+0.1 ± 4.7 in the Lira group vs +4.9 ± 5.2 in the Dula group; P = 0.013). Among the subscale scores, changes in scores for ‘Convenience’ and ‘Flexibility’ were significantly greater in the Dula group than in the Lira group (P = 0.001 and P = 0.014, respectively). Changes in the score for ‘Understanding’ tended to be greater in the Dula group than in the Lira group, but this did not reach significance (Table 2). Interestingly, changes in scores for No. 2 ‘Frequency of hyperglycemia’ tended to be lower in the Dula group than in the Lira group (+0.5 ± 1.8 in the Lira group vs −0.6 ± 1.4 in the Dula group; P = 0.069) (Table 2), although HbA1c was not improved (Table 3). This implies that patients in the Dula group tended to feel that they had better glycemic control, because lower scores are preferable for question No. 2.
Table 2.
Changes in Diabetes Treatment Satisfaction Questionnaire and Diabetes Therapy‐Related Quality of Life from baseline to week 12
| Lira group (n = 15) | Dula group (n = 16) | P‐value | |||
|---|---|---|---|---|---|
| Previous score | Changes | Previous score | Changes | ||
| DTSQ score | |||||
| Treatment satisfaction | 25.7 ± 8.1 | 0.1 ± 4.7 | 23.1 ± 5.6 | 4.9 ± 5.2 | 0.013 |
| Frequency of hyperglycemia and hypoglycemia | 5.1 ± 2.0 | −0.5 ± 1.8 | 4.9 ± 2.6 | −1.1 ± 1.7 | 0.40 |
| Subscale score | |||||
| No. 1 Current treatment | 4.7 ± 1.3 | −0.07 ± 0.96 | 4.4 ± 1.2 | 0.0 ± 1.5 | 0.89 |
| No. 2 Frequency of hyperglycemia | 2.9 ± 1.6 | 0.5 ± 1.8 | 3.6 ± 1.8 | −0.6 ± 1.4 | 0.069 |
| No. 3 Frequency of hypoglycemia | 2.3 ± 2.1 | −1.1 ± 2.1 | 1.3 ± 1.6 | −0.5 ± 1.5 | 0.66 |
| No. 4 Convenience | 4.1 ± 1.5 | 0.2 ± 1.1 | 3.4 ± 1.5 | 1.8 ± 1.3 | 0.001 |
| No. 5 Flexibility | 4.0 ± 1.7 | 0.07 ± 1.10 | 3.6 ± 1.6 | 1.3 ± 1.4 | 0.014 |
| No. 6 Understanding | 4.3 ± 1.4 | −0.3 ± 1.1 | 4.0 ± 1.1 | 0.6 ± 1.2 | 0.079 |
| No. 7 Recommend | 4.1 ± 1.8 | 0.2 ± 1.3 | 3.9 ± 1.7 | 0.6 ± 1.3 | 0.38 |
| No. 8 Continue | 4.4 ± 1.4 | 0.07 ± 1.03 | 3.8 ± 1.1 | 0.7 ± 1.1 | 0.21 |
| DTR‐QOL score | |||||
| Total score | 63.5 ± 18.0 | −3.7 ± 6.9 | 57.1 ± 16.2 | 8.9 ± 15.1 | 0.007 |
| Subscale score | |||||
| Domain 1 | 68.2 ± 22.0 | −1.5 ± 9.6 | 63.7 ± 21.3 | 9.3 ± 20.0 | 0.024 |
| Domain 2 | 61.4 ± 23.4 | −8.8 ± 13.9 | 46.9 ± 23.2 | 12.1 ± 27.8 | 0.036 |
| Domain 3 | 73.3 ± 31.0 | −1.4 ± 16.2 | 67.4 ± 28.4 | 5.2 ± 19.0 | 0.29 |
| Domain 4 | 57.2 ± 15.6 | −3.3 ± 18.8 | 54.4 ± 19.0 | 4.7 ± 24.4 | 0.16 |
Data are mean ± standard deviation. The P‐value for the comparison of the liraglutide‐ and dulaglutide‐treated groups is stated. DTR‐QOL, Diabetes Therapy‐Related Quality of Life; DTSQ, Diabetes Treatment Satisfaction Questionnaire.
Table 3.
Changes in secondary outcomes between baseline and week 12
| Lira group | Dula group | P‐value | |
|---|---|---|---|
| ΔBodyweight (kg) | −0.30 (−1.20, 0.80) | 0.65 (−0.20, 1.08) | 0.18 |
| ΔPlasma glucose (mg/dL) | 3.0 (−7.0, 17.0) | −25.5 (−64.3, 26.8) | 0.57 |
| ΔHbA1c (%) | 0.05 ± 0.41 | 0.00 ± 0.68 | 0.82 |
| ΔALT (U/L) | −1.0 (−5.0, 1.0) | −3.0 (−7.0, 1.0) | 0.62 |
Data are mean ± standard deviation or median (range). The P‐values given were generated using unpaired t‐tests or Wilcoxon U tests. ALT, alanine aminotransferase; Dula group; patients who switched to dulaglutide once weekly; HbA1c, glycated hemoglobin; Lira group, patients who continued taking liraglutide once daily.
The change in overall DTR‐QOL score was significantly greater than the change in the Lira group at week 12 (−3.7 ± 6.9 in the Lira group vs +8.9 ± 15.1 in the Dula group; P = 0.007). Among the subscale scores, changes in scores for domain 1 (burden of social activities/personal activities) and domain 2 (anxiety and dissatisfaction with treatment) were significantly better in the Dula group than in the Lira group (P = 0.024 and P = 0.036, respectively). There was no significant difference in the changes in domain 3 (hypoglycemia) scores between the groups. Changes in domain 4 (satisfaction with treatment) scores tended to be greater in the Dula group than in the Lira group (P = 0.16; Table 2).
Treatment satisfaction and the clinical characteristics
Relationships between the treatment satisfaction and the clinical characteristics were investigated in order to show the factors influencing patient satisfaction (Table 4). However, no correlations were found between the changes in DTSQ total score and the baseline characteristics or the variables in the Dula group. In the present study, half of the participants were using insulin injection therapy concomitantly, and these individuals were allocated equally to each group. For patients who were injecting insulin daily, we speculated that QOL might not be improved significantly by switching their GLP‐1 RA injection to a weekly regimen. To test this possibility, we compared the DTSQ and DTR‐QOL scores for patients who were or were not injecting insulin in the Dula group. There were no differences in these scores between patients receiving insulin injections and those who were not, although the number of patients receiving insulin in the present study might have been insufficient for comparison.
Table 4.
Relationship between changes in the Diabetes Treatment Satisfaction Questionnaire total score of the patients who switched to dulaglutide once weekly and other variables
| ρ | P‐value | |
|---|---|---|
| Age (years) | −0.30 | 0.26 |
| Disease duration (years) | −0.17 | 0.51 |
| No. concomitant medicine (n) | −0.21 | 0.44 |
| Week 0 body mass index (kg/m2) | 0.058 | 0.83 |
| Mean changes in body mass index (kg/m2) | 0.077 | 0.78 |
| Week 0 HbA1c levels (%) | 0.24 | 0.36 |
| Mean changes in HbA1c levels (%) | −0.16 | 0.54 |
The correlation coefficients were analyzed using Spearman's rank‐order correlation. HbA1c, glycated hemoglobin.
Discussion
Patient satisfaction is an important outcome in drug treatment, because it provides an indication of compliance with therapy1. We compared the satisfaction with treatment in Japanese patients with type 2 diabetes taking GLP‐1 RAs daily or weekly using the DTSQ and DTR‐QOL. This is the first investigator‐initiated randomized control study to primarily investigate treatment satisfaction with weekly administration of the GLP‐1 RA, dulaglutide, which was recently approved for clinical use in Japan. The present study shows that the improvement in the DTSQ score was significantly greater when patients were switched from liraglutide to dulaglutide. Similar results were reported for the use of exenatide using the DTSQ13. In the present study, the administration of exenatide once a week resulted in significantly greater satisfaction in terms of reduction in hyperglycemia and continuing treatment, compared with the use of exenatide twice a day. The phase III clinical trial of dulaglutide, Assessment of Weekly Administration of Dulaglutide in Diabetes (AWARD), showed a great improvement in treatment satisfaction using various types of questionnaires14. The DTSQ was used as a secondary outcome in AWARD‐1 and AWARD‐3, in which twice‐daily exenatide or metformin, respectively, were compared with dulaglutide. Significantly greater improvements were observed on the DTSQ (total score and/or hyperglycemia subscale) in the dulaglutide arm compared with both alternative therapies. However, the use of 1.5‐mg dulaglutide was not associated with a significant improvement on the questionnaires compared with the use of 1.8‐mg liraglutide in AWARD‐6. In the present study, the impact of weight on self‐perception, the ability to carry out physical activities of daily living and EuroQol 5 dimensions were used to evaluate patient satisfaction, instead of the DTSQ. These studies were subanalyses of the dulaglutide phase III clinical trial carried out by the company, which differ from the present study with regard to the use of injection‐naïve patients, and the assessment of glycemic control, weight loss, drug dose and QOL.
There have been several studies of the treatment satisfaction associated with injectable therapies for patients with type 2 diabetes13, 15, 16, 17, 18. We recently reported that a combined injection of basal insulin and a GLP‐1 RA improved the DTSQ score to a greater extent than multiple daily insulin injections in patients with type 2 diabetes19. When taken together, these findings suggest that for injectable therapies, a lower injection frequency, lower HbA1c and lower body mass were closely associated with better treatment satisfaction in patients19, 20, 21, 22. The present results show that switching from liraglutide to dulaglutide did not alter HbA1c or body mass (Table 3). This is consistent with the results of the Japanese phase III trial of dulaglutide, which showed that it was less effective than liraglutide at improving HbA1c and body mass in patients with type 2 diabetes7. Therefore, switching from liraglutide to dulaglutide could improve treatment satisfaction independent of glycemic control and body mass.
The DTR‐QOL is a recently developed questionnaire that quantitatively evaluates patient QOL with respect to their diabetes treatment, which represents an additional factor to those assessed in the DTSQ23. The present study showed that the change in overall DTR‐QOL, domain 1 and domain 2 scores in the Dula group was significantly higher than in the Lira group (P = 0.007, P = 0.024 and P = 0.036, respectively), and that domain 3 and 4 scores did not observe statistical significance between the two groups (P = 0.29 and P = 0.16, respectively). It has also been previously reported that DTR‐QOL domain 1, 2 and 4 scores are significantly associated with physical activity level24.
To establish which subgroups of patients experienced an improved QOL on switching from daily to weekly GLP‐1 RA therapy, we evaluated the associations between the DTSQ score and the baseline characteristics of the patients or changes that occurred during the study. However, none of these factors directly correlated with the change in DTSQ total score (Table 4). Consequently, we would not speculate which patients might be most satisfied by switching them to a weekly regimen. It is therefore important for us to inform all patients of the existence of GLP‐1 RAs that can be administered weekly and discuss their appropriate use.
The adverse events associated with the treatments, such as hypoglycemia and gastrointestinal symptoms, were rarely experienced during the present study and did not affect treatment satisfaction levels. The reason for this is probably that the incidences of hypoglycemia and gastrointestinal symptoms are similar for patients in each group22.
The limitations of the present study were the relatively small number of participants, the short duration and the lack of double blinding. To address these potential issues, the present findings need to be replicated in a longitudinal observation with a larger population. This study involved switching medications, but the possibility exists that the process of switching in itself conferred a psychological advantage. A randomized controlled study should therefore be carried out to investigate the treatment satisfaction associated with both drugs in GLP‐1 RA‐naïve patients.
In conclusion, switching from daily use of the GLP‐1 receptor agonist, liraglutide, to weekly use of dulaglutide improved patient satisfaction with their treatment, especially with regard to convenience and flexibility, and this occurred independent of changes in glycemic control or body mass in Japanese patients with type 2 diabetes.
Disclosure
AN has received honoraria for lectures from Mitsubishi Tanabe Pharma Co., Ono Pharmaceutical Co., Ltd. and Sanofi. YK has received honoraria for lectures from Astellas Pharma Inc., AstraZeneca, Mitsubishi Tanabe Pharma Co., Ltd., MSD, Ono Pharmaceutical Co., Ltd., Sanofi, Shionogi & Co., Ltd., Taisho Toyama Pharmaceutical Co., Ltd. and Takeda Pharmaceutical Co., Ltd. TA has received honoraria for lectures from Mitsubishi Tanabe Pharma Co., UCB Japan Co., Ltd., Chugai Pharmaceutical Co., Ltd., Astellas Pharma Inc., Takeda Pharmaceutical Co., Ltd., Pfizer Inc., Eli Lilly, Bristol‐Myers Co., Ltd., AbbVie Inc. and Eisai Co., Ltd., and has received research funding from Astellas Pharma Inc., Takeda Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Co., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Bayer Yakuhin Ltd. and Eisai Co., Ltd. HM has received honoraria for lectures from Astellas Pharma Inc., AstraZeneca, Sumitomo Dainippon Pharma Co., Ltd., Eli Lilly, Kissei, Mitsubishi Tanabe Pharma Co., MSD, Novartis Pharma, Novo Nordisk Pharma, Takeda Pharmaceutical Co., Ltd., Kowa Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd. and Sanofi, and has received research funding from Astellas Pharma Inc., AstraZeneca, Daiichi Sankyo, Sumitomo Dainippon Pharma Co., Ltd., Eli Lilly, Mitsubishi Tanabe Pharma Co., MSD, Novo Nordisk Pharma, Sanofi, Takeda Pharmaceutical Co., Ltd., Kowa Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd. and Taisho Toyama Pharmaceutical Co., Ltd. The other authors declare no conflict of interest.
J Diabetes Investig 2019; 10: 699–705
Clinical Trial Registry
University Hospital Medical Information Network
UMIN 000024552
References
- 1. Barbosa CD, Balp MM, Kulich K, et al A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Prefer Adherence 2012; 6: 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pladevall M, Williams LK, Potts LA, et al Clinical outcomes and adherence to medications measured by claims data in patients with diabetes. Diabetes Care 2004; 27: 2800–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baggio LL, Drucker DJ. Biology of incretins: GLP‐1 and GIP. Gastroenterology 2007; 132: 2131–2157. [DOI] [PubMed] [Google Scholar]
- 4. Doggrell SA. Exenetide extended‐release; clinical trials, patient preference, and economic considerations. Patient Prefer Adherence 2013; 7: 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Febunmi R, Nielsen LL, Quimbo R, et al Patient characteristics, drug adherence patterns, and hypoglycemia costs for patients with type 2 diabetes mellitus newly initiated on exenatide or insulin glargine. Curr Med Res Opin 2009; 25: 777–786. [DOI] [PubMed] [Google Scholar]
- 6. Emoto M, Terauchi Y, Ozeki A, et al A 1‐year safety study of dulaglutide in Japanese patients with type 2 diabetes on a single oral hypoglycemic agent: an open‐label, nonrandomized, phase 3 trial. Endocr J 2015; 62: 1101–1114. [DOI] [PubMed] [Google Scholar]
- 7. Miyagawa J, Odawara M, Takamura T, et al Once‐weekly glucagon‐like peptide‐1 receptor agonist dulaglutide is non‐inferior to once‐daily liraglutide and superior to placebo in Japanese patients with type 2 diabetes: a 26‐week randomized phase III study. Diabetes Obes Metab 2015; 17: 974–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gelhorn H, Bacci ED, Poon JL, et al Evaluating preferences for profiles of glucagon‐like peptide‐1 receptor agonists among injection‐naïve type 2 diabetes patients in Japan. Patient Prefer Adherence 2016; 10: 1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bradley C. The diabetes treatment satisfaction questionnaire: DTSQ In: Bradley C. (ed). Handbook of Psychology and Diabetes: A Guide to Psychological Measurement in Diabetes Research and Practice. Chur, Switzerland: Harwood Academic Publishers, 1994; 111–132. [Google Scholar]
- 10. Ishii H, Bradley C, Riazi A, et al The Japanese version of the Diabetes Treatment Satisfaction Questionnaire (DTSQ): translation and clinical evaluation. J Clin Exp Med 2000; 192: 809–814 (Japanese). [Google Scholar]
- 11. Ishii H. Development and psychometric validation of the diabetes therapy related QOL (DTR‐QOL) questionnaire. J Med Econ 2012; 15: 556–563. [DOI] [PubMed] [Google Scholar]
- 12. Ono K, Nakamura A, Kawaguchi J, et al The safety, efficacy and treatment satisfaction comparison of unchanged premixed insulin regimen plus sitagliptin with switch from the premixed insulin to once‐daily basal insulin plus sitagliptin in patients with inadequately controlled type 2 diabetes with twice‐daily premixed insulin. Int J Diabetes Clin Res 2015; 2: 4. [Google Scholar]
- 13. Best JH, Boye KS, Rubin RR, et al Improved treatment satisfaction and weight‐related quality of life with exenatide once weekly or twice daily. Diabet Med 2009; 26: 722–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu M, Van Brunt K, Vamado OJ, et al Patient‐reported outcome results in patients with type 2 diabetes treated with once‐weekly dulaglutide: data from the AWARD phase III clinical trial programme. Diabetes Obes Metab 2016; 18: 419–424. [DOI] [PubMed] [Google Scholar]
- 15. Galasso S, Facchinetti A, Bonora BM, et al Switching from twice‐daily glargine or detemir to once‐daily degludec improves glucose control in type 1 diabetes. An observational study. Nutr Metab Cardiovasc Dis 2016; 26: 1112–1119. [DOI] [PubMed] [Google Scholar]
- 16. Polonsky W, Traylor L, Wei W, et al More satisfied, but why? A pooled patient‐level analysis of treatment satisfaction following the initiation of insulin glargine vs. comparators in insulin‐naïve patients with type 2 diabetes mellitus. Diabetes Obes Metab 2014; 16: 255–261. [DOI] [PubMed] [Google Scholar]
- 17. Wysham C, Blevins T, Arakaki R, et al Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD‐1). Diabetes Care 2014; 37: 2159–2167. [DOI] [PubMed] [Google Scholar]
- 18. Umpierrez G, Tofe Povedano S, Perez Manghi F, et al Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD‐3). Diabetes Care 2014; 37: 2168–2176. [DOI] [PubMed] [Google Scholar]
- 19. Miya A, Nakamura A, Miyoshi H, et al Satisfaction of switching to combination therapy with lixisenatide and basal insulin in patients with type 2 diabetes receiving multiple daily insulin injection therapy: a randomized controlled trial. J Diabetes Investig 2018; 9: 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ishii H, Anderson JH Jr, Yamamura A, et al Improvement of glycemic control and quality‐of‐life by insulin lispro therapy: assessing benefits by ITR‐QOL questionnaires. Diabetes Res Clin Pract 2008; 81: 169–178. [DOI] [PubMed] [Google Scholar]
- 21. Bode BW, Testa MA, Magwire M, et al Patient‐reported outcomes following treatment with the human GLP‐1 analogue liraglutide or glimepiride in monotherapy: results from a randomized controlled trial in patients with type 2 diabetes. Diabetes Obes Metab 2010; 12: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Onishi Y, Oura T, Matsui A, et al Analysis of efficacy and safety of dulaglutide 0.75 mg stratified by sex in patients with type 2 diabetes in 2 randomized, controlled phase 3 studies in Japan. Endocr J 2017; 64: 553–560. [DOI] [PubMed] [Google Scholar]
- 23. Ishii H, Niiya T, Ono Y, et al Improvement of quality of life through glycemic control by liraglutide, a GLP‐1 analog, in insulin‐naïve patients with type 2 diabetes mellitus: the PAGE1 study. Diabetol Metab Syndr 2017; 9: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hayashino Y, Tsujii S, Ishii H. Association of diabetes therapy‐related quality of life and physical activity levels in patients with type 2 diabetes receiving medication therapy: the Diabetes Distress and Care Registry at Tenri (DDCRT 17). Acta Diabetol 2018; 55: 165–173. [DOI] [PubMed] [Google Scholar]


