Abstract
Aims/Introduction
The present study investigated the effect of high‐dose metformin or low‐dose metformin/linagliptin combination therapy on glycemic variability (GV) in type 2 diabetes patients with insufficient glycemic control despite low‐dose metformin monotherapy in a cross‐over study using continuous glucose monitoring.
Materials and Methods
The present study was carried out with 11 type 2 diabetes outpatients (7% < glycated hemoglobin < 10%) receiving low‐dose metformin monotherapy (500–1,000 mg). All patients were assigned to either metformin 1,500 mg monotherapy (HMET) or combination therapy of low‐dose (750 mg) metformin and linagliptin 5 mg (LMET + dipeptidyl peptidase‐4 [DPP4]). GV was evaluated by continuous glucose monitoring after >4 weeks of the initial treatment and again after cross‐over to the other treatment. GV metrics were compared between the treatments using the Wilcoxon signed‐rank test.
Results
Of the continuous glucose monitoring‐derived GV metrics for the HMET versus LMET + DPP4, mean glucose levels, standard deviations and mean amplitude of glucose excursions were not significantly different. Although the pre‐breakfast glucose levels were not significantly different among the treatments (P = 0.248), the 3‐h postprandial glucose area under the curve (>160 mg/dL) after breakfast was significantly larger with HMET versus LMET + DPP4 (9,550 [2,075–11,395] vs 4,065 [1,950–8,895]; P = 0.041).
Conclusions
A comparison of GV with HMET versus LMET + DPP4 suggested that LMET + DPP4 might reduce post‐breakfast GV to a greater degree than HMET in type 2 diabetes patients receiving low‐dose metformin monotherapy.
Keywords: Continuous glucose monitoring, Dipeptidyl peptidase‐4 inhibitor, Metformin
Introduction
The goals of diabetes treatment are to prevent the onset or progression of microangiopathy or atherosclerotic diseases as diabetic complications and to maintain a quality of life similar to healthy individuals, thereby prolonging life expectancy. However, the American Diabetes Association/European Association for the Study of Diabetes joint statement1 recommends that individual glycemic goals be determined with consideration of each patient's background characteristics, given that glycated hemoglobin (HbA1c) values of 6.5–7.0% might not be associated with meaningful reductions in macroangiopathy, as shown in the Action to Control Cardiovascular Risk in Diabetes2, Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation3 and Veterans Affairs Diabetes Trial4 studies. Because of the emphasis on individualized therapeutic strategies for diabetes patients, metformin is often recommended as the first‐line therapy, because it is less likely to be associated with hypoglycemia and weight gain, and it is inexpensive1, 5, 6, 7. Furthermore, initiating intensive glycemic control with metformin at an early stage of diabetes has been shown to lead to reductions in glucose values and HbA1c, and significant reductions in the risk of myocardial infarction, thus contributing to a better long‐term prognosis in diabetes patients in the large‐scale UK Prospective Diabetes Study 346 and UK Prospective Diabetes Study 808 clinical trials.
It is usual practice to initiate low‐dose metformin in diabetes patients to mitigate associated gastrointestinal adverse events, to titrate its dose upwards gradually when it is not sufficiently effective and to consider initiating combination therapy with another drug if metformin monotherapy fails to achieve designated glycemic goals in approximately 3 months. Multicenter double‐blind comparative studies9, 10 have shown that metformin monotherapy at doses up to 2,000 mg/day is associated with dose‐dependent reductions in fasting glucose and HbA1c. Although metformin is usually given at a regular daily dose of ≥2,000 mg in Western countries11, the daily dose was limited to 750 mg daily in Japan until 2009 due to safety concerns, so its effectiveness was inadequate and its use less widespread. However, metformin became available in Japan from 2010 for use at a maintenance dose of 750–1,500 mg daily, as well as at a maximum dose of 2,250 mg, which led to a reappraisal of its antidiabetic efficacy and widespread use in the country.
Dipeptidyl peptidase‐4 (DPP‐4) inhibitors are counted among potential therapeutic agents for use in combination with metformin in patients with inadequate glycemic control despite metformin monotherapy12. Metformin and DPP‐4 inhibitors complement each other's mechanisms of action13. Metformin activates hepatic adenosine monophosphate‐activated protein kinase and inhibits hepatic gluconeogenesis, thus lowering glucose14; DPP‐4 inhibitors lower glucose by promoting insulin secretion in a dose‐dependent manner7. DPP‐4 inhibitors are less likely to be associated with hypoglycemia and weight gain5, 7, 15, 16, 17, 18, and the combination therapy of metformin and a DPP‐4 inhibitor has been shown in a clinical trial to reduce HbA1c to a greater extent than metformin monotherapy without causing weight gain19. Of all oral hypoglycemic agents used in combination with metformin, DPP‐4 inhibitors are associated with the most significant reductions in cardiovascular events in clinical studies that evaluated the impact of metformin‐containing combination therapy on cardiovascular events20, 21, 22.
Recent studies have identified the management of postprandial hyperglycemia as the cornerstone of preventive strategy against macroangiopathy23, 24, suggesting that glycemic control is crucial to diabetes treatment, with consideration given to glycemic variability, including postprandial hyperglycemia.
However, very few clinical studies have investigated whether metformin doses should be increased or metformin should be combined with a DPP‐4 inhibitor in patients with inadequate glycemic control despite metformin monotherapy. No studies are available that offer insight into diurnal glycemic variability using continuous glucose monitoring (CGM), which allows glucose to be monitored continuously over 24 h.
Against this background, a non‐blinded, CGM‐based cross‐over study was carried out in Japanese type 2 diabetes patients with inadequate glycemic control despite low‐dose metformin monotherapy to compare their glycemic variability with the use of an increased metformin dose versus the addition of the DPP‐4 inhibitor, linagliptin.
Methods
Patients
Of all type 2 diabetes outpatients treated at the Division of Diabetes, Metabolism and Endocrinology, Jikei University School of Medicine, Tokyo, Japan, those who met the following three criteria were included in the study: (i) those taking metformin 500–1000 mg daily (in two to three divided doses) for ≥2 months; (ii) HbA1c (National Glycohemoglobin Standardization Program [%]) >7% but <10%, with an immediate glycemic variability (mean absolute glucose change) within 1.0%; and (iii) age ≥20 years but <80 years.
Patients were excluded if they met any of the following criteria: (i) type 1 diabetes; (ii) treatment with insulin therapy; (iii) treatment with oral hypoglycemic agents other than metformin; (iv) severe ketoacidosis or diabetic coma at the time of study entry; (v) serious infection, recent or upcoming surgery, or serious trauma; (vi) hepatic impairment (aspartate aminotransferase/alanine aminotransferase >2.5‐fold the upper limit of normal) or hepatic cirrhosis; (vii) renal impairment (creatinine ≥1.0 mg/dL, regardless of sex); (viii) shock, cardiac failure, myocardial infarction, pulmonary embolism, advanced lung failure or other conditions thought likely to be associated with hypoxemia; (ix) a state of malnutrition, starvation or debility, and pituitary or adrenal gland dysfunction; (x) a history of lactic acidosis; (xi) excessive alcohol intake; (xii) dehydration, diarrhea thought likely to lead to dehydration or gastrointestinal disorder, such as vomiting; (xiii) malignancy; (xiv) a history of hypersensitivity to biguanides or other drugs; (xv) pregnancy, possibility of pregnancy and lactation; and (xvi) ineligibility for any study entry, as judged by an attending physician.
Trial design
The present study was a non‐blinded cross‐over study. All study participants were randomly allocated to either metformin 1,500 mg (high‐dose metformin [HMET]) or metformin 750 mg plus linagliptin 5 mg (low‐dose metformin + DPP‐4 inhibitor; LMET + DPP4) with the minimization method, where metformin was taken three times daily and linagliptin was taken after breakfast once daily (Figure 1). All patients in both treatments were subjected to evaluation by CGM for glycemic variability after 4–12 weeks of the initial treatment. The patients were then crossed over to the other treatment and again assessed for glycemic variability by CGM after 4–12 weeks of treatment. CGM was carried out in a home setting. When CGM was fitted, HbA1c was measured. During the course of the study, all patients were instructed to follow their usual lifestyle patterns and to maintain their physical activity similar to their usual levels while on both treatments. All patients were provided with standardized retort pouch meals on day 1 (dinner) as well as on day 2 of CGM assessment (breakfast, lunch and dinner). The CGM data on day 2 (from 00.00 to 24.00 hours) were used to assess the primary end‐points.
Figure 1.
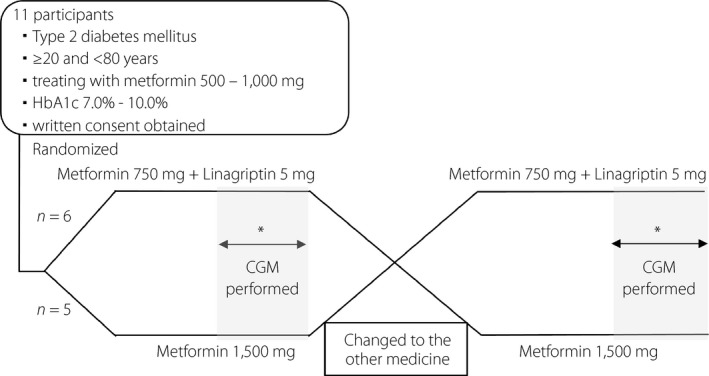
Study design. *Each patient was fitted with a continuous glucose monitoring (CGM) device after at least 4 weeks of treatment in the outpatient clinic, and 24 h data were collected for comparison under the same retort pouch meals. HbA1c, glycated hemoglobin.
CGM assessments were carried out using iPro™2 Professional CGM System and Medtronic Enlite® sensors (Medtronic Minimed, Northridge, CA, USA). All patients were blinded to these measurements, and recorded data were downloaded using the Medtronic Care‐Link™ iPro software after devices were removed from the patients. Medisafe Fit® blood glucose meters (Terumo Corporation, Tokyo, Japan) was used for self‐monitoring of blood glucose, with all patients instructed to measure blood glucose at least four times a day (before each meal and at bedtime).
Medications
All patients were given metformin monotherapy at a dose of 500–1,000 mg/day before study participation. The dose of study drug for each patient during the study was determined based on the maintenance dose of metformin (750 or 1,500 mg/day) and the regular dose of linagliptin (5 mg/day).
Use of insulin, as well as oral antidiabetic drugs other than metformin and linagliptin, was prohibited during the course of the study, and all patients who required any other drug during the study discontinued the study at that point. Whenever possible, the doses of any other concomitant drugs (e.g., antihypertensive, antiplatelet or antidyslipidemic drugs) the patients received were not altered, and they initiated no additional drugs during the study.
Meals
All eligible patients received the same retort pouch meals beginning with dinner on the day before CGM assessment. The nutritional composition of each meal was as follows: on the day before CGM assessment, dinner was 591.3 kcal with carbohydrates 65.1%; proteins 15.9%; and lipids 19.0%; on the day of CGM assessment, breakfast was 616 kcal with carbohydrates 63.6%; proteins 16.5%; and lipids 19.9%; lunch was 628 kcal with carbohydrates 69.5%; proteins 15.6%; and lipids 14.9%; and dinner was 591.3 kcal with carbohydrates 65.1%; proteins 15.9%; and lipids 19.0%.
Primary end‐points
The primary end‐points for the present study were 24‐h mean glucose levels, standard deviations (SD) of 24‐h glucose levels, coefficient of glucose variation (%CV), mean amplitude of glycemic excursions (MAGE), preprandial blood glucose levels, postprandial peak blood glucose levels, range of glucose increase from preprandial to postprandial peak glucose levels, time to peak glucose levels from preprandial glucose levels and area under the curve (AUC) measured >160 mg/dL 3 h after each meal.
Statistical analysis
Each parameter was compared using both the Wilcoxon signed‐rank test and t‐test for patients receiving HMET and those receiving LMET + DPP4, as the sample size was small.
All analyses were carried out by using SPSS 22.0 (SPSS Inc., Chicago, IL, USA; www.spss.com). All data were represented as median (interquartile range) and mean ± SD. A P‐value of <0.05 was considered to show statistical significance (two‐tailed test).
The study was approved by the ethics committee of the Jikei University School of Medicine, and conformed to the provisions of the Declaration of Helsinki (as revised in Fortaleza, Brazil, October 2013). This trial was registered at University Hospital Medical Information Network Clinical Trials Registry as UMIN000019033. Written informed consent was obtained from all patients before their study enrollment.
Results
Patient characteristics and metformin dose
The study participants comprised a total of 11 patients (8 men, 3 women; age 53.0 years [45.0–60.0 years]; duration of diabetes 4.0 years [2.0–7.0 years]; body mass index 25.8 kg/m2 [24.4–27.4 kg/m2]; HbA1c 7.6% [7.2–7.9%]; C‐peptide 2.62 ng/mL [2.19–3.59 ng/mL]; and estimated glomerular filtration rate 78.0 mL/min/1.73 m2 [71.0–85.0 mL/min/1.73 m2]). Of these, six patients received metformin 750 mg and five patients received 1,000 mg alone before their participation in the study (Table 1).
Table 1.
Patient demographics and metformin dose at baseline
| Overall | |
|---|---|
| No. patients (women) | 11 (3) |
| Age (years) | |
| Median | 53.0 (45.0–60.0) |
| Mean | 51.9 ± 9.8 |
| Duration of diabetes (years) | |
| Median | 4.0 (2.0–7.0) |
| Mean | 4.9 ± 3.3 |
| Bodyweight (kg) | |
| Median | 74.0 (66.0–79.0) |
| Mean | 73.7 ± 8.3 |
| BMI (kg/m2) | |
| Median | 25.8 (24.4–27.4) |
| Mean | 26.3 ± 3.0 |
| HbA1c (%) | |
| Median | 7.6 (7.2–7.9) |
| Mean | 7.6 ± 0.4 |
| eGFR (mL/min/1.73 m2) | |
| Median | 78.0 (71.0–85.0) |
| Mean | 78.7 ± 11.3 |
| C‐peptide (ng/mL) | |
| Median | 2.62 (2.19–3.59) |
| Mean | 2.64 ± 0.83 |
| Metformin dose | |
| 750 mg | 6 |
| 1,000 mg | 5 |
The upper row shows the median (interquartile range), and the lower row shows the mean ± standard deviation. BMI, body mass index; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin.
There was no significant difference between the treatments with regard to duration of treatment (HMET 8.0 weeks [6.9–10.0 weeks] vs LMET + DPP4 9.0 weeks [7.3–10.0 weeks], P = 0.154). The HbA1c and body mass index values were not significantly different with HMET versus LMET + DPP4 in the present study (HbA1c 7.0% [6.8–7.3%] vs 6.9% [6.7–7.0%], P = 0.076; and body mass index 26.2 kg/m2 [24.4–27.4 kg/m2] vs 25.7 kg/m2 [24.4–27.4 kg/m2], P = 0.109; Table 2).
Table 2.
Parameters for each protocol
| Metformin high dose (HMET) | Linagliptin add‐on (LMET + DPP4) | P‐value† | |
|---|---|---|---|
| BMI (kg/m2) | |||
| Median | 26.2 (24.4–27.4) | 25.7 (24.4–27.4) | 0.109 |
| Mean | 26.5 ± 3.1 | 26.3 ± 3.0 | 0.085 |
| HbA1c (%) | |||
| Median | 7.0 (6.8–7.3) | 6.9 (6.7–7.0) | 0.076 |
| Mean | 7.1 ± 0.3 | 7.0 ± 0.3 | 0.083 |
| 24‐h mean glucose levels (mg/dL) | |||
| Median | 137.5 (120.4–143.6) | 132.1 (129.6–143.5) | 0.657 |
| Mean | 135.4 ± 15.5 | 135.0 ± 14.3 | 0.946 |
| Night‐time (00.00–06.00 hours) | |||
| Median | 103.6 (86.2–114.3) | 110.1 (86.2–123.0) | 0.594 |
| Mean | 105.3 ± 25.4 | 108.8 ± 21.8 | 0.614 |
| Daytime (06.00–24.00 hours) | |||
| Median | 150.2 (131.8–156.2) | 138.1 (134.9–156.3) | 0.929 |
| Mean | 145.5 ± 16.3 | 143.8 ± 14.0 | 0.804 |
| SD of 24‐h glucose levels (mg/dL) | |||
| Median | 45.4 (31.5–50.1) | 40.7 (30.4–48.5) | 0.213 |
| Mean | 42.1 ± 13.2 | 39.1 ± 10.6 | 0.394 |
| Night‐time (00.00–06.00 hours) | |||
| Median | 11.1 (7.8–18.0) | 9.1 (4.6–16.2) | 0.657 |
| Mean | 15.0 ± 11.1 | 13.8 ± 13.7 | 0.694 |
| Daytime (06.00–24.00 hours) | |||
| Median | 43.2 (30.8–51.5) | 40.0 (32.5–50.0) | 0.374 |
| Mean | 41.7 ± 13.8 | 39.9 ± 10.1 | 0.597 |
| Coefficient of glucose variation | |||
| Median | 32.5 (24.3–36.1) | 25.3 (23.7–34.3) | 0.286 |
| Mean | 30.9 ± 8.5 | 28.9 ± 6.8 | 0.290 |
| Night‐time (00.00–06.00 hours) | |||
| Median | 12.3 (7.1–18.0) | 9.3 (4.1–17.1) | 0.594 |
| Mean | 13.5 ± 7.0 | 11.9 ± 10.3 | 0.579 |
| Daytime (06.00–24.00 hours) | |||
| Median | 28.1 (22.5–34.3) | 26.7 (23.9–32.0) | 0.657 |
| Mean | 28.4 ± 7.8 | 27.5 ± 5.1 | 0.595 |
| Mean amplitude of glycemic excursions | |||
| Median | 94.5 (67.0–130.0) | 97.0 (77.3–132.2) | 0.790 |
| Mean | 101.2 ± 35.2 | 102.2 ± 31.5 | 0.865 |
| Preprandial blood glucose levels (mg/dL) | |||
| Breakfast | |||
| Median | 118.0 (106.0–132.0) | 115.0 (102.0–127.0) | 0.248 |
| Mean | 117.5 ± 17.6 | 113.6 ± 16.2 | 0.432 |
| Lunch | |||
| Median | 109.0 (90.0–125.0) | 95.0 (89.0–113.0) | 0.756 |
| Mean | 106.6 ± 19.9 | 102.8 ± 17.2 | 0.620 |
| Dinner | |||
| Median | 98.0 (94.0–113.0) | 102.0 (97.0–108.0) | 0.689 |
| Mean | 105.0 ± 16.8 | 103.7 ± 10.4 | 0.723 |
| Postprandial blood glucose levels (mg/dL) | |||
| Breakfast | |||
| Median | 247.0 (194.0–280.0) | 222.0 (202.0–254.0) | 0.248 |
| Mean | 241.8 ± 51.2 | 226.6 ± 28.8 | 0.215 |
| Lunch | |||
| Median | 168.0 (165.0–199.0) | 177.0 (147.0–207.0) | 0.594 |
| Mean | 169.8 ± 31.2 | 178.6 ± 42.0 | 0.354 |
| Dinner | |||
| Median | 205.0 (163.0–232.0) | 199.0 (178.0–239.0) | 0.594 |
| Mean | 197.6 ± 35.8 | 208.3 ± 36.6 | 0.462 |
| Range of glucose increase from pre‐meal to postprandial peak levels (mg/dL) | |||
| Breakfast | |||
| Median | 130.0 (80.0–160.0) | 113.0 (84.0–139.0) | 0.248 |
| Mean | 125.6 ± 50.3 | 113.1 ± 29.0 | 0.280 |
| Lunch | |||
| Median | 67.0 (34.0–85.0) | 71.0 (39.0–120.0) | 0.142 |
| Mean | 63.3 ± 42.4 | 75.7 ± 46.4 | 0.146 |
| Dinner | |||
| Median | 87.0 (68.0–119.0) | 109.0 (76.0–138.0) | 0.594 |
| Mean | 92.6 ± 34.8 | 104.6 ± 42.2 | 0.367 |
| Time to peak glucose levels from pre‐meal levels (min) | |||
| Breakfast | |||
| Median | 90.0 (85.0–110.0) | 95.0 (80.0–105.0) | 0.574 |
| Mean | 95.5 ± 13.7 | 92.3 ± 14.7 | 0.612 |
| Lunch | |||
| Median | 85.0 (70.0–110.0) | 85.0 (75.0–95.0) | 1.000 |
| Mean | 89.6 ± 24.7 | 87.7 ± 20.8 | 0.871 |
| Dinner | |||
| Median | 85.0 (65.0–110.0) | 115.0 (75.0–120.0) | 0.349 |
| Mean | 90.9 ± 25.6 | 101.8 ± 23.5 | 0.334 |
| AUC >160 mg/dL 3 h after meal (mg/dL/min) | |||
| Breakfast | |||
| Median | 9,550 (2,075–11,395) | 4,065 (1,950–8,895) | 0.041* |
| Mean | 7,712 ± 5,177 | 5,004 ± 3,220 | 0.047* |
| Lunch | |||
| Median | 230 (75–1,955) | 555 (0–3,005) | 0.859 |
| Mean | 945 ± 1,374 | 2,335 ± 3,573 | 0.231 |
| Dinner | |||
| Median | 3,540 (30–6,755) | 2,635 (575–7,230) | 0.575 |
| Mean | 3,575 ± 3,529 | 4,165 ± 3,582 | 0.660 |
| Time in hypoglycemia (<70 mg/dL) during 24 h (min) | |||
| Median | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.705 |
| Mean | 10.5 ± 23.3 | 10.0 ± 22.3 | 0.967 |
The upper row shows the median (interquartile range), and the lower row shows the mean ± standard deviation. *P < 0.05. †Data were compared using the Wilcoxon signed‐rank test (upper row) and paired t‐test (lower row) with corresponding samples. AUC, area under the curve; BMI, body mass index; HbA1c, glycated hemoglobin; SD, standard deviation.
CGM data
The 24‐h mean glucose values did not differ significantly with HMET versus LMET + DPP4 (137.5 mg/dL [120.4–143.6 mg/dL] vs 132.1 mg/dL (129.6–143.5 mg/dL, P = 0.657; Table 2). Again, none of the other metrics for glycemic variability significantly differed with HMET versus LMET + DPP4 (SD 45.4 mg/dL [31.5–50.1 mg/dL] vs 40.7 mg/dL [30.4–48.5 mg/dL], P = 0.213; %CV 32.5 [24.3–36.1] vs 25.3 [23.7–34.3], P = 0.286; MAGE 94.5 [67.0–130.0] vs 97.0 [77.3–132.2], P = 0.790; Table 2).
The pre‐breakfast glucose values were not significantly different with HMET versus LMET + DPP4 at 118.0 mg/dL (106.0–132.0 mg/dL) vs 115.0 mg/dL (102.0–127.0 mg/dL, P = 0.248), respectively. Likewise, the pre‐lunch and pre‐dinner glucose values were not significantly different with HMET versus LMET + DPP4 (P = 0.756 and P = 0.689, respectively), and the glucose values after each meal were not significantly different (post‐breakfast, P = 0.248; post‐lunch, P = 0.594; and post‐dinner, P = 0.594; Table 2). The ranges of glucose increase from preprandial to postprandial peak glucose values and the times to postprandial peak glucose values were not significantly different with HMET versus LMET + DPP4.
Figure 2 shows the means ± SDs for 24‐h glycemic variability with HMET versus LMET + DPP4. Although there was no significant difference in the post‐breakfast peak glucose values and the ranges of post‐breakfast glucose increase with HMET versus LMET + DPP4 (both P = 0.248), the post‐breakfast 3‐h AUC >160 mg/dL was significantly smaller with LMET + DPP4 than with HMET (HMET vs LMET + DPP4 9,550 mg/dL·min [2,075–11,395 mg/dL·min] vs 4,065 mg/dL·min [1,950–8,895 mg/dL·min]; P = 0.041). In contrast, the post‐lunch and post‐dinner AUCs were not significantly different with HMET versus LMET + DPP4 (P = 0.859 and P = 0.575, respectively).
Figure 2.
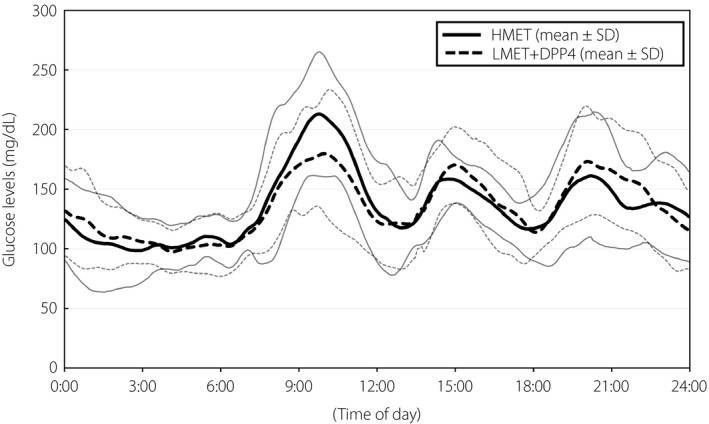
The 24‐h continuous glucose monitoring data in patients receiving high‐dose metformin (HMET) and low‐dose metformin (LMET) + dipeptidyl peptidase‐4 inhibitor (DPP4; n = 11). Curves are expressed as mean ± standard deviation (SD).
The time of hypoglycemia (<70 mg/dL) was not significantly different with HMET versus LMET + DPP4 (P = 0.705).
Discussion
In the present CGM‐based study, Japanese type 2 diabetes patients with inadequate glycemic control despite low‐dose metformin monotherapy were evaluated for glycemic variability in a cross‐over fashion, with either an increased metformin dose of 1,500 mg or metformin 750 mg combined with linagliptin 5 mg. Although there was no significant difference between the two treatment phases with regard to the metrics for glycemic variability, such as mean glucose values, MAGE, %CV and SD of glucose, the range of glucose excursions after breakfast was shown to be significantly smaller with LMET + DPP4 than with HMET.
Of note, metformin is shown to dose‐dependently reduce HbA1c9, 10, and this has been shown in a registration trial of metformin in Japan, where increasing the metformin dose to 1,500 mg reduced HbA1c by 0.57% in Japanese patients with inadequate glycemic control despite treatment with metformin 750 mg daily. Adding linagliptin to metformin has also been shown to reduce HbA1c by 0.6–0.7% compared with an add‐on placebo19, 25. Thus, the current study results, which showed no significant difference in 24‐h mean glucose values and HbA1c between HMET and LMET + DPP4, are thought to be consistent with those of earlier studies.
Apart from this, the post‐breakfast 3‐h AUC was shown to be significantly smaller with LMET + DPP4 than with HMET in the present study (HMET vs LMET + DPP4 9,550 mg/dL·min [2,075–11,395 mg/dL·min] vs 4,065 mg/dL·min [1,950–8,895 mg/dL·min], P = 0.041). Metformin is reported to enhance the expression of the preproglucagon gene, a precursor to glucagon‐like peptide‐1 (GLP‐1), in the small intestines26, as well as to inhibit bile acid reabsorption, thus accounting for the binding of increased bile acid to the L‐cell receptor in the small intestines and promoting GLP‐1 secretion27. In contrast, DPP‐4 inhibitors are shown to promote insulin secretion by dose‐dependently inhibiting the breakdown of incretins, such as GLP‐1 and gastric inhibitory polypeptide, thereby lowering blood glucose16, 28. Metformin combined with a DPP‐4 inhibitor has been shown to increase the concentration of GLP‐1 by approximately twofold compared with treatment with either drug alone, suggesting synergistic effects of the drugs combination26. Although the present study included type 2 diabetes patients with a mean duration of diabetes of 4.0 years (2.0–7.0 years); that is, those at a relatively early stage of disease whose endogenous insulin secretion likely remained relatively intact, they had HbA1c of 7.6% (7.2–7.9%) despite low‐dose metformin monotherapy. These levels suggest they likely had postprandial hyperglycemia and that LMET + DPP4 might have enhanced the postprandial concentration of active GLP‐1 to a greater extent than HMET, leading to enhanced secretion of bolus insulin and inhibition of glucagon, thus accounting for greater improvements in post‐breakfast hyperglycemia in these patients.
All type 2 diabetes patients in the present study received the same retort pouch meals during CGM assessments, with each accounting for nearly the same amount of calories and carbohydrates. Of note, of all postprandial glucose excursions seen in type 2 diabetes patients, those after breakfast are shown to be the greatest due to the influence of gluconeogenesis associated with long overnight fasting29, 30. In this study as well, although breakfast was expected to be associated with the greatest of all postprandial glucose increases due to long overnight fasting, LMET + DPP4 was thought to have led to significant reductions in post‐breakfast glycemic variability.
Results from the Diabetes Epidemiology Collaborative Analysis of Diagnostic Criteria in Europe and Diabetes Epidemiology Collaborative Analysis of Diagnostic Criteria in Asia studies suggest an association between postprandial hyperglycemia and cardiovascular death23, 24. Currently, increasing emphasis is placed on control of postprandial hyperglycemia, given that postprandial hyperglycemia represents an important risk factor for cardiovascular disease in the Asia–Pacific region as well31. Monnier et al.32 reported a strong positive correlation between oxidative stress and MAGE and postprandial glucose increases. Furthermore, the International Diabetes Federation recommend that postprandial 1‐ or 2‐h glucose levels be maintained at ≤160 mg/dL in diabetes patients33. An earlier study of type 2 diabetes patients with insufficient glycemic control despite low‐dose metformin monotherapy found LMET + DPP4 had a greater role in improving vascular endothelial function than HMET, whereas it found no difference in HbA1c reductions between the two treatments34. This appears to suggest that the smaller post‐breakfast glycemic excursions seen with LMET + DPP4 in the present study might contribute to prevention of cardiovascular events in type 2 diabetes patients with inadequate glycemic control despite low‐dose monotherapy.
In agreement with earlier reports showing that metformin and DPP‐4 inhibitors are less likely to be associated with hypoglycemia, when used alone or when combined5, 6, 7, 8, 15, 16, 17, 18, no clinically relevant hypoglycemia was reported in the present study, whereas hypoglycemia did occur with either HMET or LMET + DPP4.
Of all metformin‐related adverse events, gastrointestinal symptoms were reported to be most common, and of these, diarrhea and nausea were the most frequent. Garber et al.9 evaluated the tolerability profile of metformin and reported the discontinuation rate of metformin due to diarrhea at a dose of 500 mg/day was similar to that of the placebo, increased at a dose of 1,000 mg/day, and was no different at doses >1,000 mg/day. In the present study, of the six patients for whom the metformin dose had been increased from 750 to 1,500 mg/day, only one patient developed mild diarrhea (no difficulty continuing with metformin was reported, and the symptom resolved within 1 week), and no gastrointestinal symptoms occurred in the five patients for whom the metformin dose had been increased from 1,000 to 1,500 mg/day.
The limitations of the present study include the small number of patients enrolled and the maximum dose of metformin limited to 1,500 mg, not the 2,250 mg approved for clinical use in Japan. Again, given that this study included only those with HbA1c values 7.6% (7.2–7.9%), further study is warranted in type 2 diabetes patients with varying HbA1c values, as well as in different ethnic populations, to examine whether or how the study treatments might differ in their effects on glycemic variability in these populations. Despite these limitations, the authors believe the present pilot study represents an opportunity to provide valuable insights into the research question raised, as well as the rationale for further research in the future.
In conclusion, a comparison of glycemic variability with HMET versus LMET + DPP4 in Japanese type 2 diabetes patients with inadequate glycemic control despite low‐dose metformin monotherapy, the present study suggested a greater role for LMET + DPP4 in improving post‐breakfast glycemic excursions. It is hoped that the data presented in this study might serve as the reference data in formulating a diabetes treatment less likely to be associated with glycemic excursions.
Disclosure
Dr Rimei Nishimura has participated in speaker's bureau/advisory panels for Astellas, Astra Zeneca, Boehringer Ingelheim, Daiichi‐Sankyo, Eli Lilly, Johnson & Johnson, Kissei, Kowa, Medtronic, Novo Nordisk, Ono, Sanofi, Taisho, Takeda and Tanabe‐Mitsubishi, and served as a consultant for Abbott, Boehringer Ingelheim, Eli Lilly and Taisho. Dr Daisuke Tsujino has participated in speaker's bureau/advisory panels for Novo Nordisk, and has received research support from Boehringer Ingelheim and Takeda. Dr Kazunori Utsunomiya has received research support from Kowa, Kyowa Kirin, MSD, Ono and Tanabe‐Mitsubishi, and has participated in speaker's bureau/advisory panels for Astellas, Astra Zeneca, Kowa, MSD, Novo Nordisk and Sanofi. The other authors declare no conflict of interest.
Acknowledgments
We thank all the study participants and Kimie Shida for their assistance.
J Diabetes Investig 2019; 10: 714–722
Clinical Trial Registry
University Hospital Medical Information Network Clinical Trials Registry
UMIN000019033
References
- 1. Inzucchi SE, Bergenstal RM, Buse JB, et al Management of hyperglycemia in type 2 diabetes: a patient‐centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35: 1364–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Action to Control Cardiovascular Risk in Diabetes Study Group , Gerstein HC, Miller ME, et al Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358: 2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. ADVANCE Collaborative Group , Patel A, MacMahon S, et al Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358: 2560–2572. [DOI] [PubMed] [Google Scholar]
- 4. Duckworth W, Abraira C, Moritz T, et al Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360: 129–139. [DOI] [PubMed] [Google Scholar]
- 5. American Diabetes Association . Standards of medical care in diabetes‐2018 Abridged for primary care providers. Clin Diabetes 2018; 36: 14–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. UK Prospective Diabetes Study (UKPDS) Group . Effect of intensive blood‐glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34. Lancet 1998; 352: 854–865. [PubMed] [Google Scholar]
- 7. Nathan DM, Buse JB, Davidson MB, et al Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32: 193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Holman RR, Paul SK, Bethel MA, et al 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359: 1577–1589. [DOI] [PubMed] [Google Scholar]
- 9. Garber AJ, Duncan TG, Goodman AM, et al Efficacy of metformin in type II diabetes: results of a double‐blind, placebo‐controlled, dose‐response trial. Am J Med 1997; 103: 491–497. [DOI] [PubMed] [Google Scholar]
- 10. Sumitani S, Morita S, Utsu Y, et al Effectiveness of metformin and lifestyle interventions as an initial treatment in Japanese patients with newly diagnosed type 2 diabetes: a prospective observational study. J Med Invest 2012; 59: 166–173. [DOI] [PubMed] [Google Scholar]
- 11. Bailey CJ, Turner RC. Metformin. N Engl J Med 1996; 334: 574–579. [DOI] [PubMed] [Google Scholar]
- 12. Ahrén B. Novel combination treatment of type 2 diabetes DPP‐4 inhibition + metformin. Vasc Health Risk Manag 2008; 4: 383–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Graefe‐Mody EU, Padula S, Ring A, et al Evaluation of the potential for steady‐state pharmacokinetic and pharmacodynamic interactions between the DPP‐4 inhibitor linagliptin and metformin in healthy subjects. Curr Med Res Opin 2009; 25: 1963–1972. [DOI] [PubMed] [Google Scholar]
- 14. Zhou G, Myers R, Li Y, et al Role of AMP‐activated protein kinase in mechanism of metformin action. J CIin Invest 2001; 108: 1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brunton S. Beyond glycemic control: treating the entire type 2 diabetes disorder. Postgrad Med 2009; 121: 68–81. [DOI] [PubMed] [Google Scholar]
- 16. Green BD, Flatt PR, Bailey CJ. Dipeptidyl peptidase IV (DPP IV) inhibitors: a newly emerging drug class for the treatment of type 2 diabetes. Diabetes Vasc Dis Res 2006; 3: 159–165. [DOI] [PubMed] [Google Scholar]
- 17. Chahal H, Chowdhury TA. Gliptins: a new class of oral hypoglycaemic agent. QJM 2007; 100: 671–677. [DOI] [PubMed] [Google Scholar]
- 18. Drucker DJ, Nauck MA. The incretin system: glucagon‐like peptide‐1 receptor agonists and dipeptidyl peptidase‐4 inhibitors in type 2 diabetes. Lancet 2006; 368: 1696–1705. [DOI] [PubMed] [Google Scholar]
- 19. Deacon CF, Holst JJ. Linagliptin, a xanthine‐based dipeptidyl peptidase‐4 inhibitor with an unusual profile for the treatment of type 2 diabetes. Expert Opin Investig Drugs 2010; 19: 133–140. [DOI] [PubMed] [Google Scholar]
- 20. Yu OH, Yin H, Azoulay L. The combination of DPP‐4 inhibitors versus sulfonylureas with metformin after failure of first‐line treatment in the risk for major cardiovascular events and death. Can J Diabetes 2015; 39: 383–389. [DOI] [PubMed] [Google Scholar]
- 21. Seong JM, Choi NK, Shin JY, et al Differential cardiovascular outcomes after dipeptidyl peptidase‐4 inhibitor, sulfonylurea, and pioglitazone therapy, all in combination with metformin, for type 2 diabetes: a population‐based cohort study. PLoS ONE 2015; 10: e0124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ou SM, Shih CJ, Chao PW, et al Effects on clinical outcomes of adding dipeptidyl peptidase‐4 inhibitors versus sulfonylureas to metformin therapy in patients with type 2 diabetes mellitus. Ann Intern Med 2015; 163: 663–672. [DOI] [PubMed] [Google Scholar]
- 23. The DECODE study group on behalf of the European Diabetes Epidemiology Group . Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. Lancet 1999; 354: 617–621. [PubMed] [Google Scholar]
- 24. Nakagami T, DECODA Study Group . Hyperglycaemia and mortality from all causes and from cardiovascular disease in five populations of Asian origin. Diabetologia 2004; 47: 385–394. [DOI] [PubMed] [Google Scholar]
- 25. Taskinen MR, Rosenstock J, Tamminen I, et al Safety and efficacy of linagliptin as add‐on therapy to metformin in patients with type 2 diabetes: a randomized, double‐blind, placebo‐controlled study. Diabetes Obes Metab 2011; 13: 65–74. [DOI] [PubMed] [Google Scholar]
- 26. Migoya EM, Bergeron R, Miller JL, et al Dipeptidyl peptidase‐4 inhibitors administered in combination with metformin result in an additive increase in the plasma concentration of active GLP‐1. Clin Pharmacol Ther 2010; 88: 801–808. [DOI] [PubMed] [Google Scholar]
- 27. Mulherin AJ, Oh AH, Kim H, et al Mechanisms underlying metformin‐induced secretion of glucagon‐like peptide‐1 from the intestinal L cell. Endocrinology 2011; 152: 4610–4619. [DOI] [PubMed] [Google Scholar]
- 28. Pratley RE, Salsali A. Inhibition of DPP‐4: a new therapeutic approach for the treatment of type 2 diabetes. Curr Med Res Opin 2007; 23: 919–931. [DOI] [PubMed] [Google Scholar]
- 29. Boden G, Chen X, Urbain JL. Evidence for a circadian rhythm of insulin sensitivity in patients with NIDDM caused by cyclic changes in hepatic glucose production. Diabetes 1996; 45: 1044–1050. [DOI] [PubMed] [Google Scholar]
- 30. Monnier L, Colette C, Rabasa‐Lhoret R, et al Morning hyperglycemic excursions: a constant failure in the metabolic control of non‐insulin‐using patients with type 2 diabetes. Diabetes Care 2002; 25: 737–741. [DOI] [PubMed] [Google Scholar]
- 31. Sheu WH, Rosman A, Mithal A, et al Addressing the burden of type 2 diabetes and cardiovascular disease through the management of postprandial hyperglycaemia: an Asian‐Pacific perspective and expert recommendations. Diabetes Res Clin Pract 2011; 92: 312–321. [DOI] [PubMed] [Google Scholar]
- 32. Monnier L, Mas E, Ginet C, et al Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006; 295: 1681–1687. [DOI] [PubMed] [Google Scholar]
- 33. International Diabetes Federation Guideline Development Group . Guideline for management of postmeal glucose in diabetes. Diabetes Res Clin Pract 2014; 103: 256–268. [DOI] [PubMed] [Google Scholar]
- 34. Shigiyama F, Kumashiro N, Miyagi M, et al Linagliptin improves endothelial function in patients with type 2 diabetes: a randomized study of linagliptin effectiveness on endothelial function. J Diabetes Investig 2017; 8: 330–340. [DOI] [PMC free article] [PubMed] [Google Scholar]


