Abstract
Aims/Introduction
High plasma 3‐carboxy‐4‐methyl‐5‐propyl‐2‐furanpropanoic acid (CMPF) levels are significantly associated with type 2 diabetes mellitus, which is usually accompanied by metabolic syndrome and non‐alcoholic fatty liver disease (NAFLD) with increased triglyceride levels. Thus, we hypothesized that elevated CMPF levels might be related to lipid metabolism and NAFLD risk.
Materials and Methods
Serum CMPF levels were determined using an enzyme‐linked immunosorbent assay in a total of 466 individuals, including 116 controls with no NAFLD or type 2 diabetes mellitus, 53 individuals with NAFLD but no type 2 diabetes mellitus, 151 individuals with type 2 diabetes mellitus but no NAFLD, and 146 individuals with both NAFLD and type 2 diabetes mellitus. The associations with age, blood pressure, lipid profiles, body mass index and liver injury marker levels were examined, and a meta‐analysis of non‐diabetic and diabetic groups was carried out to detect the combined effects.
Results
The CMPF concentration in NAFLD patients was significantly lower than individuals without NAFLD in both the non‐diabetic group (P < 0.05) and diabetic group (P < 0.01), and correlated negatively with several parameters of liver function and the adiposity index. Meta‐analysis showed that serum CMPF levels was associated with decreased risk of NAFLD after combining the results (odds ratio 0.677, 95% confidence interval 0.552–0.831, P < 0.001). Additionally, the CMPF concentration was independently negatively associated with triglycerides and high‐density lipoprotein cholesterol in the meta‐analysis. Multiple stepwise regression analysis showed that body mass index, high‐density lipoprotein cholesterol, triglyceride level, age, sex and fasting plasma glucose were independently associated with CMPF (all P < 0.05).
Conclusions
The results suggest that serum CMPF levels are negatively related to lipid metabolism and could be used to predict NAFLD development.
Keywords: 3‐Carboxy‐4‐methyl‐5‐propyl‐2‐furanpropanoic acid, Non‐alcoholic fatty liver disease, Triglycerides
Introduction
The compound, 3‐carboxy‐4‐methyl‐5‐propyl‐2‐furanpropanoic acid (CMPF), is a furan fatty acid metabolite that was first identified in urine and is a protein‐bound uremic toxin1. CMPF has been reported to enhance reactive oxygen species production in human kidney 2 cells and induce renal cellular damage2. Several recent studies have reported that CMPF plays an important role in diabetes progression by contributing to β‐cell failure and decreasing glucose‐stimulated insulin secretion3, 4, 5, 6. CMPF has also been reported to inhibit mitochondrial respiration7, glutathione S‐transferase8 and drug metabolism in the liver9. Cumulatively, these findings suggest that CMPF is detrimental to healthy tissue function (including renal function, hepatocellular function and glycometabolism). However, studies in the field of nutrition have a converse viewpoint. Furan fatty acids are not produced de novo in many animals, including humans10. CMPF is a metabolite derived from furan fatty acids, is found in marine animals and is a possible biomarker of fish intake, which suggests healthier dietary choices11. A prospective cohort study including 76 participants12 showed that fish oil intake increased serum CMPF levels, and decreased triglyceride levels were found in a type 2 diabetes mellitus cohort. Another study of 106 patients13 showed that 12 weeks of fish consumption increased the CMPF concentration in plasma and did not harm glucose metabolism. In addition, in vitro experiments using human embryonic kidney 293 cells and mouse primary hepatocytes found that CMPF had no intrinsic cytotoxicity in either cell line, and did not inhibit CYP3A4, CYP2D6 or CYP2C95. Furthermore, a study of 516 participants6 showed an independent and inverse association between CMPF and triglycerides. Cumulatively, these findings show an important role of CMPF as a harmless metabolite involved in the regulation of lipid metabolism, and suggest that it is not cytotoxic to the liver. Although the clinically relevant and animal‐based studies of CMPF have assessed glycometabolism, the association of lipid metabolism and the prevalence of fatty liver has not been evaluated. More recently, Prentice et al.14 found that CMPF could increase whole‐body lipid metabolism and ameliorate steatosis in mice, but the CMPF levels in humans who suffer from NAFLD have not been reported.
Non‐alcoholic fatty liver disease (NAFLD) is increasingly recognized as a liver disease component of metabolic syndrome15. The global prevalence of NAFLD is approximately 25%16. A recent analysis of studies involving >8.5 million patients from 22 countries showed that 51.34% of patients with NAFLD are overweight or obese, 22.51% have received a diagnosis of type 2 diabetes mellitus, 69.16% have hyperlipidaemia and 42.54% have metabolic syndrome17. However, the causes and pathogenesis of NAFLD remain elusive. The objectives of the present study were to investigate whether altered serum CMPF levels are specifically associated with NAFLD in either type 2 diabetes mellitus patients or individuals with normal glucose tolerance (NGT). Furthermore, we also explored the association of serum CMPF levels with lipid profiles in these individuals.
Methods
Ethical considerations
The Medical Ethics Committee of the Shanghai Fengxian District Central Hospital approved this study, which conforms to the provision of the Declaration of Helsinki (as revised in Fortaleza, Brazil, October 2013).
Participants
The present study recruited 466 participants (including 297 type 2 diabetes mellitus patients and 169 individuals with NGT) of Chinese origin (Han Chinese; 250 men and 216 women) from Shanghai Fengxian District Central Hospital. All participants provided written informed consent before participating in this study. A 75‐g oral glucose tolerance test was carried out to determine the NGT. The classification of NGT (3.9 mmol/L ≤ fasting plasma glucose [FPG] < 5.6 mmol/L and 3.9 mmol/L ≤ 2‐h plasma glucose < 7.8 mmol/L) was based on the American Diabetes Association 2003 criteria. All of the 297 type 2 diabetes mellitus patients were inpatients taking oral hypoglycemic drugs and receiving insulin therapy. The diagnosis guidelines for NAFLD proposed by the Asia‐Pacific Working Party were used to characterize individuals with NAFLD18. NAFLD was clinically defined by manifestations on abdominal ultrasonography by experienced radiologists. All participants had routine biochemical blood analyses and physical examinations. Individuals with the following conditions were excluded from the present study: a prior history of specific diseases that could result in fatty liver, habitual drinking, acute or chronic inflammatory disease, renal failure, biliary obstructive diseases, acute or chronic viral hepatitis, cirrhosis, known hyperthyroidism or hypothyroidism, current treatment with systemic corticosteroids and the presence of cancer. Pregnant women were also excluded from this study.
Clinical measurements
Participants’ weight (in light clothing) and height (without shoes) were measured, and the body mass index (BMI) was calculated as weight in kilograms divided by the square of the height in metres. All blood samples were collected from the antecubital vein after 8 h of overnight fasting. After clotting, the blood serum samples were separated from the blood specimens by centrifugation for 15 min at approximately 1,000 g, and the aliquots were stored at −80°C until CMPF analysis. Triglycerides (TG), blood total cholesterol (TC), low‐density lipoprotein cholesterol (LDL‐c), high‐density lipoprotein cholesterol (HDL‐c) and liver injury markers levels were measured using an automatic biochemical analyser (DXC 800; Beckman Coulter, Brea, CA, USA). The hemoglobin A1c level was measured using high‐pressure liquid chromatography (HLC‐723G7; Tosoh, Tokyo, Japan)
Serum CMPF measurement
Serum concentrations of CMPF were quantified using an enzyme‐linked immunosorbent assay kit (catalogue number: BG‐HUM104400; Novatein Biosciences, Inc., Woburn, MA, USA) as described previously19. This assay has high sensitivity and excellent specificity for detection of human CMPF. No significant cross‐reactivity or interference between CMPF and analogs was observed.
Statistical analysis
Normally distributed data are shown as the mean ± standard deviation. Variables that were not normally distributed were loge‐transformed before analysis and are expressed as medians with the interquartile ranges. An exclusion of missing data was adopted in our analyses because of the missing percent under 10% in each variable. Student's unpaired t‐test was used to compare groups. Pearson's correlation and linear regression analyses were used to evaluate the correlations between the loge‐transformed serum CMPF levels and clinical parameters.
A logistic regression analysis was used to evaluate the association of loge‐transformed CMPF levels with NAFLD in the non‐diabetic and diabetic groups. Combined point estimates and odds ratio (ORs) from the NGT and type 2 diabetes mellitus groups were calculated by using Comprehensive Meta‐Analysis Software (v2.2.057; Biostat, Englewood, NJ, USA) with a fixed or random effects model after testing for heterogeneity, which was carried out by calculating the I‐squared (I 2) value. The I 2 value of 50% corresponded to cut‐off points for low and high degrees of heterogeneity. The fixed effects model was used for low degrees of heterogeneity, while the random effects model was used for high degrees of heterogeneity. IBM SPSS (version 22.0; Armonk, NY, USA) was used for statistical analyses unless otherwise specified. GraphPad Prism software (version 6.02; La Jolla, CA, USA) was used to plot the histograms. All two‐tailed P‐values <0.05 were considered significant.
Results
Clinical characteristics of participants
The anthropometric parameters and clinical characteristics of the participants are summarized in Table 1. No significant differences were found with respect to sex, systolic blood pressure, and hemoglobin A1c between the NAFLD and non‐NAFLD groups. Lower HDL‐c levels were observed in NAFLD patients than in participants without NAFLD (P < 0.001). In the non‐diabetic group, NAFLD patients had significantly higher BMI, diastolic blood pressure, aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ‐glutamyltransferase and triglyceride levels than participants without NAFLD (all P < 0.05). In the diabetic group, NAFLD patients had significantly higher BMI, diastolic blood pressure, AST, ALT, γ‐glutamyltransferase, FPG, triglyceride, TC and LDL‐c levels than participants without NAFLD (all P < 0.05). In addition, the NAFLD group was younger than the non‐NAFLD group.
Table 1.
Anthropometric parameters and clinical characteristics of the participants
| Variables | Control subjects (n = 116) | NAFLD only (n = 53) | P‐value* | Diabetes only (n = 151) | NAFLD and diabetes (n = 146) | P‐value** |
|---|---|---|---|---|---|---|
| Sex, female/male (n) | 62/54 | 25/28 | 0.452 | 69/82 | 60/86 | 0.426 |
| Age (years) | 56.00 ± 9.27 | 57.73 ± 9.38 | 0.263 | 63.54 ± 11.67 | 58.00 ± 12.48 | <0.001 |
| BMI (kg/m2) | 23.48 ± 2.56 | 26.04 ± 2.98 | <0.001 | 23.11 ± 2.86 | 26.57 ± 3.36 | <0.001 |
| SBP (mmHg) | 126.55 ± 14.64 | 130.75 ± 15.48 | 0.091 | 139.51 ± 20.61 | 139.73 ± 21.33 | 0.931 |
| DBP (mmHg) | 80.10 ± 8.94 | 84.07 ± 8.35 | 0.007 | 78.67 ± 10.35 | 82.27 ± 11.68 | 0.006 |
| FPG (mmol/L) | 5.23 ± 0.29 | 5.26 ± 0.23 | 0.512 | 8.11 ± 3.23 | 9.30 ± 2.80 | 0.001 |
| Triglycerides (mmol/L)† | 0.99 (0.72, 1.33) | 1.58 (1.17, 2.16) | <0.001 | 1.13 (0.76, 1.64) | 1.99 (1.46, 2.84) | <0.001 |
| TC (mmol/L) | 4.87 ± 0.94 | 5.04 ± 0.92 | 0.266 | 4.75 ± 1.22 | 5.13 ± 1.21 | 0.008 |
| HDL‐c (mmol/L) | 1.40 ± 0.34 | 1.19 ± 0.29 | <0.001 | 1.18 ± 0.28 | 1.04 ± 0.25 | <0.001 |
| LDL‐c (mmol/L) | 2.89 ± 0.76 | 3.09 ± 0.76 | 0.105 | 3.07 ± 0.92 | 3.33 ± 0.86 | 0.016 |
| HbA1c (%) | 5.35 ± 0.35 | 5.37 ± 0.37 | 0.840 | 10.05 ± 2.44 | 9.82 ± 1.97 | 0.390 |
| AST (U/L)† | 21.00 (18.00, 24.00) | 23.00 (21.00,28.00) | 0.030 | 18.00 (15.00, 22.00) | 21.00 (17.00, 26.00) | <0.001 |
| ALT (U/L)† | 14.00 (11.00, 20.00) | 18.00 (15.00,25.00) | <0.001 | 17.00 (12.00, 21.50) | 23.00 (16.00, 33.00) | <0.001 |
| GGT (U/L)† | 19.00 (14.00, 31.00) | 34.00 (21.00,46.00) | <0.001 | 20.00 (16.00, 29.50) | 31.00 (20.00, 49.00) | <0.001 |
| CMPF (μmol/L)† | 140.31 (49.55, 281.47) | 90.90 (41.73,153.20) | 0.028 | 215.73 (114.11,360.46) | 153.75 (85.31, 270.65) | 0.001 |
Data presented as the mean ± standard deviation or median (interquartile range). P‐value* indicates the comparison of non‐alcoholic fatty liver disease (NAFLD)‐only and control participants; P value** indicates the comparison of diabetes‐only participants and participants with NAFLD and diabetes. †Log transformed before analysis. ALT, alanine aminotransferase; AST, aspartate transaminase; BMI, body mass index; DBP, diastolic blood pressure; FPG, fasting plasma glucose; GGT, γ‐glutamyltransferase; HbA1c, hemoglobin A1C; HDL‐c, high‐density lipoprotein cholesterol; LDL‐c, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol.
Comparison of CMPF levels between the NAFLD and non‐NAFLD groups
The circulating CMPF levels in the four subgroups are presented in both Table 1 and Figure 1. The CMPF serum levels were elevated in the diabetes‐only group compared with those in the NAFLD and diabetes group (P = 0.001). The CMPF levels were also elevated in the control group compared with those in the NAFLD‐only group (P < 0.05). At the same time, the CMPF levels were higher in diabetes mellitus than NGT in both the control (P < 0.001) and NAFLD groups (P < 0.01). We then analyzed the association of CMPF with the risk of NALFD by logistic regression in each the NGT or type 2 diabetes mellitus group (Table 2). We carried out a meta‐analysis combining the two groups’ ORs (95% confidence interval [CI]) using a fixed‐effects model, which was determined by heterogeneity testing. The models that were not adjusted or adjusted for age and sex showed the CMPF levels were significantly associated with NAFLD (OR 0.677, 95% CI 0.552–0.831, P < 0.001 for not adjusted; OR 0.701, 95% CI 0.567–0.867, P = 0.001 for age and sex adjusted). However, the results became not significant when BMI entered the models.
Figure 1.
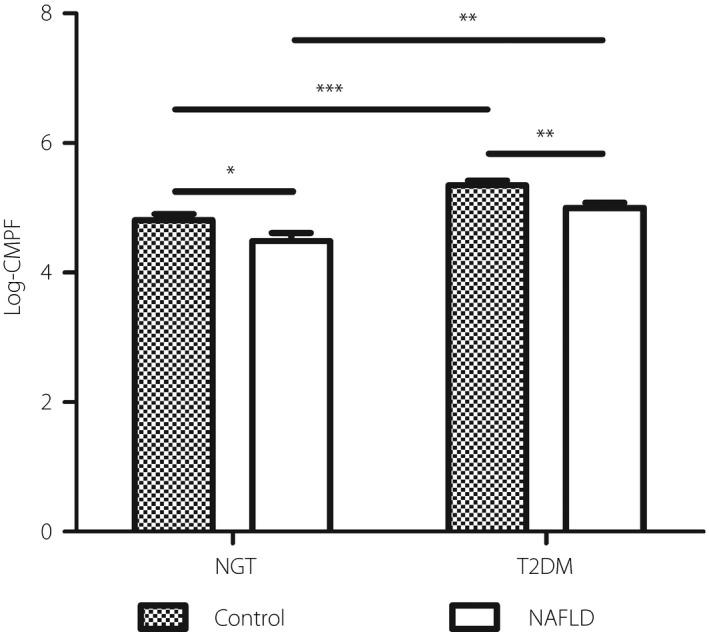
Loge transformed 3‐carboxy‐4‐methyl‐5‐propyl‐2‐furanpropanoic acid (CMPF) levels (mean ± standard error of the mean) in the four groups, namely the control group, non‐alcoholic fatty liver disease (NAFLD) only, diabetes only, and NAFLD and diabetes. The CMPF levels were significantly higher in participants without NAFLD in both the type 2 diabetes mellitus and normal glucose tolerance (NGT). *P < 0.05. **P < 0.01. ***P < 0.001.
Table 2.
Associations between 3‐carboxy‐4‐methyl‐5‐propyl‐2‐furanpropanoic acid and non‐alcoholic fatty liver disease
| Not adjusted | Adjusted for BMI | Adjusted for age and sex | Adjusted for age, sex and BMI | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P‐value | OR (95% CI) | P‐value | OR (95% CI) | P‐value | OR (95% CI) | P‐value | |
| NGT group | 0.716 (0.512, 1.003) | 0.051 | 0.826 (0.576, 1.185) | 0.300 | 0.692 (0.488, 0.983) | 0.040 | 0.787 (0.542, 1.142) | 0.207 |
| T2DM group | 0.655 (0.506, 0.849) | 0.001 | 0.804 (0.593, 1.091) | 0.161 | 0.707 (0.542, 0.924) | 0.011 | 0.854 (0.627, 1.165) | 0.320 |
| Meta‐analysis† | 0.677 (0.552, 0.831) | <0.001 | 0.813 (0.644, 1.026) | 0.082 | 0.701 (0.567, 0.867) | 0.001 | 0.826 (0.651, 1.048) | 0.116 |
| Heterogeneity tests | I 2 = 0 | I 2 = 0 | I 2 = 0 | I 2 = 0 | ||||
The meta‐analysis† shown represents the association of 3‐carboxy‐4‐methyl‐5‐propyl‐2‐furanpropanoic acid and non‐alcoholic fatty liver disease by combining the two groups with a fixed effects model. I 2 was used to assess heterogeneity. BMI, body mass index; NGT, normal glucose tolerance; OR, odds ratio; T2DM, type 2 diabetes mellitus.
Association of serum CMPF levels with quantitative traits related to NAFLD
We then examined the associations between serum CMPF levels and clinical parameters related to NAFLD and metabolism. As shown in Figure 2, CMPF was significantly correlated with LDL‐c, triglyceride, TC, ALT and AST levels (r = −0.144, P = 0.014 for LDL‐c; r = −0.193, P = 0.001 for triglycerides; r = −0.217, P < 0.001 for TC; r = −0.144, P = 0.014 for ALT; r = −0.153, P = 0.009 for AST) in type 2 diabetes mellitus patients. However, CMPF was only significantly correlated with triglyceride levels (r = −0.177, P = 0.021) in the NGT group. Additional adjustments for age, sex and BMI by meta‐analysis of the two groups (Table 3) showed that CMPF was still negatively associated with triglyceride levels (B ± SE = −0.075 ± 0.028, P = 0.008). However, CMPF was negatively associated with HDL‐c (B ± SE = −0.032 ± 0.014, P = 0.027). Furthermore, multiple stepwise regression analysis was carried out to determine variables with independent associations with serum CMPF (Table 4). In addition to age, sex and BMI, the regression model also included triglycerides, HDL‐c and FPG. These parameters were significantly associated with CMPF in the simple correlation analyses. The results showed that BMI, age, HDL‐c, TG, sex and FPG were independently associated with serum CMPF levels in order (B ± SE = −0.052 ± 0.014, P < 0.001 for BMI; B ± SE = 0.013 ± 0.004, P = 0.002 for age; B ± SE = −0.641 ± 0.162, P < 0.001 for HDL‐c; B ± SE = −0.281 ± 0.087, P = 0.001 for TG; B ± SE = 0.234 ± 0.093, P = 0.032 for sex; B ± SE = 0.037 ± 0.016, P = 0.024 for FPG).
Figure 2.
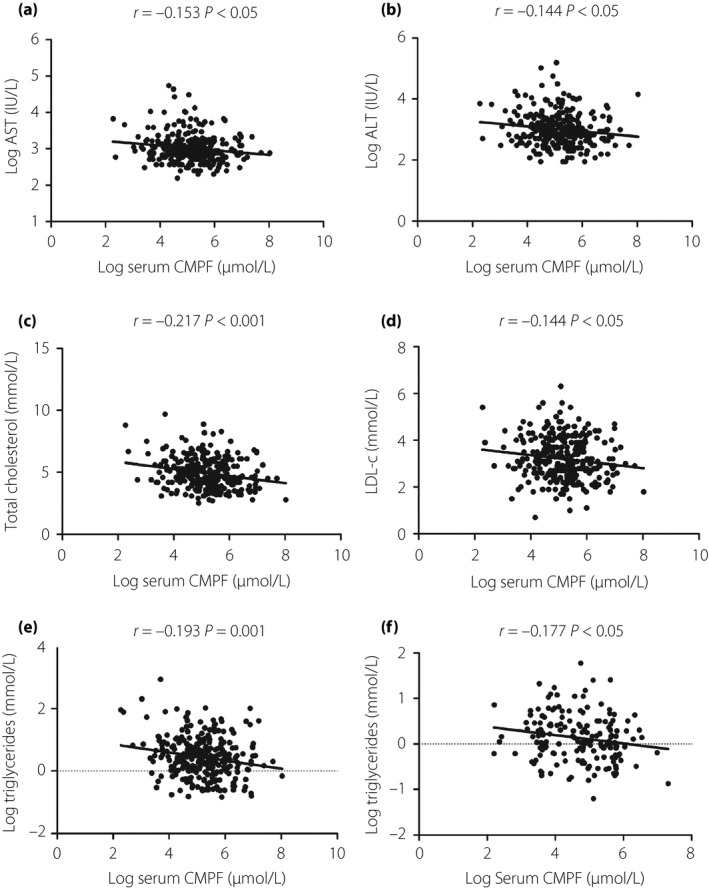
(a–e) Correlation analysis of 3‐carboxy‐4‐methyl‐5‐propyl‐2‐furanpropanoic acid (CMPF) levels with aspartate aminotransferase (AST), alanine aminotransferase (ALT), triglyceride (TC), low‐density lipoprotein cholesteraol (LDL‐c) and triglyceride (TG) levels in type 2 diabetes mellitus patients, and (f) the correlation analysis of CMPF levels with TG levels in the normal glucose tolerance group.
Table 3.
Correlation of serum 3‐carboxy‐4‐methyl‐5‐propyl‐2‐furanpropanoic acid levels with anthropometric parameters and biochemical indexes in 466 participants
| T2DM group n = 297 | NGT group n = 169 | Meta‐analysis | Heterogeneity tests | ||||
|---|---|---|---|---|---|---|---|
| B ± SE† | P‐value | B ± SE† | P‐value | B ± SE‡ | P‐value | I 2 (%) | |
| LDL‐c (mmol/L) | −0.120 ± 0.057 | 0.037 | 0.030 ± 0.063 | 0.632 | −0.047 ± 0.075 | 0.527 | 67.92 |
| HDL‐c (mmol/L) | −0.041 ± 0.017 | 0.015 | −0.009 ± 0.027 | 0.725 | −0.032 ± 0.014 | 0.027 | 0.59 |
| TC (mmol/L) | −0.256 ± 0.076 | 0.001 | 0.088 ± 0.077 | 0.252 | −0.089 ± 0.176 | 0.615 | 90.61 |
| Triglycerides (mmol/L)§ | −0.068 ± 0.039 | 0.082 | −0.083 ± 0.041 | 0.046 | −0.075 ± 0.028 | 0.008 | 0 |
| SBP (mmHg) | −0.220 ± 1.312 | 0.867 | 2.210 ± 1.139 | 0.054 | 1.166 ± 0.086 | 0.175 | 48.88 |
| DBP (mmHg) | −0.421 ± 0.723 | 0.561 | 1.454 ± 0.693 | 0.037 | 0.528 ± 0.937 | 0.573 | 71.47 |
| GGT (U/L)§ | −0.056 ± 0.045 | 0.210 | −0.013 ± 0.050 | 0.794 | −0.037 ± 0.033 | 0.272 | 0 |
| ALT (U/L)§ | −0.022 ± 0.033 | 0.508 | −0.008 ± 0.041 | 0.851 | −0.016 ± 0.026 | 0.521 | 0 |
| AST (U/L)§ | −0.050 ± 0.025 | 0.046 | 0.007 ± 0.025 | 0.786 | −0.022 ± 0.018 | 0.224 | 61.53 |
†The B ± standard error (SE) value shown indicates the association of 3‐carboxy‐4‐methyl‐5‐propyl‐2‐furanpropanoic acid with anthropometric parameters and biochemical indexes separately in the two groups by linear regression after adjustment for age, sex and body mass index. ‡The B ± SE value shown represents the association of ‐carboxy‐4‐methyl‐5‐propyl‐2‐furanpropanoic acid with anthropometric parameters and biochemical indexes by combining the two groups with a fixed‐effects model for high‐density lipoprotein cholesterol (HDL‐c), triglyceride (TG), γ‐glutamyltransferase (GGT) and alanine aminotransferase (ALT), and a random‐effects model for low‐density lipoprotein cholesterol (LDL‐c), total cholesterol (TC), systolic blood pressure (SBP), diastolic blood pressure (DBP) and aspartate transaminase (AST). I 2 was used to assess heterogeneity. §Log transformed before analysis.
Table 4.
Multiple stepwise regression analysis showing variables independently associated with the serum 3‐carboxy‐4‐methyl‐5‐propyl‐2‐furanpropanoic acid levels
| Independent variables | B | SE | Standardized β | P |
|---|---|---|---|---|
| BMI (kg/m2) | −0.052 | 0.014 | −0.177 | <0.001 |
| Age (years) | 0.013 | 0.004 | 0.146 | 0.002 |
| HDL‐c (mmol/L) | −0.641 | 0.162 | −0.208 | <0.001 |
| Triglycerides (mmol/L)† | −0.281 | 0.087 | −0.174 | 0.001 |
| Sex (1 = male, 2 = female) | 0.234 | 0.093 | 0.118 | 0.012 |
| FPG (mmol/L) | 0.037 | 0.016 | 0.110 | 0.024 |
The analysis also included aspartate transaminase, alanine aminotransferase, γ‐glutamyltransferase, low‐density lipoprotein cholesterol and total cholesterol, which were all excluded in the final model. †Log transformed before analysis. BMI, body mass index; FPG, fasting plasma glucose; HDL‐c, high‐density lipoprotein cholesterol; SE, standard error.
Discussion
This is the first study to examine the associations between serum CMPF levels and NAFLD in both type 2 diabetes mellitus patients and individuals with NGT. In the case–control comparison of each study population, we found that CMPF was substantially higher in the non‐NAFLD control group than in NAFLD patients. Additionally, the circulating CMPF level was independently and negatively associated with triglycerides in the meta‐analysis after adjusting for type 2 diabetes mellitus status. The present study suggests that CMPF plays an important role in glycolipid metabolism by enhancing fatty acid utilization and reducing glucose metabolism. These findings are consistent with the results of prior studies using rodent islets3.
CMPF levels have been found to be elevated in type 2 diabetes mellitus and GDM patients using a metabolomics approach, and this elevation was associated with dangerous effects on glucose‐induced insulin secretion in a rodent model4. Consistent with this study, the present research showed that serum CMPF levels were elevated in type 2 diabetes mellitus patients, but not individuals with NGT. Furthermore, the same team found that elevated CMPF caused the formation of advanced glycation end‐products, which are known to impair insulin secretion20, and increased fatty acid oxidation3. This result might explain why CMPF showed an independent negative association with triglycerides in previous studies6, 12 of individuals with disturbances in carbohydrate metabolism. However, we found that the negative association between CMPF and triglycerides was also present in individuals with NGT in addition to type 2 diabetes mellitus patients. We suggest a potential link among islets, CMPF and lipid metabolism in Chinese individuals.
We found a difference in the association of CMPF levels with several parameters separately in type 2 diabetes mellitus patients and individuals with NGT. Five biochemical parameters including LDL‐c, triglycerides, TC, ALT and AST were negatively associated with CMPF levels in type 2 diabetes mellitus patients, and only the TG level was negatively associated in individuals with NGT. In addition, a positive association with age (consistent with the results of renal failure patients21) and a negative association with BMI were also found in both groups. Therefore, the present data suggest that serum CMPF levels in various concentration ranges have different effects. The elevated serum CMPF levels in type 2 diabetes mellitus patients might have a reduced impact on lipid profiles and liver injury markers.
The relevance of CMPF and liver function in humans has not been reported. In the present study, elevated CMPF levels in type 2 diabetes mellitus patients showed a negative correlation with AST and ALT levels, which are well‐accepted non‐invasive biochemical markers of liver injury. We excluded individuals with cirrhosis, biliary obstructive disease, hepatitis and alcoholism. Thus, the elevations of AST and ALT levels in such individuals were likely due to NAFLD. We carried out a logistic regression analysis of NAFLD in both the type 2 diabetes mellitus and NGT groups. After adjusting for age and sex, a protective effect of CMPF was found in type 2 diabetes mellitus patients. However, this effect was also found in the NGT group. We removed the effect of glucose metabolism and found that the CMPF level had a protective role in the prevalence of NAFLD in Chinese individuals by a meta‐analysis. However, the adjustments for BMI in the logistic regression showed discrepancies in the serum CMPF levels. BMI affects the NAFLD process22 and might mask the effect of CMPF. Additionally, collinearity might exist between CMPF and BMI, as we found that the CMPF level was negatively associated with BMI. The relationship between CMPF and BMI has not been reported in previous studies. The negative association was found in both type 2 diabetes mellitus patients and the NGT group in the present study. Furthermore, together with the negative association between CMPF and triglycerides, the present results suggested that CMPF impacts lipid metabolism and might protect against NAFLD development.
We also identified a negative association between HDL‐c and CMPF after adjusting for age, sex and BMI by a meta‐analysis of the type 2 diabetes mellitus and NGT groups. Multiple stepwise regressions of all participants also showed the same outcome. The present data show a divergent association, as we know that the serum HDL‐c levels are often negatively related to TG levels, and CMPF was independently negatively associated with HDL‐c and triglycerides. However, the present results were consistent with another study showing that CMPF treatment reduced circulating TG and HDL‐c levels in mice23. The negative relationships of CMPF levels with both HDL‐c and TG levels were not in conflict, since the different transcription factors and rate‐limiting enzyme were affected by CMPF in TG or cholesterol biosynthesis23. A study showed that a high baseline log (TG)/HDL‐C ratio predicts fast progression of islet β‐cell dysfunction24, so the negative association between CMPF and HDL‐c might be an explanation for elevated CMPF levels in type 2 diabetes mellitus patients. Overall, this result suggests contradictory effects on lipid metabolism, and further studies are required to assess the role of CMPF.
The present study had several limitations, including the relatively limited sample size. Our study did not address the cause–effect relationship between CMPF and dyslipidemia or liver injury. In addition, the dietary intake was not recorded. Therefore, further prospective studies that record dietary intake are warranted to determine whether elevated serum CMPF is protective against dyslipidemia, obesity or NAFLD, or if it is a compensatory decrease in response to these diseases.
In conclusion, serum CMPF levels were lower in NAFLD patients than in controls in both the type 2 diabetes mellitus and NGT groups. Serum CMPF levels were independently associated with TG levels in Chinese individuals. The present data show that the serum concentration of CMPF in humans plays an important role in lipid metabolism and might be protective against NAFLD progression.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
We thank all of the study participants. We also appreciate the contributions the nurses and doctors at the Department of Endocrinology and Metabolism of Fengxian Central Hospital made to this study. This work was supported by a grant from the Shanghai Municipal Commission of Health and Family Planning, China (No. 201640272, No. ZK2015A02).
J Diabetes Investig 2019; 10: 793–800
References
- 1. Niwa T. Removal of protein‐bound uraemic toxins by haemodialysis. Blood Purif 2013; 35: 20–25. [DOI] [PubMed] [Google Scholar]
- 2. Miyamoto Y, Iwao Y, Mera K, et al A uremic toxin, 3‐carboxy‐4‐methyl‐5‐propyl‐2‐furanpropionate induces cell damage to proximal tubular cells via the generation of a radical intermediate. Biochem Pharmacol 2012; 84: 1207–1214. [DOI] [PubMed] [Google Scholar]
- 3. Liu Y, Prentice KJ, Eversley JA, et al Rapid elevation in CMPF may act as a tipping point in diabetes development. Cell Rep 2016; 14: 2889–2900. [DOI] [PubMed] [Google Scholar]
- 4. Prentice KJ, Luu L, Allister EM, et al The furan fatty acid metabolite CMPF is elevated in diabetes and induces beta cell dysfunction. Cell Metab 2014; 19: 653–666. [DOI] [PubMed] [Google Scholar]
- 5. Nagy E, Liu Y, Prentice KJ, et al Synthesis and characterization of urofuranoic acids: in vivo metabolism of 2‐(2‐Carboxyethyl)‐4‐methyl‐5‐propylfuran‐3‐carboxylic acid (CMPF) and effects on in vitro insulin secretion. J Med Chem, 2017; 60: 1860–1875. [DOI] [PubMed] [Google Scholar]
- 6. Zhang S, Chen P, Jin H, et al Circulating 3‐carboxy‐4‐methyl‐5‐propyl‐2‐furanpropanoic acid (CMPF) levels are associated with hyperglycemia and beta cell dysfunction in a Chinese population. Sci Rep 2017; 7: 3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Niwa T, Aiuchi T, Nakaya K, et al Inhibition of mitochondrial respiration by furancarboxylic acid accumulated in uremic serum in its albumin‐bound and non‐dialyzable form. Clin Nephrol 1993; 39: 92–96. [PubMed] [Google Scholar]
- 8. Mabuchi H, Nakahashi H. Inhibition of hepatic glutathione S‐transferases by a major endogenous ligand substance present in uremic serum. Nephron 1988; 49: 281–283. [DOI] [PubMed] [Google Scholar]
- 9. Sun H, Huang Y, Frassetto L, et al Effects of uremic toxins on hepatic uptake and metabolism of erythromycin. Drug Metab Dispos 2004; 32: 1239–1246. [DOI] [PubMed] [Google Scholar]
- 10. Xu L, Sinclair AJ, Faiza M, et al Furan fatty acids – Beneficial or harmful to health? Prog Lipid Res 2017; 68: 119–137. [DOI] [PubMed] [Google Scholar]
- 11. Savolainen O, Lind MV, Bergstrom G, et al Biomarkers of food intake and nutrient status are associated with glucose tolerance status and development of type 2 diabetes in older Swedish women. Am J Clin Nutr 2017; 106: 1302–1310. [DOI] [PubMed] [Google Scholar]
- 12. Zheng JS, Lin M, Imamura F, et al Serum metabolomics profiles in response to n‐3 fatty acids in Chinese patients with type 2 diabetes: a double‐blind randomised controlled trial. Sci Rep 2016; 6: 29522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lankinen MA, Hanhineva K, Kolehmainen M, et al CMPF does not associate with impaired glucose metabolism in individuals with features of metabolic syndrome. PLoS ONE 2015; 10: e0124379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Prentice KJ, Wendell SG, Liu Y, et al CMPF, a metabolite formed upon prescription omega‐3‐acid ethyl ester supplementation. Prevents and reverses steatosis. EBioMedicine 2018; 27: 200–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chalasani N, Younossi Z, Lavine JE, et al The diagnosis and management of non‐alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012; 55: 2005–2023. [DOI] [PubMed] [Google Scholar]
- 16. Younossi ZM, Loomba R, Anstee QM, et al Diagnostic modalities for non‐alcoholic fatty liver disease (NAFLD), non‐alcoholic steatohepatitis (NASH) and associated fibrosis. Hepatology 2018; 68: 349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Younossi ZM, Koenig AB, Abdelatif D, et al Global epidemiology of nonalcoholic fatty liver disease‐meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016; 64: 73–84. [DOI] [PubMed] [Google Scholar]
- 18. Chitturi S, Farrell GC, Hashimoto E, et al Non‐alcoholic fatty liver disease in the Asia‐Pacific region: definitions and overview of proposed guidelines. J Gastroenterol Hepatol 2007; 22: 778–787. [DOI] [PubMed] [Google Scholar]
- 19. Yi J, Jin H, Zhang R, et al Increased serum 3‐carboxy‐4‐methyl‐5‐propyl‐2‐furanpropanoic acid (CMPF) levels are associated with glucose metabolism in Chinese pregnant women. J Endocrinol Invest 2018; 41: 663–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Coughlan MT, Yap FY, Tong DC, et al Advanced glycation end products are direct modulators of beta‐cell function. Diabetes 2011; 60: 2523–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rroji M, Eloot S, Dhondt A, et al Association of advanced age with concentrations of uraemic toxins in CKD. J Nephrol 2016; 29: 81–91. [DOI] [PubMed] [Google Scholar]
- 22. Diehl AM, Day Cause C. Pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med 2017; 377: 2063–2072. [DOI] [PubMed] [Google Scholar]
- 23. Mohan H, Brandt SL, Kim JH, et al 3‐carboxy‐4‐methyl‐5‐propyl‐2‐furanpropanoic acid (CMPF) prevents high fat diet‐induced insulin resistance via maintenance of hepatic lipid homeostasis. Diabetes Obes Metab 2018. 10.1111/dom.13483 [DOI] [PubMed] [Google Scholar]
- 24. Zhou M, Li Z, Min R, et al Log (TG)/HDL‐C ratio as a predictor of decreased islet beta cell function in patients with type 2 diabetes: 6‐year cohort study. J Diabetes 2015; 7: 689–698. [DOI] [PubMed] [Google Scholar]


