Abstract
Plasma markers have been continuously advocated as pointers to estimate the long-term risk of cardiovascular disease in the general population. We examined the relationship between plasma high-sensitivity C-reactive protein (hs-CRP), homocysteine (Hcy), high-sensitivity cardiac troponin T (hs-cTnT), N-terminal prohormone of brain natriuretic peptide (NT-proBNP), 25-Hydroxyvitamin D (25OHD), glycosylated hemoglobin A1c (HbA1c), and serum uric acid (SUA) levels and hypertension in middle and old aged population. A total of 2624 Chinese (62.02 ± 5.73 years old) were recruited into a population-based, cross-sectional study. Plasma hs-CRP, Hcy, HbA1c, and SUA levels were significantly higher in the hypertension group compared with control in the entire population and men (P = 0.05 for all). We observed a positive association between the highest quartiles of Hcy, NT-proBNP, HBA1c concentrations, and the prevalence of hypertension, OR (95% CI) = 1.48 (1.16–1.90), 1.62 (1.27–2.07) and 1.94 (1.49–2.52), respectively. The multivariable-adjusted OR of hypertension for the fourth versus the first quartile of homocysteine were 2.00 and 1.39 in men and women, respectively. In conclusion, our study found an independent and robust association between elevated Hcy, NT-ProBNP, and HBA1c levels and prevalence of hypertension in the middle-aged and elderly Chinese population. A follow-up study is necessary to endorse the observed association.
Subject terms: Prognostic markers, Risk factors
Introduction
Hypertension carries significant weight in a global health perspective, and it is highly prevalent in mainland China1. Despite significant advancement in the management of hypertension, fundamental pathogenic mechanisms are not yet modified by currently existing treatment modalities. In addition to the continuing surge in the prevalence of hypertension attributed to failure to implement effective counteractions to reduce hypertension associated burden, recent studies persisted to inform the positive relationship between high blood pressure and cardiovascular events (CVE) and mortality2. Thus, for the prevention and management of elevated blood pressure (BP), reliable information about the biomarkers of hypertension is essential.
Earlier studies have recognized the role of inflammatory pathways activation as an important pathological event in the development of hypertension3. This growing evidence suggests that systemic inflammation endorses endothelial damage, which further contributes to physiological alterations in the endothelium4. Moreover, the pro-inflammatory pathways are well-known for their tremendous influence at lipid metabolism, suggesting that there is an interaction between inflammation and dyslipidemia5. Inversely, lipids have been reported to influence an inflammatory reaction, and an unhealthy lifestyle with increased cholesterol consumption can aggravate the inflammatory reaction, eventually leading to the expansion of metabolic syndrome (MetS)6. MetS is an independent risk factor in the pathogenesis of several CVDs, including hypertension7. C-reactive protein (CRP), a plasma marker that indicates inflammation, has been proposed to link with an elevated risk of hypertension. An increase in MetS associated risk factors, such as excess cholesterol and obesity, predisposes to insulin resistance and increases the risk of CVE and mortality1. Epidemiologic studies reported an inverse association between circulating levels of 25-hydroxyvitamin D (25-OHD)- a biomarker of vitamin D status, and metabolic syndrome8,9. Another published article highlighted that low vitamin D level depletes the intracellular concentrations of calcium, and interferes with the level of insulin secretion by beta cells which consequently impairs glucose tolerance10. With insulin resistance, there is an increased level of inflammation, oxidative stress, vascular adhesion molecules expression, and diminished levels of nitric oxide, which result in persistent hypertension due to an increase in vascular hardening11. Interestingly, a recent meta-analysis has provided firm evidence about the relations between lower circulating 25-OHD levels and hypertension12. However, there has been a recent debate about the role of low 25-OHD in the development of hypertension. Besides, Hemoglobin A1c (HbA1c)-a marker recommended to determine long-term blood glucose levels, has been reported as a clue in predicting CVD risks including peripheral artery disease, stroke and heart attack among individuals with diabetes mellitus. A shred of existing evidence reported that masked hypertension is associated with an increased risk of CVD in diabetic patients, and the presence of diabetes is an independent risk factor for central blood pressure increase13.
The contribution of neurohormones in the progression of hypertension has led to new treatment modalities such as β-blockers and angiotensin-converting enzyme (ACE) inhibitors. N-terminal prohormone of brain natriuretic peptide (NT-proBNP), a cleavage product of brain natriuretic peptide (BNP), is the gold standard for the diagnosis of heart failure and also it is an established marker for the prediction of cardiovascular events14. Recently, a study has demonstrated that NT-proBNP mirrors the detrimental effect of high blood pressure on subclinical organ damage denoted by aortic stiffness, left ventricular hypertrophy (LVH), or renal damage15. Like the NT-proBNP, high sensitivity cardiac troponin T (hs-TnT) has also been extensively studied in heart failure16. A previous study reported elevated levels of hs-cTnT in prehypertension stages, and an increase in hs-cTnT level was reported as an effective predictor of prehypertension17. To the extent of our knowledge so far, the mechanisms underlying increased levels of hs-cTnT in hypertensive subjects without ischemic heart disease are not fully elucidated. However, a previous study hypothesized that increased blood pressure may induce increased diastolic and systolic wall stress, damage/reshape coronary microvascular structures, or exert increased extravascolic compressive strength18. Surprisingly, studies conducted by Milwidsky et al. showed an independent association between hs-TnT and MetS19.
Homocysteine (Hcy), a non-proteinogenic α-amino acid, is a hemostatic marker of coagulation activation and endothelial dysfunction. Increased Hcy levels can be genetically inherited and/or acquired20. For instance, methionine metabolism from endogenous protein or diet degradation substantiates Hcy formation21, which is further cleared from the body through the kidneys. A previous study has demonstrated that the noxious effect of methionine residues causes alterations in the vascular endothelium22. Hcy increases endothelial cell damage, reduces vessels flexibility, and influences hemostasis23, indicating that increased Hcy adds to inflammation and vascular remodeling during the pathogenesis of hypertension. Evidence available suggests a possible connection between hyperhomocysteinemia and hypertension24. However, to validate whether Hcy should be considered as a biomarker or a risk factor, replication of consistent findings is required from various studies.
In general, biomarkers can grossly be utilized for risk stratification and for evaluation of therapeutic responses in CVDs. To the best of our knowledge, the pathophysiological mechanisms of hypertension require precise and accurate profiling of biomarkers. This is crucial in the effective management and monitoring of the disease process of hypertension. However, population-based studies that focus on the relationship between a number of plasma markers that include inflammatory, glycemic and coagulation markers and hypertension are limited, and the few studies on the association among the aforementioned plasma markers and hypertension have reported contradictory results25–32. These studies had several limitations; some enrolled a wide range of age or non-Chinese population, others were focused on either men or women. The current study assists to highlight a set of specific markers for hypertension in middle and old age population in northeast China. Thus, our findings add reliable information on plasma biomarkers for hypertension in middle and old age categories to the existing literature. Also, the present study is based on baseline data for a newly established prospective cohort study, and documentation of the baseline plasma marker levels could serve as reference values for the future. Considering the role of inflammation, arterial stiffness, oxidative stress and metabolic syndrome in the development of hypertension, and the involvement of the aforementioned biomarkers with either the redox of hypertension or risk factors of hypertension, we hypothesized that increased plasma Hcy, NT-proBNP, hs-TnT, HbA1c, SUA, and hs-CRP levels are associated with the prevalence of hypertension. Therefore, the purpose of this study was to investigate whether the most recent routine inflammatory (hs-CRP), cardiac (Hcy, NT-proBNP, and hs-TnT) and glycemic markers (HbA1c and SUA) are associated with BP readings and prevalence of hypertension in middle and old age population in North China.
Methods
Study design
We conducted a baseline population-based comparative cross-sectional study based on a prospective cohort study of the relative factors of heart failure among elderly people (PCSRFHFEP) data. The PCSRFHFEP study is a newly established prospective cohort study, currently registered in Dalian, China (ChiCTR-1900021163) to assess the risk factors for heart failure among the elderly population. In this study, we determined serum levels of hs-CRP, Hcy, HbA1c, hs-cTnT, NT-proBNP, SUA and 25-OHD in 2624 participants. We further performed a gender-specific investigation to examine the relationship between plasma markers and hypertension.
Participants
This study consists of 2764 Chinese participants enrolled between January 2017 and February 2018 in the PCSRFHFEP registries of Dalian City, Liaoning Province of China. We invited the retired workers of Dalian Port Authority to enroll for the PCSRFHFEP cohort study. Individuals above 50 years old with complete data, regardless of their gender status were eligible for the study. The eligible participants had a complete physical examination at Dalian Port Hospital. Exclusion criteria included: physician-diagnosed CVDs (history of stroke, congestive heart failure, coronary artery disease, myocardial infarction, and valvular heart disease), pacemaker implantation, severe cognitive or communication impairment, renal failure or insufficiency, liver cirrhosis, malignancy, active infectious/inflammatory disease and/or chronic obstructive pulmonary disease. One hundred and forty individuals did not fulfill the inclusion criteria, thus were excluded. Finally, 2624 individuals (1135 controls and 1489 cases of hypertension) were included in this study. This study was approved by First Affiliated Hospital of Dalian Medical University (approval number: LCKY2016-31), and informed consent was obtained prior to the data and specimen collection. The study protocol conformed to the law protecting personal data and agreed with the guidelines of Helsinki declaration. Cases of hypertension in the present study met at least one of the following conditions as described previously25: (1) systolic blood pressure (SBP) of >140 mmHg; (2) a diastolic blood pressure (DBP) of >90 mmHg; or (3) self-reported history of hypertension with the current use of blood pressure-reducing medication. Figure 1 demonstrates the flow chart of the recruitment and classification (Fig. 1).
Figure 1.
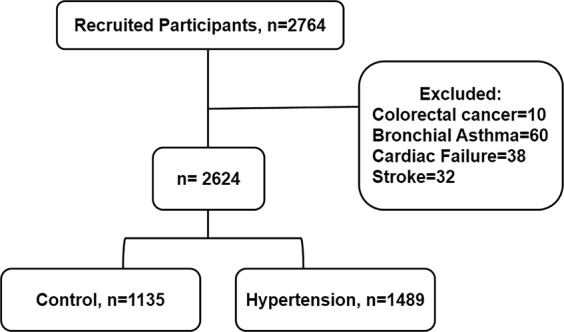
The flow chart of the recruitment and classification.
Baseline covariates
Basal data and various clinical details related to cardiovascular diseases were recorded following a detailed interview and physical examination. We gathered data on demography, health-related lifestyle, disease history, and use of any medication or drug. BP was measured twice using standard mercury sphygmomanometers with cuffs by trained physicians (measured from the brachial artery while the participant is seated in a chair for at least 5 min). If the first two records were quite different, additional measurements were made to minimize white coat hypertension. Diabetes mellitus was defined as current use of oral antidiabetic medications or insulin, fasting serum glucose >6.5 mmol/L after fasting for a minimum of 8 h, and/or a self-reported history of diabetes. Smoking exposure, alcohol consumption, and medication use were self-reported.
Biological sampling and biochemical measurements
Blood samples were biochemically examined for the concentration of hs-CRP, Hcy, hs-cTnT, NT-proBNP, 25-OHD, and HBA1c. The investigators and laboratory personnel were not aware of the subjects’ health status, and all samples were treated identically throughout the processing and analysis. Plasma hs-CRP levels were measured by a nephelometric assay on a Behring Nephelometer II (BNII) analyzer (SIEMENS BNII). Plasma hs-TnT, NT-proBNP and 25-OHD concentrations were assayed using an electrochemiluminescence immunoassay (Roche Cobas e601). Serum HbA1c concentrations were determined by using high-performance liquid chromatography (Primer Hb9210TM-HPLC HBA1C analysis). Hcy levels were determined by using an enzymatic cycling method (Hitechi7600 series automatic analyzer). High-density lipoprotein cholesterol (HDL-C), triglycerides, low-density lipoprotein cholesterol (LDL), and serum total cholesterol were measured enzymatically.
Data analyses
Study participants were compared by interquartile range or proportions of major hypertension risk factors, using student-t-test and Mann-Whitney-U test for normally and non-normally distributed data, respectively; and chi-square (χ2) test for categorical or ordinal data. The study population was stratified into quartiles based on plasma markers. Two-way analysis of variance (ANOVA) was used to detect if there was an interaction effect between genders and DM with hypertension status on the levels of plasma markers. Multiple linear regression analysis was employed to predict the systolic and diastolic BP levels (as continuous outcomes) based on the levels of markers’ concentration. Preliminary analyses were conducted to ensure no violation of assumptions of normality, homoscedasticity, independence, and linearity. Multicollinearity was assessed for all variables using tolerances and the variance inflation factors (VIF). Multicollinearity was detected when circulating biomarkers, BMI, lipid profiles and DM status were considered as an explanatory variable. The VIF of total cholesterol was greater than 10, indicating that there were high correlations among the independent (predictor) variables. However, neither assumptions were violated nor multicollinearity was detected following exclusion of total cholesterol from the model. As such, the multiple linear regression analysis was adjusted for age, gender, physical activity, cigarette status, BMI, alcohol use, triglycerides, HDL, LDL, creatinine, and DM. Binary logistic regression models were estimated, and the OR at 95% CIs were used to approximate the associated risk for hypertension according to quartiles of plasma markers, with the lowest quartile serving as the reference category. Model 1 was adjusted for age and gender. Model 2 was adjusted for age, gender, cigarette status, alcohol use, and physical activity. Model 3 was adjusted for the covariates in model 2, followed BMI, TC, HDL, LDL, TG, creatinine, and DM. Further, we ran a gender-specific analysis and sensitivity analysis to examine the relationship between plasma markers and hypertension. We performed all statistical analyses using SPSS software (IBM SPSS) version 21.
Results
Baseline characteristics
This study included 2624 individuals (1135 controls and 1489 cases) aged between 50 and 84 years. Baseline characteristics of participants are presented in Table 1. The values of the median SBP, DBP, creatinine, triglyceride, and LDL were significantly higher in the hypertension group compared with control (P < 0.01). Conversely, the median value of total cholesterol was not statistically different between hypertension and control group. The proportion of participants who were diagnosed with diabetes was significantly higher in the hypertension group compared with control. There was no statistically significant difference in physical activity between the two groups. Only 314(21.1%) of the hypertension cases were using antihypertensive drugs.
Table 1.
Baseline characteristics of the participants, N = 2624.
| Characteristic | Control (n = 1135) | Hypertension (n = 1489) | P |
|---|---|---|---|
| Male, N(%) | 436(38.4%) | 669(44.5%) | 0.001 |
| Age, years (Median) | 62(58–65) | 62(59–65) | 0.016 |
| BMI, mean ± SD, kg/m2 | 23.91 ± 3.02 | 25.48 ± 3.16 | <0.001 |
| Smoking status | |||
| Never | 823(72.5%) | 975(65.5%) | <0.001 |
| Former | 100(8.8%) | 232(15.6%) | |
| Current | 212(18.7%) | 282(18.9%) | |
| Alcohol use, N (%) | |||
| Never | 840(74%) | 1001(67.2%) | 0.001 |
| Former | 72(6.3%) | 111(7.5%) | |
| Current | 223(19.6%) | 377(25.3%) | |
| SBP(mmHg) | 128(120–134) | 155(145–170) | <0.001 |
| DBP(mmHg) | 77(71–81) | 90(83–97) | <0.001 |
| Diabetes, N(%) | 59(5.2%) | 121(8%) | 0.005 |
| Fasting glucose (mg/dl) | 5.53(5.16–6.05) | 5.58(5.36–6.57) | <0.001 |
| Physical activity | |||
| Never | 124(10.9%) | 154(10.3%) | 0.858 |
| Sometimes | 516(45.5%) | 648(46.1%) | |
| Often | 495(43.6%) | 653(43.5%) | |
| Total Cholesterol, mean ± SD, mmol/L | 5.39(4.72–6.02) | 5.43(4.80–6.06) | 0.201 |
| TG, mean ± SD, mmol/L | 1.16(0.84–1.62) | 1.37(0.99–1.94) | <0.001 |
| HDL-c, mean ± SD, mmol/L | 1.45(1.22–1.71) | 1.36(1.16–1.60) | <0.001 |
| LDL-c, mean ± SD, mmol/L | 3.3(2.76–3.87) | 3.38(2.89–3.96) | 0.008 |
| Creatinine, mean ± SD, umol//l | 62.62(57.10–73.50) | 64.48(57.17–75.01) | <0.001 |
| Antihypertensive use, N(%)# | 314(21.1%) | ||
| β-blockers | — | 59(4%) | |
| ACEI | — | 10(0.7%) | |
| ARB | — | 63(4.2%) | |
| Calcium antagonists | — | 136(9.1%) | |
| Diuretics | — | 4(0.3%) | |
| Other Antihypertensive | — | 70(4.2%) | |
TG, Triglycerides; HDL-c, High-density lipoprotein; LDL-c, Low-density lipoprotein; ACEI, Angiotensin-converting enzyme inhibitors; ARB, Angiotensin receptor blockers.
#The figures and percentile for anti-hypertensive drugs describe the N(%) within hypertension group.
Comparison of plasma markers and the interaction effect of gender and DM with hypertension status on the levels of the plasma markers
Plasma markers including, hs-CRP, Hcy, SUA, and HbA1c were statistically significantly higher in the hypertension group than in the control in the entire population, men and women (Table 2). Also, the concentrations of NT-proBNP were significantly higher in the hypertension group than in the control group in women. However, we found no statistically significant differences in 25-OHD levels between hypertension and control group in the entire population, men, and women.
Table 2.
Comparison of the plasma markers between hypertension and control group.
| General population | Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control (n = 1135) | Hypertension (n = 1489) | P | Control (n = 436) | Hypertension (n = 669) | P | Control (n = 699) | Hypertension (n = 820) | P | |
| hs-CRP (mg/L) | 0.86(0.47–1.71) | 1.13(0.60–2.08) | <0.001 | 0.90(0.48–1.90) | 1.11(0.61–2.16) | 0.001 | 0.84(0.45–1.65) | 1.15(0.59–2.03) | <0.001 |
| Hcy (µmol/L) | 9.11(7.80–11.36) | 9.75(8.14–12.45) | <0.001 | 10.44(8.80–12.49) | 11.24(9.14–14.17) | <0.001 | 8.54(7.45–10.17) | 8.85(7.64–10.68) | 0.004 |
| NT-proBNP (ng/mL) | 49.57(30.12–77.49) | 51.32(31.37–87.57) | 0.096 | 43.05(24.98–73.27) | 44.59(26.53–79.39) | 0.360 | 54.50(34.41–80.09) | 57.89(36.55–91.93) | 0.034 |
| 25-OHD (nmol/L) | 13.74(9.92–18.63) | 14.16(9.80–19.85) | 0.168 | 16.23(11.65–23.01) | 16.74(12.25–22.52) | 0.356 | 12.46(8.89–16.64) | 12.13(8.49–16.94) | 0.733 |
| HBA1C (%) | 4.99(4.66–5.43) | 5.19(4.77–5.84) | <0.001 | 5.03(4.64–5.44) | 5.17(4.76–5.85) | <0.001 | 4.98(4.67–5.43) | 5.22(4.80–5.82) | <0.001 |
| hsTnT (ng/mL) | 7.75(5.65–10.05) | 8.04(6.01–10.36) | 0.027 | 8.53(6.27–11.35) | 9.17(6.73–11.84) | 0.104 | 7.24(5.31–9.25) | 7.26(5.55–9.50) | 0.438 |
| SUA (µmol/L) | 297.85(253.47–352.47) | 321.68(265.01–376.28) | <0.001 | 342.81(297.02–394.23) | 357.34(308.57–409.68) | 0.004 | 274.95(239.40–313.81) | 287.06(246.15–339.70) | <0.001 |
Note: Plasma markers are expressed as Median (25% quartiles–75% quartiles). hs-CRP = high-sensitivity C-reactive protein; hs-cTnT, high-sensitivity cardiac troponin T; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; 25OHD, 25-Hydroxyvitamin D; SUA, serum uric acid and HbA1c, glycosylated hemoglobin A1c.
A two-way ANOVA was considered to examine the effect of sex and DM with hypertension status on biomarker levels. There was a statistically significant interaction between the effects of DM and hypertension status on HBA1c levels, F (1, 2620) = 4.771, P = 0.025. Also, there were statistically significant differences in the NT-proBNP, hcy, hs-TnT, 25-OHD and SUA levels between male and female gender (p < 0.05). In contrast, no significant interaction between gender and hypertension status on plasma marker levels (P > 0.05) was observed. The effect of gender and DM with hypertension status on biomarker levels is presented in Table 3.
Table 3.
The effect of gender, DM, and Hypertension status on biomarker levels.
| Biomarkers | The effect of gender and Hypertension status | The effect of DM and Hypertension status | ||||
|---|---|---|---|---|---|---|
| Type III Sum of Squares | F | P-value | Type III Sum of Squares | F | P-value | |
| hs-CRP (mg/L) | 5.142 | 0.181 | 0.670 | 2.900 | 0.102 | 0.749 |
| HCY (µmol/L) | 77.729 | 3.025 | 0.082 | 0.672 | 0.024 | 0.877 |
| NT-proBNP (ng/mL) | 16966.308 | 0.498 | 0.481 | 27752.051 | 0.813 | 0.367 |
| 25-OHD (nmol/L) | 8.937 | 0.162 | 0.687 | 13.547 | 0.226 | 0.634 |
| HBA1c (%) | 0.695 | 0.530 | 0.467 | 4.771 | 5.004 | 0.025 |
| SUA (µmol/L) | 161.331 | 0.033 | 0.856 | 9940.076 | 1.663 | 0.197 |
| hs-TnT (ng/mL) | 70.356 | 0.690 | 0.406 | 0.003 | 0.000 | 0.996 |
Note: Degree of freedom (DF) for the effect of gender and hypertension status was 1 whereas DF for the effect of DM and Hypertension status was 2.
The relationship between diastolic and systolic BP and plasma markers
In adjusted linear regression analysis, there was a positive association between DBP and Hcy (β = 0.073 µmol/L, 95% CI = 0.080–0.258; P < 0.001), 25-OHD (β = 0.039 nmol/L, 95% CI = 0.002–0.124; P = 0.044), and HBA1c (β = 0.046%, 95% CI = 0.032–0.963; P = 0.036) concentrations per 1 mmHg increase in DBP. Similarly, the findings of the present study demonstrated a positive association between SBP and Hcy (β = 0.076 µmol/L, 95% CI = 0.156–0.475; P < 0.001), HBA1c (β = 0.098%, 95% CI = 1.02–2.69; P < 0.001), hs-TnT (β = 0.055 ng/mL, 95% CI = 0.031–0.207; P = 0.008), and SUA (β = 0.048 µmol/L, 95% CI = 0.001–0.026; P = 0.037) per 1 mmHg increase in DBP. Table 4 depicts the relationship between plasma markers and diastolic and systolic blood pressure.
Table 4.
Association between the diastolic and systolic blood pressure and plasma markers.
| Biomarkers | Entire Population | Men | Women | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Β | 95% CI(Lower Bound- Upper Bound) | P | β | 95% CI(Lower Bound- Upper Bound) | P | β | 95% CI(Lower Bound- Upper Bound) | P | |
| DBP | |||||||||
| hs-CRP (mg/L) | −0.010 | −0.108–0.061 | 0.587 | −0.035 | −0.172–0.040 | 0.225 | 0.013 | −0.102–0.176 | 0.600 |
| HCY (µmol/L) | 0.073 | 0.080–0.258 | 0.000 | 0.092 | 0.064–0.272 | 0.002 | 0.050 | 0.002–0.343 | 0.047 |
| NT-proBNP (ng/mL) | 0.023 | −0.001–0.004 | 0.229 | 0.013 | −0.002–0.003 | 0.652 | 0.077 | 0.006–0.027 | 0.002 |
| 25-OHD (nmol/L) | 0.039 | 0.002–0.124 | 0.044 | 0.048 | −0.014–0.154 | 0.104 | 0.026 | −0.042–0.137 | 0.299 |
| HBA1c (%) | 0.046 | 0.032–0.963 | 0.036 | 0.025 | −0.460–0.942 | 0.500 | 0.062 | 0.070–1.325 | 0.029 |
| hs-TnT (ng/L) | 0.034 | −0.008–0.090 | 0.100 | 0.053 | −0.006–0.098 | 0.086 | −0.004 | −0.154–0.131 | 0.873 |
| SUA (µmol/L) | 0.037 | −0.001–0.013 | 0.101 | 0.043 | −0.003–0.017 | 0.182 | 0.022 | −0.006–0.014 | 0.426 |
| SBP | |||||||||
| hs-CRP (mg/L) | −0.029 | −0.272–031 | 0.119 | −0.037 | −0.304–0.067 | 0.210 | −0.029 | −0.405–0.101 | 0.239 |
| HCY (µmol/L) | 0.076 | 0.156–0.475 | 0.000 | 0.113 | 0.174–0.536 | 0.000 | 0.030 | −0.123–0.499 | 0.237 |
| NT-proBNP (ng/mL) | 0.019 | −0.002–0.007 | 0.323 | −0.014 | −0.005–0.003 | 0.648 | 0.123 | 0.030–0.069 | 0.000 |
| 25-OHD (nmol/L) | 0.002 | −0.104–0.116 | 0.911 | −0.001 | −0.149–0.146 | 0.980 | −0.007 | −0.187–0.139 | 0.775 |
| HBA1c (%) | 0.098 | 1.017–2.687 | 0.000 | 0.083 | 0.193–2.63 | 0.023 | 0.108 | 1.092–3.378 | 0.000 |
| hs-TnT (ng/L) | 0.055 | 0.031–0.207 | 0.008 | 0.069 | 0.013–0.196 | 0.025 | 0.041 | −0.064–0.454 | 0.141 |
| SUA (µmol/L) | 0.048 | 0.001–0.026 | 0.037 | 0.025 | −0.010–0.024 | 0.431 | 0.047 | −0.002–0.035 | 0.083 |
Adjusted for age, gender, cigarette status, Alcohol use, physical activity, BMI, high-density lipoprotein, LDL-C, triglycerides, creatinine, and Diabetes Mellitus.
Note: The plasma markers Beta estimates represents 1 unit the plasma biomarker measured in.
The relationship between plasma markers and hypertension in the general population
After we compared the concentration of plasma markers between the control and hypertension group, we further calculated the adjusted OR to investigate the relationship between the plasma markers and hypertension. When the values of plasma markers were treated as continuous data, the adjusted OR and 95% CI for hypertension in the entire population for hcy and HBA1C were 1.02(1.01–1.04; P = 0.010) and 1.24(1.12–1.36; P < 0.001), respectively. Also, statistically significant associations between hcy and HBA1c concentrations and prevalence of hypertension were discovered in men and women. In addition, there was a statistically significant relationship between NT-proBNP levels and presence of hypertension in women (OR = 1.04, 95% CI: 1.02–1.06, P = 0.001). The association between the plasma markers and hypertension in the adjusted regression analyses based on the continuous variables is shown in Table 5. A similar result was observed when plasma markers included in regression models as quartiles. There was a positive association between the higher quartiles of CRP and the presence of hypertension after adjusting for lifestyle-related variables. However, this association was not maintained after adjustment for other explanatory variables such as lipid profile, BMI, and DM. The result of the present study showed that there exist positive associations between the higher quartiles of Hcy, NT-proBNP, and HBA1c concentrations and the presence of hypertension. (Table 6).
Table 5.
ORs (95% CIs) of Hypertension when plasma markers used as continuous variables.
| Biomarkers | Entire population | Men | Women | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| hs-CRP (mg/L) | 0.997 | 0.98–1.01 | 0.681 | 1.00 | 0.98–1.02 | 0.985 | 0.99 | 0.97–1.02 | 0.460 |
| HCY (µmol/L) | 1.02 | 1.01–1.04 | 0.010 | 1.03 | 1.01–1.05 | 0.008 | 1.01 | 1.00–1.04 | 0.038 |
| NT-proBNP (ng/mL) | 1.00 | 1.00–1.00 | 0.669 | 1.00 | 1.00–1.00 | 0.963 | 1.04 | 1.02–1.06 | 0.001 |
| 25-OHD (nmol/L) | 1.00 | 0.99–1.01 | 0.887 | 1.00 | 0.99–1.02 | 0.783 | 1.00 | 0.98–1.01 | 0.545 |
| HBA1C (%) | 1.24 | 1.12–1.36 | <0.001 | 1.23 | 1.07–1.43 | 0.005 | 1.25 | 1.10–1.43 | 0.001 |
| hs-TnT (ng/L) | 1.00 | 0.99–1.01 | 0.984 | 1.00 | 0.99–1.01 | 0.745 | 0.99 | 0.97–1.02 | 0.438 |
| SUA (µmol/L) | 1.00 | 1.00–1.00 | 0.350 | 1.00 | 1.00–1.00 | 0.900 | 1.00 | 1.00–1.00 | 0.380 |
OR indicates odds ratio; CI, Confidence Interval; hs-CRP, high-sensitivity C-reactive protein; HbAC1, Glycosylated hemoglobin; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; 25OHD, 25-Hydroxyvitamin D; hs-TnT, high-sensitivity Troponin T.
Adjusted for Age and gender, cigarette status, Alcohol use, physical activity, and BMI, total serum cholesterol, high-density lipoprotein, LDL-C, triglycerides, creatinine, and Diabetes Mellitus.
Table 6.
Risk of Hypertension in the entire participants according to plasma marker quartiles.
| Plasma Marker | Plasma markers | |||
|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
| hs-CRP | ≤0.50 | 0.51–1.01 | 1.02–1.94 | ≥1.95 |
| Model 1 | 1.000(ref.) | 1.35(1.08–1.67)‡ | 1.94(1.54–2.43)‡ | 1.83(1.46–2.30)‡ |
| Model 2 | 1.000(ref.) | 1.17(0.93–1.47) | 1.51(1.19–1.91)† | 1.30(1.02–1.66)* |
| Model 3 | 1.000(ref.) | 1.10(0.88–1.39) | 1.41(1.11–1.80)* | 1.17(0.91–1.51) |
| Hcy | ≤7.97 | 7.98–9.47 | 9.48–11.92 | ≥1.93 |
| Model 1 | 1.000(ref.) | 1.09(0.87–1.35) | 1.22(0.97–1.53) | 1.59(1.26–2.01)‡ |
| Model 2 | 1.000(ref.) | 1.06(0.84–1.33) | 1.17(0.92–1.47) | 1.52(1.19–1.94)‡ |
| Model 3 | 1.000(ref.) | 1.09(0.87–1.37) | 1.17(0.92–1.47) | 1.48(1.16–1.90)† |
| hs-TnT | ≤5.82 | 5.83–7.92 | 7.93–10.23 | ≥10.24 |
| Model 1 | 1.000(ref.) | 1.15(0.91–1.46) | 1.19(0.93–1.51) | 1.21(0.94–1.55) |
| Model 2 | 1.000(ref.) | 1.16(0.91–1.48) | 1.16(0.91–1.49) | 1.08(0.83–1.39) |
| Model 3 | 1.000(ref.) | 1.16(0.91–1.49) | 1.15(0.90–1.48) | 1.05(0.81–1.37) |
| NT-proBNP | ≤30.75 | 30.75–50.77 | 50.78–83.79 | ≥83.80 |
| Model 1 | 1.000(ref.) | 1.13(0.90–1.41) | 1.06(0.85–1.32) | 1.31(1.04–1.65)* |
| Model 2 | 1.000(ref.) | 1.25(0.99–1.57) | 1.18(0.93–1.49) | 1.53(1.21–1.95)* |
| Model 3 | 1.000(ref.) | 1.28(1.02–1.62) | 1.24(0.98–1.56) | 1.62(1.27–2.07)‡ |
| 25-OHD | ≤9.85 | 9.86–13.95 | 13.96–13.39 | ≥19.40 |
| Model 1 | 1.000(ref.) | 0.81(0.65–1.01) | 0.85(0.68–1.07) | 0.99(0.79–1.25) |
| Model 2 | 1.000(ref.) | 0.80(0.64–1.01) | 0.84(0.66–1.06) | 0.95(0.75–1.21) |
| Model 3 | 1.000(ref.) | 0.81(0.65–1.03) | 0.85(0.67–1.08) | 0.97(0.76–1.24) |
| HBA1C (%) | ≤4.72 | 4.73–5.09 | 5.10–5.66 | ≥5.67 |
| Model 1 | 1.000(ref.) | 1.08(0.87–1.34) | 1.21(0.97–1.51) | 2.31(1.84–2.90)‡ |
| Model 2 | 1.000(ref.) | 1.08(0.87–1.35) | 1.21(0.97–1.50) | 2.31(1.84–2.91)‡ |
| Model 3 | 1.000(ref.) | 1.06(0.85–1.33)‡ | 1.11(0.88–1.39) | 1.94(1.49–2.52)‡ |
| Model 4 | 1.000(ref.) | 1.06(0.85–1.34) | 1.10(0.88–1.39) | 1.96(1.51–2.56)‡ |
| SUA # | ||||
| Model 1 | 1.000(ref.) | 0.99(0.80–1.23) | 1.08(0.87–1.35) | 1.71(1.37–2.14) |
| Model 2 | 1.000(ref.) | 0.90(0.72–1.12) | 0.87(0.69–1.09) | 1.23(0.97–1.56) |
| Model 3 | 1.000(ref.) | 0.87(0.69–1.09) | 0.83(0.66–1.05) | 1.11(0.87–1.41) |
*P < 0.05; †P < 0.01; ‡P < 0.001.
#The cutoff points for the Serum Uric Acid were calculated for men and women separately, and the values are described in Tables 4 and 5.
hs-CRP = high-sensitivity C-reactive protein; hs-cTnT, high-sensitivity cardiac troponin T; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; 25OHD, 25-Hydroxyvitamin D; SUA, serum uric acid and HbA1c, glycosylated hemoglobin A1c.
Model 1: Adjusted for age and gender.
Model 2: Adjusted for age, gender, smoking, Alcohol use, physical activity.
Model 3: Adjusted for the above plus total serum cholesterol, high-density lipoprotein, LDL-C, triglycerides and creatinine, BMI and Diabetes Mellitus.
Model 4: Sensitivity analysis for HBA1c markers.
The relationship between plasma markers and hypertension in Men
We observed a significant relationship between serum Hcy and HbA1c levels and presence of hypertension after adjusting for multiple confounding factors, including age, cigarette status, alcohol use, physical activity, serum TC, HDL, LDL, TG, and creatinine (Model 2, in Table 7). The association between Hcy and HBA1c levels and presence of hypertension persisted even after BMI and diabetes mellitus were added to the model (Model 3 in Table 7).
Table 7.
Risk of Hypertension in men according to plasma marker quartiles.
| Plasma Marker | Plasma markers | |||
|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
| hs-CRP | ≤0.55 | 0.56–1.04 | 1.05–2.07 | ≥2.08 |
| Model 1 | 1.000(ref.) | 1.23(0.87–1.73) | 1.66(1.17–2.36)† | 1.70(1.20–2.42)† |
| Model 2 | 1.000(ref.) | 1.12(0.79–1.59) | 1.44(1.00–2.07) | 1.41(0.98–2.04) |
| Model 3 | 1.000(ref.) | 1.05(0.74–1.51) | 1.36(0.94–1.96) | 1.30(0.90–1.90) |
| Hcy | ≤8.94 | 8.95–10.92 | 10.93–13.48 | ≥13.49 |
| Model 1 | 1.000(ref.) | 1.11(0.79–1.56) | 1.36(0.96–1.92) | 2.03(1.42–2.90)‡ |
| Model 2 | 1.000(ref.) | 1.07(0.75–1.52) | 1.37(0.96–1.96) | 2.03(1.40–2.94)‡ |
| Model 3 | 1.000(ref.) | 1.02(0.71–1.45) | 1.36(0.95–1.96) | 2.00(1.37–2.92)‡ |
| hs-TnT | ≤6.48 | 6.49–8.95 | 8.96–11.72 | ≥11.73 |
| Model 1 | 1.000(ref.) | 1.00(0.70–1.43) | 1.16(0.80–1.66) | 1.33(0.91–1.94) |
| Model 2 | 1.000(ref.) | 1.06(0.74–1.53) | 1.17(0.80–1.70) | 1.36(0.92–2.00) |
| Model 3 | 1.000(ref.) | 1.05(0.73–1.53) | 1.09(0.75–1.60) | 1.25(0.84–1.86) |
| NT-proBNP | ≤26.08 | 26.09–43.50 | 43.51–76.49 | ≥76.50 |
| Model 1 | 1.000(ref.) | 1.08(0.76–1.53) | 1.08(0.76–1.54) | 1.15(0.80–1.67) |
| Model 2 | 1.000(ref.) | 1.10(0.77–1.57) | 1.15(0.80–1.65) | 1.33(0.91–1.95) |
| Model 3 | 1.000(ref.) | 1.15(0.80–1.66) | 1.23(0.85–1.77) | 1.40(0.95–2.07) |
| 25-OHD | ≤12.04 | 12.05–16.50 | 16.51–22.69 | ≥22.70 |
| Model 1 | 1.000(ref.) | 1.07(0.75–1.51) | 1.33(0.94–1.90) | 1.01(0.72–1.43) |
| Model 2 | 1.000(ref.) | 1.10(0.77–1.57) | 1.42(0.99–2.05) | 1.09(0.76–1.56) |
| Model 3 | 1.000(ref.) | 1.09(0.76–1.56) | 1.40(0.97–2.02) | 1.07(0.74–1.54) |
| HbA1c (%) | ≤4.71 | 4.72–5.10 | 5.11–5.70 | ≥5.71 |
| Model 1 | 1.000(ref.) | 1.14(0.81–1.60) | 1.16(0.82–1.64) | 1.96(1.37–2.81)† |
| Model 2 | 1.000(ref.) | 1.19(0.84–1.69) | 1.17(0.82–1.67) | 1.81(1.25–2.63)† |
| Model 3 | 1.000(ref.) | 1.09(0.76–1.56) | 1.07(0.74–1.53) | 1.77(1.15–2.73)† |
| Model 4 | 1.000(ref.) | 1.10(0.77–1.57) | 1.01(0.70–1.46) | 1.81(1.18–2.77)† |
| SUA | ≤303.36 | 303.37–353.11 | 353.12–403.82 | ≥403.83 |
| Model 1 | 1.000(ref.) | 0.99(0.70–1.39) | 1.11(0.79–1.58) | 1.37(0.96–1.95) |
| Model 2 | 1.000(ref.) | 0.92(0.65–1.31) | 0.99(0.69–1.43) | 1.12(0.77–1.64) |
| Model 3 | 1.000(ref.) | 0.86(0.60–1.24) | 0.93(0.64–1.34) | 0.98(0.67–1.45) |
*P < 0.05; †P < 0.01;‡P < 0.001.
hs-CRP, high-sensitivity C-reactive protein; Hcy, Homocysteine; hs-cTnT, high-sensitivity cardiac troponin T; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; 25OHD, 25-Hydroxyvitamin D; SUA, Serum uric acid; and HbA1c glycosylated hemoglobin A1c.
Model 1: Adjusted for age, cigarette status, Alcohol use, physical activity.
Model 2: Adjusted for the above plus total serum cholesterol, high-density lipoprotein, LDL-C, triglycerides, and creatinine
Model 3: Adjusted for the above plus BMI and Diabetes Mellitus.
Model 4: Sensitivity analysis for HBA1c.
The relationship between plasma markers and hypertension in Women
We observed a positive and significant relationship between HbA1c, hs-CRP, Hcy, and NT-proBNP levels, and presence of hypertension after multivariate adjustment for potential confounders, including age, gender, cigarette status, alcohol use, physical activity, LDL, TC, HDL, TG, and creatinine (Model 1 and 2, in Table 8). The relationship between Hcy, NT-proBNP, and HbA1c concentrations and hypertension persisted even after adjusting for BMI and diabetes mellitus (Model 3 in Table 8). The fourth quartile of NT-proBNP concentrations was significantly associated with hypertension, with OR (95% CI) of 1.43(1.07–1.92), 1.63(1.20–2.21), and 1.85(1.35–2.53), for models 1, 2 and 3, respectively. In the fully adjusted multivariate analysis, the OR and 95% CI of hypertension for the participants in Q2, Q3, and Q4 compared to participants in the first quartile of HbA1c were 1.32(0.99–1.74), 1.79(1.33–2.41), and 2.37(1.70–3.30), respectively.
Table 8.
Risk of Hypertension in women according to plasma marker quartiles in adjusted models.
| Plasma Marker | Plasma markers | |||
|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
| hs-CRP | ≤0.53 | 0.54–1.00 | 1.01–1.85 | ≥1.86 |
| Model 1 | 1.000(ref.) | 1.31(0.99–1.75) | 1.82(1.36–2.43)‡ | 1.94(1.45–2.59)‡ |
| Model 2 | 1.000(ref.) | 1.15(0.86–1.54) | 1.60(1.19–2.16)† | 1.53(1.13–2.08)† |
| Model 3 | 1.000(ref.) | 1.02(0.75–1.38) | 1.26(0.93–1.72) | 1.04(0.75–1.45) |
| Hcy | ≤7.55 | 7.76–8.70 | 8.71–10.45 | ≥10.46 |
| Model 1 | 1.000(ref.) | 1.21(0.91–1.61) | 1.21(0.91–1.61) | 1.53(1.15–2.05)† |
| Model 2 | 1.000(ref.) | 1.23(0.92–1.64) | 1.23(0.92–1.65) | 1.53(1.13–2.07)† |
| Model 3 | 1.000(ref.) | 1.18(0.87–1.59) | 1.19(0.88–1.62) | 1.39(1.02–1.90)* |
| hs-TnT | ≤5.45 | 5.46–7.26 | 7.27–9.42 | ≥9.43 |
| Model 1 | 1.000(ref.) | 1.26(0.91–1.74) | 1.05(0.76–1.45) | 1.27(0.92–1.77) |
| Model 2 | 1.000(ref.) | 1.29(0.92–1.79) | 1.04(0.75–1.45) | 1.27(0.91–1.78) |
| Model 3 | 1.000(ref.) | 1.34(0.96–1.88) | 1.02(0.73–1.43) | 1.18(0.84–1.68) |
| NT-proBNP | ≤35.66 | 35.67–56.22 | 56.23–87.06 | ≥87.07 |
| Model 1 | 1.000(ref.) | 1.11(0.83–1.48) | 0.96(0.72–1.27) | 1.43(1.07–1.92)* |
| Model 2 | 1.000(ref.) | 1.22(0.90–1.63) | 1.07(0.80–1.44) | 1.63(1.20–2.21)† |
| Model 3 | 1.000(ref.) | 1.29(0.95–1.75) | 1.14(0.84–1.54) | 1.85(1.35–2.53)‡ |
| 25-OHD | ≤8.60 | 8.61–12.30 | 12.21–16.83 | ≥16.84 |
| Model 1 | 1.000(ref.) | 0.85(0.64–1.14) | 0.77(0.58–1.03) | 0.96(0.72–1.28) |
| Model 2 | 1.000(ref.) | 0.87(0.65–1.17) | 0.80(0.59–1.07) | 0.95(0.70–1.28) |
| Model 3 | 1.000(ref.) | 0.84(0.62–1.14) | 0.76(0.56–1.03) | 0.86(0.63–1.17) |
| HBA1C (%) | ≤4.72 | 4.73–5.09 | 5.10–5.11 | ≥5.12 |
| Model 1 | 1.000(ref.) | 2.45(1.79–3.34) | 1.36(1.02–1.81)* | 1.01(0.76–1.35)‡ |
| Model 2 | 1.000(ref.) | 1.06(0.80–1.42) | 1.34(1.00–1.78)* | 2.21(1.61–3.05)‡ |
| Model 3 | 1.000(ref.) | 1.32(0.99–1.74) | 1.79(1.33–2.41)‡ | 2.37(1.70–3.30)‡ |
| Model 4 | 1.000(ref.) | 1.32(1.00–1.75) | 1.83(1.36–2.48)‡ | 2.35(1.63–3.39)‡ |
| Uric Acid | ≤243.55 | 243.56–281.03 | 281.04–328.26 | ≥328.27 |
| Model 1 | 1.000(ref.) | 0.96(0.72–1.28) | 0.99(0.75–1.32) | 1.89(1.41–2.53)‡ |
| Model 2 | 1.000(ref.) | 0.89(0.66–1.19) | 0.88(0.65–1.18) | 1.56(1.13–2.13)† |
| Model 3 | 1.000(ref.) | 0.87(0.64–1.17) | 0.76(0.56–1.04) | 1.20(0.87–1.67) |
*P < 0.05; †P < 0.01;‡P < 0.001.
hs-CRP, high-sensitivity C-reactive protein; Hcy, Homocysteine; hs-cTnT, high-sensitivity cardiac troponin T; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; 25OHD, 25-Hydroxyvitamin D; SUA, Serum uric acid; and HbA1c glycosylated hemoglobin A1c.
Model 1: Adjusted for age, cigarette status, Alcohol use, physical activity.
Model 2: Adjusted for the above plus total serum cholesterol, high-density lipoprotein, LDL-C, triglycerides, and creatinine.
Model 3: Adjusted for the above plus BMI and Diabetes Mellitus.
Model 4: Sensitivity analysis for HBA1c.
Sensitivity analysis
To investigate whether there was a possibility that the interaction effect between hypertension status and DM on plasma HBA1c levels might have influenced the predictive power of HBA1C values for hypertension, we calculated the multivariable-adjusted odds ratios and 95% CIs in non-diabetes individuals (N = 2444). Those individuals with a high HBA1c level had a greater likelihood of having hypertension. This relationship persisted even after full adjustment for potential confounders. The OR and 95% CI for the sensitivity analysis is included in Tables 6, 7 and 8 for the entire population, men, and women, respectively.
Discussion
In this cross-sectional study, we report significant positive associations between plasma markers including, Hcy, NT-proBNP, and HbA1c, and the prevalence of hypertension in middle and old age population. These associations were independent of potential confounders; age, gender, BMI, diabetes, alcohol use, cigarette status, physical activity, TC, HDL, LDL, TG, and creatinine.
Our study reported that elevated Hcy confers a corresponding increase in DBP, SBP, and prevalence of hypertension. The results of the current study were robust to Hcy distribution and were similar when Hcy were included in regression models in quartiles. It has been previously demonstrated that hyperhomocysteinemia contributes to endothelial cell injury, increases the rigidity of vessels, and eventually influences the process of normal blood flow of haemostasis adversely33, indicating that elevated Hcy adds to inflammation and vascular remodeling during the initiation and progression of hypertension. In our study, the participants in the fourth quartile of Hcy had a significantly increased prevalence of hypertension, confirming that the risk of hypertension increases with an increase of Hcy. This finding highlights the potential role of total Hcy in the risk stratification of hypertension.
In the past, HbA1c has not been considered as a risk marker for hypertension. However, HbA1c is the most significant regulatory marker established for diabetic care. In the present study, we observed a significant and positive relationship between HbA1c values and hypertension, even after multivariate adjustment for conventional cardiovascular risk factors. According to a previous cohort study, HbA1c values are positively linked to systolic BP dipping percent among adults who were both obese and pre-diabetic but were absent in obese adults who were free of pre-diabetic34, highlighting that HbA1c is closely linked with BMI. In the present study, BMI was significantly higher in the hypertension group compared with control. However, our study controls the differences in total cholesterol level between hypertension and control groups. Thus, the strong association of HbA1c with hypertension may not be limited to cholesterol levels. The multivariable odds ratios of hypertension across the 2nd to 4th compared to the 1st quartiles of HBA1c were continuously surged in models 1, 2 and 3. This association marginally diminished, but still maintained its significance in the highest quartiles of serum HBA1c when DM and BMI were added into the regression models. This suggests that the strong inter-relationships between these two independent variables carry significant weight on the regression model results. Furthermore, there was a significant interaction between hypertension status and DM on HBA1c levels in our study. However, this interaction did not significantly influence the predictive power of HBA1c values for hypertension. This is corroborated in the multivariable-adjusted model in non-diabetes individuals showing a positive association between elevated HBA1c levels and hypertension. Bearing in mind that HBA1c is closely linked with multiple features of metabolic syndrome which relate with insulin resistance including, obesity and hyperlipidemia, pre-diabetes, and DM, a follow-up study is essential to confirm the positive, independent and robust association of elevated HBA1c with the presence of hypertension.
It has been previously reported that there exists a positive association between C-reactive protein and hypertension risk in women35. However, we observed no significant association between hs-CRP marker and hypertension, an observation consistent with a previous study25. Likewise, plasma hs-cTnT has been suggested to complement other diagnostic biomarkers in predicting prehypertension. Also, an elevated hs-cTnT has been indicated as a predictor of cardiovascular outcome in hypertensive individuals and the general population, which made it an important biomarker in patients with acute cardiac ischemia36. A recent study reported that hs-cTnT level positively correlates with prehypertension and underscored hs-cTnT as an independent predictor of prehypertension17. In our study, the hs-cTnT values were markedly higher in hypertension compared with the control group. However, we found no significant relationship between hs-cTnT levels and hypertension, particularly after adjustment for potential confounding variables.
NT-proBNP concentrations have been suggested to connect with diastolic dysfunction and left ventricular hypertrophy in hypertensive individuals37, extending the clinical application of this biomarker beyond that of heart failure. The randomized TEAMSTA Protect I- Trial reported that NT-proBNP concentrations were significantly reduced 6 months after therapy initiation from 64.8 to 53.3 ng/L38, suggesting its association with cardiovascular risk reduction. The present results showed obviously elevated NT-proBNP concentrations in the hypertension group compared with controls. A previous report from Atherosclerosis Risk in Communities Study (ARICS) concluded that persons with raised NT-proBNP were at greater risk of hypertension39. Our study demonstrated a positive association between NT-proBNP concentrations and SBP and DBP values, and hypertension prevalence, particularly in the general population and in women but not in men. In addition, a survey from the Uranosaki cohort study reported a positive association of NT-proBNP concentration with SBP among females40. This finding suggests that the outcome of our data in the general population was influenced by gender. Therefore, gender-specific evaluation is strictly required during NT-proBNP analysis. The findings, which are particular for women, highlight that future studies are needed to explore the underlying biological mechanisms of the observed association.
A previous study reported that depleted serum 25-OHD was negatively linked with hypertension among Finnish men41. Also, a recent study concluded that lower 25OHD was associated with higher blood pressure especially in those who use blood pressure medications42. However, our data reported no significant relationship between 25OHD values and presence of hypertension.
The present study is the first observational survey that determined the level of a number of CVD markers simultaneously and explored the relationship between these markers and hypertension in the middle-aged and elderly Chinese population in Dalian, Northeast China. However, several limitations can be stated for this study. The main drawback of our study is its cross-sectional nature due to which we are unable to ascribe causality to the observed relationship. The HbA1c values were astonishingly low and our study did not have control over the potential modification effect of small dense of low-density lipoprotein, Apo-lipoproteins, and BMI, which may deviate the true predictive power of HBA1c. However, we considered the interaction analysis between hypertension and diabetes status on HBA1c levels. Also, the use of echocardiographic data would have strengthened the validity of our associations; for instance, differences in left ventricular ejection fraction (LVEF) could be of importance when looking at the concentrations of NT-proBNP, hs-TnT, and HBA1c.
In conclusion, we found strong independent associations between homocysteine and HBA1c concentrations and prevalence of hypertension and high BP levels in the middle-aged and elderly Chinese population. The gender-specific data replicated positive associations between homocysteine, NT-ProBNP, and HBA1c concentrations and prevalence of hypertension in women, even after full adjustment for the potential confounding variables. Also, the relationship between homocysteine and HBA1c levels and the presence of hypertension in men was robust to potential confounding variables. For the use of these plasma markers in the prevention and management of high BP, future studies to explore the underlying biological mechanisms of the observed association, and longitudinal studies to validate the relationship are needed.
Acknowledgements
This work was supported by grants from Dalian high-level Talents Innovation and Entrepreneurship Projects (2015R019), China National Natural Science Funds (81630009), and Chang Jiang Scholar Program (T2011160).
Author Contributions
T.H.H. involved in conception and design of the study, data analysis, result interpretation, report writing and organizing the manuscript. M.L., X.Y. and Y.X. were contributed in data collection, study design, and revising the manuscript critically. L.H.H. involved in the study design, data organization and coordination, and revised the manuscript critically. All authors read and approved the final manuscript and agreed to be accountable for all aspects of the work.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Li Ma, Email: mali_lele@sina.com.
Hui-Hua Li, Email: hhli1935@aliyun.com.
References
- 1.Li D, et al. Hypertension burden and control in mainland China: Analysis of nationwide data 2003–2012. Int J Cardiol. 2015;184:637–44. doi: 10.1016/j.ijcard.2015.03.045. [DOI] [PubMed] [Google Scholar]
- 2.Sadeghi M, et al. Cardiovascular disease events and its predictors in women: Isfahan Cohort Study (ICS) J. Cardiovasc Thorac Res. 2017;9:158–163. doi: 10.15171/jcvtr.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bello-Klein A, et al. Role of Redox Homeostasis and Inflammation in the Pathogenesis of Pulmonary Arterial Hypertension. Curr Med Chem. 2018;25:1340–1351. doi: 10.2174/0929867325666171226114838. [DOI] [PubMed] [Google Scholar]
- 4.Renna NF, de Las Heras N, Miatello RM. Pathophysiology of Vascular Remodeling in Hypertension. Int J Hypertens. 2013;2013:808353. doi: 10.1155/2013/808353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Diepen JA, Berbée JF, Havekes LM, Rensen PC. Interactions between inflammation and lipid metabolism: relevance for efficacy of antiinflammatory drugs in the treatment of atherosclerosis. Atherosclerosis. 2013;228:306–15. doi: 10.1016/j.atherosclerosis.2013.02.028. [DOI] [PubMed] [Google Scholar]
- 6.Patel S, et al. Anti-inflammatory effects of apolipoprotein A-I in the rabbit. Atherosclerosis. 2010;212:392–7. doi: 10.1016/j.atherosclerosis.2010.05.035. [DOI] [PubMed] [Google Scholar]
- 7.Cheung BM, et al. Relationship between the metabolic syndrome and the development of hypertension in the Hong Kong Cardiovascular Risk Factor Prevalence Study-2 (CRISPS2) Am J Hypertens. 2008;21:17–22. doi: 10.1038/ajh.2007.19. [DOI] [PubMed] [Google Scholar]
- 8.Pinelli NR, et al. Serum 25-hydroxy vitamin d and insulin resistance, metabolic syndrome, and glucose intolerance among Arab Americans. Diabetes Care. 2010;33:1373–5. doi: 10.2337/dc09-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richard CS, Kate ES, Sudarshan R. Metabolic syndrome: A review of the role of vitamin D in mediating susceptibility and outcome. World J Diabetes. 2015;6:896–911. doi: 10.4239/wjd.v6.i7.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jalali G, et al. Relationship Between Vitamin D Deficiency and Metabolic Syndrome in Renal Transplant Patients in Mashhad, Iran, Shiraz. E-Med J. 2017;18:e40985. [Google Scholar]
- 11.Varbo A, et al. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol. 2013;61:427–36. doi: 10.1016/j.jacc.2012.08.1026. [DOI] [PubMed] [Google Scholar]
- 12.Ke L, Mason RS, Kariuki M, Mpofu E, Brock KE. Vitamin D status and hypertension: a review. Integr Blood Press Control. 2015;8:13–35. doi: 10.2147/IBPC.S49958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang L, Qin B, Zhang X, Chen Y, Hou J. Association of central blood pressure and cardiovascular diseases in diabetic patients with hypertension. Medicine (Baltimore). 2017;96:e8286. doi: 10.1097/MD.0000000000008286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zelt JGE, et al. N-Terminal Pro B-Type Natriuretic Peptide and High-Sensitivity Cardiac Troponin T levels are related to the extent of hibernating myocardium in patients with ischemic heart failure. Can J Cardiol. 2017;33:1478–1488. doi: 10.1016/j.cjca.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Courand PY, Harbaoui B, Bècle C, Mouly-Bertin C, Lantelme P. Plasma NT-proBNP mirrors the deleterious cardiovascular and renal continuum in hypertension. Eur J Prev Cardiol. 2017;24:452–459. doi: 10.1177/2047487316683070. [DOI] [PubMed] [Google Scholar]
- 16.Rajtar-Salwa R, Hładij R, Dimitrow PP. Elevated Level of Troponin but Not N-Terminal Probrain Natriuretic Peptide Is Associated with Increased Risk of Sudden Cardiac Death in Hypertrophic Cardiomyopathy Calculated According to the ESC Guidelines 2014. Dis Markers. 2017;2017:9417908. doi: 10.1155/2017/9417908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Askin L, Yesiltepe Y. High-Sensitivity Cardiac Troponin T levels in prehypertensive patients. Clin Exp Hypertens. 2017;2017:1–5. doi: 10.1080/10641963.2017.1377216. [DOI] [PubMed] [Google Scholar]
- 18.Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–40. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 19.Milwidsky A, et al. Metabolic syndrome is associated to high-sensitivity cardiac troponin T elevation. Biomarkers. 2018;2018:1–6. doi: 10.1080/1354750X.2018.1528630. [DOI] [PubMed] [Google Scholar]
- 20.Mao X, et al. Folic acid and vitamins D and B12 correlate with homocysteine in Chinese patients with type-2 diabetes mellitus, hypertension, or cardiovascular disease. Medicine. 2016;95:e2652. doi: 10.1097/MD.0000000000002652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fowdar JY, Lason MV, Szvetko AL, Lea RA, Griffiths LR. Investigation of homocysteine-pathway-related variants in essential hypertension. Int J Hypertens. 2012;2012:190923. doi: 10.1155/2012/190923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joob B, Wiwanitkit V. Homocysteine and masked hypertension. Anatol J Cardiol. 2015;15:85. doi: 10.5152/akd.2014.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maki KC, et al. Vitamin D intake and status are associated with lower prevalence of metabolic syndrome in U.S. adults: National Health and Nutrition Examination Surveys 2003–2006. Metab Syndr Relat Disord. 2012;10:363–372. doi: 10.1089/met.2012.0020. [DOI] [PubMed] [Google Scholar]
- 24.He LM, Gao CY, Wang Y, Wang H, Zhao HY. Red cell distribution width and homocysteine act as independent risk factors for cardiovascular events in newly diagnostic essential hypertension. Oncotarget. 2017;8:102590–102599. doi: 10.18632/oncotarget.21964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sesso HD, et al. Plasma Inflammatory Markers and the Risk of Developing Hypertension in Men. J Am Heart Assoc. 2015;4:e001802. doi: 10.1161/JAHA.115.001802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bautista LE, Vera LM, Arenas IA, Gamarra G. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-alpha) and essential hypertension. J Hum Hypertens. 2005;19:149–154. doi: 10.1038/sj.jhh.1001785. [DOI] [PubMed] [Google Scholar]
- 27.Chuang SY, et al. C-reactive protein predicts systolic blood pressure and pulse pressure but not diastolic blood pressure: the Cardiovascular Disease Risk Factors Two-Township Study. Am J Hypertens. 2013;26:657–664. doi: 10.1093/ajh/hps095. [DOI] [PubMed] [Google Scholar]
- 28.Fox ER, et al. Association of plasma B-type natriuretic peptide concentrations with longitudinal blood pressure tracking in African Americans: findings from the Jackson Heart Study. Hypertension. 2013;61:48–54. doi: 10.1161/HYPERTENSIONAHA.112.197657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uçar H, et al. High-sensitivity cardiac troponin T levels in newly diagnosed hypertensive patients with different left ventricle geometry. Blood Press. 2014;23:240–47. doi: 10.3109/08037051.2013.840429. [DOI] [PubMed] [Google Scholar]
- 30.Wu H, et al. Association of total homocysteine with blood pressure in a general population of Chinese adults: a cross-sectional study in Jiangsu province, China. BMJ Open. 2018;8:e021103. doi: 10.1136/bmjopen-2017-021103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sundström J, et al. Plasma homocysteine, hypertension incidence, and blood pressure tracking: the Framingham Heart Study. Hypertension. 2003;42:1100–5. doi: 10.1161/01.HYP.0000101690.58391.13. [DOI] [PubMed] [Google Scholar]
- 32.Cheung BM, et al. C-reactive protein as a predictor of hypertension in the Hong Kong Cardiovascular Risk Factor Prevalence Study (CRISPS) cohort. J Hum Hypertens. 2012;26:108–116. doi: 10.1038/jhh.2010.125. [DOI] [PubMed] [Google Scholar]
- 33.Zhang S, et al. Association between serum homocysteine and arterial stiffness in elderly: a community-based study. J Geriatr Cardiol. 2014;11:32–8. doi: 10.3969/j.issn.1671-5411.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lane-Cordova AD, et al. Hemoglobin A1c and C-reactive protein are independently associated with blunted nocturnal blood pressure dipping in obesity-related prediabetes. Hypertens Res. 2018;41:33–38. doi: 10.1038/hr.2017.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sesso HD, et al. C-Reactive Protein and the Risk of Developing Hypertension. JAMA. 2003;290:2945–2951. doi: 10.1001/jama.290.22.2945. [DOI] [PubMed] [Google Scholar]
- 36.Griffiths ME, Malan L, Delport R, Cockeran M, Reimann M. Troponin T release is associated with silent myocardial ischaemia in black men: The SABPA Study. Eur J Prev Cardiol. 2017;24:942–950. doi: 10.1177/2047487317694465. [DOI] [PubMed] [Google Scholar]
- 37.Di Stasio E, et al. NT-proBNP: a marker of preclinical cardiac damage in arterial hypertension. Clin Chim Acta. 2011;412:1106–11. doi: 10.1016/j.cca.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 38.Jagodzinski A, et al. Cardiovascular Biomarkers in Hypertensive Patients with Medical Treatment-Results from the Randomized TEAMSTA Protect I Trial. Clin Chem. 2017;63:1877–1885. doi: 10.1373/clinchem.2017.275289. [DOI] [PubMed] [Google Scholar]
- 39.Bower JK, et al. N-Terminal Pro-Brain Natriuretic Peptide (NT-proBNP) and Risk of Hypertension in the Atherosclerosis Risk in Communities (ARIC) Study. Am J. Hypertens. 2015;28:1262–1266. doi: 10.1093/ajh/hpv026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanaka A, et al. N-terminal pro-brain natriuretic peptide and associated factors in the general working population: a baseline survey of the Uranosaki cohort study. Sci Rep. 2017;7:5810. doi: 10.1038/s41598-017-06090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ke L, et al. Hypertension, pulse, and other cardiovascular risk factors and vitamin D status in Finnish men. Am J Hypertens. 2013;26:951–6. doi: 10.1093/ajh/hpt051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ke L, et al. Hypertension and other cardiovascular risk factors are associated with vitamin D deficiency in an urban Chinese population: A short report. J. Steroid Biochem Mol Biol. 2017;173:286–291. doi: 10.1016/j.jsbmb.2016.11.011. [DOI] [PubMed] [Google Scholar]


