Abstract
Anti-programmed cell death protein 1 (PD1) antibodies are in wide use for the treatment of various cancers. PD1 antibody-based immunotherapy, co-administration of nivolumab and ipilimumab, is one of the optimal immunotherapies, especially in advanced melanoma with high tumor mutation burden. Since this combined therapy leads to a high frequency of serious immune-related adverse events (irAEs) in patients with advanced melanoma, biomarkers are needed to evaluate nivolumab efficacy to avoid serious irAEs caused by ipilimumab. This study analyzed baseline serum levels of CXCL5, CXCL10, and CCL22 in 46 cases of advanced cutaneous melanoma treated with nivolumab. Baseline serum levels of CXCL5 were significantly higher in responders than in non-responders. In contrast, there were no significant differences in baseline serum levels of CXCL10 and CCL22 between responders and non-responders. These results suggest that baseline serum levels of CXCL5 may be useful as a biomarker for identifying patients with advanced cutaneous melanoma most likely to benefit from anti-melanoma immunotherapy.
Keywords: baseline levels of CXCL5, melanoma, nivolumab, prediction of efficacy, nivolumab and ipilimumab combined therapy
Introduction
Anti-programmed cell death protein 1 (PD-1) antibodies such as nivolumab and pembrolizumab are in wide use for the treatment of various cancers, including advanced melanoma (1, 2), but cost-effective analyses of their use are sometimes controversial (3). Therefore, biomarkers for the evaluation of the efficacy of anti-PD1 antibody therapy are needed. Previous clinical studies suggested that the efficacy of nivolumab monotherapy is ~40% in the Caucasian population (2, 4), which contains a high ratio of superficial spreading melanoma (SSM) with high levels of tumor mutation burden (TMB) (5). In contrast, in the Japanese population, there is a high ratio of acral lentiginous melanoma (ALM) and mucosal melanoma (6), which have low levels of TMB (5). The efficacies of nivolumab and pembrolizumab in Japan have been reported to be 34.1% and 24.1%, respectively (7, 8), suggesting that another drug that could enhance the anti-tumor immune response in melanoma is needed.
Ipilimumab is a fully humanized immunoglobulin (Ig)G1 monoclonal antibody that blocks cytotoxic T-lymphocyte antigen (CTLA-4) and is one of the promising drugs that enhance the anti-tumor immune response for patients with advanced melanoma with or without BRAF gene mutation in combination with nivolumab (1, 4, 9). Indeed, the efficacy of this combination therapy for advanced melanoma has been reported to be 57.8% (4), and, therefore, combination therapy with nivolumab and ipilimumab is recommended by the NCCN guideline for cutaneous melanoma as a first-line therapy (10). This combination therapy achieves a high efficacy rate even for the treatment of brain metastases of melanoma (11). In addition, Blank et al. reported that the efficacy of co-administration of nivolumab and ipilimumab does not parallel TMB (12). In addition to co-administration of nivolumab and ipilimumab, sequential administration of nivolumab and ipilimumab with a planned switch leads to high efficacy in the treatment of advanced melanoma (4, 9). On the other hand, both co-administration of nivolumab and ipilimumab and sequential administration of nivolumab and ipilimumab with a planned switch lead to a high frequency of serious immune-related adverse events (irAEs), such as hepatitis, colitis, polyneuropathy, etc., in patients with advanced melanoma (1, 4, 11). Therefore, determining the efficacy of nivolumab monotherapy before starting first-line immune therapy for melanoma is important.
CXCL5 is a chemokine that can recruit not only neutrophils, but also CXCR2+ myeloid-derived suppressor cells (MDSCs) and CXCR2+ monocytes that can be precursors of tumor-associated macrophages (TAMs) (13–15). Indeed, Soler-Cardona et al. reported that CXCL5-overexpressing melanomas had significantly increased lymph node metastases of melanoma (15) caused by the recruitment of immunosuppressive PD-L1-expressing neutrophils, leading to interference with systemic activation of the anti-tumor immune system using poly (I: C) (14). In another report, the recruitment of CXCR2-expressing MDSCs played significant roles in the development of colitis-associated colon cancer (13). These reports suggested the production of CXCL5 in the cancer stroma of melanoma.
In addition to autoimmune-related chemokines, chronic inflammatory chemotactic factors such as CXCL10 are also important for the recruitment of immunosuppressive cells such as regulatory T cells (Tregs) and MDSCs. Jiang et al. reported that, compared to patients with stable disease, advanced melanoma patients had increased levels of IL-1β and CXCL10 in the serum associated with accumulation of monocytic (Mo)-MDSCs and Tregs in peripheral blood, which correlated with the progression-free survival of these patients (16). In addition, other reports also suggested that serum CXCL10 levels were correlated with disease activity in advanced melanomas (17) and angiosarcomas (18). These reports suggested that serum CXCL10 levels may represent disease activity in advanced melanoma.
Not only MDSCs, but Tregs are also important for tumor progression in melanoma (19, 20). Indeed, Johansenn et al. previously reported that Tregs at the tumor sites were correlated with tumor progression in melanoma (20). More recently, Ha et al. reported the significance of high CTLA4 expression for Tregs, leading to selective depletion of Tregs in melanoma, which might be an important tool in designing cancer immunotherapy (21). In addition, as described above, the reduction of CCL22 by TAMs decreases Tregs in the tumor site, which enhances the therapeutic effects of immune therapy in the mouse melanoma model (22). Taken together, these reports suggest that serum CCL22 may be correlated with the efficacy of immune therapy.
From the above findings, in this report, the baseline serum levels of TAM-associated chemokines were investigated in 46 advanced melanoma patients treated with nivolumab.
Patients and Methods
Ethics Statement for Human Experiments
The protocol for this human study was approved by the ethics committee of Tohoku University Graduate School of Medicine, Sendai, Japan (Permit No: 2017-1-064). All methods were performed in accordance with the relevant guidelines and regulations. All patients provided their written, informed consent.
Patients
Data from patients treated with nivolumab were collected from eight institutes in Japan. Patients were eligible if they had unresectable stage III melanoma, or if the patients had stage IV melanoma with accessible cutaneous, subcutaneous, and/or nodal lesions (staging was performed according to the AJCC Staging Manual, 7th edition, 2011). All patients received 2 mg/kg of nivolumab followed by a 3-week rest period or 3 mg/kg of nivolumab followed by 2 weeks of rest, both of which are approved dosing schedules in Japan. Serum was obtained from patients before the administration of nivolumab. The response to nivolumab was assessed according to Response Evaluation Criteria In Solid Tumors.
Baseline Serum Levels of CXCL5 and CXCL10
Before nivolumab administration, the serum was stored, and serum levels of CXCL5, CXCL10, and CCL22 were then analyzed by enzyme-linked immunoassay (ELISA) according to the protocol provided by the manufacturer (R&D Systems, Minneapolis, MN).
Statistical Methods
Receiver operating characteristic (ROC) curves were used to calculate cut-off values for serum levels of CXCL5, CXCL10, and CCL22 and areas under the curves (AUCs). Cut-offs were determined using Youden's index 12 (sensitivity + specificity −1) to determine the point of the maximum index value (23). ROC curves were established to evaluate serum levels of CXCL5 and CXCL10 in patients administered nivolumab. All statistical analyses were performed using JMP version 14.1 software (SAS Institute, Tokyo, Japan). For a single comparison of two groups, the Mann-Whitney U-test was used. The level of significance was set at p < 0.05.
Results
Patients
Data were collected from 46 melanoma patients treated with nivolumab (Table 1). The mean patient age was 67 years (range, 33–93 years). Of the patients with melanoma, 58.7% were males, and 41.3% were females. The most common primary tumor site was the extremities (41.3%), followed by mucosal origin (30.4%), trunk (15.2%), head and neck (10.9%), and unknown origin (2.2%).
Table 1.
Characteristics and serum levels of CXCL5, CXCL10, and CCL22 in patients with cutaneous melanoma.
| Age (y) | Sex | Location | Efficacy | CXCL5 (pg/ml) | CXCL10 (pg/ml) | CCL22 (pg/ml) | |
|---|---|---|---|---|---|---|---|
| 1 | 51–60 | M | Trunk | SD | 226.9 | 69.31 | 290.9 |
| 2 | 31–40 | F | Extremities | PD | 307.7 | 212.8 | 814.6 |
| 3 | 61–70 | F | Vagina | PD | 237.6 | 117.9 | 611.2 |
| 4 | 61–70 | M | Extremities | PR | 497.5 | 144.4 | 314.6 |
| 5 | 61–70 | M | Extremities | PR | 332.6 | 72.13 | 401.5 |
| 6 | 81–90 | F | Extremities | PR | 434.8 | 355.5 | 977.3 |
| 7 | 61–70 | M | Trunk | PD | 862.1 | 113.0 | 891.5 |
| 8 | 81–90 | F | Extremities | PD | 433.9 | 186.1 | 1340 |
| 9 | 71–80 | M | Head and neck | SD | 461.3 | 97.21 | 615.7 |
| 10 | 81–90 | F | Trunk | PD | 314.9 | 74.89 | 637.2 |
| 11 | 91–100 | M | Extremities | PD | 423.4 | 122.2 | 582.6 |
| 12 | 71–80 | M | Extremities | SD | 471.9 | 84.24 | 1031 |
| 13 | 61–70 | M | Extremities | PD | 222.6 | 202.5 | 448.7 |
| 14 | 61–70 | F | Vagina | SD | 667.8 | 322.7 | 548.3 |
| 15 | 71–80 | M | Trunk | PR | 502.8 | 358.7 | 603.8 |
| 16 | 71–80 | F | Extremities | PR | 408.6 | 550.1 | 523.0 |
| 17 | 81–90 | F | Unknown | SD | 940 | 266.1 | 701.2 |
| 18 | 71–90 | M | Nasal cavity | SD | 332.5 | 188.1 | 840.2 |
| 19 | 61–70 | M | Nasal cavity | PD | 162.9 | 1001 | 788.0 |
| 20 | 61–70 | M | Paranasal | PD | 292.1 | 247.1 | 678.2 |
| 21 | 61–70 | F | Vagina | PD | 292.4 | 368.2 | 497.1 |
| 22 | 61–70 | F | Vagina | PD | 271.6 | 386.9 | 475.7 |
| 23 | 51–60 | F | Conjunctiva | SD | 380.9 | 336.7 | 355.5 |
| 24 | 81–90 | M | Digestive duct | PD | 237.4 | 208.1 | 438.3 |
| 25 | 61–70 | F | Digestive duct | SD | 5026 | 336.8 | 987.9 |
| 26 | 61–70 | F | Trunk | PD | 474.3 | 245.3 | 84.71 |
| 27 | 71–80 | M | Extremities | PD | 494.1 | 116.7 | 630.4 |
| 28 | 51–60 | F | Head and neck | PD | 370.5 | 93.53 | 983.2 |
| 29 | 31–40 | M | Trunk | SD | 501.8 | 97.59 | 857.8 |
| 30 | 31–40 | F | Extremities | PR | 407 | 138.7 | 963.1 |
| 31 | 71–80 | M | Extremities | SD | 529.6 | 108.4 | 637.7 |
| 32 | 31–40 | M | Extremities | PD | 687.1 | 147.1 | 845.1 |
| 33 | 71–80 | F | Extremities | SD | 544.7 | 104.6 | 935.6 |
| 34 | 71–80 | M | Head and neck | PD | 701.3 | 77.79 | 918.5 |
| 35 | 41–50 | M | Extremities | PD | 655.4 | 432.3 | 617.5 |
| 36 | 71–80 | F | Extremities | PR | 465.3 | 184.8 | 1065 |
| 37 | 61–70 | M | Trunk | PR | 555.2 | 105.7 | 915.5 |
| 38 | 41–50 | M | Head and neck | SD | 740.9 | 65.85 | 830.3 |
| 39 | 41–50 | M | Extremities | PD | 723.8 | 54.08 | 944.6 |
| 40 | 61–70 | F | Head and neck | SD | 410.7 | 62.46 | 470.7 |
| 41 | 71–80 | F | Extremities | PR | 1196 | 71.03 | 606.1 |
| 42 | 61–70 | M | Digestive duct | PR | 564 | 190.4 | 498.5 |
| 43 | 61–70 | F | Palate | PR | 1600 | 51.84 | 619.5 |
| 44 | 51–60 | F | Extremities | CR | 687.3 | 80.09 | 701.3 |
| 45 | 61–70 | M | Paranasal | CR | 1142 | 192 | 211.3 |
| 46 | 61–70 | F | Vagina | CR | 4939 | 68.73 | 573 |
Changes of CXCL5, CXCL10, and CCL22 serum levels in each patient (n = 46) before the administration of nivolumab were examined by ELISA. Data for each donor represent the means of duplicate assays.
CR, complete response.
PR, partial response.
SD, stable disease.
PD, progress disease.
Efficacy and Adverse Events (AEs) of Nivolumab 3 Months After First Administration
In patients with advanced melanoma, complete response (CR) was seen in 3 patients (6.5%; 95% confidence interval [CI], 0–13.0%), partial response (PR) was seen in 11 patients (23.9%; 95%CI, 0–47.8%), stable disease (SD) was seen in 13 patients (28.3%; 95%CI, 0–56.6%), and progressive disease (PD) was seen in 25 patients (41.3%; 95%CI, 0–82.6%). The objective response rate 3 months after first administration was thus 30.4% (95%CI, 0–60.8%). Tumor responses of individual patients are listed in Table 1. The incidence of AEs was 41.3% (Grade 4: 2.2%, Grade 3: 19.6%, Grade 2: 17.4%, Grade 1: 2.2%) (Table 2).
Table 2.
Immune-related adverse events in patients with cutaneous melanoma.
| Adverse events | Grade | |
|---|---|---|
| 1 | N.A. | N.A. |
| 2 | N.A. | N.A. |
| 3 | N.A. | N.A. |
| 4 | Bursitis | 3 |
| 5 | Hypophisitis | 4 |
| 6 | Radiation dermatitis | 3 |
| 7 | N.A. | N.A. |
| 8 | Thyroid dysfunction | 2 |
| 9 | N.A. | N.A. |
| 10 | N.A. | N.A. |
| 11 | N.A. | N.A. |
| 12 | N.A. | N.A. |
| 13 | Thyroid dysfunction | 2 |
| 14 | Thyroid dysfunction | 2 |
| 15 | Psoriasiform dermatitis | 3 |
| 16 | N.A. | N.A. |
| 17 | CIDP | 3 |
| 18 | N.A. | N.A. |
| 19 | Psoriasiform dermatitis | 3 |
| 20 | N.A. | N.A. |
| 21 | N.A. | N.A. |
| 22 | N.A. | N.A. |
| 23 | N.A. | N.A. |
| 24 | N.A. | N.A. |
| 25 | Rheumarthritis | 3 |
| 26 | Hypophisitis | 2 |
| 27 | N.A. | N.A. |
| 28 | Diarrhea | 2 |
| 29 | Abdominal pain | 2 |
| 30 | Hypophisitis | 1 |
| 31 | N.A. | N.A. |
| 32 | Diarrhea | 2 |
| 33 | N.A. | N.A. |
| 34 | N.A. | N.A. |
| 35 | N.A. | N.A. |
| 36 | N.A. | N.A. |
| 37 | N.A. | N.A. |
| 38 | N.A. | N.A. |
| 39 | Diarrhea | 2 |
| 40 | N.A. | N.A. |
| 41 | N.A. | N.A. |
| 42 | N.A. | N.A. |
| 43 | Hypophisitis | 3 |
| 44 | IDDM | 3 |
| 45 | N.A. | N.A. |
| 46 | IDDM | 3 |
CIDP, chronic inflammatory demyelinating polyneuropathy.
IDDM, insulin dependent diabetes mellitus.
Serum Levels of CXCL5, CXCL10, and CCL22
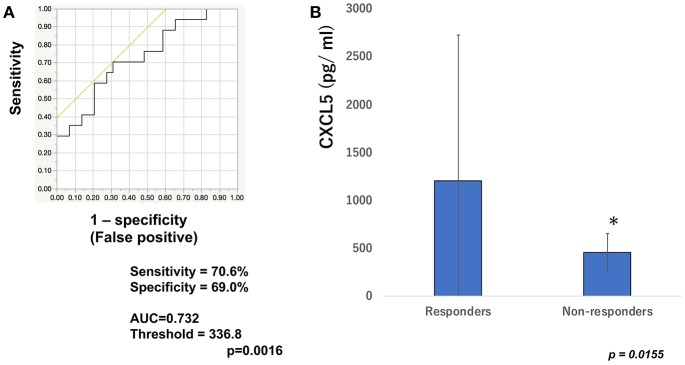
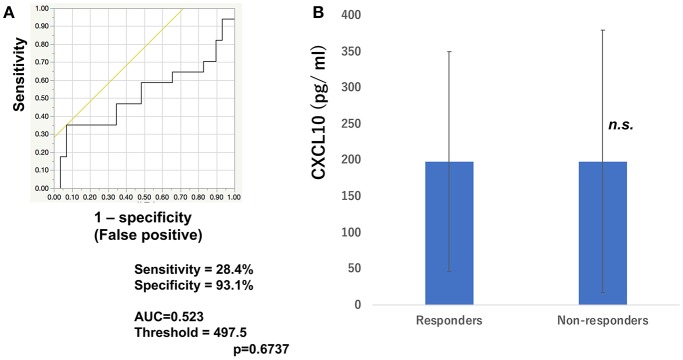
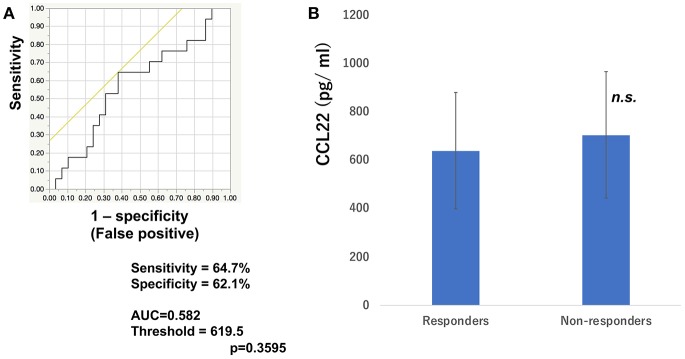
To determine whether baseline serum levels of CXCL5, CXCL10, and CCL22 may be associated with early response in melanoma patients treated with nivolumab, their levels were evaluated in 46 patients with advanced melanoma treated using nivolumab. Increases in baseline serum CXCL5 and efficacy 3 months after the first administration of nivolumab in each patient are shown in Table 1. The threshold value of CXCL5 at baseline to distinguish responders from non-responders was 497.5 pg/ml. The sensitivity and specificity of the baseline serum CXCL5 in advanced melanoma were 70.6 and 69.0%, respectively (p = 0.0016; Figure 1A). High baseline serum levels of CXCL5 were correlated with objective response to nivolumab in patients with advanced melanoma (Figure 1B). On the other hand, there were no significant relationships between serum levels of CXCL10 (Figure 2A) and CCL22 (Figure 3A) and the objective response to nivolumab in patients with advanced melanoma (CXCL10: p = 0.674, CCL22: p = 0.360). The threshold values of CXCL10 and CCL22 at baseline to distinguish responders from non-responders were 336.8 and 619.5 pg/ml, respectively. There were no significant differences in serum CXCL10 and CCL22 levels in patients with objective response and non-responding patients (Figures 2B, 3B). Baseline serum CXCL5, CXCL10, and CCL22 levels in each patient are shown in Table 1. There were no significant relationships between serum levels of CXCL5 (p = 0.0703), CXCL10 (p = 0.1748), and CCL22 (p = 0.2207) and irAEs in patients with nivolumab-treated advanced melanoma.
Figure 1.
Serum levels of CXCL5 and the ROC curve in melanoma. The ROC curve was used to calculate cut-offs for CXCL5 serum levels and the AUC. Cut-offs were determined to distinguish responders from non-responders using Youden's index (A). Mean serum levels of CXCL5 in responders (n = 16) and non-responders (n = 30) at day 0 (B). *p < 0.05 (n.s, not significant).
Figure 2.
Serum levels of CXCL10 and the ROC curve in melanoma. The ROC curve was used to calculate cut-offs for CXCL10 serum levels and the AUC. Cut-offs were determined to distinguish responders from non-responders using Youden's index (A). Mean serum levels of CXCL10 in responders (n = 16) and non-responders (n = 30) at day 0 (B). (n.s, not significant).
Figure 3.
Serum levels of CCL22 and the ROC curve in melanoma. The ROC curve was used to calculate cut-offs for CCL22 serum levels and the AUC. Cut-offs were determined to distinguish responders from non-responders using Youden's index (A). Mean serum levels of CCL22 in responders (n = 16) and non-responders (n = 30) at day 0 (B). (n.s, not significant).
Discussion
As previously reported, increased levels of soluble(s) CD163 at 6 weeks could predict the efficacy of nivolumab monotherapy 2–3 months after its first administration for the treatment of advanced cutaneous melanoma (24). Indeed, the sensitivity and specificity of serum sCD163 for the prediction of efficacy of nivolumab in cutaneous melanoma were 84.6 and 87.0%, respectively (p = 0.0030). Moreover, the absolute serum levels of sCD163 (baseline levels of sCD163 compared with day 42) were significantly increased in advanced melanoma patients who developed irAEs (24). This report concludes that the absolute serum levels of sCD163 are useful for the prediction of irAEs in melanoma patients, especially in combination with the absolute value of CXCL5 (25). Since serum sCD163 and CXCL5 are, at least in part, derived from CD163+ TAMs that are activated by periostin (24, 26), and chemokine profiles from TAMs are determined by the stimulation of stromal factors (27), spontaneously produced TAM-related factors could be detected in serum from melanoma patients (17, 25, 27). Notably, CD163+ M2 macrophages could be activated by periostin, leading to the production of characteristic chemokines, such as CXCL5, CXCL10, and CCL22, (28) that are correlated with recruitment of both immunosuppressive cells and immune-reactive anti-tumor cells (25). On the other hand, PD-1 expression is a key factor in maintaining TAMs as M2-polarized, and blockade of PD-1/PD-L1 leads to conversion of TAMs into M1-polarized activated macrophages (29). Since CD163+ TAMs are activated by anti-PD1 antibody (29), the TAM-related chemokines such as sCD163 and CXCL5 are important to evaluate the recruitment of anti-PD1 antibody in the tumor microenvironment.
From the above findings, in this report, we hypothesized that baseline serum levels of TAM-related chemokines, CXCL5, CXCL10, and CCL22, might be correlated with the efficacy of nivolumab in patients with advanced melanomas. To prove this hypothesis, serum levels of CXCL5, CXCL10, and CCL22 were analyzed in 46 cases of advanced melanoma treated with nivolumab. Baseline serum levels of CXCL5 were significantly increased in the response group compared to the non-response group in melanoma. In contrast, no significant differences in baseline serum levels of CXCL10 and CCL22 were seen between the nivolumab response and non-response groups. This discrepancy might be caused by the different sources of CXCL10 and CCL22, such as dendritic cells and endothelial cells that express lower levels of PD1 (29), leading to no effect of anti-PD1 antibody on the production of these chemokines in melanoma patients. Since CXCL5 is also reported as a biomarker for various T helper 17 cell-mediated autoimmune disorders (30–32), the high serum levels of CXCL5 might be correlated with the anti-tumor immune response of anti-PD1 antibody that could also induce autoimmune-like responses such as interstitial pneumonia, autoimmune-like colitis, and hepatitis (33). Taken together, CXCL5 may represent a predictive biomarker for evaluating the efficacy of nivolumab 3 months after its first administration for advanced melanoma. The present study suggested that CXCL5 may be a useful biomarker for the selection of those melanoma patients most likely to benefit from anti-melanoma immunotherapy using nivolumab and ipilimumab combined therapy. Because this was a pilot study, future independent studies with larger patient cohorts are needed to confirm the present findings.
Data Availability
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
The protocol for this human study was approved by the ethics committee of Tohoku University Graduate School of Medicine, Sendai, Japan (Permit No: 2017-1-064). All methods were performed in accordance with the relevant guidelines and regulations. All patients provided their written, informed consent.
Author Contributions
TFuj designed the research study. YS, TFuj, KT, CL, and YK gathered and analyzed the ELISA data. TFuj, YK, RA, AO, YF, KY, SM, HU, YY, HH, TFun, YN, RT, HO, NW and AH treated the patients and acquired the clinical data and samples. TFuj wrote the manuscript. TFuj and SA supervised the study.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study was partially funded by the Japan Agency for Medical Research and Development (2017-U-059 and 18cm0106434h0001).
References
- 1.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. (2017) 377:1345–56. 10.1056/NEJMoa1709684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. (2015) 372:320–30. 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- 3.Tarhini A, Benedict A, McDermott D, Rao S, Ambavane A, Gupte-Singh K, et al. Sequential treatment approaches in the management of BRAF wild-type advanced melanoma: a cost-effectiveness analysis. Immunotherapy. (2018) 10:1241–52. 10.2217/imt-2018-0085 [DOI] [PubMed] [Google Scholar]
- 4.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. (2015) 373:23–34. 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayward NK, Wilmott JS, Waddell N, Johansson PA, Field MA, Nones K, et al. Whole-genome landscapes of major melanoma subtypes. Nature. (2017) 545:175–80. 10.1038/nature22071 [DOI] [PubMed] [Google Scholar]
- 6.Ishihara K, Saida T, Yamamoto A. Japanese Skin Cancer Society Prognosis and Statistical Investigation Committee. Updated statistical data for malignant melanoma in Japan. Int J Clin Oncol. (2001) 6:109–16. 10.1007/PL00012091 [DOI] [PubMed] [Google Scholar]
- 7.Yamazaki N, Kiyohara Y, Uhara H, Uehara J, Fujimoto M, Takenouchi T, et al. Efficacy and safety of nivolumab in Japanese patients with previously untreated advanced melanoma: a phase II study. Cancer Sci. (2017) 108:1223–30. 10.1111/cas.13241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamazaki N, Takenouchi T, Fujimoto M, Ihn H, Uchi H, Inozume T, et al. Phase 1b study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in Japanese patients with advanced melanoma (KEYNOTE-041). Cancer Chemother Pharmacol. (2017) 79:651–60. 10.1007/s00280-016-3237-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weber JS, Gibney G, Sullivan RJ, Sosman JA, Slingluff CL, Jr, Lawrence DP, et al. Sequential administration of nivolumab and ipilimumab with a planned switch in patients with advanced melanoma (CheckMate 064): an open-label, randomised, phase 2 trial. Lancet Oncol. (2016) 17:943–55. 10.1016/S1470-2045(16)30126-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Melanoma Version 2. (2019). Available online at: https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf (accessed March 12, 2019).
- 11.Tawbi HA, Forsyth PA, Algazi A, Hamid O, Hodi FS, Moschos SJ, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. (2018) 379:722–30. 10.1056/NEJMoa1805453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blank CU, Rozeman EA, Fanchi LF, Sikorska K, van de Wiel B, Kvistborg P, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med. (2018) 24:1655–61. 10.1038/s41591-018-0198-0 [DOI] [PubMed] [Google Scholar]
- 13.Katoh H, Wang D, Daikoku T, Sun H, Dey SK, Dubois RN, et al. CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell. (2013) 24:631–44. 10.1016/j.ccr.2013.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forsthuber A, Lipp K, Andersen L, Ebersberger S, Graña-Castro, Ellmeier W, et al. CXCL5 as regulator of neutrophil function in cutaneous melanoma. J Invest Dermatol. (2019) 139:186–94. 10.1016/j.jid.2018.07.006 [DOI] [PubMed] [Google Scholar]
- 15.Soler-Cardona A, Forsthuber A, Lipp K, Ebersberger S, Heinz M, Schossleitner K, et al. CXCL5 facilitates melanoma cell-neutrophil interaction and lymph node metastasis. J Invest Dermatol. (2018) 138:1627–35. 10.1016/j.jid.2018.01.035 [DOI] [PubMed] [Google Scholar]
- 16.Jiang H, Gebhardt C, Umansky L, Beckhove P, Schulze TJ, Utikal J, et al. Elevated chronic inflammatory factors and myeloid-derived suppressor cells indicate poor prognosis in advanced melanoma patients. Int J Cancer. (2015) 136:2352–60. 10.1002/ijc.29297 [DOI] [PubMed] [Google Scholar]
- 17.Sato Y, Fujimura T, Kambayashi Y, Tanita K, Tono H, Hashimoto A, et al. Two cases of dabrafenib and trametinib therapy-failed advanced melanoma successfully controlled by nivolumab monotherapy. J Dermatol. (2018) 45:1105–8. 10.1111/1346-8138.14508 [DOI] [PubMed] [Google Scholar]
- 18.Fujimura T, Sato Y, Kambayashi Y, Tanita K, Tsukada A, Terui T, et al. Three patients with advanced cutaneous angiosarcoma treated with eribulin: investigation of serum soluble CD163 and chemokine (C-X-C motif) ligand 10 as possible biomarkers predicting the biological behaviour of angiosarcoma. Br J Dermatol. (2018) 179:1392–5. 10.1111/bjd.16676 [DOI] [PubMed] [Google Scholar]
- 19.Weber R, Fleming V, Hu X, Nagibin V, Groth C, Altevogt P, et al. Myeloid-derived suppressor cells hinder the anti-cancer activity of immune checkpoint inhibitors. Front Immunol. (2018) 9:1310. 10.3389/fimmu.2018.01310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansen LL, Lock-Andersen J, Hviid TV. The pathophysiological impact of HLA class Ia and HLA-G expression and regulatory T cells in malignant melanoma: a review. J Immunol Res. (2016) 2016:6829283. 10.1155/2016/6829283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ha D, Tanaka A, Kibayashi T, Tanemura A, Sugiyama D, Wing JB, et al. Differential control of human Treg and effector T cells in tumor immunity by Fc-engineered anti-CTLA-4 antibody. Proc Natl Acad Sci USA. (2019) 116:609–18. 10.1073/pnas.1812186116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kakizaki A, Fujimura T, Furudate S, Kambayashi Y, Yamauchi T, Yagita H, et al. Immunomodulatory effect of peritumoral administration of interferon-beta on melanoma through tumor-associated macrophages. Oncoimmunology. (2015) 4:e1047584 10.1080/2162402X.2015.1047584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Youden WJ. Index for rating diagnostic tests. Cancer. (1950) 3:32–5. [DOI] [PubMed] [Google Scholar]
- 24.Fujimura T, Sato Y, Tanita K, Kambayashi Y, Otsuka A, Fujisawa Y, et al. Serum level of soluble CD163 may be a predictive marker of the effectiveness of nivolumab in patients with advanced cutaneous melanoma. Front Oncol. (2018) 8:530. 10.3389/fonc.2018.00530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujimura T, Sato Y, Tanita K, Kambayashi Y, Otsuka A, Fujisawa Y, et al. Serum soluble CD163 and CXCL5 could be predictive markers for immune related adverse event in patients with advanced melanoma treated with nivolumab. Oncotarget. (2018) 9:15542–51. 10.18632/oncotarget.24509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen TO, Schmidt H, Møller HJ, Høyer M, Maniecki MB, Sjoegren P, et al. Macrophage markers in serum and tumor have prognostic impact in American Joint Committee on Cancer stage I/II melanoma. J Clin Oncol. (2009) 27:3330–37. 10.1200/JCO.2008.19.9919 [DOI] [PubMed] [Google Scholar]
- 27.Fujimura T, Kambayashi Y, Fujisawa Y, Hidaka T, Aiba S. Tumor-associated macrophages: therapeutic targets for skin cancer. Front Oncol. (2018) 8:3. 10.3389/fonc.2018.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furudate S, Fujimura T, Kakizaki A, Kambayashi Y, Asano M, Watabe A, et al. The possible interaction between periostin expressed by cancer stroma and tumor-associated macrophages in developing mycosis fungoides. Exp Dermatol. (2016) 25:107–12. 10.1111/exd.12873 [DOI] [PubMed] [Google Scholar]
- 29.Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. (2017) 545:495–9. 10.1038/nature22396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rumble JM, Huber AK, Krishnamoorthy G, Srinivasan A, Giles DA, Zhang X, et al. Neutrophil-related factors as biomarkers in EAE and MS. J Exp Med. (2015) 212:23–35. 10.1084/jem.20141015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buckland J. Rheumatoid arthritis: citrullination alters the inflammatory properties of chemokines in inflammatory arthritis. Nat Rev Rheumatol. (2014) 10:446. 10.1038/nrrheum.2014.112 [DOI] [PubMed] [Google Scholar]
- 32.Fujimura T, Kakizaki A, Furudate S, Aiba S. A possible interaction between periostin and CD163+ skin-resident macrophages in pemphigus vulgaris and bullous pemphigoid. Exp Dermatol. (2017) 26:1193–8. 10.1111/exd.13157 [DOI] [PubMed] [Google Scholar]
- 33.Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev. (2016) 44:51–60. 10.1016/j.ctrv.2016.02.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.