Abstract
Aims/Introduction
The relationship between glycemic variability (GV) and diabetic complications has gained much interest and remains under debate. Furthermore, the association of GV with diabetic complications has not been examined in latent autoimmune diabetes of the adult (LADA). Therefore, we evaluated the relationships among several metrics of GV with diabetic retinopathy (DR) in patients with LADA and type 2 diabetes mellitus.
Materials and Methods
A total of 192 patients with LADA and 2,927 patients with type 2 diabetes mellitus were enrolled. After continuous glucose monitoring for 72 h, three metrics of GV including standard deviation, coefficient of variation and mean amplitude of glycemic excursions were calculated. DR was assessed by fundus photography performed with a digital non‐mydriatic camera.
Results
The prevalence of DR was 20.3 and 26.4% in LADA and type 2 diabetes mellitus patients (P < 0.001), respectively. Generally, LADA patients had fewer cardiometabolic risk factors than type 2 diabetes mellitus patients, and all GV metrics were significantly higher in LADA than in type 2 diabetes mellitus. In the multivariate logistic regression analysis, no metrics for GV were identified as independent risk factors of DR (standard deviation: P = 0.175; coefficient of variation: P = 0.769; mean amplitude of glycemic excursions: P = 0.388) in LADA. However, the standard deviation was significantly associated with DR (OR 1.15, P = 0.017) in patients with type 2 diabetes mellitus after adjusting for confounders. The independent relationships of coefficient of variation and mean amplitude of glycemic excursions with DR (P = 0.194 and P = 0.251, respectively) did not reach statistical significance in type 2 diabetes mellitus.
Conclusions
GV is more strongly associated with DR in type 2 diabetes than in LADA, suggesting that different glucose‐lowering strategies should be used for these two types of diabetes.
Keywords: Diabetic retinopathy, Glycemic variability, Latent autoimmune diabetes of the adult
Introduction
Latent autoimmune diabetes in adults (LADA) refers to adult diabetes patients who are initially non‐insulin requiring, but have type 1 diabetes mellitus‐associated autoantibodies and who often progress to insulin dependency. LADA manifests as a wide spectrum of heterogeneous clinical and metabolic phenotypes that are midway between those of classic type 1 diabetes mellitus and type 2 diabetes mellitus. It is estimated that LADA accounts for 4–14% of patients with a diagnosis of type 2 diabetes mellitus, making it more common than classic childhood‐onset type 1 diabetes mellitus in some ethnic groups1.
During recent years, glycemic variability (GV) has gained much research interest as a contributor, beyond glycated hemoglobin A1c (HbA1c), to the development of diabetes‐related complications2. Mechanistic and clinical studies suggested that glucose fluctuations might incur oxidative stress and endothelial dysfunction3, 4, 5, 6, which have deleterious effects on both the microvasculature and macrovasculature. Recently, several epidemiology studies investigating the associations of GV and diabetic complications were reported, and the results were inconsistent. Of note, no study examined the effect of GV on the risk of diabetic complications in patients with LADA. Additionally, the relationships between short‐term indices of GV (as measured by continuous glucose monitoring [CGM]) and diabetic retinopathy (DR) in individuals with type 2 diabetes mellitus are largely unknown, with only one study reported with a small sample size7.
Therefore, the aim of the present study was to evaluate the link between several short‐term GV measurements, as assessed by CGM, and the prevalence of DR in patients with LADA and type 2 diabetes mellitus.
Methods
Study Populations
In the present study, LADA patients were consecutively enrolled, and type 2 diabetes mellitus patients were randomly selected from the patients admitted to the Department of Endocrinology and Metabolism of Shanghai Jiao Tong University Affiliated Sixth People's Hospital, Shanghai, China, between July, 2005, and December, 2015. Diabetes was diagnosed according to the 1999 World Health Organization criteria8. LADA was defined according to the Chinese consensus on LADA as follows: (i) aged ≥18 years at the onset of diabetes; (ii) positivity for autoantibodies to glutamic acid decarboxylase and/or islet antigen 2; and (iii) insulin independence for 6 months after diagnosis. Diabetes patients negative for autoantibodies to glutamic acid decarboxylase and autoantibodies to islet antigen 2 were defined as type 2 diabetes mellitus. All patients were aged 18–75 years and were taking a stable antidiabetic regimen for the previous 3 months. Those with acute complications of diabetes including diabetic ketoacidosis, hyperglycemic hyperosmolar state or severe and recurrent hypoglycemic events during the past 3 months were excluded from the study. The study protocol was approved by the ethics committees of Shanghai Jiao Tong University Affiliated Sixth People's Hospital in accordance with the principles of the Helsinki Declaration. Before participation, written informed consent was obtained from each participant.
CGM Parameters
All participants were continuously monitored for 72 h with a retrospective CGM system (Medtronic Inc., Northridge, CA, USA), as previously described9. After the monitoring, GV metrics were calculated with computer software. Measures of intraday GV included the standard deviation (SD) of sensor glucose values, glucose coefficient of variation (CV) and the mean amplitude of glycemic excursions (MAGE). CV was calculated as SD divided by the mean of sensor glucose readings. MAGE was calculated as the arithmetic mean of the differences between consecutive peaks and nadirs. Only excursions of >1 SD of the mean glycemic values were selected for the calculation of MAGE. All participants adhered to the original therapy regimen during the 72‐h CGM period and followed a standard dietary pattern at the same time.
Anthropometric and Biochemical Measurements
All patients underwent anthropometric measurements including height, weight and blood pressure. Body mass index was calculated as weight (kg) divided by the square of height (m). Blood pressure (BP) was measured twice using a standard mercury sphygmomanometer and the average was documented. Venous blood samples were collected after an overnight fast. Biochemical parameters including fasting plasma glucose, HbA1c, C‐peptide, triglycerides (TG), total cholesterol, high‐density lipoprotein cholesterol and low‐density lipoprotein cholesterol were assayed, as previously reported10.
Assessment of DR
For each patient, fundus photography was carried out for both eyes with a digital non‐mydriatic camera (Canon CR6‐45NM; Canon, Lake Success, NY, USA) following a standardized protocol. The status of DR was diagnosed by an ophthalmologist blinded to the characteristics of patients according to the International Classification of Diabetic Retinopathy11. When binoculus DRs were graded differently, the advanced one was selected.
Statistical Analysis
Continuous variables that followed a normal distribution were compared using Student's t‐test, whereas those with skewed distributions were compared by the Mann–Whitney U‐test. The χ2‐test was used to determine the differences between groups for categorical variables. In the univariate logistic regression analysis, the associations of potential clinical risk factors, including GV metrics, with DR were evaluated. Those variables with P‐values <0.25, as well as GV metrics, were considered for entry into a multivariate logistic regression model to examine the predictors of DR. The Cochran–Armitage trend test was used to evaluate the increasing prevalence of DR as a function of quartiles of GV metrics. To avoid multicollinearity, separate multivariate logistic regression models were constructed for SD (model 1), CV (model 2) and MAGE (model 3), respectively. The correlations among the GV metrics can be found in Table S1. Statistical analyses were carried out using SPSS software version 17.0 (SPSS Inc., Chicago, IL, USA). A P‐value of <0.05 (two‐tailed) was considered statistically significant.
Results
Clinical characteristics of the patients are shown in Table 1. A total of 2,927 type 2 diabetes mellitus and 192 LADA patients were finally included in the analyses. The prevalence of DR was 26.4 and 20.3% in type 2 diabetes mellitus and LADA patients (P < 0.001), respectively. By comparison with type 2 diabetes mellitus patients, LADA patients were leaner, had a more favorable blood pressure (including systolic and diastolic BP) and lipid profile (including TG, high‐density lipoprotein cholesterol and low‐density lipoprotein cholesterol), lower fasting C‐peptide and higher GV (including SD, CV and MAGE) during CGM. In addition, patients with LADA were more likely to be treated with insulin and statins, and had lower propensity to receive renin–angiotensin aldosterone system inhibitors than patients with type 2 diabetes mellitus. For type 2 diabetes mellitus patients, the age, duration, systolic BP, TG, HbA1c, use of oral antidiabetic agents, insulin and renin–angiotensin aldosterone system inhibitors, and all four GV metrics differed significantly between those with and without DR. For LADA patients, significant differences in sex, duration, systolic and diastolic BP, and the use of calcium‐channel blockers were observed between individuals with and without DR.
Table 1.
Clinical characteristics of participants
| Parameters | Type 2 diabetes mellitus | LADA | ||||
|---|---|---|---|---|---|---|
| Total (n = 2,927) | Non‐DR (n = 2,155) | DR (n = 772) | Total (n = 192) | Non‐DR (n = 153) | DR (n = 39) | |
| Age (years) | 57.7 ± 10.1 | 57.3 ± 10.4 | 58.8 ± 9.1** | 56.4 ± 10.1 | 56.3 ± 10.2 | 56.8 ± 9.7 |
| Sex, male (%) | 42.70 | 42.40 | 43.70 | 52.1* | 54.20 | 43.6** |
| Diabetes duration (years) | 7.7 ± 6.3 | 6.6 ± 5.8 | 10.8 ± 6.6** | 7.7 ± 6.4 | 7.0 ± 6.2 | 10.6 ± 6.2** |
| BMI (kg/m2) | 25.1 ± 3.4 | 25.1 ± 3.4 | 24.9 ± 3.4 | 23.2 ± 5.2* | 23.1 ± 5.7 | 23.2 ± 2.5 |
| SBP (mmHg) | 131.5 ± 17.2 | 130.0 ± 16.3 | 135.6 ± 18.9** | 127.2 ± 16.6* | 125.0 ± 14.4 | 136.4 ± 21.6** |
| DBP (mmHg) | 80.4 ± 9.5 | 80.2 ± 9.4 | 81 ± 9.6 | 77.6 ± 9.1* | 76.7 ± 8.6 | 81.2 ± 10.3** |
| TC (mmol/L) | 4.8 ± 1.1 | 4.7 ± 1.1 | 4.8 ± 1.2 | 4.6 ± 1.0 | 4.6 ± 1.0 | 4.9 ± 1.2 |
| TG (mmol/L) | 2.0 ± 1.8 | 2.0 ± 1.9 | 1.8 ± 1.6** | 1.2 ± 1.2* | 1.2 ± 1.3 | 1.2 ± 0.8 |
| HDL‐C (mmol/L) | 1.1 ± 0.3 | 1.1 ± 0.3 | 1.1 ± 0.3 | 1.4 ± 0.4* | 1.4 ± 0.4 | 1.4 ± 0.5 |
| LDL‐C (mmol/L) | 3.2 ± 1.0 | 3.2 ± 1.0 | 3.2 ± 1.0 | 2.8 ± 0.9* | 2.7 ± 0.8 | 2.9 ± 1.1 |
| HbA1c (%) | 8.9 ± 2.1 | 8.8 ± 2.2 | 9.2 ± 2.0** | 9.0 ± 2.0 | 8.9 ± 2.1 | 9.4 ± 1.9 |
| Fasting C‐p (ng/mL) | 1.9 ± 1.3 | 2.1 ± 1.3 | 1.6 ± 1.0** | 0.9 ± 1.0* | 0.9 ± 1.0 | 0.8 ± 1.1 |
| Diabetes treatment (%) | ||||||
| OAD | 55.3 | 58.2 | 47.9** | 54.5 | 55.7 | 50.0 |
| Insulin | 68.0 | 62.8 | 81.2** | 93.0* | 92.6 | 94.7 |
| Anti‐hypertensive agents (%) | ||||||
| RAAS inhibitors | 40.9 | 39.4 | 45.0** | 26.6* | 25.0 | 33.3 |
| Calcium‐channel blockers | 17.6 | 17.1 | 19.2 | 12.6 | 9.5 | 25.9** |
| Beta‐blockers | 7.8 | 8.1 | 7.2 | 7.7 | 6.9 | 11.1 |
| Diuretics | 2.7 | 2.3 | 3.7 | 2.1 | 2.6 | 0.0 |
| Lipid‐lowering agents (%) | ||||||
| Statins | 24.0 | 23.4 | 25.8 | 35.0* | 35.3 | 33.3 |
| Fibrates | 10.8 | 11.5 | 9.0 | 8.4 | 10.3 | 0.0 |
| GV metrics | ||||||
| SD (mmol/L) | 2.3 ± 0.9 | 2.2 ± 0.9 | 2.4 ± 0.9** | 2.8 ± 0.9* | 2.8 ± 0.9 | 2.9 ± 0.9 |
| CV (%) | 25.5 ± 8.5 | 25.2 ± 8.3 | 26.5 ± 9.0** | 30.7 ± 9.0* | 31.3 ± 9.5 | 28.9 ± 7.0 |
| MAGE (mmol/L) | 5.8 ± 2.5 | 5.7 ± 2.4 | 6.1 ± 2.5** | 7.4 ± 2.7* | 7.4 ± 2.8 | 7.4 ± 2.5 |
Data are mean ± standard deviation or percentage unless otherwise indicated. *P < 0.05 for LADA versus type 2 diabetes mellitus; **P < 0.05 for diabetic retinopathy (DR) versus non‐DR in either type 2 diabetes mellitus or latent autoimmune diabetes of the adult (LADA). BMI, body mass index; C‐p, C‐peptide; CV, coefficient of variation; DBP, diastolic blood pressure; GV, glycemic variability; HbA1c, glycated hemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; INS, insulin; LDL‐C, low‐density lipoprotein cholesterol; MAGE, mean amplitude of glycemic excursions; OAD, oral anti‐diabetic agents; RAAS, renin–angiotensin–aldosterone system; SBP, systolic blood pressure; SD, standard deviation; TC, total cholesterol; TG, triglyceride.
Univariate logistic regression was carried out to assess the crude relationship of potential risk factors with DR (Table 2). For type 2 diabetes mellitus, the age, duration, systolic BP, TG, HbA1c and all metrics of GV were significantly associated with DR (all P < 0.05). In LADA, however, only the duration, systolic BP and diastolic BP were identified as significant risk factors for DR (all P < 0.05), whereas the relationship between GV metrics and DR did not reach statistical significance (all P ≥ 0.177).
Table 2.
Associations of potential risk factors with diabetic retinopathy in type 2 diabetes mellitus and latent autoimmune diabetes of the adult analyzed by univariate logistic regression
| Variables | Type 2 diabetes mellitus | LADA | ||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Age | 1.02 (1.01–1.02) | <0.001 | 1.00 (0.97–1.04) | 0.782 |
| Sex | 1.06 (0.90–1.25) | 0.480 | 0.65 (0.32–1.32) | 0.236 |
| Duration | 1.11 (1.10–1.13) | <0.001 | 1.09 (1.03–1.15) | 0.002 |
| BMI | 0.98 (0.95–1.00) | 0.092 | 1.00 (0.93–1.08) | 0.926 |
| SBP | 1.02 (1.01–1.02) | <0.001 | 1.04 (1.02–1.06) | 0.001 |
| DBP | 1.01 (1.00–1.02) | 0.072 | 1.06 (1.01–1.10) | 0.012 |
| TC | 1.03 (0.96–1.11) | 0.400 | 1.31 (0.93–1.85) | 0.125 |
| TG | 0.93 (0.88–0.98) | 0.011 | 0.99 (0.74–1.33) | 0.964 |
| HDL‐C | 1.29 (0.99–1.67) | 0.055 | 1.66 (0.72–3.80) | 0.232 |
| LDL‐C | 1.02 (0.94–1.11) | 0.657 | 1.14 (0.77–1.69) | 0.500 |
| HbA1c | 1.08 (1.04–1.13) | <0.001 | 1.13 (0.95–1.34) | 0.158 |
| SD[Link] | 1.30 (1.19–1.41) | <0.001 | 1.05 (0.72–1.53) | 0.788 |
| CV[Link] | 1.16 (1.07–1.26) | <0.001 | 0.75 (0.49–1.14) | 0.177 |
| MAGE[Link] | 1.21 (1.11–1.31) | <0.001 | 0.97 (0.66–1.44) | 0.888 |
Odds ratios and P‐values were calculated for each one standard deviation (SD) increase in SD, coefficient of variation (CV) or mean amplitude of glycemic excursions (MAGE). BMI, body mass index; CI, confidence interval; DBP, diastolic blood pressure; DR, diabetic retinopathy; HbA1c, glycated hemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; LADA, latent autoimmune diabetes of the adult; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride.
Patients were subsequently stratified according to the quartiles of SD, CV and MAGE in LADA and type 2 diabetes mellitus, respectively. As shown in Figure 1, the prevalence of DR increased with ascending quartiles of SD and MAGE in type 2 diabetes mellitus (all P for trend ≤0.001), but not in LADA (all P for trend >0.05).
Figure 1.
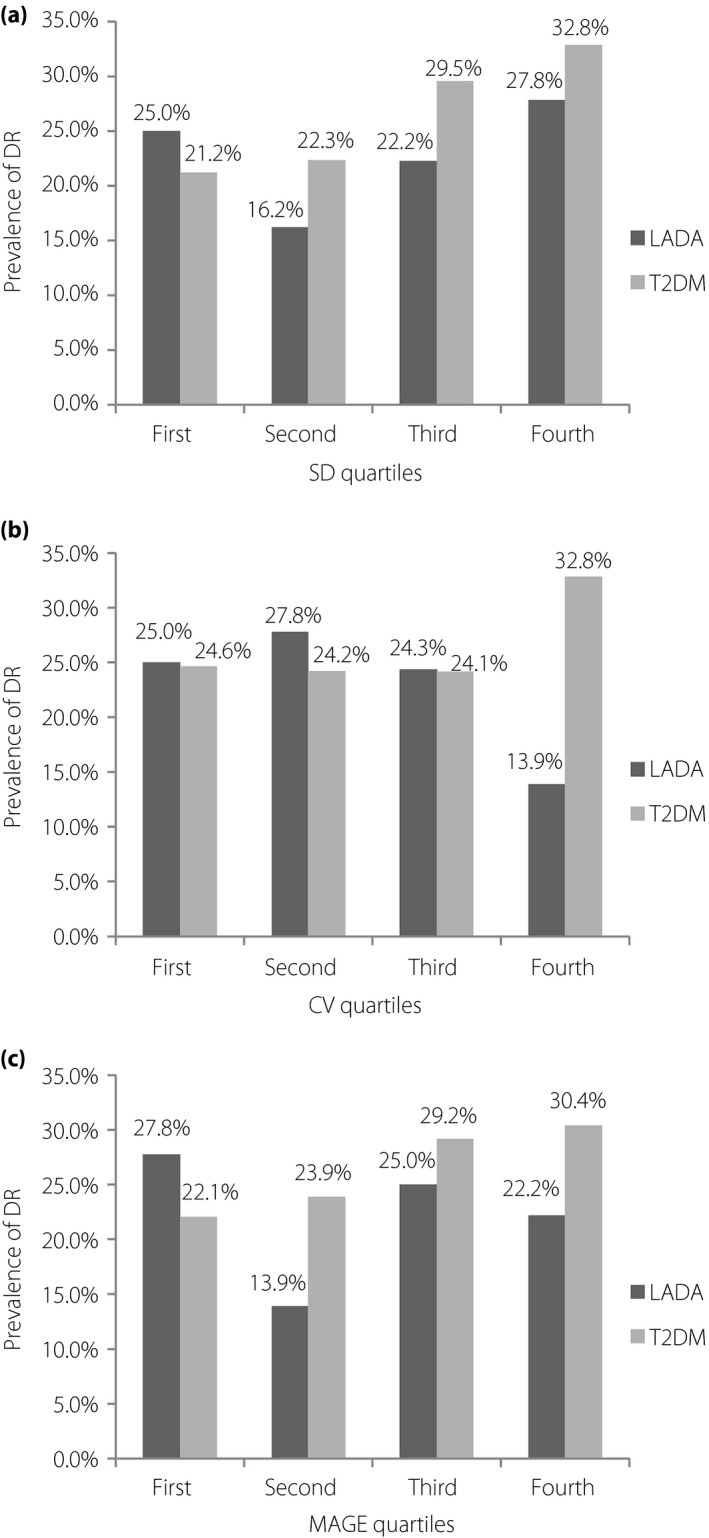
The prevalence of diabetic retinopathy (DR) according to quartiles of glycemic variability metrics in latent autoimmune diabetes of the adult (LADA) and type 2 diabetes mellitus (T2DM). (a) The cut‐off values for standard deviation (SD) quartiles were 1.6, 2.2 and 2.8 mmol/L in type 2 diabetes mellitus patients, and 2.2, 2.7 and 3.3 mmol/L in LADA patients. (b) The cut‐off values for coefficient of variation (CV) quartiles were 19.5, 24.6 and 31.1% in type 2 diabetes mellitus and 23.4, 30.4 and 36.3% in LADA. (c) The cut‐off values for mean amplitude of glycemic excursions (MAGE) quartiles were 4.0, 5.5 and 7.3 mmol/L in type 2 diabetes mellitus and 5.3, 6.8 and 9.2 mmol/L in LADA.
Based on the results of the univariate analyses, variables with P‐values <0.25 were included in the multivariate logistic regression model (Table 3,4). We found that SD was independently associated with DR (OR 1.15, P = 0.017) in type 2 diabetes mellitus after adjusting for age, duration, body mass index, systolic BP, diastolic BP, TG, high‐density lipoprotein cholesterol and HbA1c. However, the relationships of CV and MAGE with DR (P = 0.194 and P = 0.251, respectively) were not significant. For LADA, no metrics of GV were identified as independent risk factors of DR (SD: P = 0.175; CV: P = 0.769; MAGE: P = 0.388) after adjustment of covariates.
Table 3.
Independent associations of potential risk factors with diabetic retinopathy in type 2 diabetes mellitus
| Variables | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Age | 0.98 (0.97–0.99) | 0.001 | 0.98 (0.97–0.99) | 0.001 | 0.98 (0.97–0.99) | 0.001 |
| Duration | 1.11 (1.09–1.13) | <0.001 | 1.12 (1.10–1.14) | <0.001 | 1.12 (1.10–1.14) | <0.001 |
| BMI | 0.99 (0.96–1.02) | 0.584 | 0.99 (0.96–1.02) | 0.516 | 0.99 (0.96–1.02) | 0.552 |
| SBP | 1.02 (1.01–1.03) | <0.001 | 1.02 (1.01–1.03) | <0.001 | 1.02 (1.01–1.03) | <0.001 |
| DBP | 1.00 (0.98–1.01) | 0.492 | 1.00 (0.98–1.01) | 0.495 | 1.01 (0.98–1.01) | 0.476 |
| TG | 0.93 (0.87–0.99) | 0.030 | 0.93 (0.87–0.99) | 0.031 | 0.93 (0.87–0.99) | 0.027 |
| HDL‐C | 0.91 (0.65–1.27) | 0.574 | 0.91 (0.65–1.28) | 0.594 | 0.92 (0.66–1.30) | 0.640 |
| HbA1c | 1.08 (1.02–1.14) | 0.004 | 1.10 (1.05–1.16) | <0.001 | 1.10 (1.04–1.15) | <0.001 |
| SD* | 1.15 (1.03–1.29) | 0.017 | / | / | / | / |
| CV* | / | / | 1.07 (0.97–1.19) | 0.194 | / | / |
| MAGE* | / | / | / | / | 1.07 (0.96–1.19) | 0.251 |
Model 1 is adjusted for age, duration, body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), triglyceride (TG), high‐density lipoprotein cholesterol (HDL‐C), glycated hemoglobin A1c (HbA1c) and standard deviation (SD); model 2 is adjusted for age, duration, BMI, SBP, DBP, TG, HDL‐C, HbA1c and coefficient of variation (CV); model 3 is adjusted for age, duration, BMI, SBP, DBP, TG, HDL‐C, HbA1c and mean amplitude of glycemic excursions (MAGE). *Odds ratios and P‐values were calculated for each one standard deviation increase in SD, CV or MAGE. CI, confidence interval.
Table 4.
Independent associations of potential risk factors with diabetic retinopathy in latent autoimmune diabetes of the adult
| Variables | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Sex | 0.26 (0.08–0.82) | 0.021 | 0.24 (0.08–0.75) | 0.014 | 0.26 (0.08–0.84) | 0.023 |
| Duration | 1.10 (1.01–1.20) | 0.034 | 1.11 (1.02–1.22) | 0.018 | 1.10 (1.01–1.20) | 0.036 |
| BMI | 0.98 (0.80–1.19) | 0.827 | 0.98 (0.81–1.20) | 0.860 | 0.99 (0.81–1.21) | 0.922 |
| SBP | 1.03 (0.99–1.07) | 0.152 | 1.03 (0.99–1.07) | 0.221 | 1.03 (0.99–1.07) | 0.199 |
| DBP | 1.01 (0.93–1.10) | 0.789 | 1.01 (0.93–1.10) | 0.762 | 1.01 (0.93–1.10) | 0.811 |
| TC | 1.06 (0.60–1.88) | 0.828 | 1.11 (0.63–1.94) | 0.729 | 1.08 (0.62–1.90) | 0.780 |
| HDL‐C | 0.55 (0.14–2.23) | 0.405 | 0.73 (0.19–2.85) | 0.654 | 0.62 (0.15–2.51) | 0.506 |
| HbA1c | 1.30 (0.98–1.73) | 0.070 | 1.34 (1.02–1.76) | 0.038 | 1.30 (0.98–1.73) | 0.071 |
| SD* | 1.43 (0.85–2.39) | 0.175 | / | / | / | / |
| CV* | / | / | 0.92 (0.54–1.59) | 0.769 | / | / |
| MAGE* | / | / | / | / | 1.27 (0.74–2.21) | 0.388 |
Model 1 is adjusted for age, duration, body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), total cholesterol (TC), high‐density lipoprotein cholesterol (HDL‐C), glycated hemoglobin A1c (HbA1c) and standard deviation (SD); model 2 is adjusted for age, duration, BMI, SBP, DBP, TC, HDL, HbA1c and coefficient of variation (CV); model 3 is adjusted for age, duration, BMI, SBP, DBP, TC, HDL, HbA1c and MAGE. *ORs and P‐values were calculated for each one standard deviation increase in SD, CV or mean amplitude of glycemic excursions (MAGE). CI, confidence interval.
Discussion
There is emerging evidence that GV is a contributor to the development of diabetes‐related complications. For example, an in vitro study showed that intermittent high glucose increased nitrotyrosine, 8‐hydroxydeoxyguanosine (two markers of oxidative stress) and apoptosis levels in human umbilical vein endothelial cells compared with a stable high glucose condition6. In vivo, Horvath et al.4 reported that exaggerated alterations in blood glucose (“glycemic swings”) stimulated nitrotyrosine production and endothelial dysfunction in streptozotocin‐induced diabetic rats. Furthermore, the extent of endothelial dysfunction in rats with “glycemic swings” was even more pronounced than in untreated diabetic rats. Using the hyperglycemic clamp, Ceriello et al.3 found that oscillating glucose between 5 and 15 mmol/L every 6 h for 24 h led to further deterioration of endothelial dysfunction and oxidative stress compared with constant high glucose (10 or 15 mmol/L) in both healthy individuals and patients with type 2 diabetes.
To date, numerous epidemiology studies examined the relationship between GV and diabetic complications in type 1 diabetes mellitus and type 2 diabetes mellitus patients, and the results were conflicting12, 13, 14, 15, 16, 17. Of note, the broad concept of GV generally includes both long‐term and short‐term GV. The latter refers to intraday or interday GV, and is often measured by self‐monitoring of blood glucose or CGM. Currently, information on the association between short‐term GV and DR is limited7, 13, 14, 15, 18, 19. In type 1 diabetes mellitus, four studies using data from self‐monitoring of blood glucose reported negative findings13, 14, 15, 18, and one small study using CGM data observed a positive result19. However, there is only one study that investigated the relationship between short‐term GV and DR in patients with type 2 diabetes mellitus7. In that study, 33 patients with type 2 diabetes mellitus and 35 with type 1 diabetes mellitus were enrolled and underwent CGM for 72 h. The authors reported that two parameters of GV (SD and continuous overlapping net glycemic action calculated every 2 h) showed a bivariate association with DR, although the significance did not remain in the multivariate analysis. Furthermore, their study was hampered by the small sample size. Additionally, patients with type 1 diabetes mellitus or type 2 diabetes mellitus were combined in the analysis to increase statistical power, thereby resulting in the heterogeneity of participants. In the present study comprising 2,927 type 2 diabetes mellitus patients, we found SD, as assessed by CGM, was significantly associated with DR after adjusting for confounders, indicating that GV might modify the risk of DR independent of HbA1c.
To the best of our knowledge, this is the first study to examine the link between GV and diabetes‐related complications in patients with LADA. Consistent with previous studies20, 21, 22, we found that patients with LADA had fewer cardiometabolic risk factors, as evidenced by lower body mass index, lower blood pressure and better lipid profiles compared with individuals with type 2 diabetes mellitus. In addition, the prevalence of DR in LADA was significantly lower than in type 2 diabetes mellitus (20.3 vs 26.4%, P < 0.001). This finding, in light of the fact that patients with LADA had significantly higher levels of all GV metrics than patients with type 2 diabetes mellitus, suggests different roles of GV in type 2 diabetes mellitus and LADA in the development of DR. Indeed, the GV metrics did not relate to prevalent DR in either the univariate or multivariate logistic regression analysis in LADA. A possible explanation for the non‐significant relationship between GV and DR could be the intrinsic heterogeneity of LADA. Different titers and combinations of autoantibodies were shown to discriminate clinically distinct groups of LADA20, 23. For example, LADA patients with higher titers of autoantibodies to glutamic acid decarboxylase manifested an insulin‐deficient phenotype, which is characteristic of classic type 1 diabetes mellitus22, whereas patients who are positive for only autoantibodies to islet antigen 2 do not differ from patients with type 2 diabetes mellitus in terms of clinical features24. Although the present findings together with the results of other studies7 support the link between GV and DR in type 2 diabetes mellitus, most previous studies in type 1 diabetes mellitus failed to observe a significant association of short‐term GV with DR13, 14, 15, 18. This phenomenon might partially account for the null association between GV and DR in LADA. Notably, it would be interesting to determine the association of GV with DR in different subgroups of LADA in the future.
A few limitations of the present study should be recognized. First, the sample size of patients with LADA was relatively small compared with that of type 2 diabetes mellitus, potentially limiting the statistical power in this group of individuals. Second, the cross‐sectional nature of this study precluded the possibility to examine the cause–effect relationship between GV and the development of DR. Third, the participants of the present study were recruited from the inpatients of our hospital, who presumably had worse metabolic control than outpatients. Therefore, the present findings might not be generalized to patients under a primary care setting.
In conclusion, the present study provides evidence that intraday GV, as assessed by CGM, is associated with the presence of DR in type 2 diabetes mellitus patients, but not in LADA patients, suggesting that GV should be minimized to decrease the risk of DR in type 2 diabetes mellitus, whereas achieving an optimal HbA1c without increasing the risk of hypoglycemia might be the primary goal in the treatment of LADA. Future well‐designed studies with larger sample sizes are warranted to confirm the effect of GV on DR in clinically distinct subgroups of LADA.
Disclosure
The authors declare no conflict of interest.
Supporting information
Table S1 | Correlation coefficients among the metrics of glycemic variability in type 2 diabetes mellitus and latent autoimmune diabetes of the adult.
Acknowledgments
This work was funded by the Shanghai Municipal Education Commission–Gaofeng Clinical Medicine Grant Support (20161430). We thank all of the involved clinicians, nurses and technicians for dedicating their time and skills to the completion of this study.
J Diabetes Investig 2019; 10: 753–759
References
- 1. Laugesen E, Østergaard JA, Leslie RD. Latent autoimmune diabetes of the adult: current knowledge and uncertainty. Diabet Med 2015; 32: 843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hirsch IB. Glycemic Variability and Diabetes Complications: does It Matter? Of Course It Does!. Diabetes Care 2015; 38: 1610–1614. [DOI] [PubMed] [Google Scholar]
- 3. Ceriello A, Esposito K, Piconi L, et al Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 2008; 57: 1349–1354. [DOI] [PubMed] [Google Scholar]
- 4. Horvath EM, Benko R, Kiss L, et al Rapid ‘glycaemic swings’ induce nitrosative stress, activate poly(ADP‐ribose) polymerase and impair endothelial function in a rat model of diabetes mellitus. Diabetologia 2009; 52: 952–961. [DOI] [PubMed] [Google Scholar]
- 5. Monnier L, Mas E, Ginet C, et al Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006; 295: 1681–1687. [DOI] [PubMed] [Google Scholar]
- 6. Quagliaro L, Piconi L, Assaloni R, et al Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H‐oxidase activation. Diabetes 2003; 52: 2795–2804. [DOI] [PubMed] [Google Scholar]
- 7. Sartore G, Chilelli NC, Burlina S, et al Association between glucose variability as assessed by continuous glucose monitoring (CGM) and diabetic retinopathy in type 1 and type 2 diabetes. Acta Diabetol 2013; 50: 437–442. [DOI] [PubMed] [Google Scholar]
- 8. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998; 15: 539–553. [DOI] [PubMed] [Google Scholar]
- 9. Mo Y, Zhou J, Li M, et al Glycemic variability is associated with subclinical atherosclerosis in Chinese type 2 diabetic patients. Cardiovasc Diabetol 2013; 12: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jia WP, Pang C, Chen L, et al Epidemiological characteristics of diabetes mellitus and impaired glucose regulation in a Chinese adult population: the Shanghai Diabetes Studies, a cross‐sectional 3‐year follow‐up study in Shanghai urban communities. Diabetologia 2007; 50: 286–292. [DOI] [PubMed] [Google Scholar]
- 11. Wilkinson CP, Ferris FL 3rd, Klein RE, et al Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003; 110: 1677–1682. [DOI] [PubMed] [Google Scholar]
- 12. Cardoso CRL, Leite NC, Moram CBM, et al Long‐term visit‐to‐visit glycemic variability as predictor of micro‐ and macrovascular complications in patients with type 2 diabetes: the Rio de Janeiro Type 2 Diabetes Cohort Study. Cardiovasc Diabetol 2018; 17: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kilpatrick ES, Rigby AS, Atkin SL. The effect of glucose variability on the risk of microvascular complications in type 1 diabetes. Diabetes Care 2006; 29: 1486–1490. [DOI] [PubMed] [Google Scholar]
- 14. Kilpatrick ES, Rigby AS, Atkin SL. Effect of glucose variability on the long‐term risk of microvascular complications in type 1 diabetes. Diabetes Care 2009; 32: 1901–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lachin JM, Bebu I, Bergenstal RM, et al Association of Glycemic Variability in Type 1 Diabetes With Progression of Microvascular Outcomes in the Diabetes Control and Complications Trial. Diabetes Care 2017; 40: 777–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Su G, Mi S, Tao H, et al Association of glycemic variability and the presence and severity of coronary artery disease in patients with type 2 diabetes. Cardiovasc Diabetol 2011; 10: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang X, Zhao X, Dorje T, et al Glycemic variability predicts cardiovascular complications in acute myocardial infarction patients with type 2 diabetes mellitus. Int J Cardiol 2014; 172: 498–500. [DOI] [PubMed] [Google Scholar]
- 18. Service FJ, O'Brien PC . The relation of glycaemia to the risk of development and progression of retinopathy in the Diabetic Control and Complications Trial. Diabetologia 2001; 44: 1215–1220. [DOI] [PubMed] [Google Scholar]
- 19. Soupal J, Skrha J Jr, Fajmon M, et al Glycemic variability is higher in type 1 diabetes patients with microvascular complications irrespective of glycemic control. Diabetes Technol Ther 2014; 16: 198–203. [DOI] [PubMed] [Google Scholar]
- 20. Buzzetti R, Di Pietro S, Giaccari A, et al High titer of autoantibodies to GAD identifies a specific phenotype of adult‐onset autoimmune diabetes. Diabetes Care 2007; 30: 932–938. [DOI] [PubMed] [Google Scholar]
- 21. Hawa MI, Kolb H, Schloot N, et al Adult‐onset autoimmune diabetes in Europe is prevalent with a broad clinical phenotype: action LADA 7. Diabetes Care 2013; 36: 908–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tuomi T, Carlsson A, Li H, et al Clinical and genetic characteristics of type 2 diabetes with and without GAD antibodies. Diabetes 1999; 48: 150–157. [DOI] [PubMed] [Google Scholar]
- 23. Lohmann T, Kellner K, Verlohren HJ, et al Titre and combination of ICA and autoantibodies to glutamic acid decarboxylase discriminate two clinically distinct types of latent autoimmune diabetes in adults (LADA). Diabetologia 2001; 44: 1005–1010. [DOI] [PubMed] [Google Scholar]
- 24. Buzzetti R, Spoletini M, Zampetti S, et al Tyrosine phosphatase‐related islet antigen 2(256‐760) autoantibodies, the only marker of islet autoimmunity that increases by increasing the degree of BMI in obese subjects with type 2 diabetes. Diabetes Care 2015; 38: 513–520. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Correlation coefficients among the metrics of glycemic variability in type 2 diabetes mellitus and latent autoimmune diabetes of the adult.


