Abstract
Aims/Introduction
To examine the association between adult‐onset diabetes and life‐course bodyweight changes.
Materials and Methods
In a cross‐sectional study, 17,398 Japanese female nurses aged ≥30 years completed a self‐administered questionnaire in 2001–2007. Bodyweight indices were calculated for three life stages: birthweight (adjusted for gestational age), body mass index (BMI) at age 18 years and current BMI. Odds ratios for being diagnosed with adult‐onset diabetes were calculated according to the combined bodyweight categories of two life stages: at birth and age 18 years; and at age 18 years and the survey (current). Path analysis was carried out to decompose the effect of each bodyweight index into direct and mediating effects.
Results
After adjustment for age at survey and parental diabetes history, “low” birthweight (<25th percentile), when combined with either “low” or “high” BMI (≥75th percentile) at age 18 years, had significant odds ratios (2.32, 95% confidence interval [CI] 1.22–4.44; 3.69, 95% CI 2.12–6.42, respectively) compared with the group of “middle” category (25th–74th percentile) at both life stages. The combination of “low” BMI at age 18 years and “high” current BMIs showed the highest odds ratio (7.97, 95% CI 3.97–16.00). Among women without parental diabetes history, “low” BMI at age 18 years showed a significantly high odds ratio (2.25, 95% CI 1.01–4.99), even when combined with the “middle” category of current BMI. Path analysis showed that both birthweight and BMI at age 18 years had a negative direct effect on adult‐onset diabetes.
Conclusions
Underweight at adolescence, as well as overweight, is a potential risk factor for adult‐onset diabetes among Japanese women.
Keywords: Birthweight, Bodyweight changes, Thinness
Introduction
Type 2 diabetes is a major global medical burden1. Although adult obesity is an established risk factor for type 2 diabetes, accumulating evidence shows that bodyweight at earlier life stages might also plays a role2. Low birthweight has been associated with an increased risk of adult type 2 diabetes3, 4, and this association has recently been confirmed in a Japanese large‐scale female cohort, too5. The risk might be further amplified by rapid catch‐up weight gain in childhood6. However, the long‐term effect of childhood or adolescent obesity is not yet clear. Some systematic reviews have shown that overweight or obesity in childhood/adolescence has adverse consequences on cardiometabolic morbidity7, 8, but other studies have not9, 10. Also, a recent study of body mass index (BMI; kg/m2) trajectory proposed two pathways of childhood BMI and adult type 2 diabetes, one is characterized by low BMI through infancy followed by a rapid increase in BMI in childhood, and the other is characterized by low BMI at birth followed by low BMI in childhood11.
As a methodological issue, the association between childhood/adolescent obesity and adult diabetes was attenuated or reversed when adult BMI was adjusted9, 10, 12. Because adult BMI is possibly part of the causal path from childhood obesity and later disease, adjustment of adult BMI could introduce an overadjustment10, 13, 14. Bodyweights at childhood and later in life can also be part of the long path from birth to adulthood. Therefore, a life‐course approach combining bodyweights at multiple life stages is required. A recently proposed path analysis has been shown to be useful, and was used to elucidate the effect of infant velocity on adult insulin resistance mediated by adult BMI and waist circumference15, 16, 17, 18.
The Japan Nurses’ Health Study (JNHS) is an ongoing cohort study of female nurses. The baseline survey included information on bodyweight at three life stages: birth, age 18 years and age at survey. The data allow us to analyze the combined effect of bodyweights by a two‐step approach: from birth to adolescence and from adolescence to adulthood. The data are also suitable for path analysis to decompose the effect of each bodyweight on adult disease into direct and mediating effects. Using the baseline data of this cohort, we aimed to examine the association between adult‐onset diabetes and bodyweight at different phases in the life‐course.
Methods
Data
The JNHS is an ongoing prospective cohort study in female nurses19. The present cross‐sectional study used the JNHS cohort's baseline data that were collected from 49,927 female nurses between 2001 and 2007 using a self‐administered questionnaire. After applying the following exclusion criteria, the analytic cohort consisted of 17,398 women: age at survey <30 years or unknown (2,179 women); current pregnancy (944 women); unknown diabetes status (53 women); development of diabetes before age 30 years (37 women); unknown birthweight (19,328 women); unknown mother's gestational age at participant's birth (8,907 women); an extremely high (>99.9%) or low (<0.1%) percentile of z‐score of birthweight corrected for gestational age (584 women); unknown current bodyweight (227 women) or bodyweight age 18 years (270 women). Ethical approval for the study was obtained from the ethics review committees of the Faculty of Medicine, Gunma University and the National Institute of Public Health. This study was carried out in accordance with the 1964 Helsinki Declaration and its later amendments.
Variables
History of diabetes, height at survey, bodyweight at three time‐points (birth, age 18 years and at survey), mother's gestational age at participant's birth and maternal or paternal history of diabetes (i.e., diabetes history of participants’ mothers or fathers) were used as variables. History of a diabetes diagnosis was determined by the question. “Have you ever been diagnosed with diabetes by a physician?” For this question, gestational diabetes was explicitly excluded. We excluded participants diagnosed with diabetes before age 30 years and defined remaining cases as “adult‐onset diabetes” cases.
Bodyweight indices were calculated for three life stages: birthweight, BMI at age 18 years and current BMI (i.e., at the survey). Birthweight was corrected for gestational age. Specifically, z‐score was determined based on the mean and standard deviation (SD) of fetal weights on the fetal growth curve20. To ensure comparability, the BMI at age 18 years and current BMIs were also converted into z‐scores using the mean and SD according to 10‐year age group data from the National Nutrition Survey Japan in 1982 and 2004, respectively. These survey years were selected because the middle year of the JNHS baseline survey period was 2004 and the median age at survey was 40 years.
Statistical Analysis
Two sets of analyses were carried out to examine the association between adult‐onset diabetes and bodyweight at different phases of the life‐course. First, odds ratios were calculated for adult‐onset diabetes according to the combined categories of bodyweight indices by a two‐step approach (i.e., combination of birth and age 18 years; combination of age 18 years and current). Specifically, each z‐score of birthweight or BMI was classified into three categories: z < −0.67 (<25th percentile: “low”), z ≥ −0.67 and z < +0.67 (25th–74th percentile: “middle”), and z ≥ +0.67 (≥75th percentile: “high”). For the combination of the categories of two life stages, the participants were categorized into nine groups (3 × 3). Then, logistic regression analysis was carried out with adult‐onset diabetes as the dependent variable and the combined categories of birthweight or BMI as the independent variables. The middle category (25th–74th percentile) was used as the reference of odds ratios. Age at survey and a parental history of diabetes were adjusted.
Second, a path analysis was carried out to decompose the effect of each bodyweight index into direct and mediating effects15, 16, 17, 18. Path analysis is an extension of regression analysis that estimates the association between all variables, and makes it possible to assess the direct and indirect effects of each variable21. The direct effect is the part of the effect not mediated through other included variables measured at a later point of time, whereas the indirect effect is the mediating effect operating through other included variables18. Logistic regression was used to model the association between bodyweight indices and adult‐onset diabetes, and results are shown as odds ratios associated with a 1‐point increase in the z‐score of each bodyweight index.
Parental diabetes, especially maternal diabetes, is associated with an offspring's heavier weight22, 23, 24, which makes the causal pathways more complex. Therefore, we carried out an analysis excluding the participants with a parental history of diabetes (adjusted for age only). Logistic analysis with combined birthweight or BMI categories was carried out using SAS (SAS Institute Inc., Cary, NC, USA); path analysis was carried out using Stata 13 (StataCorp LP, College Station, TX, USA).
Results
Table 1 shows participants’ characteristics according to birthweight (corrected for gestational age). BMI at age 18 years was significantly greater in the groups with a higher birthweight z‐score (linear trend P < 0.001). When converted into z‐score, both BMI at age 18 years and current BMI were significantly greater in the groups with a higher z‐score of birthweight (linear trend P < 0.001). The proportion of women diagnosed with adult‐onset diabetes was significantly smaller in the groups with a higher birthweight z‐score, ranging from 0.7% to 1.5% (linear trend, P < 0.001).
Table 1.
Baseline characteristics of participants according to percentile of birthweight
| Birthweight corrected for gestational age† | Percentile | All | |||
|---|---|---|---|---|---|
| <25 percentile (z‐score < −0.67) | 25–49 percentile (−0.67 ≤ z‐score < 0) | 50–74 percentile (0 ≤ z‐score < 0.67) | 75 percentile ≤ (0.67 ≤ z‐score) | ||
| No. participants (%) | 4,292 (24.7) | 4,864 (28.0) | 4,054 (23.3) | 4,188 (24.1) | 17,398 (100.0) |
| Mean age at survey, years (SD) | 40.9 (7.1) | 41.4 (7.3) | 39.0 (6.4) | 38.6 (6.6) | 40.0 (7.0)* |
| Mean birthweight, g (SD) | 2,559 (264) | 2,921 (179) | 3,088 (237) | 3,460 (393) | 3,000 (423)* |
| Range of birthweight (g) | 900–2,920 | 1,350–3,180 | 1,180–3,440 | 1,100–4,350 | 900–4,350 |
| Mean gestational age, weeks (SD) | 39.7 (1.5) | 39.8 (1.3) | 39.5 (1.5) | 39.3 (1.9) | 39.6 (1.6) |
| Mean BMI at age 18 years (SD) | 20.7 (2.4) | 21.0 (2.4) | 21.1 (2.4) | 21.3 (2.5) | 21.0 (2.4)* |
| Mean current BMI (SD) | 21.8 (3.1) | 21.9 (3.0) | 21.8 (3.1) | 21.9 (3.1) | 21.8 (3.1) |
| Mean z‐score of BMI at age 18 years (SD) | −0.02 (0.81) | 0.07 (0.81) | 0.11 (0.82) | 0.18 (0.85) | 0.08 (0.83)* |
| Mean z‐score of current BMI (SD) | −0.03 (0.94) | −0.01 (0.91) | 0.05 (0.95) | 0.09 (0.96) | 0.02 (0.94)* |
| History of adult‐onset diabetes (%) | 1.5 | 1.0 | 0.8 | 0.7 | 1.0* |
| History of father having diabetes (%) | 14.0 | 13.1 | 13.2 | 12.4 | 13.2 |
| History of mother having diabetes (%) | 7.8 | 8.0 | 7.6 | 9.3 | 8.2* |
*Significant trend according to percentile categories (P < 0.05). †The z‐score of birthweight was calculated based on the fetal growth curve for the Japanese general population (Unno, N. 2012). The z‐scores of −0.67, 0 and +0.67 correspond to the 25th, 50th and 75th percentiles, respectively. Note that the ranges of birthweight in the four categories overlapped, because the birthweight was corrected for gestational age. Note that the ranges of current BMI in the four categories were not consecutive, because the z‐score calculation was carried out according to age groups. BMI, body mass index; SD, standard deviation.
Table 2 shows participants’ characteristics according to BMI at age 18 years. The z‐scores of birthweight and current BMI were significantly higher, as the BMI at age 18 years z‐score was higher (linear trend, P < 0.001). The proportion of women diagnosed with adult‐onset diabetes was higher, as the age 18 BMI z‐score was higher (linear trend P = 0.006), whereas the proportion seemed slightly lower in the groups with intermediate age 18 BMI z‐score categories (1.1%, 0.7%, 0.8% and 1.7% from lowest to highest categories).
Table 2.
Baseline characteristics of participants according to percentile of body mass index at age 18 years
| BMI at age 18 years† | Percentile | All | |||
|---|---|---|---|---|---|
| <25 percentile | 25–49 percentile | 50–74 percentile | 75 percentile ≤ | ||
| (z‐score < −0.67) | (−0.67 ≤ z‐score < 0) | (0 ≤ z‐score < 0.67) | (0.67 ≤ z‐score) | ||
| No. participants (%) | 2,863 (16.5) | 5,702 (32.8) | 5,346 (30.7) | 3,487 (20.0) | 17,398 (100.0) |
| Mean age at survey, years (SD) | 39.7 (6.8) | 39.7 (6.9) | 40.2 (6.9) | 40.6 (7.3) | 40.0 (7.0)* |
| Mean birthweight, g (SD) | 2,927 (420) | 2,986 (415) | 3,025 (421) | 3,046 (431) | 3,000 (423)* |
| Mean BMI at age 18 years (SD) | 17.8 (0.8) | 19.8 (0.6) | 21.7 (0.6) | 24.6 (2.0) | 21.0 (2.4)* |
| Range of BMI at age 18 years | 13.6–18.8 | 18.8–20.8 | 20.8–22.8 | 22.8–43.9 | 13.6–43.9 |
| Mean current BMI (SD) | 20.2 (2.4) | 21.1 (2.5) | 22.1 (2.7) | 24.0 (3.6) | 21.8 (3.1)* |
| Mean z‐score of birthweight corrected for gestational age[Link] (SD) | −0.10 (1.04) | 0.03 (1.04) | 0.13 (1.05) | 0.18 (1.04) | 0.07 (1.05)* |
| Mean z‐score of current BMI[Link] (SD) | −0.49 (0.70) | −0.20 (0.72) | 0.10 (0.81) | 0.70 (1.18) | 0.02 (0.94)* |
| History of adult‐onset diabetes (%) | 1.1 | 0.7 | 0.8 | 1.7 | 1.0* |
| History of father having diabetes (%) | 13.0 | 12.4 | 13.7 | 13.7 | 13.2 |
| History of mother having diabetes (%) | 7.4 | 7.5 | 7.8 | 10.4 | 8.2* |
Significant trend according to percentile categories (P < 0.05). †The z‐score of body mass index (BMI) at age 18 years was calculated based on the mean and standard deviation of Japanese females aged 18 years in the general population (Japan National Nutrition Survey, 1982). Note that the ranges of current BMI in the four categories were not consecutive, because the z‐score calculation was carried out according to age groups. SD, standard deviation.
Table 3 shows participants’ characteristics according to current (at survey) BMI. The z‐scores of birthweight and BMI at age 18 years were significantly higher, as the current BMI z‐score was higher (linear trend, P < 0.001). The proportion of women diagnosed with adult‐onset diabetes was significantly higher, as the current BMI z‐score was higher (linear trend, P < 0.001).
Table 3.
Baseline characteristics of participants according to percentile of current body mass index
| Current BMI† | Percentile | All | |||
|---|---|---|---|---|---|
| <25 percentile | 25–49 percentile | 50–74 percentile | 75 percentile ≤ | ||
| (z‐score < −0.67) | (−0.67 ≤ z‐score < 0) | (0 ≤ z‐score < 0.67) | (0.67 ≤ z‐score) | ||
| No. participants (%) | 3,668 (21.1) | 6,256 (36.0) | 4,148 (23.8) | 3,326 (19.1) | 17,398 (100.0) |
| Mean age at survey, years (SD) | 40.1 (7.1) | 39.8 (6.9) | 40.2 (7.0) | 40.2 (7.0) | 40.0 (7.0) |
| Mean birthweight, g (SD) | 2,966 (421) | 2,997 (411) | 3,020 (426) | 3,021 (440) | 3,000 (423)* |
| Mea BMI at age 18 years (SD) | 19.6 (1.8) | 20.7 (2.0) | 21.5 (2.2) | 22.7 (3.0) | 21.0 (2.4)* |
| Mean current BMI (SD) | 18.6 (1.0) | 20.7 (1.0) | 22.7 (1.2) | 26.5 (2.9) | 21.8 (3.1)* |
| Range of current BMI | 14.5–21.0 | 19.0–23.3 | 21.0–25.6 | 23.0–50.8 | 14.5–50.8 |
| Mean z‐score of birthweight corrected for gestational age (SD)[Link] | −0.02 (1.05) | 0.06 (1.02) | 0.12 (1.06) | 0.12 (1.09) | 0.07 (1.05)* |
| Mean z‐score of BMI at age 18 years[Link] (SD) | −0.44 (0.86) | −0.07 (0.94) | 0.21 (1.01) | 0.68 (1.33) | 0.06 (1.09)* |
| History of adult‐onset diabetes (%) | 0.3 | 0.4 | 1.0 | 2.7 | 1.0* |
| History of father having diabetes (%) | 11.0 | 12.5 | 14.3 | 15.4 | 13.2* |
| History of mother having diabetes (%) | 6.3 | 7.3 | 8.5 | 11.5 | 8.2* |
Significant trend according to percentile categories (P < 0.05). †The z‐score of current (at survey) body mass index (BMI) was calculated based on the age‐specific mean and standard deviation of the Japanese female general population (Japan National Nutrition Survey 2004). Note that the ranges of current BMI in the four categories were not consecutive, because the z‐score calculation was carried out according to age groups. SD, standard deviation.
Figure 1a shows the odds ratio for adult‐onset diabetes according to the combined categories of birthweight (corrected for gestational age) and BMI at age 18 years z‐scores, adjusted for age and parental history of diabetes. “Low” (<25th percentile) birthweight category combined with either “low” or “high” (≥75th percentile) BMI at age 18 years categories had significantly high odds ratios (3.69, 95% confidence interval [CI] 2.12–6.42; 2.32, 95% CI 1.22–4.44, respectively). The combination of “middle” (25th–74th percentile) birthweight and “high” BMI at age 18 years was also significant (2.02, 95% CI 1.22–3.36).
Figure 1.
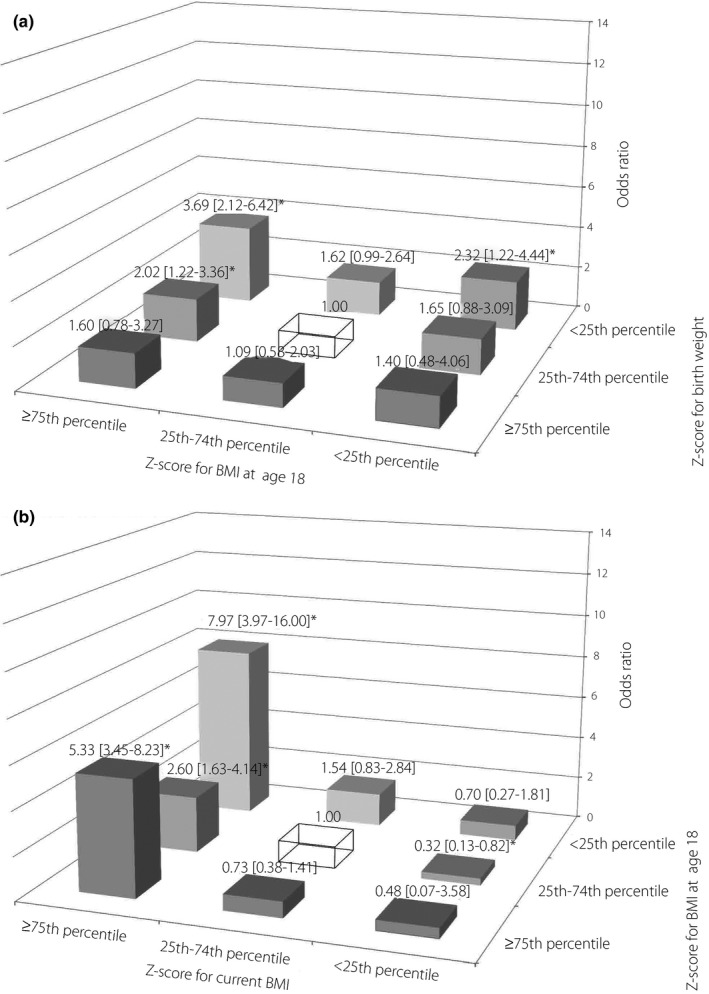
Odds ratios for being diagnosed with adult‐onset diabetes according to the combined categories of bodyweight indices at two different life stages (entire analytic cohort). The reference group was a combination of a birthweight z‐score in the 25th–74th percentile and a body mass index (BMI) z‐score at age 18 years in the 25th–74th percentile (top), and a combination of BMI z‐scores at age 18 in the 25th–74th percentile and current BMI z‐score in the 25th–74th percentile (bottom), shown as a transparent box. Adjusted for age at survey (continuous) and a history of diabetes in the father or mother. (a) Birthweight (corrected for gestational age) and BMI at age 18 years. (b) BMI at age 18 years and current BMI. *Statistically significant (P < 0.05).
Figure 1b shows the result for the combined BMI at age 18 years and current BMI categories. The “high” current BMI category was significantly associated with adult‐onset diabetes regardless of BMI at age 18 years categories; with the highest odds ratio of 7.97 (95% CI 3.97–16.00) for the combination of “low” BMI at age 18 years and “high” current BMI. The model adjusted for age alone gave almost the same results as Figure 1 (Table S1).
Figure 2a shows the result of the combined age‐adjusted odds ratio after excluding women with a history of parental diabetes. A significant odds ratio was observed for only the combination of “low” birthweight and “low” BMI at age 18 years (3.68, 95% CI 1.79–7.57). Unlike the result for the entire analytic cohort, no significant odds ratio was observed for the category of high BMI at age 18 years.
Figure 2.
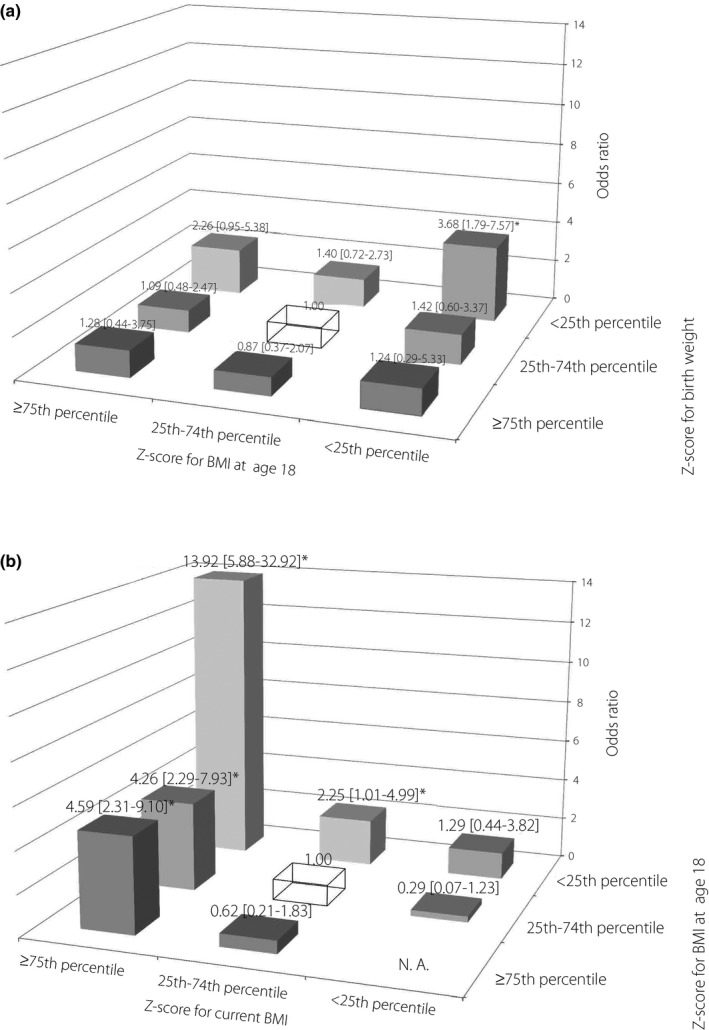
Odds ratios for being diagnosed with adult‐onset diabetes according to the combined categories of body weight indices at two different life stages (participants without parental history of diabetes). The reference group was a combination of a birthweight z‐score in the 25th–74th percentile and a body mass index (BMI) z‐score at age 18 in the 25th–74th percentile (top), and a combination of BMI z‐scores at age 18 in the 25th–74th percentile and current BMI z‐score in the 25th–74th percentile (bottom), shown as a transparent box. Adjusted for age at survey (continuous). (a) Birthweight (corrected for gestational age) and BMI at age 18. (b) BMI at age 18 years and current BMI. *Statistically significant (P < 0.05).
Figure 2b shows the results for the combination of BMI at age 18 years and current BMI among women without parental diabetes history. Similar to the result for the entire analytic cohort, the “high” current BMI was significantly associated with adult‐onset diabetes regardless of the BMI at age 18 years. A remarkably high odds ratio was observed for the combination of “low” BMI at age 18 years and “high” current BMI categories (13.92, 95% CI 5.88–32.92). The “low” BMI at age 18 years showed a significantly high odds ratio (2.25, 95% CI 1.01–4.99), even when combined with the “middle” category of current BMI.
Figure 3 shows the results of path analysis, which decomposed the effect of each bodyweight index into direct and mediating effects. For the entire analytic cohort (Figure 3a), the direct effect of bodyweight on adult‐onset diabetes at three different life stages showed a negative‐to‐positive shift, with an odds ratio of 0.74 (95% CI 0.63–0.86) for birthweight, 0.96 (95% CI 0.80–1.14) for BMI at age 18 years and 2.07 (95% CI 1.81–2.35) for current BMI. Birthweight had a significant positive mediating effect on BMI at age 18 years (odds ratio 1.07, 95% CI 1.06–1.09). BMI at age 18 years had an even larger mediating effect on current BMI (odds ratio 1.73, 95% CI 1.70–1.75). When the analysis was limited to women without parental history of diabetes (Figure 3b), the direct effect of birthweight, BMI at age 18 years and current BMI was 0.71 (95% CI 0.57–0.88), 0.64 (95% CI 0.48–0.85) and 2.31 (95% CI 1.94–2.74), respectively. Unlike the result of the entire analytic cohort, the direct effect of BMI at age 18 years was significantly negative.
Figure 3.

Direct and mediating effects of birthweight and body mass index (BMI) at three different life stages on adult‐onset diabetes for (a) the entire analytic cohort and (b) participants without parental history of diabetes. The effect of each bodyweight index was expressed as the odds ratio associated with a 1‐point increase in z‐score. The z‐score was calculated based on the growth curve for gestational age (for birthweight) or on the Japan National Nutrition Survey (for BMI at age 18 years and current BMI). Adjusted for age at survey (continuous). For the entire analytic cohort, a history of diabetes in the father or mother was additionally adjusted. *The marked plot indicates that the odds ratio was statistically significantly higher or lower than one (P < 0.05).
Discussion
The present analysis combining multiple bodyweight indices showed that a low birthweight combined with low or high BMI at age 18 years was associated with adult‐onset diabetes. In addition, it was shown that the combination of low BMI at age 18 years and high current BMI exhibited the strongest association. Among women without parental diabetes history, low BMI at age 18 years was significantly associated with adult‐onset diabetes, even when it was combined with the middle category of current BMI. Our path analysis identified a negative direct effect of birthweight on adult‐onset diabetes, which showed that birthweight was inversely associated with adult‐onset diabetes, not mediated by BMIs in later life. The effect of current BMI on adult‐onset diabetes was significantly positive, which was consistent with existing knowledge. For women without parental diabetes history, the direct effect of BMI at age 18 years was significantly negative, and the mediating effect of BMI at age 18 years on current BMI was significantly positive, which indicated that BMI at adolescence had an independent inverse association with adult‐onset diabetes, separately from the positive path through BMI in later life. This finding suggests that underweight at adolescence, as well as overweight, is a potential risk factor for developing adult‐onset diabetes.
There has been limited evidence regarding the adverse effect of low BMI at adolescence. The Nurses’ Health Study II reported a link between low adolescent BMI and an increased risk of gestational diabetes25. They found a U‐shaped relationship between gestational diabetes and BMI at age 18 years, and explained the elevated risk by the greater subsequent weight gain. In the present study, although weight gain from adolescence to adulthood was strongly associated with adult‐onset diabetes, another possibility was proposed that adolescent thinness itself could have an adverse effect on the subsequent glucose metabolism. The distribution of BMI among Japanese nurses was shifted toward a thin body compared with that of American nurses25; the prevalence of obesity (BMI ≥30) at age 18 years was just 0.5% in our analytic cohort. It is possible that the inverse association observed in the present study reflected the descending part of the U‐shaped curve. Eriksson et al.11 proposed two pathways of life‐course BMI and adult type 2 diabetes: one is through low BMI during infancy followed by a rapid increase in BMI in childhood, and the other is through continued low BMI at birth and in childhood. The negative effect of adolescent BMI observed in the present study might have reflected the second pathway. An association between low BMI at adolescence and gestational diabetes has also been reported among Japanese women26. Proposed biological mechanisms were insulin resistance caused by nutritional deficiency27, 28 or reduced skeletal muscle mass29. These explanations can be applied to adult‐onset diabetes as well.
Evidence on the link between childhood or adolescent bodyweight and subsequent diabetes is inconsistent among systematic reviews7, 8, 9, 10. The association between childhood/adolescent obesity and adult diabetes was attenuated or reversed when adult BMI was adjusted9, 10, 12. Because adult BMI might be on the causal path from childhood obesity and later disease, adjustment for adult BMI could introduce overadjustment10, 13, 14. By a path analysis, we separated the direct and mediating effects of BMI at age 18 years. As a result, adolescent BMI was inversely associated with adult‐onset diabetes at least among women without a parental history of diabetes. This supports the possibility of the adverse effect of adolescent low BMI itself.
The association between low BMI at age 18 years and adult‐onset diabetes was strongly observed among women without a parental history of diabetes. The interaction between each bodyweight z‐score and parental diabetes was significant for BMI at age 18 years only (birthweight: P = 0.5821; age 18 BMI: P = 0.0005; current BMI: P = 0.5681). In the path analysis for women with parental history of diabetes, the association between BMI at age 18 years and adult‐onset diabetes was positive (significant among women with mother's history of diabetes; Figure S1). There might be differences in body composition and fat distribution between women with and without parental diabetes history. However, because of the limited number of participants with a parental history of diabetes, further studies are required to elucidate the path from parental diabetes to the offspring's bodyweight and diabetes.
Birthweight was inversely associated with adult‐onset diabetes in the present results. There is firm evidence, mostly from Western countries, that intrauterine growth restriction is associated with an increased risk of adult‐onset diabetes2, 4. We recently confirmed a similar association among the same Japanese female large‐scale cohort5. That previous report showed that even among women with normal low BMI (18.5–20.9), the prevalence of adult‐onset diabetes was significantly higher in women with a low birthweight, which was consistent with the independent negative effect of birthweight shown in our path analysis.
Underweight and obesity are defined in Japan as BMI <18.5 and BMI ≥25, respectively (the definitions of the Japan Society for the Study of Obesity). This definition of obesity is different from the definition by the World Health Organization, where a BMI of 30 is used as the cut‐off30, 31. When those classifications were applied to the present data, the proportions of underweight and obesity were 12.8% (<18.5) and 5.6% (BMI ≥25), and 0.5% (BMI ≥30), respectively, at age 18 years, and corresponding figures were 9.2% and 13.4%, and 2.0%, respectively at the baseline. We used our percentile‐based classifications for the comparability with birthweight and also in order to ensure a balanced number of participants in each category.
Regarding the generalizability to males, though we do not have direct evidence, previous literature showed no sex difference in the association of adult BMI and weight change with diabetes32, 33. From the public health perspective, our finding is more important for females, because the prevalence of underweight is higher in females (especially in young females) than in males in Japan (4.4% for males, 11.6% for females and 20.7% for females aged 20–29 34).
Several limitations of the present study warrant mention. First, our use of a cross‐sectional design hampers the assignation of causation. The effects of BMI at age 18 years and current BMI might have been overestimated by recall bias, but a substantial systematic bias is unlikely, given that these variables showed opposite directions in their association with diabetes. Current bodyweight might have been influenced by weight loss after a diabetes diagnosis, but we consistently observed a strong positive association between current BMI and diabetes diagnosis. Regarding birthweight, it preceded the onset of adult diabetes, and the association between birthweight and diabetes is not sufficiently established to have biased the participants’ reports. The fetal growth curve we used was not developed from the same birth cohort as our participants. However, standardized fetal growth curves are available only for recent birth cohorts35, and we confirmed that our result did not change when we used older35 or newer version36.
Second, we determined diabetes status using a self‐reported history of diagnosis. A sensitivity analysis using a dependent variable of adult‐onset diabetes defined by a combination of a self‐reported fasting plasma glucose level of ≥126 mg/dL and anti‐diabetic medication did not change our main results. A study that examined the validity of self‐reported diabetes in a general Japanese population reported a sensitivity of 70.4% and a specificity of 97.3%37. In general, limited sensitivity in disease classification is unlikely to bias relative risk estimates, if specificity is very high38. Because the present study's participants were healthcare professionals, we assume that their responses were more accurate than those of the general population. Another issue is the status of impaired glucose tolerance. As we did not have data for glucose tolerance tests, those with impaired glucose tolerance were classified as non‐diabetes in the present analysis. The validity of self‐reported birthweight was confirmed in a subsample of the participants, as described in our previous study. The accuracy of recall of current and past bodyweight was confirmed for general populations39, 40.
Third, the participants of the present study were nurses, who might have different risk profiles than the general population; however, the mean z‐scores of BMI standardized to the general population showed only a small deviation. The prevalence of diabetes observed in the present study was slightly lower than the general population41, probably because the participants were recruited from working nurses. However, it is unlikely that healthcare professionals differ in how bodyweight affects adult‐onset diabetes.
Finally, our analytic cohort was just 35% of the 49,927 total participants. The percentage of adult‐onset diabetes among the analytic cohort was slightly smaller than the excluded participants (1.0% vs 1.4%). The majority of the excluded participants had an unknown birthweight (84%); therefore, we carried out a sensitivity analysis by treating these women as having either a −1 SD or +1 SD z‐score (−0.67 or 0.67, respectively) for birthweight. There was no marked change in the inverse association with adult‐onset diabetes.
In conclusion, being underweight at adolescence, as well as overweight at adolescence, is a potential risk factor for adult‐onset diabetes among Japanese women.
Disclosure
The authors declare no conflict of interest.
Supporting information
Figure S1 ¦ Direct and mediating effects of bodyweight indices at three different life stages on adult‐onset diabetes, among women with parental history of diabetes.
Table S1 ¦ Age‐adjusted odds ratios for being diagnosed with adult‐onset diabetes according to the combined categories of birthweight and body mass index at two different life stages (entire analytic cohort).
Acknowledgments
The authors sincerely thank Dr Takehiro Sugiyama at the National Center for Global Health and Medicine, Japan, for his contributions to the present study. This work was supported by the Japan Society for the Promotion of Science Grants‐in‐Aid for Scientific Research (24501367 and 18390195), and the Japan Society for Menopause and Women's Health (JMWH Bayer Grant).
J Diabetes Investig 2019; 10: 827–836
References
- 1. Lozano R, Naghavi M, Foreman K, et al Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aboderin I, Kalache A, Ben‐Shlomo Y, et al Life Course Perspectives on Coronary Heart Disease, Stroke and Diabetes: Key Issues and Implications for Policy and Research. Geneva: World Health Organization, 2002. [Google Scholar]
- 3. Harder T, Rodekamp E, Schellong K, et al Birth weight and subsequent risk of type 2 diabetes: a meta‐analysis. Am J Epidemiol 2007; 165: 849–857. [DOI] [PubMed] [Google Scholar]
- 4. Whincup PH, Kaye SJ, Owen CG, et al Birth weight and risk of type 2 diabetes: a systematic review. JAMA 2008; 300: 2886–2897. [DOI] [PubMed] [Google Scholar]
- 5. Katanoda K, Noda M, Goto A, et al Impact of birth weight on adult‐onset diabetes mellitus in relation to current body mass index: The Japan Nurses’ Health Study. J Epidemiol 2017; 27: 428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Forsen T, Eriksson J, Tuomilehto J, et al The fetal and childhood growth of persons who develop type 2 diabetes. Ann Intern Med 2000; 133: 176–182. [DOI] [PubMed] [Google Scholar]
- 7. Kelsey MM, Zaepfel A, Bjornstad P, et al Age‐related consequences of childhood obesity. Gerontology 2014; 60: 222–228. [DOI] [PubMed] [Google Scholar]
- 8. Reilly JJ, Kelly J. Long‐term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes 2011; 35: 891–898. [DOI] [PubMed] [Google Scholar]
- 9. Lloyd LJ, Langley‐Evans SC, McMullen S. Childhood obesity and risk of the adult metabolic syndrome: a systematic review. Int J Obes 2012; 36: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Park MH, Falconer C, Viner RM, et al The impact of childhood obesity on morbidity and mortality in adulthood: a systematic review. Obes Rev 2012; 13: 985–1000. [DOI] [PubMed] [Google Scholar]
- 11. Eriksson JG, Osmond C, Kajantie E, et al Patterns of growth among children who later develop type 2 diabetes or its risk factors. Diabetologia 2006; 49: 2853–2858. [DOI] [PubMed] [Google Scholar]
- 12. Tirosh A, Shai I, Afek A, et al Adolescent BMI trajectory and risk of diabetes versus coronary disease. N Engl J Med 2011; 364: 1315–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lucas A, Fewtrell MS, Cole TJ. Fetal origins of adult disease‐the hypothesis revisited. BMJ 1999; 319: 245–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tu YK, West R, Ellison GT, et al Why evidence for the fetal origins of adult disease might be a statistical artifact: the “reversal paradox” for the relation between birth weight and blood pressure in later life. Am J Epidemiol 2005; 161: 27–32. [DOI] [PubMed] [Google Scholar]
- 15. Slining MM, Kuzawa CW, Mayer‐Davis EJ, et al Evaluating the indirect effect of infant weight velocity on insulin resistance in young adulthood: a birth cohort study from the Philippines. Am J Epidemiol 2011; 173: 640–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tilling K, Davies N, Windmeijer F, et al Is infant weight associated with childhood blood pressure? Analysis of the Promotion of Breastfeeding Intervention Trial (PROBIT) cohort. Int J Epidemiol 2011; 40: 1227–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bjerregaard LG, Rasmussen KM, Michaelsen KF, et al Effects of body size and change in body size from infancy through childhood on body mass index in adulthood. Int J Obes 2014; 38: 1305–1311. [DOI] [PubMed] [Google Scholar]
- 18. Kirkegaard H, Stovring H, Rasmussen KM, et al How do pregnancy‐related weight changes and breastfeeding relate to maternal weight and BMI‐adjusted waist circumference 7 y after delivery? Results from a path analysis. Am J Clin Nutr 2014; 99: 312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hayashi K, Mizunuma H, Fujita T, et al Design of the Japan Nurses’ Health Study: a prospective occupational cohort study of women's health in Japan. Ind Health 2007; 45: 679–686. [DOI] [PubMed] [Google Scholar]
- 20. Unno N. A study on enhancement of community perinatal care and appropriate distribution of medical resources. Report of the Health and Labour Sciences Research Grants. 2012; 301–340 (Japanese).
- 21. Bentler PM, Stein JA. Structural equation models in medical research. Stat Methods Med Res 1992; 1: 159–181. [DOI] [PubMed] [Google Scholar]
- 22. Savona‐Ventura C, Chircop M. Birth weight influence on the subsequent development of gestational diabetes mellitus. Acta Diabetol 2003; 40: 101–104. [DOI] [PubMed] [Google Scholar]
- 23. Gillman MW, Rifas‐Shiman S, Berkey CS, et al Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics 2003; 111: e221–e226. [DOI] [PubMed] [Google Scholar]
- 24. Lawlor DA, Fraser A, Lindsay RS, et al Association of existing diabetes, gestational diabetes and glycosuria in pregnancy with macrosomia and offspring body mass index, waist and fat mass in later childhood: findings from a prospective pregnancy cohort. Diabetologia 2010; 53: 89–97. [DOI] [PubMed] [Google Scholar]
- 25. Yeung EH, Hu FB, Solomon CG, et al Life‐course weight characteristics and the risk of gestational diabetes. Diabetologia 2010; 53: 668–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yachi Y, Tanaka Y, Nishibata I, et al Low BMI at age 20 years predicts gestational diabetes independent of BMI in early pregnancy in Japan: Tanaka Women's Clinic Study. Diabet Med 2013; 30: 70–73. [DOI] [PubMed] [Google Scholar]
- 27. Kirii K, Mizoue T, Iso H, et al Calcium, vitamin D and dairy intake in relation to type 2 diabetes risk in a Japanese cohort. Diabetologia 2009; 52: 2542–2550. [DOI] [PubMed] [Google Scholar]
- 28. Pittas AG, Dawson‐Hughes B, Li T, et al Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care 2006; 29: 650–656. [DOI] [PubMed] [Google Scholar]
- 29. DeFronzo RA, Jacot E, Jequier E, et al The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 1981; 30: 1000–1007. [DOI] [PubMed] [Google Scholar]
- 30. Takahashi H, Mori M. [Characteristics and significance of criteria for obesity disease in Japan 2011]. Nihon Rinsho. 2013;71:257–261 (Japanese). [PubMed] [Google Scholar]
- 31. WHO Expert Consultation . Appropriate body‐mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004; 363: 157–163. [DOI] [PubMed] [Google Scholar]
- 32. Nanri A, Mizoue T, Takahashi Y, et al Association of weight change in different periods of adulthood with risk of type 2 diabetes in Japanese men and women: the Japan Public Health Center‐Based Prospective Study. J Epidemiol Community Health 2011; 65: 1104–1110. [DOI] [PubMed] [Google Scholar]
- 33. Waki K, Noda M, Sasaki S, et al Alcohol consumption and other risk factors for self‐reported diabetes among middle‐aged Japanese: a population‐based prospective study in the JPHC study cohort I. Diabet Med 2005; 22: 323–331. [DOI] [PubMed] [Google Scholar]
- 34. National Health and Nutrition Survey Report 2016 [Internet]. Ministry of Health, Labour and Welfare; 2017 [cited Aug. 17, 2018]. Available from: https://www.mhlw.go.jp/bunya/kenkou/eiyou/h28-houkoku.html (Japanese). [Google Scholar]
- 35. Standardization of ultrasound measurement and standard values for Japanese fetus [Internet]. Terminology and Diagnostic Criteria Committee, The Japan Society of Ultrasonics in Medicine; 2003. [cited Dec. 19, 2017]. Available from: http://www.jsog.or.jp/public/shusanki/kijunchi.pdf (Japanese). [Google Scholar]
- 36. Itabashi K, Miura F, Uehara R, et al New Japanese neonatal anthropometric charts for gestational age at birth. Pediatr Int 2014; 56: 702–708. [DOI] [PubMed] [Google Scholar]
- 37. Goto A, Morita A, Goto M, et al Validity of diabetes self‐reports in the Saku diabetes study. J Epidemiol 2013; 23: 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Greenland S, Lash TL. Bias analysis In: Rothman KJ, Greenland S, Lash TL. (eds). Modern Epidemiology, 3rd edn Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2008; 345–380. [Google Scholar]
- 39. Tamakoshi K, Yatsuya H, Kondo T, et al The accuracy of long‐term recall of past body weight in Japanese adult men. Int J Obes Relat Metab Disord 2003; 27: 247–252. [DOI] [PubMed] [Google Scholar]
- 40. Casey VA, Dwyer JT, Berkey CS, et al Long‐term memory of body weight and past weight satisfaction: a longitudinal follow‐up study. Am J Clin Nutr 1991; 53: 1493–1498. [DOI] [PubMed] [Google Scholar]
- 41. Charvat H, Goto A, Goto M, et al Impact of population aging on trends in diabetes prevalence: a meta‐regression analysis of 160,000 Japanese adults. J Diabetes Investig 2015; 6: 533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 ¦ Direct and mediating effects of bodyweight indices at three different life stages on adult‐onset diabetes, among women with parental history of diabetes.
Table S1 ¦ Age‐adjusted odds ratios for being diagnosed with adult‐onset diabetes according to the combined categories of birthweight and body mass index at two different life stages (entire analytic cohort).


