Abstract
Introduction: Poor oral health has been implicated as an independent risk factor for the development of COPD, but few studies have evaluated the association between oral health and COPD exacerbations. We aimed to determine if poor oral health is associated with COPD exacerbations and/or worse respiratory health.
Methods: We performed a case-control study of oral health among COPD exacerbators and non-exacerbators. Cases (exacerbators) had experienced ≥1 exacerbation in the previous 12 months, while controls (non-exacerbators) had no exacerbations in the previous 24 months. We excluded those with <4 teeth. We evaluated the global oral health assessment, Oral Health Impact Profile (OHIP-5), dental symptoms/habits, and St. George’s Respiratory Questionnaire (SGRQ). In a subset, we performed blinded dental exams to measure bleeding on probing, probing depth, clinical attachment loss, periodontitis severity, plaque index, gingival index, and carries risk. We evaluated associations between oral health and COPD exacerbations using logistic regression. Linear regression was used to assess relationships between oral health and SGRQ scores.
Results: Screened non-exacerbators (n=118) were significantly more likely to have <4 teeth, compared to screened exacerbators (n=100) (44% vs 30%, respectively; p=0.046). After excluding those with <4 teeth, there were 70 cases and 66 controls. Self-reported oral health and objective dental exam measures did not vary significantly between cases vs controls. However, the odds of severe COPD exacerbations requiring hospitalizations and/or emergency department visits trended higher in those with worse dental exam compared to those with better dental exam. Worse OHIP-5 was strongly associated with worse SGRQ scores.
Conclusions: Oral health status was not related to COPD exacerbations, but was associated with self-reported respiratory health. Non-exacerbators were more likely to be edentate or have ≤4 teeth compared to exacerbators. Larger studies are needed to address oral health as a potential method to improve respiratory health in patients with COPD.
Keywords: pulmonary disease, chronic obstructive, oral health, periodontitis
Introduction
COPD exacerbations are a major cause of morbidity, seriously impair quality of life, and can result in irreversible loss of lung function.1–3 A severe COPD exacerbation requiring hospitalization is associated with high mortality both in the hospital and after discharge.2 Prevention of COPD exacerbations is an important aspect of COPD management.4 Therefore, exploration of risk factors and identification of patients who are susceptible to COPD exacerbations is needed.
Approximately 50% of COPD exacerbations are attributed to bacterial infections. Published studies have demonstrated increased microbial biomass and microbial diversity in COPD patients compared to healthy adults.5–7 In addition, oral and nasal bacteria have been identified in the COPD lung tissue microbiota, suggesting aspiration of oral secretions as a major source of the COPD lung microbiota.5–7 This is consistent with observations that COPD patients are prone to aspiration of oral secretions due to reduced laryngotracheal mechanosensitivity8 and decreased airway clearance from impaired mucociliary function.9 These findings highlight the potential impact of oral health in COPD. Thus, we hypothesize that COPD exacerbations are likely associated with poor oral health status.
Periodontal disease and poor oral health have been associated with a number of systemic diseases, including COPD. A meta-analysis evaluating 14 observational studies demonstrated an association between periodontal disease and COPD,10 but few studies have evaluated the association between oral health and COPD exacerbations.11–14 In this study, we compared the oral health status of COPD exacerbators and non-exacerbators to determine if COPD exacerbations are related to worse oral health.
Methods
Using a case-control design, we administered questionnaires via phone interview or in-person visits at the Minneapolis Veterans Affairs Health Care System. Comprehensive dental examination was performed on a subset of participants.
Study participants
Participants with COPD were recruited from the Minneapolis Veterans Affairs Health Care System. Inclusion criteria included participants between 40 and 80 years of age with COPD. We defined COPD in standard fashion as recommended by the American College of Physicians, American College of Chest Physicians, and American Thoracic Society15 – respiratory symptoms with spirometry-confirmed airflow obstruction defined as FEV1/FVC <0.70 in those with ≥10 pack-year smoking history – and further included only those with FEV1 <70% of predicted normal. Exclusion criteria included history of asthma, presence of <4 teeth, and undergoing active treatment with chemotherapy for malignancy (exceptions: radiation or hormonal therapy for prostate or breast cancer, and non-metastatic skin cancer). Cases were defined as having at least one COPD exacerbation in the previous 12 months. COPD exacerbations were defined as taking antibiotics and/or oral corticosteroids for respiratory symptoms, or hospitalization or emergency department visit for respiratory illness. Severe exacerbations were defined as requiring an emergency department visit and/or hospitalization for the exacerbation. Controls had COPD but with no exacerbations in the previous 24 months. Case or control status was verified by medical chart review and participant interviews.
The study was approved by the Minneapolis VA Institutional Review Board. All study participants provided informed consent before participating in the study. Participants who completed the questionnaires by phone provided verbal informed consent, while participants who had dental examinations and in-person visits provided written informed consent. Both methods of informed consent were reviewed and approved by the Minneapolis VA Institutional Review Board.
Questionnaires
Oral health questionnaire
Our oral health questionnaire assessed demographic information, history of COPD exacerbations, inhaler use, dental symptoms, dental care habits, a 1-item global oral health status assessment, and the 5-item version of the Oral Health Impact Profile (OHIP) (Table S1). OHIP is the most widely used oral health-related quality of life (OHRQoL) instrument to assess the impact of oral health disorders and dental interventions.16 OHIP captures four correlated aspects of patient-perceived OHRQoL: oral function, orofacial pain, orofacial appearance, and psychological impact. The responses were classified using the Likert scale with five choices ranging from “never” (1) to “very often” (5), with a score of 5 reflecting the most severe oral health for each item, and the overall score ranging from 4 to 20. There is no established minimal clinically important difference for the OHIP.
Table S1.
Oral health questionnaire
| Global oral health assessment | |||||
| Overall, how would you rate the health of your teeth and gums? | Excellent | Very good | Good | Fair | Poor |
| OHIP-5 | |||||
| In the last month: | Never | Hardly ever | Occasionally | Fairly often | Very often |
| Have you had difficulty chewing any foods because of problems with your teeth, mouth, dentures, or jaw? | |||||
| Have you had painful aching in your mouth? | |||||
| Have you felt uncomfortable about the appearance of your teeth, mouth, dentures, and jaws? | |||||
| Have you felt that there has been less flavor in your food because of problems with your teeth, mouth, dentures, or jaws? | |||||
| Have you had difficulty doing your usual jobs because of problems with your teeth, mouth, dentures, or jaws? | |||||
| Dental symptoms | |||||
| Yes | No | ||||
| Do you think you might have gum disease? (symptoms of gum disease include bad breath that won’t go away, red or swollen gums, tender or bleeding gums, painful chewing, loose teeth, sensitive teeth, and receding gums or longer appearing teeth). | |||||
| Have you ever had any teeth become loose on their own, without an injury? | |||||
| Do your gums bleed after you brush your teeth? | |||||
| Do you have dry mouth? | |||||
| Dental habits | |||||
| Do you have removable dentures? | Yes | No | |||
| If yes, are your dentures: | Partial denture | Full denture | |||
| How often do you brush or clean your teeth or dentures? | Never | <1x per day | 1x per day | 2x per day | >2x per day |
| How long has it been since you last saw a dental specialist (dentist or orthodontist)? | <6 months ago | 6–12 months ago | 1–2 years ago | >2 years ago | Never |
| Do you use any of the following to clean your teeth or dentures? (check all that apply) | Tooth-brush | Dental floss | Mouth-wash | Denture cleanser | |
St. George’s Respiratory Questionnaire (SGRQ)
The SGRQ is a 50-item questionnaire developed to assess respiratory health status in patients with obstructive lung diseases. The questions evaluate three domains: symptoms, activity, and impact. The SGRQ is scored on a scale of 0–100, with higher scores reflecting worse respiratory health status. The minimum clinically important difference in the SGRQ is widely accepted as 4 units.17
Dental examination
All participants were offered a comprehensive dental examination. Comprehensive dental examinations were performed on a subset (27 cases and 29 controls) of participants by two dentists. The dentists were blinded to the case-control status of the participants. Assessments included periodontitis severity, bleeding on probing (BOP), gingival index (GI), plaque index (PI), and carries risk assessment (CRA).
Periodontitis severity (mild, moderate, and severe) was determined by probing depth (PD, scored as ≤3, >3 to <5, ≥5 to <7, and 7+ mm) and clinical attachment loss (CAL, scored as <1, 1 to 2, or 5+ mm) based on the involvement of at least 30% of the entire dentition, according to the American Academy of Periodontology Task Force Report (Table S2).18 PD evaluations were carried out by measuring pocket depths using a periodontal probe at six points per tooth. The probe, positioned parallel to the long axis of the tooth at each site, is inserted until the probe tip encounters the resistance of the junctional epithelium. The probe is then moved up and down in short strokes and forward in 1-mm increments. CAL was the measured distance between cemento-enamel junction and the free gingival margin.19 PD and CAL measurements were rounded up to the nearest millimeter. The higher category of severity was used if PD and CAL were in two different categories.
Table S2.
Periodontitis severity based on probing depth (PD) and clinical attachment loss (CAL)
| Periodontitis severity | PD (mm) | CAL (mm) |
|---|---|---|
| Mild | >3 to <5 | 1–2 |
| Moderate | ≥5 to <7 | 3 to 4 |
| Severe | ≥7 | ≥5 |
BOP was assessed by probing gently along the wall of soft tissue of the gingival sulcus and was scored as yes or no for presence or absence of bleeding, respectively.
GI was used to assess evidence of inflammation in the gingival tissues characterized by redness, swelling, and BOP. Each of the four gingival areas (buccal, mesial, distal, and lingual) of the tooth was scored from 0 to 3 based on Löe’s Gingival Index System: 0 – normal gingiva, 1 – mild inflammation, 2 – moderate inflammation, and 3 – severe inflammation.20 The GI score for the tooth was calculated by adding the four scores then dividing by four. The final GI recorded was the sum of all values from each tooth divided by the number of teeth examined.20
PI indicates soft deposits and calculi at the gingival margin and interproximally. The Modified Plaque Scoring System was used to record PI measurements and was scored as 0 – no plaque, 1 – separate flecks of plaque at the cervical margin of the tooth, 2 – a thin continuous band of plaque up to 1 mm at the cervical margin of the tooth, 3 – a band of plaque wider than 1 mm covering less than one-third of the crown of the tooth, 4 – plaque covering at least one-third but less than two-thirds of the crown of the tooth, and 5 – plaque covering two-thirds or more of the crown of the tooth.21
The American Dental Association Carries Risk Assessment Form was used to evaluate the overall dental carries risk categorized as low, moderate, or high.
Statistical analysis
We used Chi-square tests for categorical variables and two-sample t-tests for continuous variables to test the differences in demographic and clinical characteristics between cases and controls. We used logistic regression models to estimate the associations between oral health and COPD exacerbation (case:control) status, adjusting for potential confounders including inhaler use (inhaled corticosteroids and anticholinergic inhalers) and FEV1 % predicted. The variables used for adjustment in the models were based on the baseline characteristics that were found to be significantly different between exacerbators and non-exacerbators (Table 1). Additionally, we fit a logistic regression model with the outcome of severe vs mild exacerbation for each dental exam measure (periodontitis severity, BOP, PD, CAL PI, GI, and CRA). All but BOP were ordinal scales where higher values indicated worse oral health. We modeled these scales as continuous predictors to assess for trends between worse oral health and exacerbation severity. For this association between dental exam measures and severity of COPD exacerbations, only unadjusted logistic regression analyses were performed due to the small sample size. Multivariate linear regression model was used to assess relationships between oral health and SGRQ scores with adjustment for the same covariates.
Table 1.
Baseline characteristics by COPD exacerbation status
| COPD exacerbation status | |||
|---|---|---|---|
| Exacerbator (n=70) | Non-exacerbator (n=66) | p-values | |
| Age (years), mean (SD) | 66.8 (7.2) | 67.5 (5.5) | 0.511 |
| Sex | |||
| Male | 67 (96%) | 65 (98%) | 0.620 |
| Race | |||
| White | 62 (89%) | 58 (88%) | >0.999 |
| Black or African American | 5 (7.1%) | 7 (11%) | 0.682 |
| American Indian or Alaska Native | 3 (4.3%) | 1 (1.5%) | 0.620 |
| Education | 0.349 | ||
| Some high school | 4 (5.7%) | 1 (1.5%) | |
| High school graduate or GED | 20 (29%) | 23 (35%) | |
| Some college credit | 23 (33%) | 18 (27%) | |
| Trade/technical training | 13 (19%) | 17 (26%) | |
| College graduate | 4 (5.7%) | 6 (9.1%) | |
| Some postgraduate work | 2 (2.9%) | 0 (0%) | |
| Postgraduate degree | 4 (5.7%) | 1 (1.5%) | |
| BMI (kg/m2) | 0.216 | ||
| <18.5 | 1 (1.4%) | 0 (0%) | |
| 18.5–24.9 | 11 (16%) | 11 (17%) | |
| 25–29.9 | 24 (34%) | 14 (21%) | |
| ≥30 | 34 (49%) | 41 (62%) | |
| Tobacco | |||
| Active smoker | 18 (26%) | 17 (26%) | >0.999 |
| Pack years, mean (SD)a | 50 (24) | 47 (21) | 0.409 |
| Chew or snuff | 2 (2.9%) | 1 (1.5%) | >0.999 |
| Spirometryb | 0.026 | ||
| FEV1 50–70% predicted | 24 (35%) | 37 (56%) | |
| FEV1 30–50% predicted | 36 (52%) | 26 (39%) | |
| FEV1 <30% predicted | 9 (13%) | 3 (4.5%) | |
| Comorbidities | |||
| Diabetes | 16 (23%) | 22 (33%) | 0.242 |
| GERD | 23 (33%) | 20 (30%) | 0.892 |
| Alcohol use | 29 (41%) | 23 (35%) | 0.430 |
| Inhaler use | |||
| Inhaled corticosteroid | 57 (81%) | 32 (48%) | <0.001 |
| Anticholinergic inhalers | 59 (84%) | 27 (41%) | <0.001 |
Notes: aOne non-exacerbator was missing this measure. Percentages are of non-missing. bOne exacerbator was missing this measure. Percentages are of non-missing.
Sample size calculation
We initially planned to enroll 360 participants (120 cases and 240 controls) to provide 83% power to detect an odds ratio of 2.0 at a two-sided significance level of 0.05. We anticipated that 80% of screened participants would be eligible and provide complete data, so we planned to screen 150 cases and 300 controls. However, due to an unexpectedly high proportion of participants being ineligible due to having ≤4 teeth, especially amongst potential controls, we decided to perform an interim analysis of our primary outcome OHIP-5 data and re-evaluate feasibility of this observational study. This analysis indicated futility, so we halted enrollment at 70 cases and 66 controls.
Results
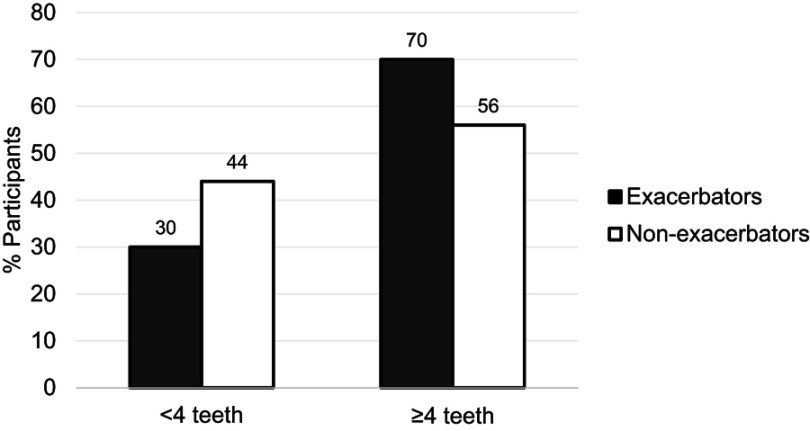
We screened 100 COPD exacerbators and 118 non-exacerbators. Of these, only 70 (70%) and 66 (56%), respectively, met the inclusion criteria of having ≥4 teeth (p=0.046 for difference in proportion with this exclusion criteria between cases and controls) (Figure 1). Dental examinations were performed on 27 (39%) exacerbators and 29 (44%) non-exacerbators.
Figure 1.
Percent of screened participants with <4 teeth vs ≥4 teeth, by COPD exacerbation status. Non-exacerbators (n=118) were significantly more likely to have <4 teeth compared to exacerbators (n=110) (p=0.046).
The baseline characteristics of the participants that met eligibility are summarized in Table 1 according to COPD exacerbation status. As expected, there were significant differences between cases and controls in inhaler use (both inhaled corticosteroids and anticholinergics were more commonly used in cases) and FEV1 % predicted; therefore, inhaler use and FEV1 % predicted were included as covariates in adjusted models. Exacerbators tended to have greater inhaler use and lower FEV1 % predicted compared to non-exacerbators, consistent with previously published studies.3,4,22–24 No other statistically significant differences were found between cases and controls including the oral health questionnaire responses, dental exam measurements, and SGRQ scores, shown in Tables 2–4, respectively.
Table 2.
Oral health questionnaire responses based on COPD exacerbation status
| COPD exacerbation status | p-value | ||
|---|---|---|---|
| Exacerbator (n=70) | Non-exacerbator (n=66) | ||
| Global oral health status | 0.30 | ||
| Poor | 17 (24%) | 21 (32%) | |
| Fair | 24 (34%) | 25 (38%) | |
| Good | 22 (31%) | 12 (18%) | |
| Very good | 3 (4.3%) | 6 (9.1%) | |
| Excellent | 4 (5.7%) | 2 (3.0%) | |
| Self-reported gum disease | 34 (49%) | 33 (50%) | 0.94 |
| Oral symptoms | |||
| Loose teeth | 28 (40%) | 32 (48%) | 0.37 |
| Bleeding gums | 17 (24%) | 22 (33%) | 0.30 |
| Dry mouth | 48 (69%) | 33 (50%) | 0.02 |
| Dentures | |||
| Partial denture | 20 (29%) | 17 (26%) | 0.09 |
| Full denture (upper or lower) | 4 (5.7%) | 10 (15%) | 0.49 |
| Frequency of teeth brushing or denture cleaninga | 0.75 | ||
| Never | 1 (1.5%) | 1 (1.5%) | |
| Less than once a day | 11 (16%) | 11 (17%) | |
| Once a day | 33 (49%) | 26 (39%) | |
| Two times per day | 21 (31%) | 24 (36%) | |
| More than two times per day | 2 (2.9%) | 4 (6.1%) | |
| Time since last dental visitb | 0.36 | ||
| <6 months ago | 24 (36%) | 30 (45%) | |
| 6–12 months ago | 14 (21%) | 8 (12%) | |
| 1–2 years ago | 11 (16%) | 7 (11%) | |
| >2 years ago | 18 (27%) | 21 (32%) | |
| Teeth/denture cleaning | |||
| Toothbrush | 66 (94%) | 60 (91%) | 0.46 |
| Dental floss | 38 (54%) | 32 (48%) | 0.56 |
| Mouthwash | 36 (51%) | 28 (42%) | 0.33 |
| Denture cleanser solution | 16 (23%) | 16 (24%) | 0.81 |
Notes: aTwo frequent exacerbators are missing this measure. Percentages are of non-missing. bThree frequent exacerbators are missing this measure. Percentages are of non-missing.
Table 4.
St. George’s Respiratory Questionnaire (SGRQ) scores based on COPD exacerbation status
| COPD exacerbation status | ||
|---|---|---|
| SGRQ | Exacerbator (n=70), mean (SD) | Non-exacerbator (n=66), mean (SD) |
| Symptom | 56 (23) | 49 (22) |
| Activity | 70 (22) | 65 (22) |
| Impact | 40 (20) | 30 (19) |
| Total | 52 (18) | 44 (18) |
The unadjusted and adjusted odds ratios (OR) for self-reported oral health status (Table 5) and dental exam measures (Table 6) did not vary significantly between exacerbators and non-exacerbators. However, there was a trend towards higher odds of exacerbations in those with “dry mouth” in both unadjusted and adjusted models (unadjusted OR 2.18; 95% CI 1.09–4.43; p-value 0.03 and adjusted OR 2.29; 95% CI 0.99–5.44; p-value 0.05). Self-reported “bleeding gums” was less likely among exacerbators compared to non-exacerbators (adjusted OR 0.39; 95% CI 0.15–0.97; p-value 0.04).
Table 5.
Association between oral health measures and COPD exacerbation status. Unadjusted and adjusted odds ratios of COPD exacerbator (n=70) and non-exacerbator (n=66) status for the oral health measures
| Unadjusted OR (95% CI) | p-value | Adjusted ORa (95% CI) | p-value | |
|---|---|---|---|---|
| Oral Health Impact Profile-5 | ||||
| Difficulty chewing | 0.93 (0.72, 1.20) | 0.58 | 0.88 (0.64, 1.20) | 0.42 |
| Painful ache in the mouth | 0.96 (0.71, 1.31) | 0.80 | 0.99 (0.69, 1.44) | 0.96 |
| Uncomfortable about appearance | 0.97 (0.77, 1.22) | 0.80 | 0.96 (0.72, 1.27) | 0.76 |
| Less flavor | 1.00 (0.77, 1.29) | 0.97 | 0.85 (0.62, 1.16) | 0.30 |
| Difficulty doing jobs | 0.87 (0.50, 1.46) | 0.59 | 1.18 (0.62, 2.22) | 0.59 |
| Oral symptoms | ||||
| Global oral health status | 1.19 (0.86, 1.65) | 0.29 | 1.17 (0.8, 1.74) | 0.42 |
| Self-reported gum disease | 0.94 (0.48, 1.85) | 0.87 | 1.25 (0.55, 2.89) | 0.59 |
| Loose teeth | 0.71 (0.36, 1.40) | 0.32 | 0.93 (0.41, 2.14) | 0.87 |
| Bleeding gums | 0.64 (0.30, 1.35) | 0.24 | 0.39 (0.15, 0.97) | 0.04 |
| Dry mouth | 2.18 (1.09, 4.43) | 0.03 | 2.29 (0.99, 5.44) | 0.05 |
| Partial denturesb | 1.00 (0.46, 2.18) | 0.19 | 0.92 (0.36, 2.39) | 0.39 |
| Full denturesb | 0.34 (0.09, 1.10) | 0.37 (0.08, 1.51) | ||
| Oral care habits | ||||
| Brush once per dayc | 1.27 (0.49, 3.31) | 0.52 | 0.96 (0.29, 3.07) | 0.99 |
| Brush twice or more per dayc | 0.82 (0.31, 2.18) | 1.01 (0.30, 3.40) | ||
| Dental visit 6–12 months agod | 2.19 (0.80, 6.30) | 1.40 (0.39, 5.19) | 0.43 | |
| Dental visit 1–2 years agod | 1.96 (0.67, 6.08) | 0.33 | 1.88 (0.54, 6.97) | |
| Dental visit >2 years agod | 1.07 (0.47, 2.46) | 0.67 (0.24, 1.80) |
Notes: aAdjustments were for inhaler use (inhaled corticosteroids and anticholinergic inhalers) and FEV1 % predicted. Reference categories: b’no dentures’, c’brush less than once per day’, and d’dental visit less than 6 months ago’. For the categorical variables, the overall p-value for the variable is listed for the first category, but applies to all categories.
Table 6.
Association between dental exam measures and COPD exacerbation status. Unadjusted and adjusted odds ratios of COPD exacerbator (n=27) and non-exacerbator (n=29) status for the dental exam measures
| Dental exam | Unadjusted OR (95% CI) | p-value | Adjusted ORa (95% CI) | p-value |
|---|---|---|---|---|
| Periodontitis severity | 0.78 (0.39, 1.52) | 0.46 | 0.73 (0.33, 1.56) | 0.42 |
| Probing depth | 0.82 (0.38, 1.68) | 0.59 | 1.07 (0.46, 2.50) | 0.88 |
| Clinical attachment loss | 1.36 (0.58, 3.32) | 0.49 | 1.54 (0.57, 4.29) | 0.39 |
| Bleeding on probing | 1.27 (0.38, 4.48) | 0.70 | 1.20 (0.29, 5.18) | 0.80 |
| Plaque index | 0.90 (0.61, 1.31) | 0.58 | 0.90 (0.57, 1.39) | 0.63 |
| Gingival index | 0.74 (0.40, 1.33) | 0.32 | 0.77 (0.38, 1.51) | 0.45 |
| Carries risk assessment | 0.90 (0.45, 1.81) | 0.77 | 0.81 (0.37, 1.76) | 0.60 |
Note: aAdjustments were for inhaler use (inhaled corticosteroids and anticholinergic inhalers) and FEV1 % predicted.
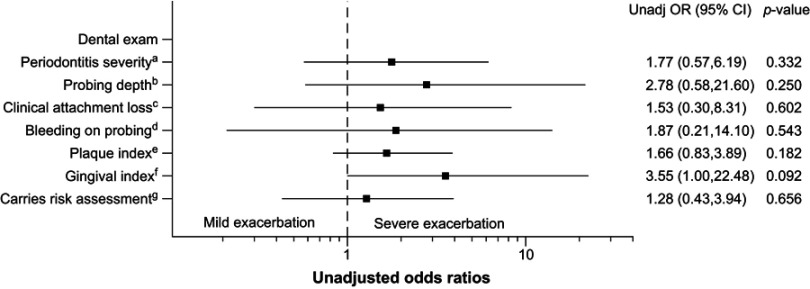
The associations between dental exam measurements (Table 3) and severe COPD exacerbations are shown in Figure 2. Of the exacerbators who underwent dental exam, 19/27 had severe exacerbations (requiring emergency department visit and/or hospitalization), while 7/27 had mild exacerbations. The unadjusted odds ratios of severe exacerbations relative to mild exacerbations trended higher in those with worse categories of measurements for periodontitis severity (mild, moderate severe), PD (≤3, >3 to <5, ≥5 to <7, ≥7), CAL (<1, 1 to 2, 3 to 4, ≥5), BOP (yes/no), PI (score 0, 1, 2, 3, 4, 5), GI (score 0, 1, 2, 3), and CRA (low, moderate, high) compared to those with better dental exam measurements. However, statistical significance was not reached. Adjustment for covariates was not performed due to the small sample size.
Table 3.
Dental exam measurements comparing COPD exacerbators vs non-exacerbators
| COPD exacerbation status | p-value | ||
|---|---|---|---|
| Exacerbator (n=27) | Non-exacerbator (n=29) | ||
| Probing Depth (mm)a | 0.36 | ||
| ≤3 | 12 (46%) | 14 (48%) | |
| >3 and <5 | 12 (46%) | 10 (34%) | |
| ≥5 and <7 | 2 (7.7%) | 4 (14%) | |
| ≥7 | 0 (0%) | 1 (3.4%) | |
| Bleeding on Probinga | 20 (77%) | 21 (72%) | 0.71 |
| Clinical attachment loss (mm)a | 0.65 | ||
| <1 | 0 (0%) | 0 (0%) | |
| 1 to 2 | 1 (3.8%) | 3 (10%) | |
| 3 to 4 | 12 (46%) | 13 (45%) | |
| ≥5 | 13 (50%) | 13 (45%) | |
| Severity of periodontitisa | 0.68 | ||
| Mild | 7 (27%) | 5 (17%) | |
| Moderate | 8 (31%) | 10 (34%) | |
| Severe | 11 (42%) | 14 (48%) | |
| Plaque indexa | 0.54 | ||
| Score 0 | 2 (7.7%) | 2 (6.9%) | |
| Score 1 | 4 (15%) | 4 (14%) | |
| Score 2 | 7 (27%) | 5 (17%) | |
| Score 3 | 5 (19%) | 5 (17%) | |
| Score 4 | 5 (19%) | 12 (41%) | |
| Score 5 | 3 (12%) | 1 (3.4%) | |
| Gingival indexa | 0.38 | ||
| Score 0 | 2 (7.7%) | 3 (10%) | |
| Score 1 | 11 (42%) | 6 (21%) | |
| Score 2 | 8 (31%) | 12 (41%) | |
| Score 3 | 5 (19%) | 8 (28%) | |
| Caries risk assessment | 0.69 | ||
| Low | 7 (26%) | 5 (17%) | |
| Moderate | 9 (33%) | 13 (45%) | |
| High | 11 (41%) | 11 (38%) | |
Notes: aData for one exacerbator is missing. Percentages are of non-missing.
Figure 2.
Association between dental exam measures and severity of COPD exacerbations. Unadjusted odds ratios of severe (n=19) vs mild (n=7) COPD exacerbations for the dental exam measures. Categories of scales – a: mild, moderate, severe; b: ≤3, >3 to <5, ≥5 to <7, ≥7; c: <1, 1 to 2, 3 to 4, ≥5; d: yes/no; e: score 0, 1, 2, 3, 4, 5; f: score 0, 1, 2, 3; and g: low, moderate, high.
The correlations between OHRQoL and respiratory health are shown in Table 7. The adjusted estimates reflect the difference in mean total SGRQ scores between the exacerbators and non-exacerbators with a 1-point difference in OHIP. Worse OHRQoL was strongly associated with worse respiratory health scores. In addition, “painful ache in the mouth” and “difficulty doing jobs” were also clinically significantly associated with worse respiratory health scores.
Table 7.
Association between oral health-related quality of life (OHIP-5) and respiratory health (total SGRQ score). Unadjusted and adjusted ß coefficients reflect the difference in mean St. George’s Respiratory Questionnaire (SGRQ) total scores between groups with a 1-point difference in the OHIP measure
| OHIP-5 | Unadjusted ß coefficient(95% CI) | p-value | Adjusted ß coefficienta (95% CI) | p-value |
|---|---|---|---|---|
| Difficulty chewing | 2.36 (0.11, 4.61) | 0.042 | 2.57 (0.39, 4.75) | 0.023 |
| Painful ache in the mouth | 5.26 (2.57, 7.95) | <0.001 | 5.43 (2.84, 8.02) | <0.001 |
| Uncomfortable about appearance | 2.95 (0.88, 5.03) | 0.006 | 3.17 (1.15, 5.19) | 0.003 |
| Less flavor | 3.89 (1.58, 6.20) | 0.001 | 3.53 (1.11, 5.94) | 0.005 |
| Difficulty doing jobs | 5.79 (1.20, 10.38) | 0.015 | 7.31 (3.08, 11.54) | <0.001 |
Note: aAdjustments were for inhaler use and FEV1 % predicted.
Discussion
We aimed to determine if COPD exacerbation status (exacerbator vs non-exacerbator) is associated with oral health. In this study, we did not find that poor oral health, as determined by self-report or by objective dental examination, was associated with COPD exacerbations. We did find a relationship between poor OHRQoL and worse respiratory health status and a possible trend towards worse dental exam measures among those with severe COPD exacerbations.
There are several observational studies linking periodontitis with COPD. A meta-analysis of 14 observational studies by Zeng et al showed an association between periodontitis and COPD diagnosis with a pooled OR of 2.09 (95% CI: 1.48–2.91).10 Published studies also show that COPD patients have fewer remaining teeth compared with healthy controls.25–28 However, few studies have evaluated the relationship between COPD exacerbation rates with periodontitis and dentition status.11–14
One cross-sectional study by Liu et al evaluating the association between oral hygiene and periodontal health in COPD exacerbations in China showed increased COPD exacerbations in those with fewer remaining teeth.11 In contrast, we found that fewer teeth were associated with non-exacerbator status. However, the definitions of “fewer” teeth and COPD exacerbation status varied between these two studies. Liu et al defined fewer teeth as ≤25 teeth vs >25 teeth while we compared <4 teeth vs ≥4 teeth (most of our patients with <4 teeth were edentate).11 Additionally, Liu et al did not find significant differences in periodontal health indices including PD, CAL, and bleeding on probing between exacerbators and non-exacerbators, similar to our findings.11
We found a trend towards more severe COPD exacerbations requiring emergency room visits and/or hospitalizations in those with worse periodontal health indices, although this association did not reach statistical significance, possibly due to small sample size. Shen et al found a strong positive correlation between incident periodontal disease and frequency of emergency room visits and hospitalizations for COPD exacerbation, which support our findings.12
We theorize that the oral microbiota and oral inflammation play key roles in this relationship between dentition status and COPD exacerbations.5–7 The oral microbiota is a major source of the lung microbiota.5–7 Lung inflammation may be mediated by aspiration of inflammatory cytokines from the oral cavity, or aspiration of a dysbiotic oral microbiota may lead to dysbiosis of the lung microbiota. The presence of diseased teeth allows for extensive biofilm formation, which then could be aspirated into the lungs, thereby contributing to COPD exacerbations.29 Full-mouth teeth extraction has been found to significantly reduce the burden of periodontopathogens.19 Absence of any teeth is likely associated with a lower burden of periodontal pathogens compared to those with the presence of potentially diseased teeth30,31 and may explain our observation that non-exacerbators were more likely to have fewer or no teeth.
In this study, we also show a relationship between poor oral health-related quality of life as measured by OHIP-5 and respiratory health status (SGRQ total score), regardless of the COPD exacerbation status. We used the OHIP, the most widely used oral health-related quality of life instrument, to assess the impact of oral health.16 Prior studies have demonstrated good correlation of the OHIP with clinical oral examination among the Veteran population.32 However, limitations related to the OHIP-5 are the absence of an established minimal clinically important difference. Similarly, Zhou et al report an association between poor periodontal health and low quality of life in COPD patients.33 Correlation of our current findings with the Global Initiative for Chronic Obstructive Lung Disease (GOLD) ABCD stages that incorporates both spirometric severity and symptom burden would have been interesting; however, we did not collect data for the Modified Medical Research Council (mMRC) Dyspnea Scale and COPD Assessment Test (CAT) scores. The observed relationship between OHIP-5 and SGRQ total score suggest the need for studies to address oral health as a potential method to improve respiratory health status in patients with COPD.
Our study has several limitations. First, case-control studies are subject to selection bias and information bias, and therefore directional causality cannot be established. In addition, questionnaire studies are susceptible to response bias. This study was also conducted in a single center, predominantly white male population with a relatively modest sample size. Larger studies with more diverse populations may provide further insight regarding the role of oral health in COPD outcomes and quality of life. We also did not meet our original target sample size, possibly limiting statistically significant findings, although we chose to close the study after interim analyses suggested that larger sample sizes would be futile for detecting differences in oral health between cases and controls. Lastly, we assessed comorbidities that are most likely to affect oral health status such as diabetes, GERD, and alcohol use, but we acknowledge that other comorbidities that we did not assess could also play a role.
Conclusions
Oral health status was not related to COPD exacerbation status, but was associated with patient-reported respiratory health status. Non-exacerbators were more likely to be edentate or have <4 teeth compared to exacerbators. Larger studies are needed to address oral health as a potential method to improve respiratory health status in patients with COPD.
Acknowledgments
Disclaimer: the views expressed in this article are those of the authors and do not reflect the views of the United States Government, the Department of Veterans Affairs, the funders, the sponsors, or any of the author’s affiliated academic institutions.
This research was supported by the National Heart, Lung and Blood Institute (NHLBI) grant T32 HL007741-23 (AKB), Flight Attendant Medical Research Institute (CHW), and Veterans Affairs Career Development Award 1IK2CX001095 (AAP). This material is also the result of work supported with resources of the Minneapolis VA Health Care System.
A version of this manuscript’s abstract was published and presented as a poster discussion at the American Thoracic Society in San Diego, CA, USA, in May 2018, and the Aspen Lung Conference in Aspen, CO, USA, in June 2018 (https://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2018.197.1_MeetingAbstracts.A2754).
Disclosure
KMK has received consulting fees from GlaxoSmithKline outside of this submitted work and reports no conflicts of interest in this work. All of the other authors report that they have no affiliations with or involvement in any organization or entity with any financial or non-financial interest in the subject matter or materials discussed in this manuscript, and have no conflicts of interest in this work.
Supplementary materials
References
- 1.Hurst JR, Anzueto A, Vestbo J. Susceptibility to exacerbation in COPD. Lancet Respir Med. 2017;5(9):e29. doi: 10.1016/S2213-2600(17)30234-5 [DOI] [PubMed] [Google Scholar]
- 2.Hoogendoorn M, Hoogenveen RT, Rutten-van Mölken MP, Vestbo J, Feenstra TL. Case fatality of COPD exacerbations: a meta-analysis and statistical modelling approach. Eur Respir J. 2011;37(3):508–515. doi: 10.1183/09031936.00043710 [DOI] [PubMed] [Google Scholar]
- 3.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi: 10.1056/NEJMoa0909883 [DOI] [PubMed] [Google Scholar]
- 4.Niewoehner DE, Lokhnygina Y, Rice K, et al. Risk indexes for exacerbations and hospitalizations due to COPD. Chest. 2007;131(1):20–28. doi: 10.1378/chest.06-1316 [DOI] [PubMed] [Google Scholar]
- 5.Pragman AA, Kim HB, Reilly CS, Wendt C, Isaacson RE. The lung microbiome in moderate and severe chronic obstructive pulmonary disease. PLoS One. 2012;7(10):e47305. doi: 10.1371/journal.pone.0047305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pragman AA, Kim HB, Reilly CS, Wendt C, Isaacson RE. Chronic obstructive pulmonary disease lung microbiota diversity may be mediated by age or inhaled corticosteroid use. J Clin Microbiol. 2015;53(3):1050. doi: 10.1128/JCM.03320-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pragman AA, Lyu T, Baller JA, et al. The lung tissue microbiota of mild and moderate chronic obstructive pulmonary disease. Microbiome. 2018;6(1):7. doi: 10.1186/s40168-018-0432-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clayton NA, Carnaby-Mann GD, Peters MJ, Ing AJ. The effect of chronic obstructive pulmonary disease on laryngopharyngeal sensitivity. Ear Nose Throat J. 2012;91(9):370, 372, 374 passim. [PubMed] [Google Scholar]
- 9.Smaldone GC, Foster WM, O’Riordan TG, Messina MS, Perry RJ, Langenback EG. Regional impairment of mucociliary clearance in chronic obstructive pulmonary disease. Chest. 1993;103(5):1390–1396. [DOI] [PubMed] [Google Scholar]
- 10.Zeng XT, Tu ML, Liu DY, Zheng D, Zhang J, Leng W. Periodontal disease and risk of chronic obstructive pulmonary disease: a meta-analysis of observational studies. PLoS One. 2012;7(10):e46508. doi: 10.1371/journal.pone.0046508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Z, Zhang W, Zhang J, et al. Oral hygiene, periodontal health and chronic obstructive pulmonary disease exacerbations. J Clin Periodontol. 2012;39(1):45–52. doi: 10.1111/j.1600-051X.2011.01808.x [DOI] [PubMed] [Google Scholar]
- 12.Shen TC, Chang PY, Lin CL, et al. Risk of periodontal diseases in patients with chronic obstructive pulmonary disease: a nationwide population-based cohort study. Medicine (Baltimore). 2015;94(46):e2047. doi: 10.1097/MD.0000000000000874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kucukcoskun M, Baser U, Oztekin G, Kiyan E, Yalcin F. Initial periodontal treatment for prevention of chronic obstructive pulmonary disease exacerbations. J Periodontol. 2013;84(7):863–870. doi: 10.1902/jop.2012.120399 [DOI] [PubMed] [Google Scholar]
- 14.Takahashi T, Muro S, Tanabe N, et al. Relationship between periodontitis-related antibody and frequent exacerbations in chronic obstructive pulmonary disease. PLoS One. 2012;7(7):e40570. doi: 10.1371/journal.pone.0040570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155(3):179–191. doi: 10.7326/0003-4819-155-3-201108020-00008 [DOI] [PubMed] [Google Scholar]
- 16.Naik A, John MT, Kohli N, Self K, Flynn P. Validation of the English-language version of 5-item Oral Health Impact Profile. J Prosthodont Res. 2016;60(2):85–91. doi: 10.1016/j.jpor.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones PW. St. George’s Respiratory Questionnaire: MCID. Copd. 2005;2(1):75–79. [DOI] [PubMed] [Google Scholar]
- 18.American Academy of Periodontology Board of Trustees.American Academy of Periodontology task force report on the update to the 1999 classification of periodontal diseases and conditions. J Periodontol. 2015;86(7):835–838. doi: 10.1902/jop.2015.157001 [DOI] [PubMed] [Google Scholar]
- 19.Barbosa VL, Angst PD, Finger Stadler A, Oppermann RV, Gomes SC. Clinical attachment loss: estimation by direct and indirect methods. Int Dent J. 2016;66(3):144–149. doi: 10.1111/idj.12218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Löe H. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967;38(6 Suppl):610–616. doi: 10.1902/jop.1967.38.6_part2.610 [DOI] [PubMed] [Google Scholar]
- 21.Turesky S, Gilmore ND, Glickman I. Reduced plaque formation by the chloromethyl analogue of victamine C. J Periodontol. 1970;41(1):41–43. doi: 10.1902/jop.1970.41.41.41 [DOI] [PubMed] [Google Scholar]
- 22.Hoogendoorn M, Feenstra TL, Hoogenveen RT, Al M, Mölken MR. Association between lung function and exacerbation frequency in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2010;5:435–444. doi: 10.2147/COPD.S13826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donaldson GC, Wedzicha JA. COPD exacerbations 0.1: epidemiology. Thorax. 2006;61(2):164–168. doi: 10.1136/thx.2005.041806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burgel PR, Nesme-Meyer P, Chanez P, et al. Cough and sputum production are associated with frequent exacerbations and hospitalizations in COPD subjects. Chest. 2009;135(4):975–982. doi: 10.1378/chest.08-2062 [DOI] [PubMed] [Google Scholar]
- 25.Wang Z, Zhou X, Zhang J, et al. Periodontal health, oral health behaviours, and chronic obstructive pulmonary disease. J Clin Periodontol. 2009;36(9):750–755. doi: 10.1111/j.1600-051X.2009.01448.x [DOI] [PubMed] [Google Scholar]
- 26.Offenbacher S, Beck JD, Barros SP, Suruki RY, Loewy ZG. Obstructive airway disease and edentulism in the atherosclerosis risk in communities (ARIC) study. BMJ Open. 2012;2(6):e001615. doi: 10.1136/bmjopen-2012-001615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barros SP, Suruki R, Loewy ZG, Beck JD, Offenbacher S. A cohort study of the impact of tooth loss and periodontal disease on respiratory events among COPD subjects: modulatory role of systemic biomarkers of inflammation. PLoS One. 2013;8(8):e68592. doi: 10.1371/journal.pone.0068592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaeckle NT, Heyman B, Criner AJ, Criner GJ. Markers of dental health correlate with daily respiratory symptoms in COPD. Chronic Obstr Pulm Dis. 2018;5(2):97–105. doi: 10.15326/jcopdf.5.2.2017.0159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marsh PD, Moter A, Devine DA. Dental plaque biofilms: communities, conflict and control. Periodontol 2000. 2011;55(1):16–35. doi: 10.1111/j.1600-0757.2009.00339.x [DOI] [PubMed] [Google Scholar]
- 30.Van Assche N, Van Essche M, Pauwels M, Teughels W, Quirynen M. Do periodontopathogens disappear after full-mouth tooth extraction? J Clin Periodontol. 2009;36(12):1043–1047. doi: 10.1111/j.1600-051X.2009.01477.x [DOI] [PubMed] [Google Scholar]
- 31.O’Donnell LE, Robertson D, Nile CJ, et al. The oral microbiome of denture wearers is influenced by levels of natural dentition. PLoS One. 2015;10(9):e0137717. doi: 10.1371/journal.pone.0137717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones JA, Kressin NR, Spiro A, et al. Self-reported and clinical oral health in users of VA health care. J Gerontol A Biol Sci Med Sci. 2001;56(1):M5562. doi: 10.1093/gerona/56.1.M55 [DOI] [PubMed] [Google Scholar]
- 33.Zhou X, Wang Z, Song Y, Zhang J, Wang C. Periodontal health and quality of life in patients with chronic obstructive pulmonary disease. Respir Med. 2011;105(1):67–73. doi: 10.1016/j.rmed.2010.06.017 [DOI] [PubMed] [Google Scholar]