Abstract
Morphine is commonly used in clinical management to alleviate moderate-to-severe pain. However, prolonged and repeated use of morphine leads to tolerance. Morphine tolerance is a challenging clinical problem that limits its clinical application in pain treatment. The mechanisms underlying morphine tolerance are still not completely understood. MicroRNAs (miRNAs) are small noncoding RNAs containing 18~22 nucleotides that modulate gene expression in a post-transcriptional manner, and their dysregulation causes various diseases. miRNAs bind to the 3ʹ-UTR (untranslated region) of target gene mRNA, inhibiting or destabilizing translation of the transcripts. Morphine causes differential miRNA upregulation or downregulation. This review will present evidence for the contribution of miRNAs to tolerance of the antinociception effect of opioids.
Keywords: microRNA, morphine tolerance, MOR, β-arrestin 2, CaMKII/NMDAR
Introduction to morphine tolerance
Morphine is used extensively in clinical practice for the treatment of acute and chronic pain as well as cancer-related pain.1 Long-term morphine treatment is usually accompanied by morphine tolerance.2 Morphine tolerance is characterized by a progressively decreasing pain control response, requiring increasing morphine dosage to achieve adequate analgesia after long-term application.3 Morphine tolerance is the major reason for pain treatment failure, and the molecular mechanisms of morphine tolerance are complicated.
Opioid receptors and morphine tolerance
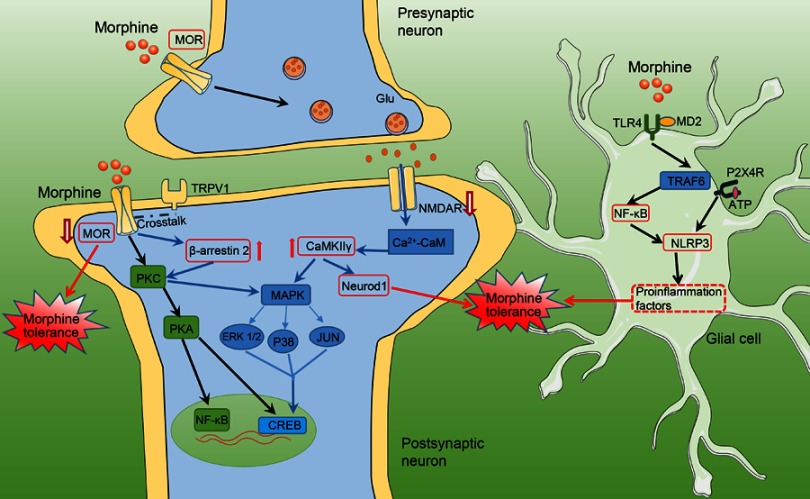
Opioid tolerance reflects changes in how systems affected by the opioid respond such as changes in receptor density or desensitization of receptors.4 The opioid receptor belongs to the G protein-coupled receptor (GPCR) family and primarily mediates the analgesic function of morphine. Morphine targets mu opioid receptor (MOR) through adenylyl cyclase (AC) and extracellular signal-regulated kinase (ERK) pathways as well as intracellular calcium storage and cell membrane ion channels to form antinociceptive tolerance.5 Endocytosis of MOR through corresponding kinases, including protein kinase C (PKC), protein kinase A (PKA), and GPCR kinases (GRKs) to promote serine or threonine phosphorylation on MOR, facilitates the development of morphine tolerance (Figure 1).6 A previous study reported that delta opioid receptor (DOR) is a key receptor in morphine antinociceptive tolerance. MOR analgesic tolerance is reduced by blockade of DORs that interact with MORs.7,8 Recent studies show that the MOR/DOR interaction in nociceptive afferent neurons in the dorsal root ganglion may contribute to morphine analgesic tolerance.9 Cyclin-dependent kinase 5 (Cdk5) phosphorylated DOR at Thr-161 accelerated the development of morphine tolerance.8 Besides, one mechanism for the role of DOR in modulating analgesia is through MOR–DOR heterodimerization.10 The DOR antagonist can increase MOR binding and signaling by occupancy of DOR and enhance morphine-mediated analgesia.10,11 Furthermore, morphine tolerance can be blockaded by genetic interruption of DOR system.12 In addition, co-administration of a κ-receptor antagonist with morphine suppressed the development of antinociceptive tolerance to morphine.13
Figure 1.
Schematic showing mechanisms underlying morphine tolerance. Schematic model showing how MOR/β-arrestin 2 and NMDAR/CaMKⅡγ-dependent signaling in neuron plays a crucial role in the promotion of morphine tolerance. Morphine induces activation of glial cells and upregulates proinflammatory cytokines via the TLR4/NF-κB pathway to facilitate morphine tolerance.
Abbreviations: MAPK, mitogen-activated protein kinase; ERK1/2, extracellular signal-regulated kinase 1/2; NeuroD 1, neurogenic differentiation factor 1; TRAF6, tumor necrosis factor receptor-associated factor 6; P2X4R, purinergic P2X4 receptors; ATP, adenosine 5'-diphosphate; NLRP3, NACHT, LRR and PYD domains-containing protein 3 inflammasome.
Synaptic connections and morphine tolerance
Recently, most cellular studies on morphine tolerance have focused on synaptic mechanisms. N-methyl-D-aspartic acid receptor (NMDAR), platelet-derived growth factor receptor β (PDGFR-β),14 and substance P precursor proteins (tachykinin precursors) in the synapse were reported indirectly participated in morphine tolerance.8,15 The presynaptic glutamate receptor, which is co-expressed with the transient receptor potential vanilloid 1 (TRPV-1), promotes glutamate release and produces long-term potentiation (LTP) to facilitate morphine tolerance.16 Studies have demonstrated that chronic morphine treatment leads to a reduction in postsynaptic K+ conductance and voltage-gated calcium channels in the periaqueductal gray (PAG).17 Besides, transcription factors, such as cAMP-response element binding (CREB)18 and nuclear factor-κB (NF-κB),19 are also regulated by morphine and participate in synaptic plasticity and the pathology of morphine tolerance.
Inflammatory factors and morphine tolerance
A large number of researchers have found that long-term morphine application results in neuroinflammatory responses, especially those mediated by toll-like receptor-4 (TLR4), in the brain and spinal cord, which are a very important cause of morphine tolerance.20 TLR4 is a key innate immune receptor, and morphine bound to myeloid differentiation factor 2 (MD-2) activates TLR4 signaling facilitating morphine tolerance.21 Recently, increasing evidence indicates that morphine tolerance is accompanied by increased glial cell activation.22 The number of inflammation-associated astrocytes and microglia was significantly increased by morphine in the spinal cord of rats; moreover, these increased cell numbers were accompanied by morphological changes.23 Pentoxifylline inhibits astrocyte activation and releases neuroinflammatory factors, such as tumor necrosis factor alpha (TNF-α), IL–1β, and IL–6), effectively reversing the development of morphine tolerance.24 Morphine activates microglial cells, upregulates microglia marker (CD-11b or Iba1) expression, acts on TLR4 and activates proinflammatory signaling to facilitate morphine tolerance.25 In addition, P2X4 and P2X7,26,27 ATP receptors, were upregulated in microglia of the spinal cord by morphine, and its antagonists prevented the development of morphine tolerance (Figure 1).
The mechanisms underlying morphine tolerance are not completely understood, and effective prevention and treatment measures are lacking. In recent years, some studies have stated that many of the mechanisms that have been implicated in opioid tolerance appear to be regulated by miRNA.28 This review discusses how abnormally expressed miRNAs promote morphine tolerance by targeting its downstream genes.
MicroRNA (miRNA) synthesis and function
miRNAs are a group of noncoding, single-stranded small RNAs approximately 18~22 nucleotides (nt) in length. When pri-miRNAs are synthesized in the cell nucleus, dicer enzymes process the pre-miRNAs into mature miRNAs, which are rapidly transferred to the cytoplasm. miRNAs guide Argonaute (AGO) proteins and recruit miRNA-induced silencing complex (miRISC) to mRNA targets.29 Negative miRNA regulation functions include direct degradation of target gene mRNA and modulation of target gene mRNA stability to indirectly inhibit target gene translation.30 When multiple 3ʹ-UTR-binding sites are present, the negative regulatory function of target genes is more obvious.31 miRNAs have also been reported to bind to mRNA-coding regions. However, the inhibitory effect of binding to the coding regions is lower than of binding to the 3ʹ-UTRs.32
Recent studies have clearly demonstrated that miRNAs are essential and critical players in mammalian development and closely associated with human genetic diseases, nervous system development, and the development and progression of certain major diseases.33–35 In opioid analgesic efficiency research, we found that miRNAs play an indispensable role in morphine tolerance, drug addiction, and opioid receptor expression.36
miRNAs participate in morphine tolerance
With the gradual increase in the number of miRNA and morphine tolerance studies, accumulating results have demonstrated that morphine-induced antinociceptive tolerance is accompanied by upregulation or downregulation of many miRNAs in vivo and in vitro and that the differentially expressed miRNAs are important regulators of morphine tolerance. A growing number of studies have reported miRNA mechanisms in morphine tolerance (Table 1).
Table 1.
miRNAs and morphine tolerance
| Name | Change | Drug studied | Tissue | Function |
|---|---|---|---|---|
| miR-16 | Decreased | Morphine | CEM ×174 cell | Binds to the MOR-1 mRNA 3’-UTR and suppress OPRM1 gene expression.74 |
| miR-103/miR-107 | Increased | Morphine/fentanyl | Mouse (prefrontal cortex)/human embryonic kidney 293(HEK293 cells)/Be(2)C cells | Downregulates polyribosome-associated MOR-1A in both Be(2)C cells and the striatum of a morphine-tolerant mice.44 |
| miR-339 | Increased | Morphine | Mouse hippocampus/mouse neuroblastoma neuro2A(N2A) cell | Inhibits the production of MOR protein by destabilizing MOR mRNA.43 |
| miR-let-7 family | Increased | Morphine | Mouse brain/HEK293 cells | Mediates movement of MOR mRNA into P-bodies, leading to translational repression.39 |
| miR-23b | Increased | Morphine | Human neuronal cell lines (NMB)/HEK293 cells | Inhibits lysome-mRNA association with MOR (mouse neuronal N2A cells).75 |
| miR-365 | Decreased | Morphine | Spinal cord (rat) | Involved in morphine tolerance development and maintenance through regulation of β-arrestin 2.51 |
| miR-219-5p | Decreased | Morphine | Spinal cord (rat) | Alleviates morphine tolerance by inhibiting the CaMKIIγ/NMDA receptor pathway.55 |
| miR-190 | Decreased | Morphine/fentanyl | Hippocampus (mouse) | A key post-transcriptional repressor of neurogenic differentiation factor NeuroD.76 |
| miR-338 | Decreased | Morphine | Spinal cord (rat) | Regulated by miR-338, CXCR4 was significantly increased, and play an important role in morphine tolerance.77 |
| miR-223-3p | Increased | Morphine | Spinal cord (rat) | Upregulates the expression of NLRP3 to facilitate morphine analgesic tolerance.64 |
| miR-375 | Increased | Morphine | Dorsal root ganglia (DRG:mouse) | Ameliorates morphine tolerance by downregulating JAK2/STAT3 expression.78 |
miRNAs regulate opioid receptor expression to accelerate morphine tolerance
let-7 is one of the earliest discovered miRNAs after lin-4. let-7 is a highly conserved miRNA. Let-7 family are encoded by 13 genomic loci in the human body37 and mainly participates in stem cell differentiation, nerve and muscle tissue development, and cell proliferation and differentiation.38 Under morphine stimulation, let-7 expression is upregulated. Validation with luciferase assays showed that the let-7 sequence has many binding sites on the 3ʹ-UTR of the MOR gene. Morphine upregulates let-7 and downregulates MOR protein expression in SH-SY5Y cells.39 Downregulation of let-7 in the mouse brain partially reversed morphine tolerance.40 Further studies showed that let-7 does not directly reduce MOR mRNA degradation; instead, it reduces the binding between ribosomes and mRNAs through a P-body to influence MOR translation and decrease MOR expression.41 These results suggest that the “star molecule” let-7 plays an important role in MOR and participates in morphine tolerance.39 Long-term morphine treatment increased miR23b expression in a dose- and time-dependent manner and repressed target MOR1 mRNA with polysomes through the MOR1 3ʹ-UTR.42 After chronic treatment of mice with μ-opioid agonists (morphine or fentanyl), miR-339-3p was increased in the hippocampus and inhibited MOR 3ʹ-UTR activity by binding to its target sequence and promoting mRNA decay.43 miR-107 and miR-103 were increased in Be(2)C cells and mouse striatum, mainly functioned as repressive elements on MOR1, and participated in morphine tolerance by repressing the expression of MOR.44 Consequently, morphine regulates MOR expression through a number of miRNAs in cell lines and in animals to aggravate morphine tolerance (Figure 2).
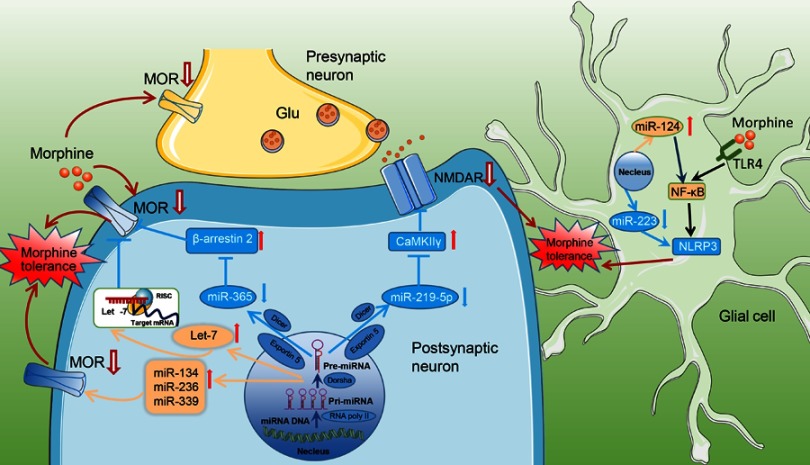
Figure 2.
Schematic diagram showing the mechanism of morphine tolerance regulation by miRNAs. Morphine induces many miRNAs. Pri-miRNAs are synthesized in the nucleus and transferred to the cytoplasm, Dicer makes pre-miRNA into mature miRNA. Differentially expressed miRNAs regulate neuroplasticity-related target protein expression and thereby participate in the development of morphine tolerance. miR-365 was significantly downregulated after morphine administration, along with its target gene β-arrestin 2, and acted indirectly on MOR to participate in morphine tolerance. In addition, morphine induced low miR-219-5p expression in the spinal cord and further upregulates CaMKIIγ to promote morphine tolerance. MOR is the primary opioid receptor and the target of miR-134/miR-339/miR-236, which are involved in morphine tolerance. Mature let-7 is exported from the nucleus into the cytosol and incorporated into RISC, a protein composed of translational machinery that recruits target mRNA to P-bodies and effectively reduces polysome-bound mRNA, resulting in translation repression. In glial cells, morphine administration altered the expression of miR-124 or miR-223, along with their target gene, which participated in morphine tolerance.
Abbreviations: RISC, RNA-induced silencing complex; miR, microRNA.
miRNAs regulate β-arrestin 2 to participate in morphine tolerance
miR-365 is present in many types of cancer cells including colorectal cancer,45 lung cancer,46 and cholangiocarcinoma cell.47 In gastrointestinal cancer, miR-365 inhibits cell cycle progression through inhibition of cyclin D1 (CCND1) to inhibit cancer development.45 miR-365 was decreased in malignant glioma cells and functioned in phosphoinositide-3-kinase regulatory subunit 3 (PIK3R3) to participate in glioma progression, and overexpression of miR-365 inhibited glioma proliferation and invasion. miR-365 was upregulated in the ischemic brain, inhibited the target gene PAX6 (a neurogenic fate determinant) expression, and exacerbated ischemic brain injury.48
Arrestins are inhibitory proteins, and β-arrestin 2 is a subtype49 that can activate GPCRs to mediate internalization and desensitization of MOR. Upregulated β-arrestin 2 functions on MOR to attenuate the analgesic effects of opioid drugs through AC, ERK pathways, intracellular calcium storage, and cell membrane ion channels. β-arrestin2-knockout mice have been reported to represent an animal model in which the morphine-induced desensitization of MOR has been significantly impaired and display an enhanced and prolonged response to morphine in pain perception.50 Our study revealed that miR-365 was significantly decreased, accompanied by high expression of β-arrestin 2, in the spinal cord of morphine group rats compared with saline group rats. Luciferase assays showed that miRNA-365 has many binding sites with the 3ʹ-UTR of the target gene β-arrestin 2 (Figure 2).51 Our research data suggest that morphine tolerance occurs via the miR-365/β-arrestin 2 pathway. In addition, miR-365 targets β-arrestin2 by inhibiting ERK/CREB activation, thus reducing IL-1 and TNF-α content, and lowering morphine analgesic tolerance.52 Therefore, miR-365 might become a potential target for prevention and treatment of morphine tolerance (Figure 2).
miRNAs aggravate morphine tolerance through CaMKII/NMDAR
Calmodulin-dependent protein kinase II (CaMKII) is a serine/threonine protein kinase composed of α, β, γ, and δ subunits. CaMKII is extensively distributed in the central and peripheral nervous systems to regulate synaptic transmission and neuronal functions. Immunofluorescence experiments have demonstrated that MOR and CaMKIIγ are co-expressed and are mainly present in neural pathways that conduct pain. Previous research showed that morphine increased the expression of CaMKII in the dorsal root ganglia (DRG) directly influencing the expression of calcition gene-related peptide (CGRP) required for the development of tolerance to morphine-induced analgesia.53 CaMKII downregulation inhibited both CREB activation induced by morphine and phosphorylation of opioid receptor and attenuated the development of morphine tolerance.54 The expression of miR-219-5p was downregulated in the spinal cords of morphine tolerance rats and acted on the downstream target gene CaMKIIγ.55 CaMKII activates CREB to promote NR1 synthesis. NMDAR activation causes calcium influx to increase calcium concentrations in the cytoplasm and recruit CaMKII to increase the Ca/CaMKII complex concentration and mediate the conduction of neuronal activity.56
NMDA participates in morphine tolerance development and neuronal plasticity within the central nervous system. NMDAR is one of the receptors that transmit excitatory neuronal signals. NMDAR primarily mediates calcium influx and transduction of downstream signaling to induce cellular internal cascade amplification and causes internalization of MOR.57 NR1 is a subunit of endogenous NMDA receptors.58 In the mouse brain chronic morphine treatment alters the expression level of NR1, which plays an important role in morphine tolerance.59 Animal studies have shown that the mRNA levels of NR1 in the striatum are significantly upregulated in morphine tolerance models to accelerate morphine tolerance development. Our group showed that morphine induces low miR-219-5p expression in the spinal cord to further upregulate the expression of target proteins, CaMKIIγ, and NR1 (Figure 2). These results suggest that morphine tolerance development is associated with the miR-219-5p/CaMKIIγ pathway.55
miRNAs alleviate morphine tolerance by controlling the expression of inflammatory factors
Chronic morphine exposure often results in increased expression of various proinflammatory cytokines such as IL-1β, IL-6, and TNF-α, in vitro and in vivo and elevates lipopolysaccharide (LPS)-induced immune response.60 Inhibition of the function of glial cells, including astrocytes and microglia, can attenuate the development of morphine analgesic tolerance.61 The ventrolateral periaqueductal gray glial contributes to morphine tolerance by activating the innate immune receptor TLR4.62 TLR4 signaling increased the expression of the NLRP3 inflammasome in microglia through NF-κB within a period of morphine-induced sensitization.63 miR-223 negatively regulated NLRP3 inflammasome expression to relieve morphine analgesic tolerance.64 Furthermore, TLR4-mediated NF-κB activation in the spinal cord is involved in the development and maintenance of morphine tolerance.65 Morphine-induced upregulation of miR-124, which directly inhibits its downstream targets NF-κB and TRAF6, plays a critical role in morphine-mediated microglia immunity suppression (Figure 2).66
Studies in miRNA clinical trials
A lot of studies involving miRNA treatments have been conducted over the years, and a small number of miRNA composites have moved into clinical application. The locked nucleic acid (LNA) drug miravirsen67,68 and a GalNAc-conjugated antimiR against miR-122, both designed to treat hepatitis C virus (HCV) infection by suppressing the function of miR-122, have undergone Phase Ⅰ trials in HCV-infected patients.69 A miR-29 mimic for patients with scleroderma and an LNA-based antimiR-155 for patients with cutaneous T-cell lymphoma are in Phase Ⅰ clinical trials.70 A growing number of studies have shown that miRNAs are involved in morphine tolerance, but potential miRNA therapeutic rarely move into clinical development. One of the challenges is that clinical drugs mainly target drug enrichment sites through target proteins, and miRNAs have diverse downstream action sites and do not have specific target proteins. Second, miRNAs are exogenous small RNAs and thus, for further clinical application, it is very important to increase miRNA lipophilicity or use specific technologies to allow miRNA to rapidly enter cells through cell membranes and exert regulatory functions. Third, potential immune-related adverse reactions and jaundice should be taken into consideration.
Conclusions
The human genome generates approximately 1,500 miRNAs. Biomedically oriented miRNA studies have generated a large amount of miRNAs information including pathway, disease, organs, and target analysis. This information can be searched on the website of miEAA miRase, miRWalk, and miRTarbase.71 Several binding bases are required between a miRNA and its target gene mRNA, and thus the association between miRNAs and target genes is a network-like relationship. One miRNA can act on many target genes to negatively regulate target protein expression and thus can participate in many pathophysiological processes in human diseases. Recently, many studies have revealed molecular mechanisms of miRNAs and target genes in morphine tolerance, differentiation, and cancer, but the biological effects of miRNAs have not been completely determined.72 Additional miRNAs, such as miR-873a-5p, will likely be found to further elucidate the mechanisms underlying the development and progression of morphine tolerance.
Increasing research has revealed that miRNAs participate in morphine tolerance development, and interference with certain miRNAs has been shown to inhibit morphine tolerance development in rodents. let-7 and miR-365, involved in the MOR and β-arrestin2 pathway, may have therapeutic potential that may be explored in future clinical trials. Researchers have reported that there are two application routes, intravenous injection and subcutaneous injection, for miRNA treatment and that miRNAs would form the basis of a new treatment approach.73 Additionally, more studies need to be carried out to examine the mechanisms by which miRNAs participate in morphine tolerance.
Currently, there is a huge gap between preclinical and clinical studies regarding the role of miRNAs in opioid tolerance and the potential implications in human subjects. Therefore, with further in-depth miRNA-related translational studies, miRNAs may become targets for drug development for the prevention and treatment of morphine tolerance.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81271244, 81471135 and 81771206) and the Natural Science Funds for Distinguished Young Scholar of Hunan Province (2017JJ1036).
Disclosure
The authors declare that they have no competing interests associated with this work.
References
- 1.Trescot AM, Glaser SE, Hansen H, Benyamin R, Patel S, Manchikanti L. Effectiveness of opioids in the treatment of chronic non-cancer pain. Pain Physician. 2008;11(2 Suppl):S181–200. [PubMed] [Google Scholar]
- 2.Manglik A, Kruse AC, Kobilka TS, et al. Crystal structure of the micro-opioid receptor bound to a morphinan antagonist. Nature. 2012;485(7398):321–326. doi: 10.1038/nature10954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ueda H, Ueda M. Mechanisms underlying morphine analgesic tolerance and dependence. Front Biosci (Landmark Ed). 2009;14:5260–5272. [DOI] [PubMed] [Google Scholar]
- 4.Bekhit MH. Opioid-induced hyperalgesia and tolerance. Am J Ther. 2010;17(5):498–510. doi: 10.1097/MJT.0b013e3181ed83a0 [DOI] [PubMed] [Google Scholar]
- 5.Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408(6813):720–723. doi: 10.1038/35047086 [DOI] [PubMed] [Google Scholar]
- 6.Sounier R, Mas C, Steyaert J, et al. Propagation of conformational changes during mu-opioid receptor activation. Nature. 2015;524(7565):375–378. doi: 10.1038/nature14680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li XY, Sun L, He J, et al. The kappa-opioid receptor is upregulated in the spinal cord and locus ceruleus but downregulated in the dorsal root ganglia of morphine tolerant rats. Brain Res. 2010;1326:30–39. doi: 10.1016/j.brainres.2010.02.070 [DOI] [PubMed] [Google Scholar]
- 8.Xie WY, He Y, Yang YR, et al. Disruption of Cdk5-associated phosphorylation of residue threonine-161 of the delta-opioid receptor: impaired receptor function and attenuated morphine antinociceptive tolerance. J Neurosci. 2009;29(11):3551–3564. doi: 10.1523/JNEUROSCI.0415-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X, Bao L. Interaction and regulatory functions of mu- and delta-opioid receptors in nociceptive afferent neurons. Neurosci Bull. 2012;28(2):121–130. doi: 10.1007/s12264-012-1206-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomes I, Gupta A, Filipovska J, Szeto HH, Pintar JE, Devi LA. A role for heterodimerization of mu and delta opiate receptors in enhancing morphine analgesia. Proc Natl Acad Sci U S A. 2004;101(14):5135–5139. doi: 10.1073/pnas.0307601101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Portoghese PS, Akgun E, Lunzer MM. Heteromer induction: an approach to unique pharmacology? ACS Chem Neurosci. 2017;8(3):426–428. doi: 10.1021/acschemneuro.7b00002 [DOI] [PubMed] [Google Scholar]
- 12.Zhu Y, King MA, Schuller AG, et al. Retention of supraspinal delta-like analgesia and loss of morphine tolerance in delta opioid receptor knockout mice. Neuron. 1999;24(1):243–252. [DOI] [PubMed] [Google Scholar]
- 13.Tsuji M, Yamazaki M, Takeda H, et al. The novel kappa-opioid receptor agonist TRK-820 has no affect on the development of antinociceptive tolerance to morphine in mice. Eur J Pharmacol. 2000;394(1):91–95. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Barker K, Shi S, Diaz M, Mo B, Gutstein HB. Blockade of PDGFR-beta activation eliminates morphine analgesic tolerance. Nat Med. 2012;18(3):385–387. doi: 10.1038/nm.2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen HJ, Xie WY, Hu F, Zhang Y, Wang J, Wang Y. Disruption of delta-opioid receptor phosphorylation at threonine 161 attenuates morphine tolerance in rats with CFA-induced inflammatory hypersensitivity. Neurosci Bull. 2012;28(2):182–192. doi: 10.1007/s12264-012-1216-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou HY, Chen SR, Chen H, Pan HL. Opioid-induced long-term potentiation in the spinal cord is a presynaptic event. J Neurosci. 2010;30(12):4460–4466. doi: 10.1523/JNEUROSCI.5857-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson-Poe AR, Jeong HJ, Vaughan CW. Chronic morphine reduces the readily releasable pool of GABA, a presynaptic mechanism of opioid tolerance. J Physiol. 2017;595(20):6541–6555. doi: 10.1113/JP274157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chao J, Nestler EJ. Molecular neurobiology of drug addiction. Annu Rev Med. 2004;55:113–132. doi: 10.1146/annurev.med.55.091902.103730 [DOI] [PubMed] [Google Scholar]
- 19.Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2(2):119–128. doi: 10.1038/35053570 [DOI] [PubMed] [Google Scholar]
- 20.Eidson LN, Inoue K, Young LJ, Tansey MG, Murphy AZ. Toll-like receptor 4 mediates morphine-induced neuroinflammation and tolerance via soluble tumor necrosis factor signaling. Neuropsychopharmacology. 2017;42(3):661–670. doi: 10.1038/npp.2016.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Loram LC, Ramos K, et al. Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc Natl Acad Sci U S A. 2012;109(16):6325–6330. doi: 10.1073/pnas.1200130109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eidson LN, Murphy AZ. Persistent peripheral inflammation attenuates morphine-induced periaqueductal gray glial cell activation and analgesic tolerance in the male rat. J Pain. 2013;14(4):393–404. doi: 10.1016/j.jpain.2012.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song P, Zhao ZQ. The involvement of glial cells in the development of morphine tolerance. Neurosci Res. 2001;39(3):281–286. [DOI] [PubMed] [Google Scholar]
- 24.Harada S, Nakamoto K, Tokuyama S. The involvement of midbrain astrocyte in the development of morphine tolerance. Life Sci. 2013;93(16):573–578. doi: 10.1016/j.lfs.2013.08.009 [DOI] [PubMed] [Google Scholar]
- 25.Merighi S, Gessi S, Varani K, Fazzi D, Stefanelli A, Borea PA. Morphine mediates a proinflammatory phenotype via mu-opioid receptor-PKCvarepsilon-Akt-ERK1/2 signaling pathway in activated microglial cells. Biochem Pharmacol. 2013;86(4):487–496. doi: 10.1016/j.bcp.2013.05.027 [DOI] [PubMed] [Google Scholar]
- 26.Zhou D, Chen ML, Zhang YQ, Zhao ZQ. Involvement of spinal microglial P2X7 receptor in generation of tolerance to morphine analgesia in rats. J Neurosci. 2010;30(23):8042–8047. doi: 10.1523/JNEUROSCI.5377-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horvath RJ, Romero-Sandoval EA, De Leo JA. Inhibition of microglial P2X4 receptors attenuates morphine tolerance, Iba1, GFAP and mu opioid receptor protein expression while enhancing perivascular microglial ED2. Pain. 2010;150(3):401–413. doi: 10.1016/j.pain.2010.02.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dai Z, Chu H, Ma J, Yan Y, Zhang X, Liang Y. The regulatory mechanisms and therapeutic potential of MicroRNAs: from chronic pain to morphine tolerance. Front Mol Neurosci. 2018;11:80. doi: 10.3389/fnmol.2018.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hausser J, Zavolan M. Identification and consequences of miRNA-target interactions–beyond repression of gene expression. Nat Rev Genet. 2014;15(9):599–612. doi: 10.1038/nrg3765 [DOI] [PubMed] [Google Scholar]
- 30.Ambros V, Bartel B, Bartel DP, et al. A uniform system for microRNA annotation. Rna. 2003;9(3):277–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muljo SA, Kanellopoulou C, Aravind L. MicroRNA targeting in mammalian genomes: genes and mechanisms. Wiley Interdiscip Rev Syst Biol Med. 2010;2(2):148–161. doi: 10.1002/wsbm.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sethi N, Wright A, Wood H, Rabbitts P. MicroRNAs and head and neck cancer: reviewing the first decade of research. Eur J Cancer. 2014;50(15):2619–2635. doi: 10.1016/j.ejca.2014.07.012 [DOI] [PubMed] [Google Scholar]
- 33.Li X, Jin P. Roles of small regulatory RNAs in determining neuronal identity. Nat Rev Neurosci. 2010;11(5):329–338. doi: 10.1038/nrn2739 [DOI] [PubMed] [Google Scholar]
- 34.Peng C, Li L, Zhang MD, et al. miR-183 cluster scales mechanical pain sensitivity by regulating basal and neuropathic pain genes. Science. 2017;356(6343):1168–1171. doi: 10.1126/science.aam7671 [DOI] [PubMed] [Google Scholar]
- 35.Feng Y, Zou L, Yan D, et al. Extracellular MicroRNAs induce potent innate immune responses via TLR7/MyD88-dependent mechanisms. J Immunol. 2017. doi: 10.4049/jimmunol.1700730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee YS, Dutta A. MicroRNAs: small but potent oncogenes or tumor suppressors. Curr Opin Investig Drugs. 2006;7(6):560–564. [PubMed] [Google Scholar]
- 37.Roush S, Slack FJ. The let-7 family of microRNAs. Trends Cell Biol. 2008;18(10):505–516. doi: 10.1016/j.tcb.2008.07.007 [DOI] [PubMed] [Google Scholar]
- 38.Bussing I, Slack FJ, Grosshans H. let-7 microRNAs in development, stem cells and cancer. Trends Mol Med. 2008;14(9):400–409. doi: 10.1016/j.molmed.2008.07.001 [DOI] [PubMed] [Google Scholar]
- 39.He Y, Yang C, Kirkmire CM, Wang ZJ. Regulation of opioid tolerance by let-7 family microRNA targeting the mu opioid receptor. J Neurosci. 2010;30(30):10251–10258. doi: 10.1523/JNEUROSCI.2419-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He Y, Wang ZJ. Let-7 microRNAs and opioid tolerance. Front Genet. 2012;3:110. doi: 10.3389/fgene.2012.00110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee H, Han S, Kwon CS, Lee D. Biogenesis and regulation of the let-7 miRNAs and their functional implications. Protein Cell. 2016;7(2):100–113. doi: 10.1007/s13238-015-0212-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu Q, Zhang L, Law PY, Wei LN, Loh HH. Long-term morphine treatment decreases the association of mu-opioid receptor (MOR1) mRNA with polysomes through miRNA23b. Mol Pharmacol. 2009;75(4):744–750. doi: 10.1124/mol.108.053462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu Q, Hwang CK, Zheng H, et al. MicroRNA 339 down-regulates mu-opioid receptor at the post-transcriptional level in response to opioid treatment. FASEB J. 2013;27(2):522–535. doi: 10.1096/fj.12-213439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu Z, Xu J, Xu M, Pasternak GW, Pan YX. Morphine regulates expression of mu-opioid receptor MOR-1A, an intron-retention carboxyl terminal splice variant of the mu-opioid receptor (OPRM1) gene via miR-103/miR-107. Mol Pharmacol. 2014;85(2):368–380. doi: 10.1124/mol.113.089292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nie J, Liu L, Zheng W, et al. microRNA-365, down-regulated in colon cancer, inhibits cell cycle progression and promotes apoptosis of colon cancer cells by probably targeting cyclin D1 and Bcl-2. Carcinogenesis. 2012;33(1):220–225. doi: 10.1093/carcin/bgr245 [DOI] [PubMed] [Google Scholar]
- 46.Qi J, Rice SJ, Salzberg AC, et al. MiR-365 regulates lung cancer and developmental gene thyroid transcription factor 1. Cell Cycle. 2012;11(1):177–186. doi: 10.4161/cc.11.1.18576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen L, Huang X, Chen X. MiR-365 suppresses cholangiocarcinoma cell proliferation and induces apoptosis by targeting E2F2. Oncol Res. 2018. doi: 10.3727/096504018X15188352857437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mo JL, Liu Q, Kou ZW, et al. MicroRNA-365 modulates astrocyte conversion into neuron in adult rat brain after stroke by targeting Pax6. Glia. 2018;66(7):1346–1362. doi: 10.1002/glia.23308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Attramadal H, Arriza JL, Aoki C, et al. Beta-arrestin2, a novel member of the arrestin/beta-arrestin gene family. J Biol Chem. 1992;267(25):17882–17890. [PubMed] [Google Scholar]
- 50.Raehal KM, Bohn LM. The role of beta-arrestin2 in the severity of antinociceptive tolerance and physical dependence induced by different opioid pain therapeutics. Neuropharmacology. 2011;60(1):58–65. doi: 10.1016/j.neuropharm.2010.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang J, Xu W, Zhong T, et al. miR-365 targets beta-arrestin 2 to reverse morphine tolerance in rats. Sci Rep. 2016;6:38285. doi: 10.1038/srep38285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu XP, She RX, Yang YP, Xing ZM, Chen HW, Zhang YW. MicroRNA-365 alleviates morphine analgesic tolerance via the inactivation of the ERK/CREB signaling pathway by negatively targeting beta-arrestin2. J Biomed Sci. 2018;25(1):10. doi: 10.1186/s12929-018-0408-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Z, Chabot JG, Quirion R. On the possible role of ERK, p38 and CaMKII in the regulation of CGRP expression in morphine-tolerant rats. Mol Pain. 2011;7:68. doi: 10.1186/1744-8069-7-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang ZJ, Tang L, Xin L. Reversal of morphine antinociceptive tolerance by acute spinal inhibition of Ca(2+)/calmodulin-dependent protein kinase II. Eur J Pharmacol. 2003;465(1–2):199–200. [DOI] [PubMed] [Google Scholar]
- 55.Wang J, Xu W, Shao J, et al. miR-219-5p targets CaMKIIgamma to attenuate morphine tolerance in rats. Oncotarget. 2017;8(17):28203–28214. doi: 10.18632/oncotarget.15997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tavalin SJ, Colbran RJ. CaMKII-mediated phosphorylation of GluN2B regulates recombinant NMDA receptor currents in a chloride-dependent manner. Mol Cell Neurosci. 2017;79:45–52. doi: 10.1016/j.mcn.2016.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodriguez-Munoz M, Sanchez-Blazquez P, Vicente-Sanchez A, Berrocoso E, Garzon J. The mu-opioid receptor and the NMDA receptor associate in PAG neurons: implications in pain control. Neuropsychopharmacology. 2012;37(2):338–349. doi: 10.1038/npp.2011.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mori H, Mishina M. Structure and function of the NMDA receptor channel. Neuropharmacology. 1995;34(10):1219–1237. [DOI] [PubMed] [Google Scholar]
- 59.Zhu H, Brodsky M, Gorman AL, Inturrisi CE. Region-specific changes in NMDA receptor mRNA induced by chronic morphine treatment are prevented by the co-administration of the competitive NMDA receptor antagonist LY274614. Brain Res Mol Brain Res. 2003;114(2):154–162. [DOI] [PubMed] [Google Scholar]
- 60.Staikos L, Malellari L, Chang SL. Lipopolysaccharide-induced pro-inflammatory cytokines in the brain of rats in the morphine-tolerant state. J Neuroimmune Pharmacol. 2008;3(4):236–240. doi: 10.1007/s11481-008-9111-9 [DOI] [PubMed] [Google Scholar]
- 61.Wang Z, Ma W, Chabot JG, Quirion R. Cell-type specific activation of p38 and ERK mediates calcitonin gene-related peptide involvement in tolerance to morphine-induced analgesia. FASEB J. 2009;23(8):2576–2586. doi: 10.1096/fj.08-128348 [DOI] [PubMed] [Google Scholar]
- 62.Eidson LN, Murphy AZ. Blockade of Toll-like receptor 4 attenuates morphine tolerance and facilitates the pain relieving properties of morphine. J Neurosci. 2013;33(40):15952–15963. doi: 10.1523/JNEUROSCI.1609-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grace PM, Strand KA, Galer EL, et al. Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proc Natl Acad Sci U S A. 2016;113(24):E3441–3450. doi: 10.1073/pnas.1602070113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xie XJ, Ma LG, Xi K, et al. Effects of microRNA-223 on morphine analgesic tolerance by targeting NLRP3 in a rat model of neuropathic pain. Mol Pain. 2017;13:1744806917706582. doi: 10.1177/1744806917706582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bai L, Zhai C, Han K, et al. Toll-like receptor 4-mediated nuclear factor-kappaB activation in spinal cord contributes to chronic morphine-induced analgesic tolerance and hyperalgesia in rats. Neurosci Bull. 2014;30(6):936–948. doi: 10.1007/s12264-014-1483-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qiu S, Feng Y, LeSage G, et al. Chronic morphine-induced microRNA-124 promotes microglial immunosuppression by modulating P65 and TRAF6. J Immunol. 2015;194(3):1021–1030. doi: 10.4049/jimmunol.1400106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ottosen S, Parsley TB, Yang L, et al. In vitro antiviral activity and preclinical and clinical resistance profile of miravirsen, a novel anti-hepatitis C virus therapeutic targeting the human factor miR-122. Antimicrob Agents Chemother. 2015;59(1):599–608. doi: 10.1128/AAC.04220-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lanford RE, Hildebrandt-Eriksen ES, Petri A, et al. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science. 2010;327(5962):198–201. doi: 10.1126/science.1178178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van der Ree MH, de Vree JM, Stelma F, et al. Safety, tolerability, and antiviral effect of RG-101 in patients with chronic hepatitis C: a phase 1B, double-blind, randomised controlled trial. Lancet. 2017;389(10070):709–717. doi: 10.1016/S0140-6736(16)31715-9 [DOI] [PubMed] [Google Scholar]
- 70.Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16(3):203–222. doi: 10.1038/nrd.2016.246 [DOI] [PubMed] [Google Scholar]
- 71.Fan Y, Siklenka K, Arora SK, Ribeiro P, Kimmins S, Xia J. miRNet - dissecting miRNA-target interactions and functional associations through network-based visual analysis. Nucleic Acids Res. 2016;44(W1):W135–141. doi: 10.1093/nar/gkw288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rodriguez RE. Morphine and microRNA activity: is there a relation with addiction? Front Genet. 2012;3:223. doi: 10.3389/fgene.2012.00223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van Rooij E, Kauppinen S. Development of microRNA therapeutics is coming of age. EMBO Mol Med. 2014;6(7):851–864. doi: 10.15252/emmm.201100899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hou W, Li H, Jiang W, Zhang C, McNutt MA, Li G. Simian Immunodeficiency virus impacts MicroRNA-16 mediated post-transcriptional regulation of mu opioid receptor in CEM x174 cells. J Cell Biochem. 2016;117(1):84–93. doi: 10.1002/jcb.25251 [DOI] [PubMed] [Google Scholar]
- 75.Wu Q, Law PY, Wei LN, Loh HH. Post-transcriptional regulation of mouse mu opioid receptor (MOR1) via its 3‘ untranslated region: a role for microRNA23b. FASEB J. 2008;22(12):4085–4095. doi: 10.1096/fj.08-108175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zheng H, Zhang Y, Li W, Loh HH, Law PY. NeuroD modulates opioid agonist-selective regulation of adult neurogenesis and contextual memory extinction. Neuropsychopharmacology. 2013;38(5):770–777. doi: 10.1038/npp.2012.242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mei HX, Zhou MH, Zhang XW, et al. Effects of miR-338 on morphine tolerance by targeting CXCR4 in a rat model of bone cancer pain. Biosci Rep. 2017;37:2. doi: 10.1042/BSR20160517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li H, Tao R, Wang J, Xia L. Upregulation of miR-375 level ameliorates morphine analgesic tolerance in mouse dorsal root ganglia by inhibiting the JAK2/STAT3 pathway. J Pain Res. 2017;10:1279–1287. doi: 10.2147/JPR.S125264 [DOI] [PMC free article] [PubMed] [Google Scholar]