Abstract
Context
Studies in aged mice support a role for kynurenine, a tryptophan metabolite, in age-induced bone loss; however, the role of kynurenine in human bone metabolism is not well understood.
Objective
To assess whether the kynurenine level in bone marrow (BM) aspirates, directly reflecting the bone microenvironment, is associated with osteoporosis-related phenotypes and bone biochemical markers.
Design and Setting
A case-control study conducted in a clinical unit.
Participants and Main Outcome Measures
BM samples were collected from 72 patients at the time of hip surgery for either fragility hip fracture (HF) (n = 27) or for other causes (n = 45). In these samples, kynurenine was measured by liquid chromatography–tandem mass spectrometry, and the levels of tartrate-resistant acid phosphatase 5b (TRAP5b), bone-specific alkaline phosphatase (BSALP), receptor activator of nuclear factor-κB ligand (RANKL), and osteoprotegerin (OPG) were measured by immunoassay.
Results
Age was positively correlated with BM kynurenine level. After adjustment for confounders, subjects with fragility HF had a 39.7% higher BM kynurenine level than those without, and the OR per SD increment in BM kynurenine level for fragility HF was 3.80. The BM kynurenine level was inversely associated with bone mass at the total femur. Higher kynurenine concentrations were significantly associated with higher TRAP-5b and RANKL levels, but not with BSALP and OPG levels, in BM plasma.
Conclusion
These results suggest that increased kynurenine levels during aging may contribute to the bone fragility seen in the elderly through increased bone resorption, with a resultant imbalance in bone remodeling.
Increased kynurenine levels during aging may contribute to the bone fragility seen in the elderly through the stimulation of bone resorption, with a resultant imbalance in bone remodeling.
The incidence of debilitating bone fractures is increasing globally as the size of the aging population grows worldwide (1, 2). Bone loss with aging is a primary contributor to bone fragility and bone fracture, and there are multiple mechanisms underlying bone loss with aging (3, 4). These include a decreased capacity for bone formation in response to anabolic stimuli, such as exercise and mechanical loading, and increased bone resorption by osteoclasts driven by oxidative stress, cytokines, and inflammatory factors secreted by senescent cells (5, 6). Heterochronic parabiosis experiments revealed that age-associated changes in various organs and tissues, such as skeletal muscle and the brain, may result from changes in circulating factors (7). In a recent murine study, we demonstrated that the tryptophan metabolite kynurenine is increased in the circulation with age and that exogenous kynurenine can increase bone resorption and also lipid storage by bone marrow (BM) stem (stromal) cells (8). These findings are consistent with previous work demonstrating that kynurenine accumulates with age in various organs and tissues (9, 10), elevated kynurenine relative to tryptophan is associated with frailty and increased age-related mortality (11, 12), and the kynurenine pathway is implicated in age-associated diseases such as inflammation, neurodegeneration, and immunosenescence (13–15). Moreover, the aryl hydrocarbon receptor, which is activated by kynurenine (16), is now recognized as promoting aging phenotypes across species (17).
Several of the previously mentioned studies documented increases in circulating kynurenine with age in both humans and animal models; however, the BM microenvironment is a complex milieu that contains a variety of cell types with strong immunomodulatory properties (18). These various cell types may metabolize tryptophan in different ways, thus altering the balance of kynurenine and its metabolites relative to their parent molecule tryptophan. It is therefore possible that changes in kynurenine with age may differ somewhat between systemic levels and local levels in BM, although this has not been studied. Here, we provide data on kynurenine levels in human BM. In addition, we examine the relationship between bone-specific kynurenine and hip fracture (HF) to better define a pathogenic role for kynurenine in age-related bone loss.
Materials and Methods
Study subjects and protocol
The study population consisted of consecutive patients aged 65 years or older who underwent hip surgery at the Department of Orthopedics at Asan Medical Center (Seoul, Korea) between March 2013 and May 2014. All patients had hip surgery because of either fragility HF or other causes such as osteoarthritis. Fragility fractures are defined as fractures that result from mechanical forces that would not ordinarily result in fracture, known as low-level (or low-energy) trauma (19, 20). The World Health Organization has quantified this as forces equivalent to falls from standing height or less (21). Subjects who took drugs that could affect bone metabolism, such as bisphosphonates, systemic glucocorticoids, or hormone replacement therapy, for more than 6 months or within the previous 12 months before hip surgery were excluded. Subjects with diseases that might result in secondary osteoporosis, such as hyperthyroidism or rheumatoid arthritis, were also excluded. Subjects were excluded if they had a fever (oral temperature ≥38.0°C) or abnormal findings on a complete blood count of leukocytes (<4.0 or >10.0 × 109/L) or platelets (<150 or >350 × 109/L). Abnormal liver and kidney functions were reasons for exclusion as well. These criteria were used to rule out systemic illness. Finally, subjects with fractures not caused by low-energy trauma (e.g., subjects with fractures due to motor vehicle accidents or falls above standing height) were excluded. Seventy-two patients were consented from among the 112 eligible participants not meeting the exclusion criteria and for whom we could simultaneously collect blood and BM samples during hip surgery. Baseline characteristics, including sex, age, body mass index (BMI), and percentage of fragility HFs, were similar between the 72 consented and 40 refused subjects. Of the 72 patients enrolled in the study, 27 were cases with fragility HF, and 45 were controls.
A patient questionnaire was used to assess smoking (current smoker), alcohol intake (≥3 U/d), history of medication use, previous medical or surgical procedure, and reproductive status (including menstruation). This study was approved by the Asan Medical Center institutional review board. Written informed consent was provided by all enrolled participants.
Measurement of tryptophan and kynurenine in BM plasma
BM samples were collected during hip surgery, which was performed within 4 days after onset of HF in most cases. After sample centrifugation at 3000 rpm for 5 minutes at 4°C, we carefully collected the supernatants to exclude cell components. All samples with hemolysis or clotting were discarded. Fifty microliters of human BM plasma was mixed with 200 μL of chloroform/methanol (v/v, 1/2), and then internal standard solution containing 0.6 μM tryptophan-d5 (Sigma-Aldrich, St. Louis, MO) was added. The sample was centrifuged at 14,000 rpm for 15 minutes. The supernatant was collected, and 100 μL each of H2O and chloroform was added. The sample was mixed vigorously and centrifuged at 4000 rpm for 20 minutes. The aqueous phase was used for chemical derivatization using phenylisothiocyanate (Sigma-Aldrich). After the reaction, the derivatization products were extracted with 5 mM ammonium acetate in methanol and were ready for liquid chromatography–tandem mass spectrometry (LC-MS/MS) analysis.
The levels of tryptophan and kynurenine were determined by LC-MS/MS equipped with 1290 HPLC (Agilent, Waldbronn, Germany), QTRAP 5500 (AB Sciex, Toronto, ON, Canada), and a reverse phase column (Zorbax Eclipse XDB-C18, 100 × 2.0 mm). Three microliters was injected into the LC-MS/MS system and ionized with turbo spray ionization source; 0.2% formic acid in H2O and 0.2% formic acid in acetonitrile were used as mobile phase A and B, respectively. The separation gradient was as follows: hold at 0% B for 0.5 minute, 0% to 95% B for 5 minutes, 95% B for 1 minute, and 95% to 0% B for 0.5 minute and then hold at 0% B for 2.5 minutes. Liquid chromatography flow was 500 μL/min, and column temperature was kept at 50°C. Multiple reaction monitoring was used in positive ion mode, and the extracted ion chromatogram corresponding to the specific transition for each amino acid was used for quantitation. Calibration range was generally from 1 nM to 600 μM with R2 > 0.98. Data analysis was performed by using Analyst 1.5.2 software (Sciex).
Biochemical measurement
The following bone biochemical markers were measured in BM plasma samples with immunoassay kits: tartrate-resistant acid phosphatase 5b (TRAP5b; catalog no. SB-TR201A; Immunodiagnostic Systems, Scottsdale, AZ), bone-specific alkaline phosphatase (BSALP; catalog no. MBS262250; Mybiosource, San Diego, CA), osteoprotegerin (OPG; catalog no. ab100617; Abcam, Cambridge, MA), and receptor activator of nuclear factor-κB ligand (RANKL; catalog no. K1016; Immunodiagnostic Systems). The interassay and intra-assay coefficients of variation (CVs) for each assay were as follows: TRAP5b <9% and <9%, BSALP <12% and <7%, OPG <12% and <10%, and RANKL <9.3% and <3.5%, respectively.
To determine the levels of serum 25-hydroxyvitamin D3 (25-OH-D3) and creatinine, morning fasting blood samples were obtained. Serum 25-OH-D3 concentration was measured using a Cobra II Auto-γ Counting system by radioimmunoassay (Packard Instruments, Downers Grove, IL), with a lower limit of detection of 0.6 ng/mL. Serum creatinine level was measured using the Toshiba 200FR system by the Jaffe method (Toshiba Medical System Co., Tokyo, Japan), and creatinine clearance was estimated using the Cockcroft-Gault equation (22). The intra-assay and interassay CVs for these analyses were consistently <3.5%.
Bone mineral density measurement
Areal bone mineral density (BMD; g/cm2) was measured at the lumbar spine (L1 to L4), femur neck, and total femur by dual-energy X-ray absorptiometry using a Lunar densitometer (Prodigy, Madison, WI). In terms of the CVs, the precision values of the equipment were 0.67% for the lumbar spine and 1.25% for the femur neck; they were determined by measuring 17 volunteers who were not enrolled in this study. The volunteers were required to individually undergo five scans on the same day and to climb on and off the table between examinations.
Statistical analysis
All data are presented as means ± SD or as numbers and percentages unless otherwise specified. The baseline characteristics of the study population according to the status of fragility HF were compared using Student t tests for continuous variables and χ2 tests for categorical variables. The relationship between age and levels of tryptophan and kynurenine and their ratio in BM plasma was investigated using Pearson correlation analysis. The kynurenine/tryptophan ratio was multiplied by 100 through the whole analysis. The multivariable-adjusted least square means (95% CIs) of the levels of tryptophan and kynurenine and their ratio in BM plasma in terms of the presence of fragility HF were estimated and compared by using analysis of covariance. Potential confounding variables included sex, age, BMI, smoking status, alcohol intake, and serum 25-OH-D3 level, which are established factors associated with human bone health, as well as creatinine clearance, which may affect tryptophan and kynurenine levels. To generate ORs per SD increment in the levels of tryptophan and kynurenine and per increment in the kynurenine/tryptophan ratio for fragility HF, we performed multiple logistic regression analyses after adjustment for confounding variables. The associations of the levels of tryptophan and kynurenine and their ratio with BMD and bone-related parameters in BM plasma were investigated through multiple linear regression analyses. In these analyses, the levels of TRAP-5b, BSALP, RANKL, OPG, and RANKL/OPG ratio in BM plasma were log-transformed because of the skewed distribution. All statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL). P < 0.05 was considered statistically significant.
Results
Clinical characteristics of the study subjects according to their fragility HF status
Among the 27 cases with fragility HF, 20 (74.1%) and 7 (25.9%) were women and men, respectively (Table 1). Among 45 controls, 30 (66.7%) and 15 (33.3%) were women and men, respectively. The mean ages for cases and controls were 77.9 ± 9.0 years (range, 65 to 95 years) and 72.1 ± 5.4 years (range, 65 to 90 years), respectively. There were no significant differences in weight, height, BMI, smoking status, alcohol intake, and creatinine clearance between groups. The BMD values at the lumbar spine, femur neck, and total femur were significantly lower in subjects with HF than in those without HF. The levels of TRAP-5b and RANKL and the RANKL/OPG ratio in BM plasma were markedly higher in cases than in controls, whereas the differences in the levels of BSALP and OPG in BM plasma between groups did not reach statistical significance. In crude analyses, the kynurenine level and kynurenine/tryptophan ratio, but not the tryptophan level, in BM plasma were significantly higher in cases than in controls.
Table 1.
Baseline Characteristics of the Study Subjects According to Fragility HF Status
| Variables | Subjects With HF (n = 27) | Subjects Without HF (n = 45) | P |
|---|---|---|---|
| Sex, no. (%) | 0.602 | ||
| Female | 20 (74.1) | 30 (66.7) | |
| Male | 7 (25.9) | 15 (33.3) | |
| Age, y | 77.9 ± 9.0 | 72.1 ± 5.4 | 0.004 |
| Weight, kg | 56.8 ± 10.8 | 58.9 ± 9.0 | 0.373 |
| Height, cm | 155.1 ± 8.7 | 154.6 ± 8.1 | 0.802 |
| Body mass index, kg/m2 | 23.6 ± 4.3 | 24.6 ± 3.1 | 0.265 |
| Current smoker, no. (%) | 3 (11.1) | 4 (8.9) | 0.999 |
| Alcohol intake ≥3 U/d, no. (%) | 3 (11.1) | 8 (17.8) | 0.519 |
| Serum 25-OH-D3, ng/mL | 15.9 ± 9.8 | 24.6 ± 14.5 | 0.007 |
| Creatinine clearance, mL/min | 71.7 ± 39.5 | 79.7 ± 26.4 | 0.311 |
| Bone mineral density, g/cm2 | |||
| Lumbar spine | 0.827 ± 0.180 | 1.008 ± 0.243 | 0.003 |
| Femur neck | 0.624 ± 0.107 | 0.867 ± 0.184 | <0.001 |
| Total femur | 0.644 ± 0.129 | 0.860 ± 0.126 | <0.001 |
| Bone-related markers in BM plasma, median (IQR) | |||
| TRAP-5b, U/L | 4.02 (2.28–5.90) | 2.44 (1.58–3.28) | 0.003 |
| BSALP, ng/mL | 180.5 (161.4–236.2) | 203.4 (168.8–226.8) | 0.649 |
| RANKL, pg/mL | 2320.1 (105.0–41023.8) | 86.5 (45.3–418.3) | 0.018 |
| OPG, ng/mL | 507.7 (272.9–783.6) | 501.1 (374.4–842.9) | 0.941 |
| RANKL/OPG ratio | 6.22 (0.38–109.61) | 0.15 (0.07–0.87) | 0.040 |
| BM tryptophan, μM | 51.2 ± 10.4 | 46.4 ± 10.2 | 0.061 |
| BM kynurenine, μM | 2.53 ± 1.18 | 1.59 ± 0.65 | 0.001 |
| BM kynurenine/tryptophan ratio | 5.40 ± 3.54 | 3.64 ± 1.99 | 0.009 |
Values are presented as the mean ± SD unless otherwise specified. Boldface values are statistically significant.
Abbreviation: IQR, interquartile range.
Association of age with levels of tryptophan and kynurenine and their ratio in BM plasma
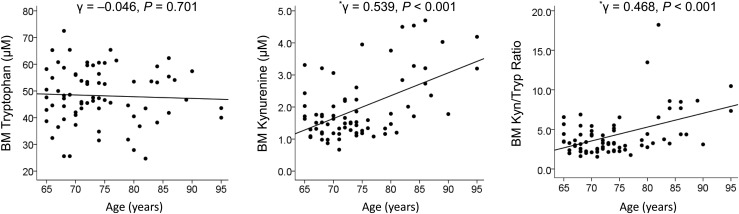
This correlation was investigated by Pearson correlation analysis with scatter plots (Fig. 1). Although there was no association between age and tryptophan level, age was positively correlated to kynurenine level and kynurenine/tryptophan ratio in BM plasma.
Figure 1.
Pearson correlation coefficients with scatter plots for the association of age with levels of tryptophan and kynurenine and their ratio in BM plasma. *Represents a statistically significant value. Kyn, kynurenine; Tryp, tryptophan.
Difference in levels of tryptophan and kynurenine and their ratio in BM plasma according to fragility HF status
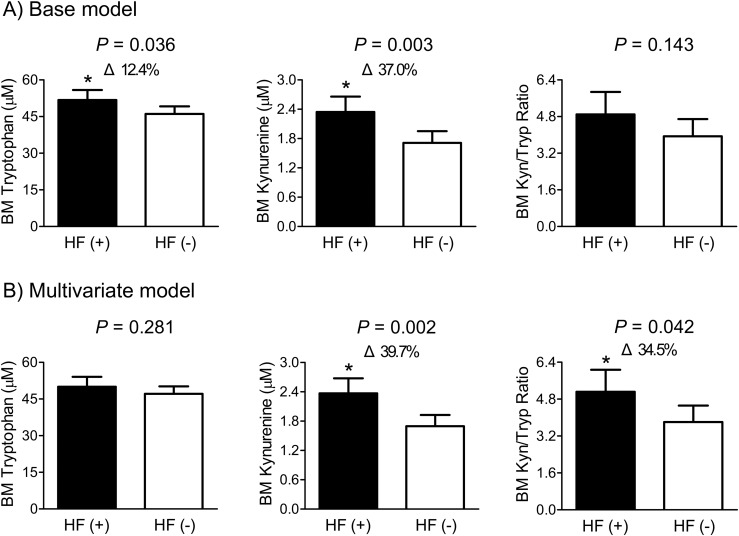
In the base adjustment model including sex, age, and BMI, the levels of tryptophan and kynurenine, but not the kynurenine/tryptophan ratio, in BM plasma were significantly higher in HF cases than in controls (Fig. 2). After additional adjustment for smoking status, alcohol intake, serum 25-OH-D3, and creatinine clearance, subjects with fragility HF had a 39.7% higher kynurenine level and a 34.5% higher kynurenine/tryptophan ratio in BM plasma than those without HF, with statistically significant difference. However, there was no significant difference in BM tryptophan level between groups in this adjustment model.
Figure 2.
Differences in the levels of tryptophan and kynurenine and their ratio in BM plasma according to fragility HF status. After adjustment for confounders, the estimated means with 95% CIs were generated and compared using analysis of covariance. (A) Base model: adjusted for sex, age, and body mass index. (B) Multivariate model: adjusted for sex, age, body mass index, smoking status, alcohol intake, serum 25-hydroxyvitamin D3, and creatinine clearance. Delta (Δ) indicates a change in the value of a variable from the control. *Represents a statistically significant difference from the control by analysis of covariance. HF (+) refers to the presence of heart failure. HF (−) refers to the absence of heart failure. Kyn, kynurenine; Tryp, tryptophan.
Risk of fragility HF according to increments in the levels of tryptophan and kynurenine and their ratio in BM plasma
After adjustment for sex, age, and BMI, the ORs per SD increment in the levels of tryptophan and kynurenine in BM plasma for fragility HF were 1.86 and 2.75, respectively (Table 2). However, in the final adjustment model additionally including smoking status, alcohol intake, serum 25-OH-D3, and creatinine clearance, only kynurenine SD increment, not tryptophan, was significantly associated, with an OR of 3.80. Each increment in kynurenine/tryptophan ratio appeared to be associated with a multivariate-adjusted OR of 1.33, but the statistical significance was marginal (P = 0.080).
Table 2.
Multiple Logistic Regression Analyses to Determine the ORs for Fragility Hip Fracture According to Levels of Tryptophan and Kynurenine and Their Ratio in BM Plasma
| Adjustment Model | OR (95% CI) per SD Increment in BM Tryptophan | P | OR (95% CI) per SD Increment in BM Kynurenine | P | OR (95% CI) per Increment in BM KTR | P |
|---|---|---|---|---|---|---|
| Base | 1.86 (1.03–3.37) | 0.039 | 2.75 (1.31–5.76) | 0.008 | 1.18 (0.92–1.52) | 0.184 |
| Multivariate | 1.50 (0.78–2.86) | 0.224 | 3.80 (1.53–9.40) | 0.004 | 1.33 (0.97–1.82) | 0.080 |
Boldface values are statistically significant. Base model: adjusted for sex, age, and body mass index. Multivariate model: adjusted for sex, age, body mass index, smoking status, alcohol intake, serum 25-hydroxyvitamin D3, and creatinine clearance.
Abbreviation: KTR, kynurenine/tryptophan ratio.
Association of the levels of tryptophan and kynurenine and their ratio in BM plasma with BMD
Although the BM kynurenine level did not have significant correlation with bone mass at the lumbar spine and femur neck, it was inversely associated with total femur BMD after adjustment for sex, age, and BMI, and the statistical significance persisted even in the final adjustment model (Table 3). However, neither tryptophan level nor kynurenine/tryptophan ratio correlated with BMD values at any site.
Table 3.
Multiple Linear Regression Analyses to Determine the Association of Levels of Tryptophan and Kynurenine and Their Ratio in BM Plasma With Bone Mineral Density
| BM Tryptophan Level |
BM Kynurenine Level |
BM KTR |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | β | P | β | SE | β | P | β | SE | β | P | |
| Base model | ||||||||||||
| Lumbar spine BMD | −0.004 | 0.003 | −0.185 | 0.127 | −0.011 | 0.035 | −0.041 | 0.760 | 0.002 | 0.014 | 0.022 | 0.866 |
| Femur neck BMD | −0.004 | 0.002 | −0.203 | 0.053 | −0.034 | 0.025 | −0.171 | 0.178 | −0.003 | 0.009 | −0.038 | 0.757 |
| Total femur BMD | −0.004 | 0.001 | −0.197 | 0.060 | −0.047 | 0.019 | −0.280 | 0.016 | −0.007 | 0.007 | −0.122 | 0.276 |
| Multivariate model | ||||||||||||
| Lumbar spine BMD | −0.004 | 0.003 | −0.161 | 0.205 | −0.001 | 0.039 | −0.006 | 0.970 | 0.004 | 0.015 | 0.037 | 0.793 |
| Femur neck BMD | −0.004 | 0.002 | −0.225 | 0.058 | −0.035 | 0.027 | −0.174 | 0.207 | −0.004 | 0.009 | −0.057 | 0.667 |
| Total femur BMD | −0.003 | 0.002 | −0.204 | 0.064 | −0.050 | 0.020 | −0.303 | 0.016 | −0.010 | 0.007 | −0.167 | 0.174 |
The Enter method was applied to this model. Boldface values are statistically significant. Base model: adjusted for sex, age, and body mass index. Multivariate model: adjusted for sex, age, body mass index, smoking status, alcohol intake, serum 25-hydroxyvitamin D3, and creatinine clearance.
Abbreviations: KTR, kynurenine/tryptophan ratio; β, unstandardized regression coefficient; β, standardized regression coefficient.
Association of levels of tryptophan and kynurenine and their ratio with bone-related parameters in BM plasma
In both base and multivariate adjustment models, a higher kynurenine concentration was significantly associated with higher levels of TRAP-5b and RANKL and higher RANKL/OPG ratio, but not with the levels of BSALP and OPG, in BM plasma (Table 4). The kynurenine/tryptophan ratio showed the positive correlation only with TRAP-5b level in BM plasma after all potential confounders were considered. However, the tryptophan level was not associated with any bone-related parameters, regardless of the adjustment models.
Table 4.
Multiple Linear Regression Analyses to Determine the Association of Levels of Tryptophan and Kynurenine and Their Ratio With Bone-Related Markers Measured in BM Plasma
| BM Tryptophan Level |
BM Kynurenine Level |
BM KTR |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | β | P | β | SE | β | P | β | SE | β | P | |
| Base model, BM plasma | ||||||||||||
| TRAP-5b | 0.007 | 0.007 | 0.111 | 0.335 | 0.226 | 0.084 | 0.350 | 0.009 | 0.051 | 0.029 | 0.224 | 0.083 |
| BSALP | −0.004 | 0.003 | −0.169 | 0.172 | −0.018 | 0.036 | −0.075 | 0.610 | 0.002 | 0.012 | 0.026 | 0.854 |
| RANKL | 0.013 | 0.034 | 0.046 | 0.703 | 1.056 | 0.405 | 0.354 | 0.011 | 0.225 | 0.141 | 0.213 | 0.114 |
| OPG | 0.003 | 0.007 | 0.050 | 0.671 | −0.105 | 0.088 | −0.164 | 0.237 | −0.040 | 0.030 | −0.174 | 0.186 |
| RANKL/OPG ratio | 0.010 | 0.035 | 0.035 | 0.776 | 1.162 | 0.412 | 0.387 | 0.006 | 0.265 | 0.144 | 0.249 | 0.069 |
| Multivariate model, BM plasma | ||||||||||||
| TRAP-5b | 0.010 | 0.008 | 0.168 | 0.198 | 0.287 | 0.091 | 0.445 | 0.002 | 0.065 | 0.032 | 0.286 | 0.046 |
| BSALP | −0.005 | 0.003 | −0.218 | 0.116 | −0.010 | 0.039 | −0.042 | 0.797 | 0.006 | 0.013 | 0.068 | 0.660 |
| RANKL | 0.019 | 0.038 | 0.066 | 0.626 | 1.055 | 0.449 | 0.353 | 0.022 | 0.211 | 0.157 | 0.199 | 0.183 |
| OPG | 0.008 | 0.008 | 0.134 | 0.298 | −0.065 | 0.095 | −0.101 | 0.499 | −0.036 | 0.032 | −0.157 | 0.270 |
| RANKL/OPG ratio | 0.011 | 0.039 | 0.037 | 0.788 | 1.120 | 0.458 | 0.373 | 0.017 | 0.247 | 0.160 | 0.232 | 0.128 |
The Enter method was applied to this model. The levels of TRAP-5b, BSALP, RANKL, OPG, and RANKL/OPG ratio were log-transformed because of their skewed distributions. Boldface numbers indicate statistically significant values. Base model: adjusted for sex, age, and body mass index. Multivariate model: adjusted for sex, age, body mass index, smoking status, alcohol intake, serum 25-hydroxyvitamin D3, and creatinine clearance.
Abbreviations: KTR, kynurenine/tryptophan ratio; β, unstandardized regression coefficient; β, standardized regression coefficient.
Discussion
The current study demonstrated that BM kynurenine levels increased with age and that this increase was associated with an increased risk of fragility HF, a decrease in total femur BMD, and an increase in markers of bone resorption (TRAP-5b and RANKL). This study measured tryptophan and kynurenine levels in a human BM aspirate. Normally, tryptophan is the amino acid present at the lowest circulating levels and is subject to many factors, including timing and composition of meals (23). Thus, we measured BM levels to avoid some of these confounding factors because, presumably, BM tryptophan and kynurenine levels more accurately reflect the concentrations to which BM stem cells and osteoclasts are directly exposed.
Tryptophan is an essential amino acid, and many of its metabolites are bioactive, including serotonin, quinolinic acid, melatonin, and nicotinamide adenine dinucleotide (13, 24). Tryptophan is an aromatic amino acid that is anabolic for bone formation, and supplementation of aromatic amino acids helps prevent bone loss in aging mice (8, 25). Tryptophan restriction results in increased lifespan, and increased lifespan is also observed with calorie and protein restriction (26, 27); however, tryptophan restriction, calorie restriction, and protein restriction all result in bone loss (8, 28).
Kynurenine is the first stable oxidation product of tryptophan breakdown and is generated either systemically through tryptophan 2,3-dioxygenase mainly in the liver or at the local cellular level by either indoleamine 2,3-dioxygenase (IDO) 1 or 2 (29, 30). IDO 1 and 2 catalyze the same reaction but differ in their affinity for tryptophan as a substrate. IDO 1 is expressed in both mesenchymal stem cells (MSCs) and macrophages and is inducible by inflammatory cytokines such as interferon γ (31). Kynurenine itself may serve as an immune modulator, inducing both T regulatory cells and T cell apoptosis (32). This raises the possibility that kynurenine is simply a biomarker for chronic inflammation rather than a pathogenic molecule itself. In fact, kynurenine levels have been shown to increase with age, associated with the increased inflammatory state seen with aging, or “inflamm-aging” (33). In a previous study, we fed 3-month-old C57BL/6 mice kynurenine for 2 months to assess the potential for direct toxic effects of kynurenine on bone turnover (8). IDO is strongly expressed in osteoclasts, and we found that feeding kynurenine to young mice resulted in accelerated bone aging, with an increase in bone resorption and increases in the marker of bone breakdown, pyridinoline, as well as increases in RANKL, which promotes osteoclast differentiation (8). These data suggest that although kynurenine levels might initially increase as a compensatory mechanism in response to inflammation, chronic elevations of this molecule are ultimately harmful for bone metabolism.
Few studies to date have examined a potential role for kynurenine in bone metabolism in a human population. A 2014 study by Apalset et al. (31) examined whether there were any correlations between hip bone mass and blood kynurenine metabolites in a population of middle-aged (46 to 49 years) vs older (71 to 74 years) community-based subjects (Hordaland Health Study). These authors found a negative correlation between the kynurenine/tryptophan ratio and BMD in the older group but not in the middle-aged group. On the other hand, in another study examining possible correlations between kynurenine metabolites and HF, these same authors did not find a correlation between the kynurenine/tryptophan ratio and HF. There was, however, a positive correlation between two kynurenine metabolites, 3-hydroxykynurenine and anthranilic acid, and HF in the older group (34). Although these two studies are important for being among the first to implicate the kynurenine pathway in the pathophysiology of aging bone and thus are broadly in agreement with the present report, there are a number of significant differences from our study. For the Hordaland Health Study, kynurenine metabolite measurements were done in blood, and no BM metabolite measurements were performed. Further, with respect to the HF study, subjects were recruited during a specified time period (1998 to 1999), but the HFs were monitored for several years afterward (2009). Thus, blood measurements were not generally done at the time of the HF.
Kynurenine has also been implicated in the pathogenesis of other age-related diseases including neurodegenerative disorders (Alzheimer’s and Parkinson disease) and sarcopenia (35–37). Thus, this makes the kynurenine pathway an attractive therapeutic target for multiple degenerative disorders, particularly of the musculoskeletal system.
In our efforts to clearly elucidate the underlying mechanisms explaining the detrimental roles of kynurenine, we measured bone biochemical markers, including TRAP-5b, BSALP, RANKL, and OPG, in BM aspirates instead of peripheral blood. Drake et al. (38) previously compared the correlation between the levels of these markers in the peripheral blood and BM plasma in humans. In contrast to a strong relationship between circulating and BM plasma levels of sclerostin, the associations between the blood and BM plasma levels of TRAP-5b, BSALP, and OPG were considerably weaker. Even RANKL was not detectable in the peripheral blood of most samples. Furthermore, the correlation between the peripheral blood and BM plasma levels of kynurenine was modest (γ = 0.427; P < 0.001) and was not present for tryptophan (P = 0.435) in the current study. Consequently, the measurement of these markers in peripheral blood may not accurately represent the bone microenvironment and thus could be inappropriate for a human mechanism study related to bone. We believe that the use of BM samples is the major strength of our clinical study.
An interesting finding in our study is that the kynurenine level in BM plasma had stronger associations with fragility HF, bone mass, and bone biochemical markers than the kynurenine/tryptophan ratio did. These results suggest that the absolute value of kynurenine per se might be physiologically important and a better indicator in terms of human bone health.
Several limitations should be considered when interpreting our data. Most importantly, this was a case-control study. Therefore, we could not determine whether a causal relationship exists between BM kynurenine level and osteoporosis-related phenotypes, although we assume that high kynurenine level may contribute to poor bone health during aging because of its consistent associations with diverse bone parameters in clinical studies and a plausible explanation from our murine study. Second, the major characteristics between cases and controls were not matched because of difficulties in obtaining BM samples. Although we adjusted these factors in the multivariate analyses as a best alternative, this may not have been enough to generate convincing conclusions. Furthermore, a relatively small sample size can limit statistical power, especially in the analyses for bone mass; however, as mentioned previously, this was a unique cohort that made mechanistic evaluation possible in a human bone microenvironment. Third, our study population consisted of subjects who visited a referral hospital for hip surgery; it therefore may not be representative of the general population and could have resulted in selection bias. Fourth, bone turnover is known to be increased after acute fracture; thus, our results might be affected by these changes. Lastly, although we attempted to consider as many confounding factors as possible, we cannot exclude the possibility that the observed associations were attributed to uncontrolled factors that affect bone metabolism and/or the kynurenine pathway, such as chronic inflammation, protein intake, or neurologic illness.
Despite these limitations, our current and published data support a pathogenic role for tryptophan degradation products in age-related bone loss. Potentially, in the aging BM microenvironment, interferon γ released from MSCs and immune cells increases expression of IDO, which depletes tryptophan, impairing MSC function and also increasing the levels of kynurenine metabolites, which then stimulate osteoclastic activity. Thus, clinically, these tryptophan metabolites may be attractive therapeutic targets to promote increased MSC anabolic functions and inhibit bone breakdown.
In conclusion, our data demonstrate that a higher BM kynurenine level was associated with an increased risk of fragility HF, lower total femur BMD, and higher levels of TRAP-5b and RANKL, but not BSALP and OPG, in BM plasma in subjects aged 65 years or older. These results clinically validate previous results of age-related bone loss observed in mice with kynurenine treatment and suggest that increased kynurenine during aging may contribute to the bone fragility seen in the elderly through the stimulation of osteoclastogenesis and the resultant imbalance in bone remodeling.
Acknowledgments
Financial Support: This paper was supported by grants from the Bio & Medical Technology Development Program of the National Research Foundation, funded by the Korean Government, MSIP (2016M3A9E8941329) (to B.-J.K.) and from the National Institute on Aging, US National Institutes of Health (AG036675) (to C.M.I.).
Disclosure Summary: M.W.H. and C.M.I. are cofounders of Gerologix, Inc. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- 25-OH-D3
25-hydroxyvitamin D3
- BM
bone marrow
- BMD
bone mineral density
- BMI
body mass index
- BSALP
bone-specific alkaline phosphatase
- CV
coefficient of variation
- HF
hip fracture
- IDO
indoleamine 2,3-dioxygenase
- LC-MS/MS
liquid chromatography–tandem mass spectrometry
- MSC
mesenchymal stem cell
- OPG
osteoprotegerin
- RANKL
receptor activator of nuclear factor-κB ligand
- TRAP5b
tartrate-resistant acid phosphatase 5b
References
- 1. Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporos Int. 1997;7(5):407–413. [DOI] [PubMed] [Google Scholar]
- 2. Johnell O, Gullberg B, Kanis JA. The hospital burden of vertebral fracture in Europe: a study of national register sources. Osteoporos Int. 1997;7(2):138–141. [DOI] [PubMed] [Google Scholar]
- 3. Drake MT, Clarke BL, Lewiecki EM. The pathophysiology and treatment of osteoporosis. Clin Ther. 2015;37(8):1837–1850. [DOI] [PubMed] [Google Scholar]
- 4. Farr JN, Khosla S. Skeletal changes through the lifespan: from growth to senescence. Nat Rev Endocrinol. 2015;11(9):513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khosla S, Farr JN, Kirkland JL. Inhibiting cellular senescence: a new therapeutic paradigm for age-related osteoporosis. J Clin Endocrinol Metab. 2018;103(4):1282–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Manolagas SC. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev. 2010;31(3):266–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rebo J, Mehdipour M, Gathwala R, Causey K, Liu Y, Conboy MJ, Conboy IM. A single heterochronic blood exchange reveals rapid inhibition of multiple tissues by old blood. Nat Commun. 2016;7(1):13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Refaey ME, McGee-Lawrence ME, Fulzele S, Kennedy EJ, Bollag WB, Elsalanty M, Zhong Q, Ding KH, Bendzunas NG, Shi XM, Xu J, Hill WD, Johnson MH, Hunter M, Pierce JL, Yu K, Hamrick MW, Isales CM. Kynurenine, a tryptophan metabolite that accumulates with age, induces bone loss. J Bone Miner Res. 2017;32(11):2182–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Braidy N, Guillemin GJ, Mansour H, Chan-Ling T, Grant R. Changes in kynurenine pathway metabolism in the brain, liver and kidney of aged female Wistar rats. FEBS J. 2011;278(22):4425–4434. [DOI] [PubMed] [Google Scholar]
- 10. Caballero B, Gleason RE, Wurtman RJ. Plasma amino acid concentrations in healthy elderly men and women. Am J Clin Nutr. 1991;53(5):1249–1252. [DOI] [PubMed] [Google Scholar]
- 11. Pertovaara M, Raitala A, Lehtimäki T, Karhunen PJ, Oja SS, Jylhä M, Hervonen A, Hurme M. Indoleamine 2,3-dioxygenase activity in nonagenarians is markedly increased and predicts mortality. Mech Ageing Dev. 2006;127(5):497–499. [DOI] [PubMed] [Google Scholar]
- 12. Valdiglesias V, Marcos-Pérez D, Lorenzi M, Onder G, Gostner JM, Strasser B, Fuchs D, Bonassi S. Immunological alterations in frail older adults: a cross sectional study. Exp Gerontol. 2018;112:119–126. [DOI] [PubMed] [Google Scholar]
- 13. Cervenka I, Agudelo LZ, Ruas JL. Kynurenines: tryptophan’s metabolites in exercise, inflammation, and mental health. Science. 2017;357(6349):eaaf9794. [DOI] [PubMed] [Google Scholar]
- 14. Tan L, Yu JT, Tan L. The kynurenine pathway in neurodegenerative diseases: mechanistic and therapeutic considerations. J Neurol Sci. 2012;323(1-2):1–8. [DOI] [PubMed] [Google Scholar]
- 15. Wu W, Nicolazzo JA, Wen L, Chung R, Stankovic R, Bao SS, Lim CK, Brew BJ, Cullen KM, Guillemin GJ. Expression of tryptophan 2,3-dioxygenase and production of kynurenine pathway metabolites in triple transgenic mice and human Alzheimer’s disease brain. PLoS One. 2013;8(4):e59749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M, Jugold M, Guillemin GJ, Miller CL, Lutz C, Radlwimmer B, Lehmann I, von Deimling A, Wick W, Platten M. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478(7368):197–203. [DOI] [PubMed] [Google Scholar]
- 17. Eckers A, Jakob S, Heiss C, Haarmann-Stemmann T, Goy C, Brinkmann V, Cortese-Krott MM, Sansone R, Esser C, Ale-Agha N, Altschmied J, Ventura N, Haendeler J. The aryl hydrocarbon receptor promotes aging phenotypes across species. Sci Rep. 2016;6(1):19618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pontikoglou C, Deschaseaux F, Sensebé L, Papadaki HA. Bone marrow mesenchymal stem cells: biological properties and their role in hematopoiesis and hematopoietic stem cell transplantation. Stem Cell Rev. 2011;7(3):569–589. [DOI] [PubMed] [Google Scholar]
- 19. Kanis JA, Oden A, Johnell O, Jonsson B, de Laet C, Dawson A. The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporos Int. 2001;12(5):417–427. [DOI] [PubMed] [Google Scholar]
- 20. Yoo JH, Moon SH, Ha YC, Lee DY, Gong HS, Park SY, Yang KH. Osteoporotic fracture: 2015 position statement of the Korean Society for Bone and Mineral Research. J Bone Metab. 2015;22(4):175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Langsetmo L, Shikany JM, Cawthon PM, Cauley JA, Taylor BC, Vo TN, Bauer DC, Orwoll ES, Schousboe JT, Ensrud KE; Osteoporotic Fractures in Men (MrOS) Research Group . The association between protein intake by source and osteoporotic fracture in older men: a prospective cohort study. J Bone Miner Res. 2017;32(3):592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. [DOI] [PubMed] [Google Scholar]
- 23. Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009;37(1):1–17. [DOI] [PubMed] [Google Scholar]
- 24. Braidy N, Grant R. Kynurenine pathway metabolism and neuroinflammatory disease. Neural Regen Res. 2017;12(1):39–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. El Refaey M, Watkins CP, Kennedy EJ, Chang A, Zhong Q, Ding KH, Shi XM, Xu J, Bollag WB, Hill WD, Johnson M, Hunter M, Hamrick MW, Isales CM. Oxidation of the aromatic amino acids tryptophan and tyrosine disrupts their anabolic effects on bone marrow mesenchymal stem cells. Mol Cell Endocrinol. 2015;410:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mirzaei H, Suarez JA, Longo VD. Protein and amino acid restriction, aging and disease: from yeast to humans. Trends Endocrinol Metab. 2014;25(11):558–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Segall P. Long-term tryptophan restriction and aging in the rat. Aktuelle Gerontol. 1977;7(10):535–538. [PubMed] [Google Scholar]
- 28. Hamrick MW, Ding KH, Ponnala S, Ferrari SL, Isales CM. Caloric restriction decreases cortical bone mass but spares trabecular bone in the mouse skeleton: implications for the regulation of bone mass by body weight. J Bone Miner Res. 2008;23(6):870–878. [DOI] [PubMed] [Google Scholar]
- 29. Badawy AA. Kynurenine pathway of tryptophan metabolism: regulatory and functional aspects. Int J Tryptophan Res. 2017;10:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fatokun AA, Hunt NH, Ball HJ. Indoleamine 2,3-dioxygenase 2 (IDO2) and the kynurenine pathway: characteristics and potential roles in health and disease. Amino Acids. 2013;45(6):1319–1329. [DOI] [PubMed] [Google Scholar]
- 31. Apalset EM, Gjesdal CG, Ueland PM, Midttun Ø, Ulvik A, Eide GE, Meyer K, Tell GS. Interferon (IFN)-γ-mediated inflammation and the kynurenine pathway in relation to bone mineral density: the Hordaland Health Study. Clin Exp Immunol. 2014;176(3):452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu X, Shin N, Koblish HK, Yang G, Wang Q, Wang K, Leffet L, Hansbury MJ, Thomas B, Rupar M, Waeltz P, Bowman KJ, Polam P, Sparks RB, Yue EW, Li Y, Wynn R, Fridman JS, Burn TC, Combs AP, Newton RC, Scherle PA. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood. 2010;115(17):3520–3530. [DOI] [PubMed] [Google Scholar]
- 33. de Bie J, Guest J, Guillemin GJ, Grant R. Central kynurenine pathway shift with age in women. J Neurochem. 2016;136(5):995–1003. [DOI] [PubMed] [Google Scholar]
- 34. Apalset EM, Gjesdal CG, Ueland PM, Øyen J, Meyer K, Midttun Ø, Eide GE, Tell GS. Interferon gamma (IFN-γ)-mediated inflammation and the kynurenine pathway in relation to risk of hip fractures: the Hordaland Health Study. Osteoporos Int. 2014;25(8):2067–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dukes A, Davis C, El Refaey M, Upadhyay S, Mork S, Arounleut P, Johnson MH, Hill WD, Isales CM, Hamrick MW. The aromatic amino acid tryptophan stimulates skeletal muscle IGF1/p70s6k/mTor signaling in vivo and the expression of myogenic genes in vitro. Nutrition. 2015;31(7-8):1018–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maddison DC, Giorgini F. The kynurenine pathway and neurodegenerative disease. Semin Cell Dev Biol. 2015;40:134–141. [DOI] [PubMed] [Google Scholar]
- 37. Strasser B, Volaklis K, Fuchs D, Burtscher M. Role of dietary protein and muscular fitness on longevity and aging. Aging Dis. 2018;9(1):119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Drake MT, Srinivasan B, Mödder UI, Peterson JM, McCready LK, Riggs BL, Dwyer D, Stolina M, Kostenuik P, Khosla S. Effects of parathyroid hormone treatment on circulating sclerostin levels in postmenopausal women. J Clin Endocrinol Metab. 2010;95(11):5056–5062. [DOI] [PMC free article] [PubMed] [Google Scholar]