Abstract
Background: Immune responses play an important role in the development of breast cancer. Trastuzumab can activate antibody-dependent cellular cytotoxicity (ADCC) in human epidermal growth factor receptor-2 (HER-2)-positive breast cancer. Many studies have demonstrated that inflammatory markers, such as the neutrophil-to-lymphocyte ratio (NLR) and absolute lymphocyte count (ALC), are associated with prognosis in breast cancer. The aim of this study was to explore whether preoperative NLR, ALC or the absolute neutrophil count (ANC) is associated with prognosis in HER-2-positive breast cancer patients who received adjuvant trastuzumab.
Patients and methods: Three hundred sixty-seven female patients with HER-2-positive invasive breast cancer who were treated with one-year adjuvant trastuzumab were analysed in this retrospective study. Preoperative haematological parameters, clinicopathological data and survival data were obtained. The cut-off points for ALC, ANC and NLR were based on the median values. Disease-free survival (DFS) and Overall survival (OS) were analysed by the Kaplan-Meier method. Multivariable Cox regression was used to determine the independent prognostic significance of ALC, ANC and NLR.
Results: Survival analysis revealed that the 3-year DFS in patients with high ALC was 89.0%, which was significantly worse than 95.0% in patients with low ALC (p=0.014). Kaplan-Meier analysis also showed that patients with low NLR had a poorer 3-year DFS than patients with high NLR (89.7% vs 94.0%, respectively; p=0.047). Multivariate analysis showed that ALC was an independent prognostic factor for DFS (HR=2.723; 95% CI=1.211–6.122; p=0.015). Neither ANC, ALC nor NLR could predict OS independently.
Conclusion: In HER-2-positive breast cancer patients who were treated with adjuvant trastuzumab, a high ALC is significantly associated with a poor DFS.
Keywords: breast cancer, HER-2 positive, trastuzumab, absolute lymphocyte count, neutrophil-to-lymphocyte ratio
Introduction
Breast cancer is a heterogeneous disease, and there are four subtypes that call for different treatment approaches: two types of estrogen receptor (ER)-positive breast cancer (luminal A like and luminal B like), human epidermal growth factor receptor-2 (HER-2)-positive tumors regardless of ER status and triple-negative tumors.1 Patients with HER-2 overexpression account for 20–25% of invasive breast cancer, for whom treatment with trastuzumab can decrease the risk of relapse greatly in the adjuvant setting.2 Trastuzumab has not only cytostatic but also cytotoxic properties and can induce antibody-dependent cellular cytotoxicity (ADCC).3
Inflammatory responses play distinct roles at different stages of tumor development, and have effect on immune surveillance and responses to therapy.4 An increasing number of studies have shown that peripheral haematologic parameters, such as the neutrophil to lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR), can predict the prognosis for breast cancer patients.5,6 Our previous retrospective study indicated that elevated preoperative NLR predicted a poor disease-free survival (DFS) in Chinese breast cancer patients, especially in those with triple-negative breast cancer, although no significant difference for DFS was observed in the HER-2-enriched subgroup.7 Meanwhile, few studies have demonstrated the prognostic and predictive values of ALC and the absolute neutrophil count (ANC) in breast cancer. However, in HER-2-positive advanced breast cancer, a recent study showed that ALC was superior to NLR and PLR for predicting progression- free survival (PFS).8 In breast cancer patients treated with neoadjuvant chemotherapy, a high lymphocyte count is predictive for a pathological complete response.9 Therefore, we sought to explore whether preoperative ANC, ALC or NLR has a prognostic role in HER-2-positive early breast cancer patients who received adjuvant trastuzumab in this study.
Patients and methods
Eligibility
Three hundred sixty-seven consecutive female patients with primary HER-2-positive invasive breast cancer were identified retrospectively in our study from January 2012 to February 2016. The last follow up on those patients was December 2017. All the patients were treated with one-year adjuvant trastuzumab at Shanghai Ruijin Hospital affiliated to Shanghai Jiao Tong University School of Medicine. The patients in the study should have complete preoperative haematological parameters and have received all phases of adjuvant treatment suggested, including chemotherapy, radiotherapy or endocrine therapy. Patients with the following characteristics were excluded: HER-2 negative breast cancer, no one-year trastuzumab treatment, stage Ⅳ breast cancer, breast cancer in situ, patients who received neoadjuvant treatment, clinical evidence of acute infection, presence of haematological disorders, chronic inflammatory or autoimmune diseases, severe renal disease or any other malignancies.
The study was conducted in accordance with the Declaration of Helsinki. The protocol was reviewed and approved by the independent ethical committee/institutional review board of Shanghai Ruijin Hospital affiliated with Shanghai Jiao Tong University School of Medicine. Written informed consent was obtained from all patients involved.
Clinical and laboratory data
Before any surgical intervention, routine blood tests were performed in all patients, and parameters such as the neutrophil count and lymphocyte count were obtained and analysed using the XN-series blood analyser (Sysmex, Japan). Other clinical characteristics of the patients, including the menopausal status, age, surgery type, comorbidities and adjuvant treatment details, were obtained from the electronic patient’s history system.
The histopathological features were reviewed and identified by two different pathologists independently, including the breast cancer histological type, tumor size, grade, number of lymph node involved, ER expression, progesterone receptor (PR) expression, CerbB-2 and Ki-67 index. More than 1% positive tumor cells with nuclear staining was deemed as ER positive or PR positive. Tumors with a HER-2 3+ status in the immunohistochemistry (IHC) assay and/or HER-2 gene overexpression confirmed by florescence in situ hybridization (FISH) were defined as HER-2 positive.
Statistical analysis
NLR was calculated as the absolute neutrophil count divided by the absolute lymphocyte count. The cut-off points for ANC, ALC and NLR were based on the median values. Patients were categorized into two cohorts according to the median ALC (low ALC cohort: ALC<1.80×109/L and high ALC cohort: ALC≥1.80×109/L). The categorical variables of the two cohorts were compared using chi-square tests or Fisher’s exact test. DFS was defined as freedom from any event as follows in the follow-up: newly diagnosed contralateral breast cancer (invasive or non-invasive); local or regional recurrence; distant organ metastasis; second primary malignancy or death from any cause. OS was defined as the proportion of patients free from any death. The Kaplan-Meier method and the log-rank test were used to analyse DFS and OS. The independent prognostic values of ALC, ANC and NLR were determined by the multivariate Cox proportional hazards model. Clinicopathological variables with p-value <0.05 in the univariate analysis and several important clinicopathological characteristics were included in the Cox proportional hazards model. The backward: LR method was chosen in the analysis. Statistical analyses were performed using SPSS (version 22.0) software (IBM Corporation, Armonk, NY, USA). A p-value <0.05 was considered to indicate statistically significance.
Results
Patients’ characteristics
Six hundred forty HER-2-positive invasive breast cancer patients were collected from our clinical database, and 367 patients who met the inclusion criteria were enrolled finally in our study. The median ALC was 1.80×109/L (range, 0.70–4.20×109/L), the median ANC was 3.20×109/L (range, 1.30–7.05×109/L) and the median NLR was 1.77 (range, 0.45–5.99). Patients were categorized in to the high ALC cohort (ALC≥3.20×109/L) and low ALC cohort (ALC<3.20×109/L), high ANC cohort (ANC≥1.80×109/L) and low ANC cohort (ANC<1.80×109/L), high NLR cohort (NLR≥1.77) and low NLR cohort (NLR<1.77). The median follow-up was 39.0 months.
Table 1 shows the patients’ clinicopathological characteristics presented by ALC categories. The median age of these patients was 53 years old, ranging from 23 to 81 years. One hundred fifty-six (42.5%) patients were premenopausal, and 57.5% of those patients were postmenopausal. Of the 367 patients, 83 received breast-conserving surgery, and 91.8% of those cases were invasive ductal carcinoma (IDC). Additionally, 207 cases were node negative, and 160 cases had one or more lymph node involved. 47.1% of those cases were ER positive and 30.5% of them were PR positive. The patients’ clinicopathological characteristics were well balanced, and there were no significant differences between the low ALC cohort and high ALC cohort.
Table 1.
Patients’ characteristics of the two ALC cohorts
| Characteristics | Overall, n(%) | ALC | p-Value | ||
|---|---|---|---|---|---|
| <1.8 | ≥1.8 | ||||
| Age | ≤50 | 146(39.8) | 75 | 71 | 0.217 |
| >50 | 221(60.2) | 99 | 122 | ||
| Menopausal status | Premenopausal | 156(42.5) | 79 | 77 | 0.287 |
| Postmenopausal | 211(57.5) | 95 | 116 | ||
| Surgery | Mastectomy | 284(77.4) | 136 | 148 | 0.736 |
| BCS | 83(22.6) | 38 | 45 | ||
| Pathology | IDC | 337(91.8) | 161 | 176 | 0.641 |
| Others | 30(8.2) | 13 | 17 | ||
| Tumors | ≤2 cm | 165(45.0) | 82 | 83 | 0.428 |
| >2 cm | 202(55.0) | 92 | 110 | ||
| Nodes involved | 0 | 207(56.4) | 94 | 113 | 0.383 |
| ≥1 | 160(43.6) | 80 | 80 | ||
| Stage | Ⅰ | 123(33.5) | 62 | 61 | 0.548 |
| Ⅱ | 167(45.5) | 74 | 93 | ||
| Ⅲ | 77(21.0) | 38 | 39 | ||
| Grade | Ⅰ | 3(0.8) | 1 | 2 | 0.163 |
| Ⅱ | 113(30.8) | 45 | 68 | ||
| Ⅲ | 229(62.4) | 119 | 110 | ||
| NA | 22(6.0) | 9 | 13 | ||
| ER | Positive | 173(47.1) | 84 | 89 | 0.679 |
| Negative | 194(52.9) | 90 | 104 | ||
| PR | Positive | 112(30.5) | 57 | 55 | 0.376 |
| Negative | 255(69.5) | 117 | 138 | ||
| Ki67 | ≤30% | 179(48.8) | 80 | 99 | 0.309 |
| >30% | 188(51.2) | 94 | 94 | ||
Abbreviations: IDC, invasive ductal carcinoma; NA, not available; BCS, breast conserving surgery; ER, estrogen receptor; PR, progestrone receptor; ALC, absolute lymphocyte count.
The adjuvant treatment details for patients are summarized in Table 2. Of those patients, 300 (81.7%) cases received epirubicin and cyclophosphamide followed by the docetaxel regimen, 41 (11.3%) cases received the docetaxel and carboplatin regimen, and 21 cases received weekly paclitaxel. Additionally, 207 patients received radiation and 160 patients received endocrine therapy.
Table 2.
Treatment for patients in the two ALC cohorts
| Characteristics | Overall, n(%) | ALC | p-value | ||
|---|---|---|---|---|---|
| <1.8 | ≥1.8 | ||||
| Chemotherapy | EC-T | 300(81.7) | 144 | 156 | 0.581 |
| TCb | 41(11.2) | 18 | 23 | ||
| P | 21(5.7) | 11 | 10 | ||
| Others | 5(1.4) | 1 | 4 | ||
| Radiation | Yes | 207(56.4) | 100 | 107 | 0.695 |
| No | 160(43.6) | 74 | 86 | ||
| Endocrine therapy | Yes | 160(43.6) | 77 | 83 | 0.810 |
| No | 207(56.4) | 97 | 110 | ||
Abbreviations: EC-T, epirubicin and cyclophosphamide followed by docetaxel; TCb, docetaxel and carboplatin; P, paclitaxel; ALC, absolute lymphocyte count.
Disease-free survival
Thirty-one events occurred in 367 patients during the follow-up. Distant metastasis of breast cancer occurred in 10 patients, local recurrence occurred in 6 patients, contralateral newly diagnosed breast cancer occurred in 2 patients, and secondary malignancies occurred in 2 patients. Nine patients died of breast cancer, 1 patient died of pancreatic cancer and 1 patient died of viral myocarditis.
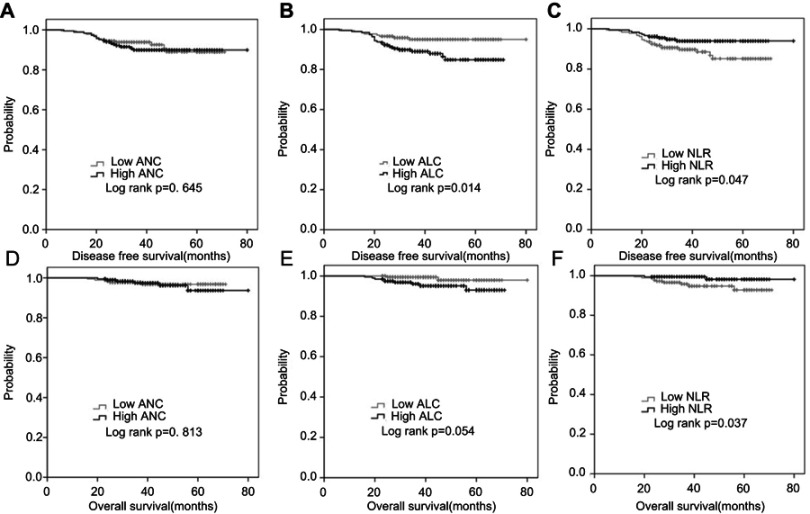
The numbers of events in each cohort are summarized in Table 3. The 3-year DFS was 93.8% in the low ANC cohort and 89.9% in the high ANC cohort (p=0.645) (Figure 1A). Eight events occurred in the low ALC cohort and 23 events occurred in the high ALC cohort. Kaplan-Meier analysis showed that patients in the low ALC cohort had significantly better 3-year DFS than patients in the high ALC cohort (95.0% vs 89.0%, p=0.014) (Figure 1B). In the high NLR cohort, there were 10 events and the 3-year DFS was 94.0%; however, in the low NLR cohort, there were 21 events and the 3-year DFS was 89.7% (p=0.047) (Figure 1C).
Table 3.
Disease free survival and overall survival for each cohort
| Cohort | No. of patients | DFS | OS | |||||
|---|---|---|---|---|---|---|---|---|
| No. of events | 3-year DFS | p-Value | No. of deaths | 3-year OS | p-Value | |||
| ANC | Low | 182 | 14 | 93.8% | 0.645 | 5 | 97.8% | 0.813 |
| High | 185 | 17 | 89.9% | 6 | 97.5% | |||
| ALC | Low | 174 | 8 | 95.0% | 0.014 | 2 | 99.4% | 0.054 |
| High | 193 | 23 | 89.0% | 9 | 96.0% | |||
| NLR | Low | 186 | 21 | 89.7% | 0.047 | 9 | 95.8% | 0.037 |
| High | 181 | 10 | 94.0% | 2 | 99.4% | |||
Abbreviations: ANC, absolute neutrophil count; ALC, absolute lymphocyte count; NLR, neutrophil to lymphocyte ratio; DFS, disease free survival; OS, overall survival.
Figure 1.
Cumulative DFS and OS curves of patients. (A) Cumulative DFS curve for the two ANC cohorts. (B) Cumulative DFS curve for the two ALC cohorts. (C) Cumulative DFS curve for the two NLR cohorts. (D) Cumulative OS curve for the two ANC cohorts. (E) Cumulative OS curve for the two ALC cohorts. (F) Cumulative OS curve for the two NLR cohorts. A p-value <0.05 was considered to indicate statistically significance.
Abbreviations: ALC, absolute lymphocyte count; NLR, neutrophil to lymphocyte ratio; DFS, disease free survival; OS, overall survival.
A multivariate Cox proportional hazards model, including age, lymph node involved, tumor size, ER status, PR status, Ki-67 index, ALC and NLR, was set up. The results showed that ALC was an independent prognostic factor for DFS in HER-2-positive breast cancer patients who received adjuvant trastuzumab treatment (HR=2.723; 95%CI=1.211–6.122; p=0.015) (Table 4).
Table 4.
Univariate and multivariate analysis for DFS
| Characteristics | Univariate | Multivariate | ||
|---|---|---|---|---|
| p-Value | HR | 95%CI | p-Value | |
| Age (>50 vs ≤50) | 0.069 | 0.485 | 0.237–0.989 | 0.047 |
| Menopausal status (pre vs post) | 0.765 | |||
| Surgery (mastectomy vs BCS) | 0.334 | |||
| Pathology (IDC vs others) | 0.799 | |||
| Tumors (≤2 cm vs >2 cm) | 0.252 | 1.365 | 0.643–2.897 | 0.417 |
| Nodes involved (negative vs positive) | 0.013 | 2.866 | 1.371–5.992 | 0.005 |
| Grade (I-II vs III) | 0.848 | |||
| ER (positive vs negative) | 0.557 | 0.725 | 0.351–1.495 | 0.383 |
| PR (positive vs negative) | 0.591 | 0.837 | 0.276–2.543 | 0.754 |
| Ki67 (≤30% vs >30%) | 0.013 | 0.404 | 0.185–0.885 | 0.023 |
| Chemotherapy (EC-T vs others) | 0.721 | |||
| Radiation (yes vs no) | 0.323 | |||
| Endocrine therapy (yes vs no) | 0.910 | |||
| ALC (≥1.8 vs <1.8) | 0.014 | 2.723 | 1.211–6.122 | 0.015 |
| NLR (≥1.77 vs<1.77) | 0.047 | 0.619 | 0.275–1.394 | 0.247 |
| ANC (≥3.2 vs <3.2) | 0.645 | |||
Abbreviations: IDC, invasive ductal carcinoma; BCS, breast conserving surgery; ER, estrogen receptor; PR, progestrone receptor; ANC, absolute neutrophil count; ALC, absolute lymphocyte count; NLR, neutrophil to lymphocyte ratio.
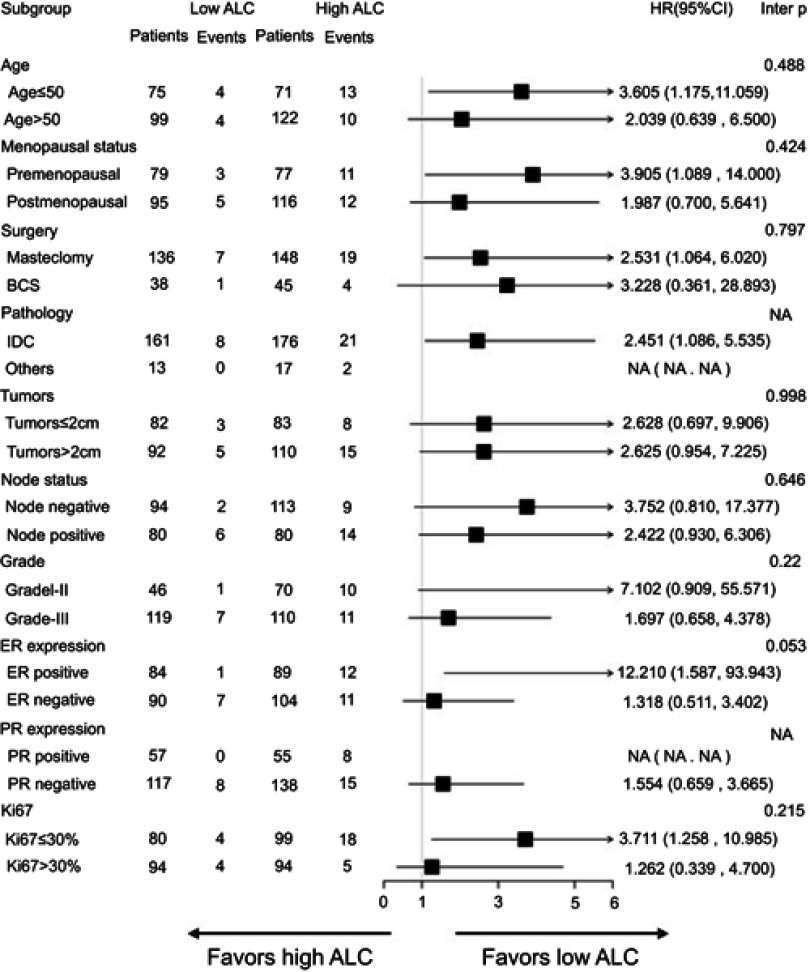
Regarding the subgroups and the P-value for interaction, Figure 2 shows a forest plot of hazard ratio for DFS. Across all subgroups, there was a trend that patients with low ALC had a better DFS, especially in breast cancer subgroups with an ER-positive status or Ki67≤30%. In ER-positive and HER-2-positive patients, the 3-year DFS was 98.5% in the low ALC cohort and 86.6% in the high ALC cohort. Additionally, in the subgroup of patients with Ki67≤30%, the 3-year DFS was 94.7% in the low ALC cohort and 84.3% in the high ALC cohort.
Figure 2.
Forest plots of subgroup analysis for disease-free survival.
Abbreviations: ALC, absolute lymphocyte count; BCS, breast-conserving surgery; IDC, invasive ductal carcinoma; ER, estrogen receptor; PR, progesterone receptor; NA, not available.
Overall survival
Overall, there were 11 deaths during the follow-up in the study. The 3-year OS was 97.8% in the low ANC cohort and 97.5% in the high ANC cohort (p=0.813) (Figure 1D). In the low ALC cohort, the 3-year OS was 99.4%; in the high ALC cohort, the 3-year OS was 96.0% (p=0.054) (Figure 1E). Kaplan-Meier analysis showed that patients in the high NLR cohort had significantly better 3-year OS than patients in the low NLR cohort (99.4% vs 95.8%, respectively; p=0.037) (Figure 1F). However, multivariate analysis showed that no factor in the analysis could predict OS independently in HER-2-positive breast cancer patients who received adjuvant trastuzumab (Table 5).
Table 5.
Univariate and multivariate analysis for OS
| Characteristics | Univariate | Multivariate | ||
|---|---|---|---|---|
| p-Value | HR | 95%CI | p-Value | |
| Age (>50 vs ≤50) | 0.089 | 0.323 | 0.094–1.108 | 0.072 |
| Menopausal status (pre vs post) | 0.811 | |||
| Surgery (mastectomy vs BCS) | 0.711 | |||
| Pathology (IDC vs others) | 0.832 | |||
| Tumors (≤2 cm vs >2 cm) | 0.218 | 2.087 | 0.542–8.039 | 0.285 |
| Nodes involved (negative vs positive) | 0.199 | 2.674 | 0.774–9.238 | 0.120 |
| Grade (I–II vs III) | 0.990 | |||
| ER (positive vs negative) | 0.930 | 0.786 | 0.236–2.617 | 0.695 |
| PR (positive vs negative) | 0.674 | 1.887 | 0.285–12.480 | 0.510 |
| Ki67 (≤30% vs >30%) | 0.791 | 0.895 | 0.263–3.041 | 0.859 |
| Chemotherapy (EC-T vs others) | 0.575 | |||
| Radiation (yes vs no) | 0.923 | |||
| Endocrine therapy (yes vs no) | 0.839 | |||
| ALC (≥1.8 vs <1.8) | 0.054 | 4.433 | 0.955–20.583 | 0.057 |
| NLR (≥1.77 vs <1.77) | 0.037 | 0.338 | 0.068–1.694 | 0.187 |
| ANC (≥3.2 vs <3.2) | 0.813 | |||
Abbreviations: IDC, invasive ductal carcinoma; BCS, breast conserving surgery; ER, estrogen receptor; PR, progestrone receptor; ANC, absolute neutrophil count; ALC, absolute lymphocyte count; NLR, neutrophil to lymphocyte ratio.
Discussion
Numerous previous studies have evaluated the prognostic value of NLR in breast cancer and showed that pretreatment high NLR was associated with adverse DFS or OS.5 Nevertheless, our retrospective study revealed that, in HER-2-positive breast cancer patients who received adjuvant one-year trastuzumab, preoperative ALC instead of NLR was an independent prognostic factor for DFS and a high ALC was significantly associated with a poor DFS.
Inflammation is a hallmark of cancer and represents a link between intrinsic and extrinsic factors contributing to tumor development.10 The trafficking of T cells into tumors is a crucial process, and higher tumor-infiltrating lymphocytes (TILs) are significantly associated with improved survival.11,12 Several inflammatory markers, such as NLR, PLR and the lymphocyte to monocyte ratio (LMR), have explored their predictive values in cancers.13,14 Our previous study indicated that an elevated preoperative NLR predicted a poor DFS, particularly in triple-negative breast cancer, but there was no significant difference observed in the HER-2-enriched subgroup.7 Additionally, a study with 187 cases also revealed that in HER-2-positive breast cancer patients receiving trastuzumab, NLR was not a predictor for DFS or OS.15
Few studies have validated the prognostic value of ALC and ANC in breast cancer. In HER-2-positive advanced breast cancer patients treated with trastuzumab and pertuzumab plus eribulin or nab-paclitaxel, a study with 51 cases demonstrated that a high baseline ALC was significantly associated with improved PFS.8 For early-stage patients, higher ALC, but not ANC, predicted a lower overall mortality and breast cancer-specific mortality in triple-negative breast cancer.16 Our study also validated that ALC was superior to NLR and ANC to predict DFS in HER-2-positive patients treated with trastuzumab, although both ALC and NLR were significantly associated with DFS in the univariate analysis. However, the results of our study were completely different from previous studies in which a low ALC was significantly associated with better DFS.
Preclinical studies have shown that trastuzumab can kill HER2-expressing tumor cells by interfering with HER2 signalling and through immune mechanisms such as ADCC and complement-dependent cytotoxicity.17 Thus, trastuzumab can induce a substantial increase in natural killer cells and CD8-positive T cells, implying that trastuzumab can activate different mechanisms to recruit lymphocytes to the tumor site.17 Higher TILs were significantly associated with a higher pathological complete response in patients receiving the neoadjuvant regimen and better overall survival in HER-2-positive advanced breast cancer.12,18 However, CD8+TILs and ALC were negatively correlated in breast cancer.19 HER-2 overexpression in ductal carcinoma was significantly associated with higher FOXP3+ TILs, and increased FOXP3+ TILs were associated with more aggressive tumor features.20 Additionally, in the tumor microenvironment, there also co-exist many other infiltrating immune cells, such as tumor-associated macrophages (TAMs) and cancer-related neutrophils (CRNs), which can enhance tumor cell invasion and metastasis.21,22 Beyond that, chemotherapy, like the taxane regimen, can also enhance trastuzumab-mediated ADCC in tumor cells.23 Therefore, the tumor immune microenvironment is very complex and influenced by numerous factors, such as treatment with trastuzumab and effect of chemotherapy.
In the subgroup analysis, we observed the tendency that, in patients with an ER-positive status or Ki67≤30%, a high ALC predicted a poor disease free survival. No sufficient evidence can explain these results. A recent study showed that ALC was correlated with DFS only in hormone receptor-positive breast cancer but not in HER-2-positive or triple-negative breast cancer.19 Regarding another prognostic marker, a meta-analysis demonstrated that ER and HER-2 positivity had a negative effect on the association between NLR and DFS.5 Many other studies also had verified the predictive and prognostic value of NLR in triple-negative breast cancer.24,25 Only a few studies have supported the prognostic value of NLR in ER-positive breast cancer. In a study concerning ER positive/HER2-negative breast cancer patients who received neoadjuvant chemotherapy, a high NLR was correlated with poor recurrence-free survival and OS.26 However, data are lacking regarding the prognostic value of ALC in ER-positive/HER-2-positive breast cancer.
This study possesses several limitations. First, this was a retrospective analysis, and the patients’ prognosis were influenced by many factors, although the patients’ characteristics and treatment were balanced. Second, the median follow-up for those patients was relatively short. Most events occurred after 18 months during the follow-up and only 6 events occurred in 18 months; thus Kaplan-Meier analysis showed that survival differences appeared after 18 months. Third, we observed that ALC was correlated with DFS, but we could not clearly identify the subtypes of lymphocytes.
Conclusion
Our retrospective study suggested that preoperative ALC is an independent prognostic factor for DFS in HER-2-positive breast cancer patients treated with adjuvant trastuzumab. In those patients, a high ALC was significantly associated with a poor DFS. Further studies should be carried out to illuminate the underlying mechanism and validate the result.
Acknowledgments
We would like to thank all patients whose information was analysed in our study. Our study was supported by the grants from the Medical Guidance Foundation of Shanghai Municipal Science and Technology Commission (Grant Number: 15411966400), Technology Innovation Act Plan of Shanghai Municipal Science and Technology Commission (Grant Number: 15411952500, 15411952501), Technology Innovation Act Plan of Shanghai Municipal Science and Technology Commission (Grant Numbers: 14411950200, 14411950201), National Natural Science Foundation of China (Grant Number: 81472462), Joint Research Project of the Emerging Cutting-edge Technology of Shanghai Shen-kang Hospital Development Center (Grant Number: SHDC12014103).
Disclosure
The authors report no conflict of interest in this work.
References
- 1.Curigliano G, Burstein HJ, Winner EP, et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol. 2017;28(8):1700–1712. doi: 10.1093/annonc/mdx308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273–1283. doi: 10.1056/NEJMoa0910383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valabrega G, Montemurro F, Aglietta M. Trastuzumab: mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann Oncol. 2007;18(6):977–984. doi: 10.1093/annonc/mdl475 [DOI] [PubMed] [Google Scholar]
- 4.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ethier JL, Desautels D, Templeton A, Shah PS, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res. 2017;19(1):2. doi: 10.1186/s13058-016-0794-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krenn-Pilko S, Langsenlehner U, Thurner EM, et al. The elevated preoperative platelet-to-lymphocyte ratio predicts poor prognosis in breast cancer patients. Br J Cancer. 2014;110(10):2524–2530. doi: 10.1038/bjc.2014.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong J, Mao Y, Chen X, et al. Elevated preoperative neutrophil-to-lymphocyte ratio predicts poor disease-free survival in Chinese women with breast cancer. Tumour Biol. 2016;37(3):4135–4142. doi: 10.1007/s13277-015-4233-1 [DOI] [PubMed] [Google Scholar]
- 8.Araki K, Ito Y, Fukada I, et al. Predictive impact of absolute lymphocyte counts for progression-free survival in human epidermal growth factor receptor 2-positive advanced breast cancer treated with pertuzumab and trastuzumab plus eribulin or nab-paclitaxel. BMC Cancer. 2018;18(1):982. doi: 10.1186/s12885-018-4242-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qian Y, Tao J, Li X, et al. Peripheral inflammation/immune indicators of chemosensitivity and prognosis in breast cancer patients treated with neoadjuvant chemotherapy. Onco Targets Ther. 2018;11:1423–1432. doi: 10.2147/OTT.S148496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339(6117):286–291. doi: 10.1126/science.1232227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slaney CY, Kershaw MH, Darcy PK. Trafficking of T cells into tumors. Cancer Res. 2014;74(24):7168–7174. doi: 10.1158/0008-5472.CAN-14-2458 [DOI] [PubMed] [Google Scholar]
- 12.Luen SJ, Salgado R, Fox S, et al. Tumour-infiltrating lymphocytes in advanced HER2-positive breast cancer treated with pertuzumab or placebo in addition to trastuzumab and docetaxel: a retrospective analysis of the CLEOPATRA study. Lancet Oncol. 2017;18(1):52–62. doi: 10.1016/S1470-2045(16)30631-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolan RD, Laird BJA, Horgan PG, McMillan DC. The prognostic value of the systemic inflammatory response in randomised clinical trials in cancer: a systematic review. Crit Rev Oncol Hematol. 2018;132:130–137. doi: 10.1016/j.critrevonc.2018.09.016 [DOI] [PubMed] [Google Scholar]
- 14.Cho U, Park HS, Im SY, et al. Prognostic value of systemic inflammatory markers and development of a nomogram in breast cancer. PLoS One. 2018;13(7):e0200936. doi: 10.1371/journal.pone.0200936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ulas A, Avci N, Kos T, et al. Are neutrophil/lymphocyte ratio and platelet/lymphocyte ratio associated with prognosis in patients with HER2-positive early breast cancer receiving adjuvant trastuzumab? J Buon. 2015;20(3):714–722. [PubMed] [Google Scholar]
- 16.Afghahi A, Purington N, Han SS, et al. Higher absolute lymphocyte counts predict lower mortality from early-stage triple-negative breast cancer. Clin Cancer Res. 2018;24(12):2851–2858. doi: 10.1158/1078-0432.CCR-17-1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bianchini G, Gianni L. The immune system and response to HER2-targeted treatment in breast cancer. The Lancet Oncology. 2014;15(2):e58–e68. doi: 10.1016/S1470-2045(13)70477-7 [DOI] [PubMed] [Google Scholar]
- 18.Inoue H, Horii R, Ito Y, Iwase T, Ohno S, Akiyama F. Tumor-infiltrating lymphocytes affect the efficacy of trastuzumab-based treatment in human epidermal growth factor receptor 2-positive breast cancer. Breast Cancer. 2017;25:268–274. [DOI] [PubMed] [Google Scholar]
- 19.Lee KH, Kim EY, Yun JS, et al. The prognostic and predictive value of tumor-infiltrating lymphocytes and hematologic parameters in patients with breast cancer. BMC Cancer. 2018;18(1):938. doi: 10.1186/s12885-018-4242-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Droeser R, Zlobec I, Kilic E, et al. Differential pattern and prognostic significance of CD4+, FOXP3+ and IL-17+ tumor infiltrating lymphocytes in ductal and lobular breast cancers. BMC Cancer. 2012;12:134. doi: 10.1186/1471-2407-12-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J, Bae JS. Tumor-associated macrophages and neutrophils in tumor microenvironment. Mediators Inflamm. 2016;2016:6058147. doi: 10.1155/2016/6058147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaul ME, Fridlender ZG. Cancer related circulating and tumor-associated neutrophils - subtypes, sources and function. FEBS J. 2018. doi: 10.1111/febs.14524 [DOI] [PubMed] [Google Scholar]
- 23.Di Modica M, Sfondrini L, Regondi V, et al. Taxanes enhance trastuzumab-mediated ADCC on tumor cells through NKG2D-mediated NK cell recognition. Oncotarget. 2016;7(1):255–265. doi: 10.18632/oncotarget.6353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pistelli M, De Lisa M, Ballatore Z, et al. Pre-treatment neutrophil to lymphocyte ratio may be a useful tool in predicting survival in early triple negative breast cancer patients. BMC Cancer. 2015;15:195. doi: 10.1186/s12885-015-1584-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jia W, Wu J, Jia H, et al. The peripheral blood neutrophil-to-lymphocyte ratio is superior to the lymphocyte-to-monocyte ratio for predicting the long-term survival of triple-negative breast cancer patients. PLoS One. 2015;10(11):e0143061. doi: 10.1371/journal.pone.0143061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koh YW, Lee HJ, Ahn JH, Lee JW, Gong G. Prognostic significance of the ratio of absolute neutrophil to lymphocyte counts for breast cancer patients with ER/PR-positivity and HER2-negativity in neoadjuvant setting. Tumour Biol. 2014;35(10):9823–9830. doi: 10.1007/s13277-014-2282-5 [DOI] [PubMed] [Google Scholar]