Abstract
Chronic hepatitis viral infection, alcoholic intoxication, and obesity cause liver fibrosis, which progresses to decompensated liver cirrhosis, a disease for which medical demands cannot be met. Since there are currently no approved anti-fibrotic therapies for established liver fibrosis, the development of novel modalities is required to improve patient prognosis. In this study, we clarified the anti-fibrotic effects of cell sheets produced from human bone marrow-derived mesenchymal stem cells (MSCs) incubated on a temperature-sensitive culture dish with the chemical compound IC-2. Orthotopic transplantation of IC-2-engineered MSC sheets (IC-2 sheets) remarkably reduced liver fibrosis induced by chronic CCl4 administration. Further, the marked production of fibrolytic enzymes such as matrix metalloproteinase (MMP)-1 and MMP-14, as well as thioredoxin, which suppresses hepatic stellate cell activation, was observed in IC-2 sheets. Moreover, the anti-fibrotic effect of IC-2 sheets was much better than that of MSC sheets. Finally, knockdown experiments revealed that MMP-14 was primarily responsible for the reduction of liver fibrosis. Here, we show that IC-2 sheets could be a promising therapeutic option for established liver fibrosis.
Subject terms: Regeneration, Liver fibrosis
Introduction
Chronic liver injury leads to liver fibrosis, which is the excessive accumulation of extracellular matrix (ECM). Moreover, advanced and established liver fibrosis results in liver cirrhosis. Especially, established liver fibrosis progresses to decompensated liver cirrhosis, for which medical needs remain unmet1–6. In addition, because liver fibrosis is strongly associated with the incidence of hepatocellular carcinoma (HCC)7, the development of anti-fibrotic therapies would not only cure liver fibrosis, but also suppress subsequent incidences of HCC.
Liver transplantation is the most effective treatment for liver cirrhosis8. However, it is not feasible for all patients because of donor scarcity. Stem cell therapy shows considerable potential as a treatment for liver disease9,10, and the therapeutic effects of mesenchymal stem cells (MSCs) have been studied in clinical research. Accordingly, MSCs are emerging as a promising cell source for the treatment of liver cirrhosis11–15. However, there is much room for improvement regarding the methodology of MSC transplantation to accomplish an effective therapeutic modality (e.g. optimal delivery route, sufficient number of MSCs, and extension of the survival of engrafted MSCs)16. Cell sheet engineering has attracted much attention to overcome these problems. This technology enables the transplantation of abundant cells without the risk of embolization or undesired cellular migration to other organs, as compared to the intravascular infusion of cell suspensions. Furthermore, transplanted cell sheets can be engrafted for a long term without losing their functions, as compared to that with the intravascular infusion of cell suspensions17. Indeed, in a myocardial infarction model, myoblast cells transplanted as a cell sheet suppressed fibrosis compared to that with cell infusion18. Therefore, tissue-engineered cell sheet transplantation should be an appropriate treatment for liver fibrosis.
Based on our observations that Wnt/β-catenin signaling is downregulated during the hepatic differentiation of MSCs and that suppression of this signaling axis causes the hepatic differentiation of MSCs19,20, we previously produced hepatic cell sheets from MSCs via treatment with hexachlorophene, a Wnt/β-catenin inhibitor, on thermoresponsive polymer-coated culture dishes21. Orthotopic transplantation of hexachlorophene-treated MSC sheets ameliorated carbon tetrachloride (CCl4)-induced acute liver injury21. In another previous report, we screened our synthetic chemical libraries to improve the performance of cell sheets, identifying IC-2, a derivative of the Wnt/β-catenin signaling inhibitor ICG-001. IC-2 proved to potently induce the differentiation of MSCs into hepatic lineages22. Recently, we reported that the therapeutic effect of IC-2-engineered cell sheets (i.e. IC-2 sheets) on acute liver injury is more potent than that of hexachlorophene-engineered MSC sheets23. In addition, it is not known whether IC-2 sheets have anti-fibrotic effects on liver fibrosis. In the present study, we clarified the anti-fibrotic effect of IC-2 sheets on liver fibrosis and its molecular mechanisms.
Results
Reversal of liver fibrosis via orthotopic transplantation of IC-2 sheets during chronic liver injury
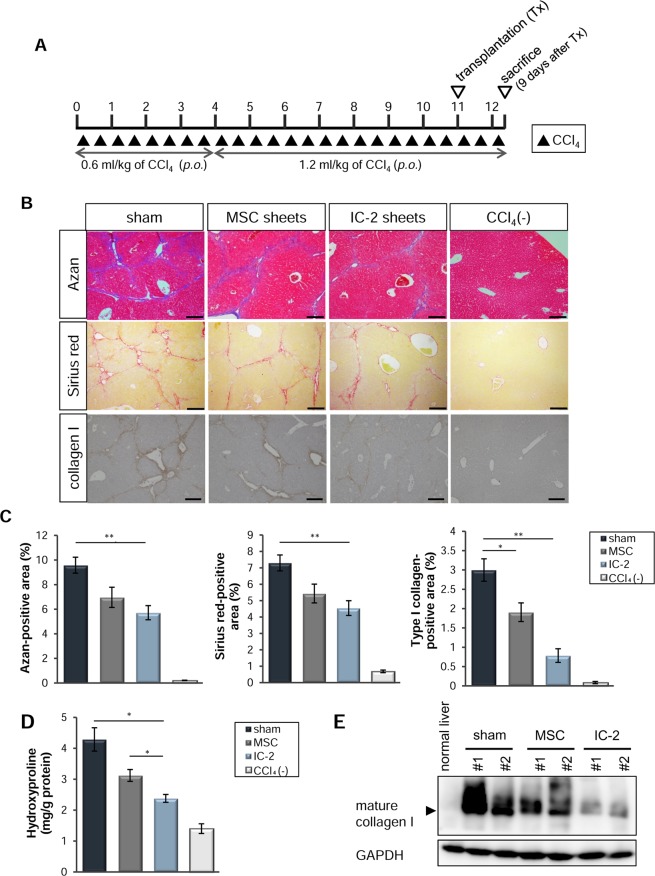
We previously reported that hepatic cells differentiated from human MSCs in the presence of a Wnt/β-catenin signaling inhibitor could ameliorate acute liver injury upon orthotopic transplantation as cell sheets21,23. Although both hexachlorophene and IC-2 induced the hepatic differentiation of MSCs, the effect of IC-2 on this process was more potent than that of hexachlorophene22,23. Moreover, IC-2 sheets potently suppressed acute liver injury compared to that with hexachlorophene sheets23. In the present study, we clarified the anti-fibrotic effect of IC-2 sheets on liver fibrosis, by comparing it to that of untreated MSC sheets (i.e. MSC sheets). We then generated a model of chronic liver injury using immunodeficient mice for the transplantation of human cells into the liver. Since B cells are indispensable for the establishment of liver fibrosis24, we used BALB/c-nu/nu mice for the CCl4-induced liver fibrosis model (Fig. 1A). Eleven weeks after the initiation of CCl4 administration, mice, which were equally divided into three groups according to liver function tests and body weights, received transplantation of three-layer IC-2 sheets and MSC sheets at two sites of the liver surface (i.e. IC-2 and MSC group, respectively). Sham-operated mice (i.e. sham group) served as a control. The continuous administration of CCl4 into three groups of mice was performed for another week, and all mice were sacrificed 9 days after transplantation. Azan staining, Sirius red staining, and immunohistochemistry for type I collagen showed that the IC-2 group exhibited a significant reduction in liver fibrosis compared to that in the sham group (Fig. 1B,C). Moreover, a remarkable reduction in hydroxyproline content was observed in the IC-2 group, compared to that in the sham and MSC group (Fig. 1D). Further, mature type I collagen was prominently decreased in the IC-2 group (Figs 1E, S1A), and only this treatment group exhibited a significant reduction in collagen content compared to that in the sham group. Type III collagen was also decreased following IC-2 sheet transplantation (Fig. S1B). Since the reduction in type III collagen was not as pronounced as that of type I collagen, the reduction in fibril contents seemed to be preferentially affected by the decrease in type I collagen through IC-2 sheet transplantation. These data showed that IC-2-treated cell sheets could consistently mediate a significant reduction in collagen fibril content. Serum alanine transaminase (ALT) was significantly decreased in the IC-2 group, whereas serum aspartate transaminase (AST) and total bilirubin were not changed (Fig. S2A–C). The number of Ki-67-positive hepatocytes was increased in the IC-2 group, and mitotic hepatocytes gradually increased in the sham, MSC, and IC-2 groups in that order (Fig. S2D–F). Improved ALT levels and the promotion of hepatocyte growth were not observed in the MSC group; therefore, MSCs gained both activities through IC-2 treatment. Our data suggest that IC-2 sheets can reduce liver fibrosis and stimulate liver regeneration.
Figure 1.
Suppression of hepatic fibrosis by IC-2 sheets on the ninth day after transplantation. (A) Protocol of the animal experiment. Abbreviations: p.o., per os; Tx, transplantation. “p.o.” indicates oral administration of CCl4. “Tx” indicates transplantation of cell sheets. (B) Micrographs of liver sections subjected to Azan staining (upper), Sirius red staining (middle), and immunohistochemistry for type I collagen (lower). MSC, mesenchymal stem cell. (C) The proportions of fibrotic areas among the groups (n = 7–9 except for n = 3 for CCl4(−) group). CCl4(−) indicates control mice without CCl4 intoxication. (D) Hydroxyproline contents in the liver (n = 6 except for n = 3 for CCl4(−) group). (E) Western blot of type I collagen in recipient liver tissues. The results are expressed as the mean ± S.E.M. Levels of significance: *P < 0.05; **P < 0.01 (one-way ANOVA followed by Games–Howell test).
Hepatic stellate cell activation is suppressed by IC-2 sheet transplantation
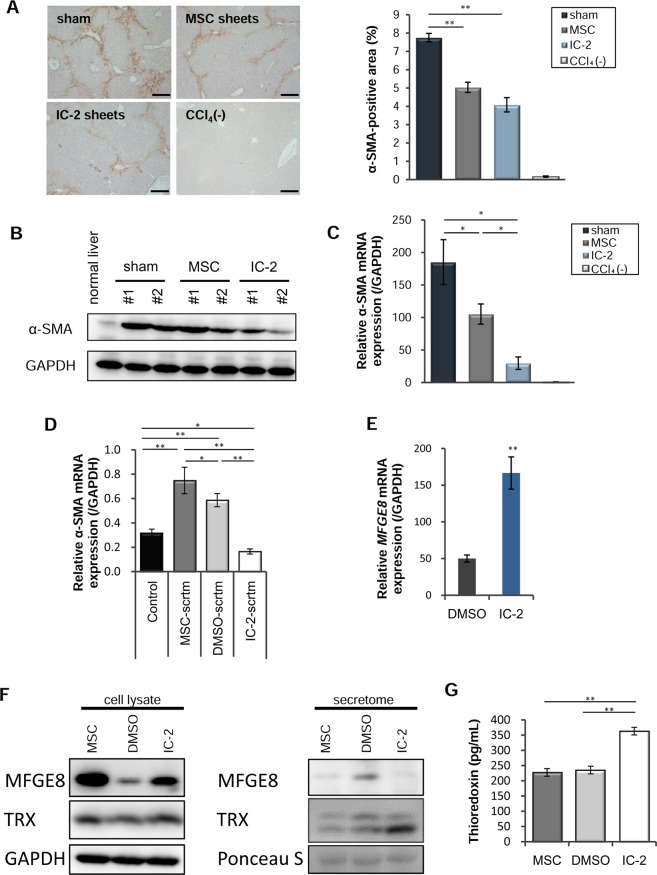
Liver fibrosis is characterized by the excessive accumulation of ECM as a result of a disruption in the balance between fibrogenesis and fibrolysis. As such, targeted anti-fibrotic therapies addressing molecules and mechanisms central to fibrogenesis and fibrolysis are required for patients with liver cirrhosis25. Fibrogenesis is regulated by activated hepatic stellate cells (HSCs), and is regarded as the main target of anti-fibrotic therapy1,2,26. To clarify whether the anti-fibrotic effect of IC-2 sheets was due to decreased fibrogenesis or increased fibrolysis, we examined the status of HSCs. Alpha-smooth muscle actin (α-SMA) is a major indicator of HSC activation. α-SMA-positive areas were markedly decreased in the MSC and IC-2 groups compared to those in the sham group (Figs 2A,B, S3). Moreover, α-SMA expression levels in the IC-2 group were decreased compared to those in the MSC group (Fig. 2C). Our results thus suggest that IC-2 sheets can suppress HSC activation in CCl4 chronically-administered mice.
Figure 2.
Reduction of hepatic stellate cell activation by IC-2 sheets. (A) Immunohistochemistry for alpha-smooth muscle actin (α-SMA) in recipient livers (left). Quantification of α-SMA-positive areas (right; n = 7–9 except for n = 3 for CCl4(−) group; CCl4(−) indicates control mice without CCl4 intoxication; mean ± S.E.M., *P < 0.05, **P < 0.01, one-way ANOVA, Games–Howell test). MSC, mesenchymal stem cell. (B) α-SMA expression in the recipient livers was measured by western blotting and (C) α-SMA expression in the recipient liver was measured by qRT-PCR analysis (n = 6 except for n = 3 for CCl4(−) group; mean ± S.E.M., *P < 0.05, one-way ANOVA, Games–Howell test). (D) qRT-PCR analysis of α-SMA in LX-2 cells after treatment with each secretome harvested from untreated, vehicle-treated, or IC-2-treated MSCs (n = 3, mean ± S.E.M., *P < 0.05, **P < 0.01, one-way ANOVA, least significant difference test). (E) MFGE-8 expression in MSCs on day 7 in vitro (n = 3, mean ± S.D., **P < 0.01, Student’s t-test). (F) MFGE8 and thioredoxin (TRX) expression in cell lysates (left) and secretomes (right), as analyzed by western blotting. GAPDH and Ponceau S were used as a loading control. (G) The secretion of TRX was determined by ELISA (n = 3, mean ± S.D., **P < 0.01, one-way ANOVA, least significant difference test).
Recently, hepatically-differentiated MSCs have been reported to ameliorate liver fibrosis through the secretion of milk-fat globule epidermal growth factor (MFGE)-827. We first examined the effect of IC-2-treated MSC secretomes on HSC activation. The addition of that from IC-2-treated MSCs decreased α-SMA expression in LX-2 human HSCs, suggesting that the secretome of IC-2-treated MSCs contains humoral factors that suppress HSC activation (Fig. 2D). Although MFGE-8 was increased in the cell lysates of MSCs treated with IC-2 (Fig. 2E,F), this marker was not increased in the secretome, contrary to our expectations (Fig. 2F). Thioredoxin (TRX) was increased in both cell lysates and the secretome of MSCs treated with IC-2 (Fig. 2F,G). In our previous report, TRX was upregulated in livers transplanted with hexachlorophene- and IC-2-treated cell sheets21,23. Moreover, the upregulation of TRX via knockdown of TRX-interacting protein was shown to suppress HSC activation28. In addition, a TRX transgene in mice had a preventive effect on thioacetamide-induced liver fibrosis29. These data suggested that TRX is a humoral factor that suppresses HSC activation. Since the activation of these cells was inhibited in both cell sheet-transplanted groups, we investigated whether the resolution of liver fibrosis was due to the indirect effects of cell sheet transplantation via the inhibition of HSC activation. However, the de novo expression of several enzymes involved in collagen synthesis and crosslinking, namely collagen 1α1, lysyl oxidase, and prolyl-4-hydroxylase, was not altered in liver tissues (Fig. S4B–D). Furthermore, regarding the de novo expression of enzymes involved in the resolution of collagen in recipient mice, matrix metalloproteinase-2 (Mmp-2) and Mmp-8 were decreased in the IC-2 group (Fig. S4E,F), whereas Mmp-13 and Mmp-14 remained unchanged (Fig. S4G,H). Mmp-1a30, a murine homologue of human MMP-1, was not detected (data not shown). These data suggest that regarding the de novo expression of enzymes involved in the resolution of type I collagen in recipient mice, no functional change in collagen degradation occurred. Our findings suggest that the suppressive effect of IC-2 sheets on liver fibrosis was not due to the de novo expression of collagen metabolism-related enzymes in recipient livers.
Next, we examined the possibility that IC-2 itself, included in the cell sheets, inhibits collagen synthesis. The Wnt/β-catenin signaling inhibitor ICG-001 was previously reported to improve liver fibrosis in mice31, and its derivative PRI-724 has been used in clinical trials for HCV-related cirrhosis32. IC-2 is also a derivative of ICG-001, and therefore, it could be an anti-fibrotic agent. First, we investigated IC-2 content during the preparation of cell sheets. As shown in Fig. S5A, IC-2 content increased with treatment time and reached 478.4 ng/sheet on day 7. Since each mouse received six cell sheets, each animal was exposed to 2.87 µg of IC-2. Assuming that IC-2 is limited to the liver, its concentration was equivalent to 2.3 µM, as the liver volume was determined to be 2.36 ml, calculating this based on an average liver weight of 2.54 g (hepatic volume (in ml) = 0.907 × liver weight (in gram) + 0.053)33. Next, we examined whether 2.3 µM of IC-2 would inhibit collagen expression in vitro. This concentration was not able to suppress collagen expression in the LX-2 hepatic stellate cell line (Fig. S5B). Therefore, we concluded that the amount of IC-2 included in the cell sheets was not sufficient to reduce liver fibrosis.
Humoral factors involved in the improvement of liver fibrosis
Since neither the promotion of fibrinolysis nor the suppression of fibrogenesis was observed in recipient livers and because a suppressive effect of IC-2 itself on fibrosis was not observed, the resolution of liver fibrosis by IC-2 sheet transplantation might have been due to the transplanted cell sheets themselves. First, we addressed how the cell sheets affect liver fibrosis based on the distance from the transplanted cell sheets. Focusing on the relationship between the Azan-positive area and the distance from the transplanted cell sheets, improved liver fibrosis was observed in IC-2-treated cell sheet-transplanted mice regardless of the depth from the cell sheets (Fig. S6A). Furthermore, the resolution of liver fibrosis was recognized in another lobe of the liver, where cell sheets were not transplanted (Fig. S6B). Because murine liver lobes are separate from each other, these data support an anti-fibrotic effect of the cell sheets originating from humoral factors via the vascular network. Interestingly, focusing on the relationship between the α-SMA-positive area and the distance from the transplanted cell sheets, the suppression of HSC activation was observed most prominently in mice transplanted with both types of cell sheets beneath the cell sheets (Fig. S6C). These data indicated that the anti-fibrotic effects of the cell sheets and the inhibitory effects of HSC activation are independent mechanisms; specifically, one comprised widespread diffusion to the liver via the host vasculature, whereas the other included the focal penetration beneath the cell sheets.
Production of MMPs in response to IC-2 treatment
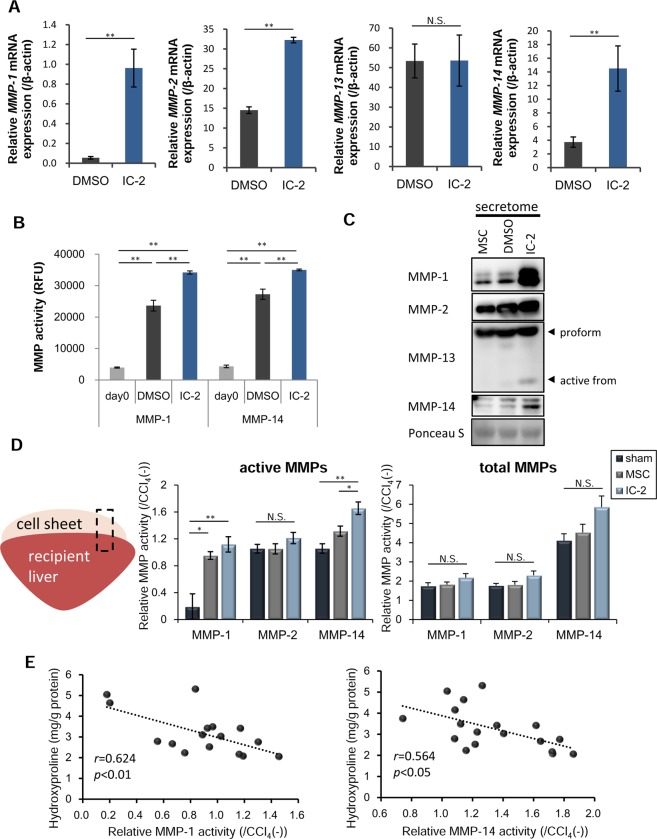
Next, we evaluated fibrolytic enzymes that originated from IC-2 sheets. We focused on several MMPs involved in the degradation of type I collagen, which comprises 60~70% of total ECM proteins in cirrhotic livers34. Interstitial collagenolytic enzymes (MMP-1, MMP-2, MMP-8, and MMP-13) and membrane type I matrix metalloproteinase (MMP-14) use type I collagen as a substrate35–37. IC-2 upregulated the mRNA expression of MMP-1, MMP-2, and MMP-14 in MSCs in vitro, whereas MMP-13 mRNA expression was not altered (Fig. 3A). MMP-8 mRNA levels were not detected in IC-2-treated MSCs (data not shown). Since MMP-1, MMP-2, and MMP-14 mRNA levels were unchanged in hexachlorophene-treated MSCs (data not shown), IC-2 appeared to have additional actions besides inhibition of the Wnt/β-catenin pathway. Among these, MMP-1 and MMP-14 protein levels were increased in IC-2-treated cells, whereas MMP-2 levels were not altered (Fig. S7). Further, the enzymatic activities of MMP-1 and MMP-14 were also increased by IC-2 treatment for 1 week (Fig. 3B). We then examined active forms of the aforementioned MMPs in culture supernatant, since MMP-14 is known to activate both MMP-2 and MMP-1338,39. Secretome analysis showed that the active forms of MMP-2 and MMP-13 were increased by IC-2 treatment. In addition to MMP-2 and MMP-13, the secretion of MMP-1 and MMP-14 was also prominently induced by IC-2 (Fig. 3C). Although MMP-14 is known as a membrane-bound type matrix metalloproteinase, the production of a soluble form was also previously reported40,41. Taken together, IC-2 enhanced the production of MMP-1 and MMP-14, which are involved in type I collagen degradation.
Figure 3.
IC-2 increases the production and secretion of matrix metalloproteinases (MMPs) in mesenchymal stem cells (MSCs). (A) mRNA expression of fibrolytic genes in MSCs on day 7 in vitro (n = 3, mean ± S.D., **P < 0.01, Student’s t-test). (B) In vitro activities of MMP-1 and MMP-14 in MSCs on day 7 (n = 3, mean ± S.D., **P < 0.01, one-way ANOVA, Tukey test). (C) Expression of MMPs in the secretome of MSCs in vitro. (D) Images of liver tissue used in the experiments and enzymatic activities of MMPs in liver tissues containing cell sheets. Data are expressed as activity relative to that in the CCl4(−) group. CCl4(−) indicates control mice without CCl4 intoxication. Images of liver tissues used in these experiments (left). MMP activities (middle) and activities of total MMPs (right), consisting of active enzyme and latent pro-enzyme (n = 6, mean ± S.E.M., *P < 0.05, **P < 0.01, one-way ANOVA, Games–Howell test). (E) Linear regression analysis of relative activities of active MMP-1 (left) or active MMP-14 (right) with respect to hepatic hydroxyproline content 9 days after transplantation (n = 12, Pearson’s correlation coefficient).
MMP-14 is mainly responsible for the resolution of liver fibrosis via IC-2 sheet transplantation
We next investigated which MMPs are involved in the resolution of fibrosis. Liver tissues containing cell sheets from the IC-2 group had higher levels of active MMP-1 and MMP-14 than those of the sham and MSC groups (Fig. 3D). However, significant changes in the activities of total MMPs were not observed among the three groups (Fig. 3D). In contrast, the relative activities of active and total MMP-1, MMP-2, and MMP-14 were not different among sham, MSC, and IC-2 groups in the mouse liver (Fig. S4I,J). Therefore, these data suggest that upregulation of MMP-1 and MMP-14 activities are mediated by transplanted IC-2-treated cell sheets. Hepatic hydroxyproline contents 1 week after transplantation were inversely correlated with relative MMP-1 and MMP-14 activity at same time point (Fig. 3E). These results suggest that MMP-1 and MMP-14 secreted from IC-2 sheets play an important role in the resolution of liver fibrosis. However, as an important problem, these MMP activities were not specific to each substrate. Therefore, we investigated the expression of MMP-1, 2, 3, 7, 8, 9, 12, 13, and 14, during the measurement of MMP activities. As a result, only MMP-1, 2, and 14 were detected in liver tissues containing transplanted cell sheets by RT-PCR analysis (Fig. S8). MMP-2 activities were not altered among the experimental groups (Fig. 3D); therefore, it was important to reveal which MMPs exert the anti-fibrotic effect of IC-2-treated MSC sheets.
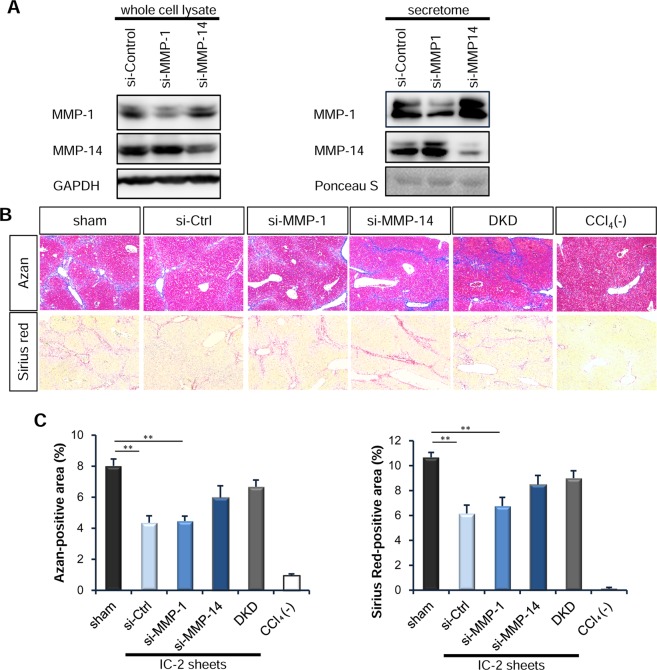
For this, we knocked down MMP-1 and/or MMP-14 through small interfering RNA (siRNA) transfection during the preparation of IC-2 sheets. Reverse transfection of each siRNA was successfully performed to suppress the expression of MMP-1 and MMP-14 in both cell lysates and secretomes (Fig. 4A). Azan and Sirius red staining were also performed to compare the anti-fibrotic effects on liver fibrosis by transfecting each siRNA into IC-2 sheets. Liver fibrosis was significantly reduced in the group transplanted with si-Ctrl-transfected IC-2 sheets compared to that in the sham group based on Azan staining (Fig. 4B). Transplantation of si-MMP-1-transfected IC-2 sheets achieved almost the same reduction in liver fibrosis as si-Ctrl-transfected IC-2 sheets. However, the anti-fibrotic effect was diminished with IC-2 sheets transfected with si-MMP-14 or both si-MMP-1 and si-MMP-14. A similar result was also obtained based on Sirius red staining (Fig. 4C). These results suggest that the fibrolytic activity of IC-2 sheets on liver fibrosis is dependent on MMP-14. Although MMP-1 possesses potent collagenolytic activity42, the fibrinolytic effect of si-MMP-1-transfected IC-2 sheets was marginal. To assess the expression of these MMPs in cell sheets over time, MMP-1 and MMP-14 levels were determined in liver tissues containing cell sheets by reverse-transcription polymerase chain reaction (RT-PCR) analysis using human-specific primers (Fig. S9A). One day after transplantation, the expression of MMP-1 and MMP-14 was markedly increased in IC-2 sheets and was somewhat diminished in MSC sheets. On 9 day after transplantation, the expression of MMP-1 became much weaker in both IC-2 and MSC groups. In contrast, the expression of MMP-14 in both groups was stable 9 days after transplantation (Fig. S9A). Furthermore, western blot analysis of the liver tissues containing cell sheets after knockdown experiments showed that MMP-1 protein became undetectable in IC-2 sheets transfected with si-Ctrl 6 days after transplantation. However, the expression of MMP-14 was still apparent in IC-2 sheets transfected with si-Ctrl or si-MMP-1 6 days after transplantation (Fig. S9B). These data suggest that the expression of MMP-1 is diminished at the earlier phase, but that robust expression of MMP-14 was maintained for at least 9 days.
Figure 4.
MMP-14 plays an important role in the anti-fibrotic effect of IC-2 sheets. (A) Downregulation of MMP-1 or MMP-14 expression through small interfering RNA (siRNA) transfection in IC-2 sheets was confirmed by western blotting. (B) Micrographs of Azan (upper) and Sirius red (lower) staining on the sixth day after transplantation in liver tissues transplanted with IC-2 sheets in which transfection of si-MMP-1 and/or si-MMP-14 was performed. (C) Measurement of the extent of fibrosis based on Azan staining (left) and Sirius red staining (right) (n = 5–6 per group except for n = 4 for DKD and n = 3 for CCl4(−) group. DKD group and CCl4(−) group indicate double knock-down of MMP-1 and MMP-14 and control mice without CCl4 intoxication, respectively (mean ± S.E.M., **P < 0.01, one-way ANOVA, Games–Howell test).
Primary human mesenchymal stem cell-engineered hepatic cell sheets with IC-2 also suppress liver fibrosis
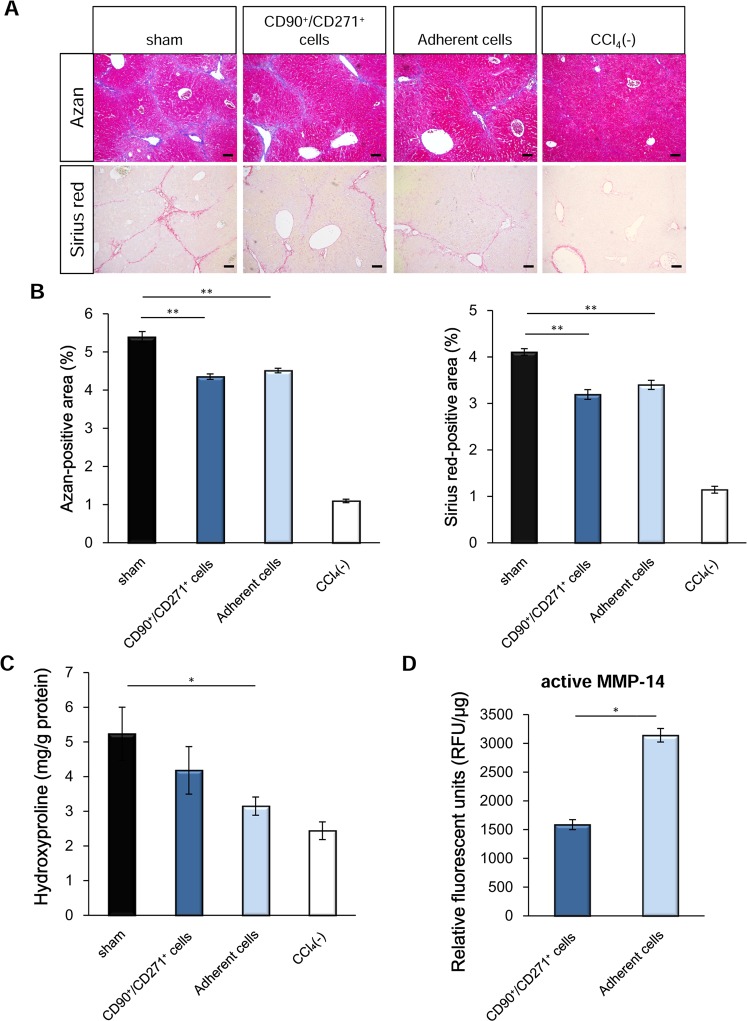
Since the aforementioned results were obtained using cell sheets manufactured from UE7T-13 cells, we assessed whether IC-2-engineered primary MSCs are also effective. It was previously reported that bone marrow-derived mononuclear cells that adhere to plastic dishes generally contain MSCs43. CD90+CD271+ bone marrow-derived mononuclear cells have been reported to include MSCs with a high number of colony-forming unit fibroblasts (CFU-Fs) and have elevated differentiation capacity toward a mesenchymal lineage44. To investigate which population of MSCs would be suitable for manufacturing IC-2 sheets, we compared the anti-fibrotic effect of IC-2 sheets derived from the two respective cell populations by Azan and Sirius red staining (Fig. 5A). Both cell populations were prepared from bone marrow-derived mononuclear cells obtained from Lonza Inc. Azan-positive areas in mouse livers transplanted with adherent cell-derived cell sheets were significantly decreased compared to those in the sham group (P < 0.01), and those in mouse livers transplanted with CD90+CD271+ cell-derived sheets were also significantly reduced (P < 0.01, Fig. 5B). Moreover, Sirius red-positive areas in both groups were significantly reduced compared to those in the sham group (Fig. 5B). Hydroxyproline contents in liver tissues transplanted with adherent cell-derived sheets were significantly decreased compared to those in the sham group, but those in CD90+CD271+ cell-derived sheets were not reduced (Fig. 5C). Furthermore, the activity of MMP-14 in adherent cell-derived sheets was significantly higher than that in CD90+CD271+ cell-derived sheets (Fig. 5D). These data suggest that adherent cells are a more suitable source than CD90+CD271+ cells for the treatment of liver fibrosis.
Figure 5.
Comparison of the anti-fibrotic effects of IC-2 sheets between CD90+/CD271+ bone marrow-derived mononuclear cells (BM-MNCs) and adherent BM-MNCs. (A) Micrographs of IC-2 liver sections subjected to Azan staining (upper) and Sirius red staining (lower). (B) Measurement of fibrotic areas based on Azan and Sirius red staining (n = 9–10 except for n = 3 for CCl4(−) group. CCl4(−) indicates control mice without CCl4 intoxication; mean ± S.E.M., *P < 0.05, one-way ANOVA, least significant difference test). (C) Hydroxyproline contents in liver tissues (n = 9–10 except for n = 3 for CCl4(−) group, mean ± S.E.M., *P < 0.05, one-way ANOVA, least significant difference test). (D) MMP-14 activity in BM-MNCs on the eleventh day after IC-2 treatment in vitro (n = 3, mean ± S.D., *P < 0.05, student’s t-test).
Finally, we examined the effects of the harvesting method and plating cell density on the activities of MMP-1 and MMP-14. With respect to cells treated with IC-2, sheet formation resulted in higher MMP-1 and MMP-14 activity than suspension cells in three individual samples (Fig. S10A,B). Especially, all MSCs showed higher MMP-1 and MMP-14 activities upon sheet formation compared to those with suspension cells. Furthermore, the activities of MMP-1 and MMP-14 in IC-2 sheets were associated with the cell density upon inoculation (Fig. S10C). These data suggest that IC-2 sheets manufactured by plating a higher cell density are suitable for the treatment of liver fibrosis.
Discussion
Effects of IC-2-engineered cell sheets on liver fibrosis
Based on our previous findings that MSC-engineered cell sheets produced with Wnt/β-catenin inhibitors could ameliorate acute liver injury21,23, we examined the anti-fibrotic effect of IC-2 sheets on CCl4-induced liver fibrosis in the present study. Orthotopic transplantation of IC-2 sheets potently reduced liver fibrosis based on Azan staining, Sirius red staining, immunohistochemistry for type I collagen, and hepatic hydroxyproline contents, when compared to that with unmanipulated MSC sheets. The expression of α-SMA indicated that HSC activation was also suppressed by IC-2 sheets. However, the mRNA expression of fibrogenic factors (e.g. collagen 1α1, lysyl oxidase, and prolyl-4-hydroxylase) was not significantly reduced de novo in mouse liver tissues. Further, higher activities of MMP-1 and MMP-14 were observed in liver tissues containing IC-2 sheets. In addition, the secretome of IC-2-treated MSCs contained abundant MMP-1 and MMP-14, suggesting that IC-2 sheets secrete these MMPs. Since MMP-1, MMP-2, and MMP-14 mRNA levels were not changed in MSCs treated with hexachlorophene, which is a Wnt/β-catenin inhibitor (data not shown), the induction of MMPs was not due to the suppression of this pathway. These data suggest that IC-2 induces MMPs independent of Wnt/β-catenin pathway inhibition.
To our knowledge, there have been no reports thus far indicating that the transplantation of cell sheets can suppress liver fibrosis in vivo. MMPs comprise a family of Zn2+-dependent endopeptidases34; therefore, MMP activity might be inhibited by ethylenediaminetetraacetic acid (EDTA) through the chelation of metal ions45. Treatment with trypsin and EDTA can suppress MMP activities, whereas cell sheets enable harvesting without the loss of MMP activities (Fig. S5A,B). MSCs exert potent anti-fibrotic effects through the secretion of MMPs when they are transplanted as cell sheets that are produced in the presence of IC-2. In addition, IC-2 sheets suppress the activation of HSCs possibly through the production of TRX. However, increases in MMPs seem to be the main reason as to why IC-2 sheets potently suppress liver fibrosis. Our findings suggest that MMP-14 is mainly responsible for the improvement of liver fibrosis.
In the present study, the reduction of type I collagen by IC-2 sheets was more efficient than the reduction of type III collagen. It is well known that MMP-14 degrades type III collagen and other ECM components in addition to type I collagen46. However, soluble MMP-14, which lacks transmembrane and cytoplasmic domains, has been reported to degrade type I collagen more efficiently than type II or III collagen47. Furthermore, the degradation of type I collagen by soluble MMP-14 was also reported to be enhanced synergistically in the presence of MMP-247. In the present study, IC-2 induced MMP-2 expression and secretion in addition to MMP-14 in MSCs (Fig. 3A,C). These data support the fact that type I collagen is more efficiently dissolved by soluble MMP-14 in the presence of MMP-2 produced by IC-2 sheets. Further type I collagen is predominant in the human cirrhotic liver34; therefore, IC-2-treated MSC sheets have potential applications for human cirrhosis.
Cell sheet technology as regenerative medicine for liver fibrosis
Tissue engineering-based cell sheet therapy is suitable for the efficient transplantation of hepatocytes or differentiated hepatic cells. In the present study, we demonstrated that the formation of cell sheets is important to increase the activities of MMP-1 and MMP-14 (Fig. S5A,B). These findings suggest that cell sheet technology is especially useful for the treatment of liver fibrosis, in addition to other advantages such as preserved cellular communication junctions, endogenous extracellular matrix, and integrative adhesive agents18. Recently, several groups including us revealed that cell sheet transplantation is useful to ameliorate acute liver failure21,23,48,49. In one study using iPSC-derived hepatocyte sheets, it was determined that the therapeutic effects of cell sheets on acute liver injury are due to hepatocyte growth factor (HGF) production48. This paper implied that iPSC-derived cell sheets contain hepatic non-parenchymal cells since these cells, but not hepatic parenchymal cells, produce HGF. The transplantation of hepatocytes into radiation-induced or partially hepatectomized livers also resulted in compensated liver functions49. Taken together, the combination of cell sheet-based technology with a single small molecule compound such as IC-2 can accelerate the resolution of liver fibrosis. However, if the etiology of cirrhosis continues, repeat transplantation would be required. Repeating cell sheet transplantation in the case of laparotomy is thought to be possible, but it is better to develop the laparoscopic transplantation of cell sheets in the future to reduce the burden on patients that is associated with the use of devices, as occurs with endoscopic cell sheet transplantation50.
In summary, IC-2 sheets possess potent anti-fibrotic activity via the induction of MMPs and the suppression of HSC activation. A novel anti-fibrotic therapy based on cell sheet technology will be a potent option for liver fibrosis in the future.
Methods
Chemical compounds and cells
IC-222,23, a derivative of ICG-001, was synthesized in house and dissolved in dimethyl sulfoxide (DMSO). The final concentration of DMSO was 0.1%. UE7T-13 human bone marrow-derived MSCs51 were used. For experiments using bone marrow mononuclear cells adherent to culture dishes and CD90+/CD271+ cells, human bone marrow mononuclear cells were purchased from Lonza Inc. (Walkersville, MD) and were used at passage five as previously described22.
Preparation of cell sheets
IC-2-treated cell sheets were manufactured as follows. UE7T-13 cells were plated on ϕ 60-mm temperature-responsive culture dishes (CellSeed Inc., Tokyo, Japan) at a cell density of 9.0 × 103 cells/cm2, treated with 15 μM IC-2 for 1 week, and used as IC-2 sheets. MSC sheets were prepared by plating UE7T-13 cells at a density of 1.8 × 104 cells/cm2, cultured without IC-2 for 4 days, and used as MSC sheets. One day before transplantation, both cell sheets were detached from ϕ 60-mm temperature-responsive culture dishes by incubating them at 20 °C for ~30 min and then at 20 °C until use.
Adherent cells were harvested from bone marrow-derived mononuclear cells from Lonza, Inc. attached to culture dishes. CD90+/CD271+ cells were sorted from bone marrow-derived mononuclear cells from Lonza Inc. with a cell sorter using anti-CD90/anti-CD271 antibodies as previously described22. Cell sheets were prepared as follows; both cell types were plated on ϕ 60-mm temperature-responsive culture dishes at a cell density of 1.8 × 104 cells/cm2 and treated with 30 μM IC-2 for 11 days. Cell sheets were harvested from thermoresponsive polymer-coated culture dishes with CellShifter (CellSeed Inc.), which were overlaid with a ϕ 30-mm support membrane, by incubating them at 20 °C for ~3 h before transplantation.
siRNA-transfected cell sheet transplantation
For siRNA-transfected cell sheet transplantation, cell sheets were created as follows. UE7T-13 cells were plated onto culture dishes at a density of 9.0 × 103 cells/cm2 and treated with 15 μM IC-2 for 7 days. Culture media were replaced 4 days after seeding. Subsequently, 9.64 × 106 IC-2-treated cells were reverse transfected with 600 pmol siRNA using the Lipofectamine RNAiMAX reagent (Thermo Fisher Scientific Inc., MA), and reseeded onto ϕ 100-mm temperature-responsive culture dishes 1 day before harvesting. Six hours after reverse transfection, cells were treated with 15 μM IC-2. Silencer® select validated siRNA (s8879 for si-MMP14, s8849 for si-MMP1, and negative control no.1 siRNA for si-control) were purchased from Thermo Fisher Scientific Inc. siRNA-transfected cell sheets were detached from ϕ 100-mm temperature-responsive culture dishes by incubating them at 20 °C for ~15 min 1 day before transplantation and were incubated at 20 °C until use.
Cell sheet transplantation and biochemical tests
All animal experiments were conducted in accordance with the ethical approval of the Tottori University Subcommittee on Laboratory Animal Care. All mice were housed under pathogen-free conditions in a temperature-controlled, illuminated (12-h daily) room with ad libitum access to water and chow.
To induce chronic liver injury, carbon tetrachloride (CCl4) dissolved in olive oil (Wako Pure Chemical Industries Ltd., Osaka, Japan) was orally administered twice per week to 7–9-week-old BALB/c-nu/nu male mice (CLEA Japan, Inc., Tokyo, Japan) at a dose of 0.6 mL/kg for 4 weeks and a dose of 1.2 mL/kg for 6 to 7 weeks. One day before transplantation, the mice were subjected to liver function tests (e.g. serum ALT, AST, and total bilirubin) and their body weights were measured. Mice were equally divided into three groups according to liver function tests and body weight. Transplantation was performed 2 days after the last CCl4 dose.
In most cases, three-layer cell sheets were transplanted at two sites on the left lateral lobe. For experiments using adherent cells and CD90+/CD271+ cells, three-layer, half-sized cell sheets were transplanted at two sites on the left lateral lobe and middle lateral lobe. For siRNA-transfection experiments, two-layer cell sheets were transplanted at one site on the left lateral lobe. CCl4 administration was continued for another week after transplantation.
Two days after the final CCl4 dose, mice were sacrificed by exsanguination under anesthesia with pentobarbital sodium and blood samples were collected from the inferior vena cava, which was followed by liver resection. Mice transplanted with siRNA-transfected cell sheets were sacrificed 6 days after transplantation. A portion of the liver was fixed in 4% paraformaldehyde and embedded in paraffin for histological analysis. The remaining liver tissues were snap-frozen in liquid nitrogen.
Blood samples were maintained overnight on ice and serum was isolated by centrifugation at 2,000 × g for 20 min. Serum aminotransferase and total bilirubin levels were measured as previously reported21.
Details of other experimental protocols are provided in the Supplemental Information.
Supplementary information
Acknowledgements
We thank S. Okazaki, T. Kawasaki, I. Noda, N. Izumi and T. Yoshida for technical assistance. The authors would like to thank Editage (www.editage.jp) for the English language review. This work was supported by the project for realization of regenerative medicine, program for Creating STart-ups from Advanced Research and Technology from the Ministry of Education, Culture, Sports, Science and Technology in Japan, and KanonCure Inc.
Author Contributions
N.I., Y.K., K.W. and T.Y. performed the experiments. H.O. and M.M. synthesized IC-2. M.O. performed pathologic analysis. H.K. performed LC/MS analysis. N.I. and G.S. designed all the experiments and wrote the manuscript. G.S. supervised all the experiments.
Competing Interests
G.S. holds more than 5% of the total shares of KanonCure Inc. and receives compensation as a member of KanonCure Inc. Y.K. is employed by KanonCure Inc. The other authors have no competing interests to declare.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-43298-0.
References
- 1.Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017;14:397–411. doi: 10.1038/nrgastro.2017.38. [DOI] [PubMed] [Google Scholar]
- 2.Friedman SL. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N. Engl. J. Med. 1993;328:1828–1835. doi: 10.1056/NEJM199304223281620. [DOI] [PubMed] [Google Scholar]
- 3.Ge PS, Runyon BA. Treatment of patients with cirrhosis. N. Engl. J. Med. 2016;375:767–777. doi: 10.1056/NEJMra1504367. [DOI] [PubMed] [Google Scholar]
- 4.Su TH, Kao JH. Unmet needs in clinical and basic hepatitis B virus research. J. Infect. Dis. 2017;16:S750–S756. doi: 10.1093/infdis/jix382. [DOI] [PubMed] [Google Scholar]
- 5.Trautwein C, Friedman SL, Schuppan D, Pinzani M. Hepatic fibrosis: concept to treatment. J. Hepatol. 2015;62:S15–24. doi: 10.1016/j.jhep.2015.02.039. [DOI] [PubMed] [Google Scholar]
- 6.Torok NJ, Dranoff JA, Schuppan D, Friedman SL. Strategies and endpoint of antifibrotic drug trials: summary and recommendations from the AASLD emerging trends conference, Chicago, June 2014. Hepatology. 2015;62:627–634. doi: 10.1002/hep.27720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang DY, Friedman SL. Fibrosis-dependent mechanisms of hepatocarcinogenesis. Hepatology. 2012;56:769–775. doi: 10.1002/hep.25670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choudhary NS, Saraf N, Saigal S, Soin AS. Liver transplantation for acute on chronic liver failure. J. Clin. Exp. Hepatol. 2017;7:247–252. doi: 10.1016/j.jceh.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stutchfield BM, Forbes SJ, Wigmore SJ. Prospects for stem cell transplantation in the treatment of hepatic disease. Liver Transpl. 2010;16:827–836. doi: 10.1002/lt.22083. [DOI] [PubMed] [Google Scholar]
- 10.Kuo TK, et al. Stem cell therapy for liver disease: parameters governing the success of using bone marrow mesenchymal stem cells. Gastroenterology. 2008;134:2111–2121. doi: 10.1053/j.gastro.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puglisi MA, et al. Therapeutic implications of mesenchymal stem cells in liver injury. J. Biomed. Biotechnol. 2011;2011:860578. doi: 10.1155/2011/860578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shiota G, Itaba N. Progress in stem cell-based therapy for liver disease. Hepatol. Res. 2017;47:127–141. doi: 10.1111/hepr.12747. [DOI] [PubMed] [Google Scholar]
- 13.Volarevic V, Nurkovic J, Arsenijevic N, Stojkovic M. Concise review: Therapeutic potential of mesenchymal stem cells for the treatment of acute liver failure and cirrhosis. Stem Cells. 2014;32:2818–2823. doi: 10.1002/stem.1818. [DOI] [PubMed] [Google Scholar]
- 14.Zhao L, Chen S, Shi X, Cao H, Li L. A pooled analysis of mesenchymal stem cell-based therapy for liver disease. Stem Cell Res. Ther. 2018;9:72. doi: 10.1186/s13287-018-0816-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee CW, Chen YF, Wu HH, Lee OK. Historical perspectives and advances in mesenchymal stem cell research for the treatment of liver diseases. Gastroenterology. 2018;154:46–56. doi: 10.1053/j.gastro.2017.09.049. [DOI] [PubMed] [Google Scholar]
- 16.Alfaifi M, Eom YW, Newsome PN, Baik SK. Mesenchymal stromal cell therapy for liver diseases. J. Hepatol. 2018;68:1272–1285. doi: 10.1016/j.jhep.2018.01.030. [DOI] [PubMed] [Google Scholar]
- 17.Ohashi K, et al. Engineering functional two- and three-dimensional liver systems in vivo using hepatic tissue sheets. Nat. Med. 2007;13:880–885. doi: 10.1038/nm1576. [DOI] [PubMed] [Google Scholar]
- 18.Memon IA, et al. Repair of impaired myocardium by means of implantation of engineered autologous myoblast sheets. J. Thorac. Cardiovasc. Surg. 2005;130:1333–1341. doi: 10.1016/j.jtcvs.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida Y, et al. A role of Wnt/beta-catenin signals in hepatic fate specification of human umbilical cord blood-derived mesenchymal stem cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293:1089–1098. doi: 10.1152/ajpgi.00187.2007. [DOI] [PubMed] [Google Scholar]
- 20.Shimomura T, et al. Hepatic differentiation of human bone marrow-derived UE7T-13 cells: effects of cytokines and CCN family gene expression. Hepatol. Res. 2007;37:1068–1079. doi: 10.1111/j.1872-034X.2007.00162.x. [DOI] [PubMed] [Google Scholar]
- 21.Itaba N, et al. Human mesenchymal stem cell-engineered hepatic cell sheets accelerate liver regeneration in mice. Sci. Rep. 2015;10:16169. doi: 10.1038/srep16169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Itaba N, et al. Identification of the small molecule compound which induces hepatic differentiation of human mesenchymal stem cells. Regen. Ther. 2015;2:32–41. doi: 10.1016/j.reth.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Itaba N, et al. Hepatic cell sheets engineered from human mesenchymal stem cells with a single small molecule compound IC-2 ameliorate acute liver injury in mice. Regen. Ther. 2018;9:45–57. doi: 10.1016/j.reth.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Novobrantseva TI, et al. Attenuated liver fibrosis in the absence of B cells. J. Clin. Invest. 2005;115:3072–3082. doi: 10.1172/JCI24798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuppan D, Ashfaq-Khan M, Yang AT, Kim YO. Liver fibrosis: Direct antifibrotic agents and targeted therapies. Matrix Biol. 2018;68:435–451. doi: 10.1016/j.matbio.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Malla A, Lotersztajn S. Cellular mechanisms of tissue fibrosis. 5. Novel insights into liver fibrosis. Am. J. Physiol. Cell Physiol. 2013;305:C789–C799. doi: 10.1152/ajpcell.00230.2013. [DOI] [PubMed] [Google Scholar]
- 27.An SY, et al. Milk fat globule-EGF factor 8, secreted by mesenchymal stem cells, protects against liver fibrosis in mice. Gastroenterology. 2017;152:1174–1186. doi: 10.1053/j.gastro.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Shimizu H, Tsubota T, Kanki K, Shiota G. All-trans retinoic acid ameliorates hepatic stellate cell activation via suppression of thioredoxin interacting protein expression. J. Cell. Physiol. 2018;233:607–616. doi: 10.1002/jcp.25921. [DOI] [PubMed] [Google Scholar]
- 29.Okuyama H, et al. Overexpression of thioredoxin prevents thioacetamide-induced hepatic fibrosis in mice. J. Hepatol. 2005;42:117–123. doi: 10.1016/j.jhep.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 30.Foley CJ, Kuliopulos A. Mouse matrix metalloproteinase-1a (Mmp1a) gives new insight into MMP function. J. Cell. Physiol. 2014;229:1875–1880. doi: 10.1002/jcp.24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akcora BÖ, Storm G, Bansal R. Inhibition of canonical WNT signaling pathway by β-catenin/CBP inhibitor ICG-001 ameliorates liver fibrosis in vivo through suppression of stromal CXCL12. Biochim. Biophys. Acta. Mol. Basis Dis. 2018;1864:804–818. doi: 10.1016/j.bbadis.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Kimura K, et al. Safety, tolerability, and preliminary efficacy of the anti-fibrotic small molecule PRI-724, a CBP/β-catenin inhibitor, in patients with hepatitis C virus-related cirrhosis: A single-center, open-label, dose escalation phase 1 trial. EBioMedicine. 2017;23:79–87. doi: 10.1016/j.ebiom.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie C, et al. Quantification of hepatic vascular and parenchymal regeneration in mice. PLoS One. 2016;11:e0160581. doi: 10.1371/journal.pone.0160581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schuppan D. Structure of the extracellular matrix in normal and fibrotic liver: collagens and glycoproteins. Semin. Liver Dis. 1990;10:1–10. doi: 10.1055/s-2008-1040452. [DOI] [PubMed] [Google Scholar]
- 35.Amar S, Smith L, Fields GB. Matrix metalloproteinase collagenolysis in health and disease. Biochim. Biophys. Acta. 2017;1864:1940–1951. doi: 10.1016/j.bbamcr.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gioia M, et al. Characterization of the mechanisms by which gelatinase A, neutrophil collagenase, and membrane-type metalloproteinase MMP-14 recognize collagen I and enzymatically process the two alpha-chains. J. Mol. Biol. 2007;368:1101–1113. doi: 10.1016/j.jmb.2007.02.076. [DOI] [PubMed] [Google Scholar]
- 37.Aimes RT, Quigley JP. Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J. Biol. Chem. 1995;270:5872–5876. doi: 10.1074/jbc.270.11.5872. [DOI] [PubMed] [Google Scholar]
- 38.Will H, Atkinson SJ, Butler GS, Smith B, Murphy G. The soluble catalytic domain of membrane type 1 matrix metalloproteinase cleaves the propeptide of progelatinase A and initiates autoproteolytic activation. Regulation by TIMP-2 and TIMP-3. J. Biol. Chem. 1996;19:17119–17123. doi: 10.1074/jbc.271.29.17119. [DOI] [PubMed] [Google Scholar]
- 39.Knäuper V, et al. Cellular activation of proMMP-13 by MT1-MMP depends on the C-terminal domain of MMP-13. FEBS Lett. 2002;532:127–130. doi: 10.1016/S0014-5793(02)03654-2. [DOI] [PubMed] [Google Scholar]
- 40.Li H, et al. Immunological characterization of cell-surface and soluble forms of membrane type 1 matrix metalloproteinase in human breast cancer cells and in fibroblasts. Mol. Carcinog. 1998;22:84–94. doi: 10.1002/(SICI)1098-2744(199806)22:2<84::AID-MC3>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 41.Toth M, et al. Cleavage at the stem region releases an active ectodomain of the membrane type 1 matrix metalloproteinase. Biochem. J. 2005;387:497–506. doi: 10.1042/BJ20041324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Du C, et al. Transplantation of human matrix metalloproteinase-1 gene-modified bone marrow-derived mesenchymal stem cell attenuates CCl4-induced liver fibrosis in rats. Int. J. Mol. Med. 2018;41:3175–3184. doi: 10.3892/ijmm.2018.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tocci A, Forte L. Mesenchymal stem cell: use and perspectives. Hematol. J. 2003;4:92–96. doi: 10.1038/sj.thj.6200232. [DOI] [PubMed] [Google Scholar]
- 44.Mabuchi Y, et al. LNGFR(+)THY-1(+)VCAM-1(hi+) cells reveal functionally distinct subpopulations in mesenchymal stem cells. Stem Cell Reports. 2013;1:152–165. doi: 10.1016/j.stemcr.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hazra S, et al. Modulation of matrix metalloproteinase activity by EDTA prevents posterior capsular opacification. Mol. Vis. 2012;18:1701–1711. [PMC free article] [PubMed] [Google Scholar]
- 46.Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol. 2011;1:3. doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohuchi E, et al. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J. Biol. Chem. 1997;272:2446–2451. doi: 10.1074/jbc.272.4.2446. [DOI] [PubMed] [Google Scholar]
- 48.Nagamoto Y, et al. Transplantation of a human iPSC-derived hepatocyte sheet increases survival in mice with acute liver failure. J. Hepatol. 2016;64:1068–1075. doi: 10.1016/j.jhep.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 49.Baimakhanov Z, et al. Efficacy of multilayered hepatocyte sheet transplantation for radiation-induced liver damage and partial hepatectomy in a rat model. Cell Transplant. 2016;25:549–558. doi: 10.3727/096368915X688669. [DOI] [PubMed] [Google Scholar]
- 50.Maeda M, et al. Endoscopic cell sheet transplantation device developed by using a 3-dimensional printer and its feasibility evaluation in a porcine model. Gastrointest. Endosc. 2015;82:147–152. doi: 10.1016/j.gie.2015.01.062. [DOI] [PubMed] [Google Scholar]
- 51.Takeda Y, et al. Can the life span of human marrow stromal cells be prolonged by bmi‐1, E6, and E7 and/or telomerase without affecting cardiomyogenic differentiation? J. Gene Med. 2004;6:833–845. doi: 10.1002/jgm.583. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.