Abstract
Aim/Objective: To assess the diagnostic performance of TrueHb® point-of-care (POC) hemometer compared with Sysmex i3 analyzer at International Hospital Kampala, Uganda.
Materials and methods: We analyzed ethylenediaminetetraacetic acid blood samples to estimate hemoglobin (Hb) levels using parallel testing with TrueHb® hemometer and Sysmex i3 analyzer. Data were analyzed to ascertain the diagnostic performance of the test assays using the Bland and Altman method. Sensitivity, specificity, positive and negative predictive values were calculated.
Results: The study enrolled 402 patients; of these, 156 (38.8%) were males. The average Hb levels were 8.7±1.8 and 13.3±2.6 g/dL for the anemic and nonanemic patients, respectively. One hundred and fifty-five participants were anemic, giving anemia prevalence of 38.56% (95% CI: 35.17–40.38). The mean difference of the TrueHb® and Sysmex i3 assays was 2.2219 (SD 1.07915), and the two devices did not show a difference in their measurements (t=−2.407, p-value 0.017, 95% CI: −0.095–0.010). Further, they showed a significant level of agreement (t=41.281; 95% CI: 2.1161–2.3277) and intraclass correlation coefficients (ICC=0.793). The sensitivity, specificity, positive and negative predictive values were 100.00%, 51.01%, 55.16% and 100.00%, respectively. The average performance turnaround time (TAT) for the TrueHb® hemometer was 2.46 mins (95% CI: 2.37–2.55).
Conclusion: TrueHb® POC hemometer is an accurate POC for Hb estimation with a good performance agreement with the Sysmex i3 analyzer. This, coupled with its utility aspects, makes it a good diagnostic tool in a high anemia burden and low-resource setting.
Keywords: anemia, hemoglobin estimation, point-of-care testing, TrueHb®, Sysmex i3, Uganda
Background
The burden of anemia remains unacceptably high in sub-Saharan Africa.1–3 It is linked to numerous life-threatening consequences like poor cognitive ability, diminished immunity and aggravated mortality.3 To avert its effect, timely and accurate determination of hemoglobin concentration has been recognized.4,5 This has taken varied determination methods such as cyanmethemoglobin, use of copper II sulfate, automated hematology analyzers and point-of-care (POC) devices as the latest invention.6–10
While the automated analyzers are considered the gold standard assay, it is greatly limited by poor electricity connections in most areas, prohibitive purchase prices, installation, trainings as well as daily maintenance costs.9–11 This phenomenon agitates the need for cheap, easy-to-use and accurate methods.5,9,10 Due to limited electricity supply in most parts of developing countries, the cyanmethemoglobin, gravimetric copper II sulfate and color code hemoglobin estimation methods are commonly used;7–9,12 however, these are inaccurate and are subjective to a high observer error.13 Cognizant to these, efforts to find affordable, sensitive, specific, user-friendly, rapid and robust, equipment-free and deliverable to end users (ASSURED) have proven ideal leading to invention of POC devices for resource-limited settings that are easy to use, are fieldable, use microvolume of blood, are fast, have shortened turnaround time(TAT), have high accuracy and are well-interpreted.6,7,11,14 Consequently, this has led to the invention of varied POC devices with diverse performance. They include hemoglobin color scale that rely on color comparison of blood absorbed on chromatography paper with a standard red-based color scale, a battery-operated photometer branded as the Hemocue® (Hemocue AB, Angelholm, Sweden) that is based on modified azide methemoglobin measurement, DiaSpect hemoglobin T system (DiaSpect Medical GmbH, Sailauf, Germany) that utilizes multichromatic sensor to measure its absorbance over a wide spectral range and the latest being TrueHb® (Wrig Nanosystems Pvt. Ltd, New Delhi, India).
TrueHb® hemometer is an in vitro diagnostic device that utilizes fresh capillary whole blood samples to screen for anemia.15 It is based on the principle of reflectance photometry following the conversion of hemoglobin in the sample to a complex with the help of proprietary set of reagents present in the strip (Wrig Nanosystems Pvt. Ltd). A previous study to validate TrueHb® has indicated a strong positive correlation with the Sysmex hematology analyzer and a better POC device for screening anemia.4
Despite the availability of numerous POC devices to screen anemia, none has become widely utilized in our settings where they are desired. They are limited by low reliability, being affected by humid environments and are very expensive to suit the requirements of a limited resource setting.10 Further, the availability of numerous POC devices has posed an impasse in the choice of an appropriate device for clinical care, research and blood banking. This study assessed the diagnostic performance of a new invention, TrueHb® POC hemometer, in comparison with Sysmex i3 analyzer to elucidate its scientific evidence and feasibility for use at International Hospital Kampala (IHK) in Uganda.
Materials and methods
Study site, design and duration
This was a cross-sectional laboratory-based study conducted during the period of March to April 2018 in the Hematology Laboratory at IHK, located on plot 4686 Kisugu, Namuwongo, a Kampala suburb. The facility started in 1999 as a private hospital offering extended medical services for an array of patients, and it runs about 100 blood samples daily from both inpatients and outpatients. Hb estimation is routinely performed as part of the complete blood count (CBC) using the Sysmex i3 analyzer. The laboratory participates in the external proficiency of National Health Laboratory Scheme, South Africa.
Study population, sample size estimation and recruitment criteria
Our study comprised both pediatric and adult patients seeking medical services at IHK whose clinical condition(s) required laboratory hematological investigations and who consented/assented to participate.
Being a validation study, we conveniently considered 402 blood samples, basing on the available logistics. Venous blood samples were collected by venipuncture according to the WHO-recommended standard operating procedures16 and were analyzed within 1 hr of collection. In situations of anticipated delays, the samples were kept refrigerated at 2–8°C and allowed to equilibrate at room temperature before analysis.
The samples were investigated for CBC using Sysmex i3 analyzer as requested by the attending clinician who was independent of the study. We excluded clotted blood samples, those from neonates patients with a known bleeding diathesis.
Laboratory analyses
Samples were assayed to estimate the hemoglobin concentration using the TrueHb® hemometer (Wrig Nanosystems Pvt. Ltd) and Sysmex i3 hematology analyzer (Sysmex Corporation Kobe, Japan) according to the manufacturers’ instructions.
The Hb levels performed by the Sysmex i3 analyzer were considered as the gold standard. Assessment of the degree of anemia was based on the cutoff values for age and gender as defined by the WHO criteria. Anemia was defined as Hb levels less than 11.0 g/dL, 11.5 g/dL and 12.0 g/dL for children aged 6–59 months, 5–11 years and 12–14 years, respectively. For adults, anemia was considered as Hb concentration less than 12.0 g/dL in nonpregnant women, 11.0 g/dL in pregnant women and 13.0 g/dL in males.17
Quality control
The performance of the TrueHb® hemometer and the Sysmex i3 hematology analyzer was standardized based on the biological control (BC)-3D three levels of low, normal and high ran daily. The function of the TrueHb® hemometer was checked daily by measuring a control sample. Strict adherence of the work instructions for each of the devices was ensured, and instruments were maintained according to manufacturer’s specifications, and the personnel involved in both the TrueHb® hemometer and the Sysmex i3 analyzer were blinded to the results.
Statistical analysis
Data were entered into a statistical software package of EPI info version 3.5.3. It was cleaned, validated and transferred to STATA 12 (College Station, TX, USA) for analysis. Proportions of participants with anemia were analyzed as the number of those with the Hb levels below the upper limit of the range for an individual’s sex and age divided by the total number of participants. Agreement between the test methods was assessed using the Bland and Altman method,18 where the mean, standard deviation and limit of agreement of paired results were calculated and accuracy was estimated by calculating bias values along with intraclass correlation coefficients (ICCs). The ICCs were interpreted as follows: <0: poor; 0.01–0.20: slight agreement; 0.21–0.40: fair agreement; 0.41–0.60: moderate agreement; 0.61–0.80: substantial agreement and 0.81–1.00: almost perfect agreement.18 Performance evaluation was based on measurement of: sensitivity=true positives (TP)/[TP+false negatives (FN)]; specificity=TN/[false positives (FP)+TN]; positive predictive value (PPV)=TP/(TP+FP); negative predictive value (NPV)=TN/(TN+FN).
Ethical approval
Ethical approval was obtained from the research and ethics committee of Clarke International University, Kampala, Uganda. Permission to use patients’ samples was sought from the director and laboratory manager of IHK. Also, we obtained patients written consent (and written assent in case of minors) for the study. For those patients who were under the age of 18 years, their parents or adult caregiver gave the written informed consent. Laboratory numbers were used during data entry to ensure the confidentiality of participants.
Results
Demographic characteristics
We enrolled 402 participants, of these, 156 (38.8%) were male and 246 (61.2%) female. Participants had varied age categories, of whom 65 (16.2%) were 1 month–5 years, 42 (10.4%) were 5–11 years, 12 (3.0%) were 12–14 years, while 283 (70.4%) were 15 years and older. The average levels of Hb were 8.7±1.8 and 13.3±2.6 g/dL for the anemic and nonanemic participants, respectively (p=0.0001).
Prevalence of anemia
Of the 402 participants, 155 had Hb levels indicative of anemia as measured using the Sysmex i3, giving a prevalence of 38.56% (95% CI: 35.17–40.38) among the different age and gender with varied clinical degrees as shown in Tables 1 and 2.
Table 1.
Clinical severity by age and gender categories using Sysmex i3 analyzer
| Age group | Anemia status, N (%) | Total | |||
|---|---|---|---|---|---|
| Normal | Mild anemia | Moderate anemia | Severe anemia | ||
| Child 6 months–5 years | 49 (19.8) | 6 (7.6) | 9 (14.5) | 1 (7.1) | 65 |
| Child 5–11 years | 24 (9.7) | 2 (2.5) | 14 (22.6) | 2 (14.3) | 42 |
| Child 12–14 years | 11 (4.5) | 0 (0.0) | 1 (1.6) | 0 (0.0) | 12 |
| >15 years – men | 80 (32.4) | 11 (15.5) | 7 (21.9) | 1 (7.1) | 99 |
| >15 years – women | 83 (33.6) | 60 (84.5) | 31 (50.0) | 10 (71.4) | 184 |
| Total | 247 | 79 | 62 | 14 | 402 |
Table 2.
Clinical severity by age and gender categories using TrueHb® hemometer
| Age group | Anemia status | Total | |||
|---|---|---|---|---|---|
| Normal | Mild anemia | Moderate anemia | Severe anemia | ||
| Child 6 months–5 years | 22 (17.5) | 11 (13.9) | 22 (15.5) | 10 (18.2) | 65 |
| Child 5–11 years | 12 (9.5) | 10 (12.7) | 11 (7.7) | 9 (16.4) | 42 |
| Child 12–14 years | 3 (2.4) | 3 (3.8) | 4 (2.8) | 2 (3.6) | 12 |
| >15 years – men | 49 (38.9) | 27 (34.2) | 16 (11.3) | 7 (12.7) | 99 |
| >15 years – women | 40 (31.7) | 28 (35.4) | 89 (62.7) | 27 (49.1) | 184 |
| Total | 126 | 79 | 142 | 55 | 402 |
Using the Sysmex i3 (Table 1), a total of 65 children were aged 6 months to 5 years. Among these, 16 had anemia, with mild, moderate and severe forms occurring in 9 (14.5%), 6 (7.6%) and 1 (7.1%), respectively. Among those aged 5–11 years, 18 children had anemia, with mild, moderate and severe forms occurring in 2 (2.5%), 14 (22.6%) and 2 (14.3%), respectively. The least number of anemic cases was among the 12–14 years age category; a single case presented with moderate form of anemia. Among men who were >15 years of age, 19 participants were anemic; of these, 11 (15.5%) had mild anemia, 7 (21.9%) had moderate anemia while 1 (7.1%) had severe anemia. The prevalence of anemia was high among women >15 years of age. Of these, 60 (84.5%) had mild anemia, 31 (50.0%) had moderate anemia and 10 (71.4%) were severely anemic.
In the TrueHb (Table 2), a total of 65 children were aged 6 months to 5 years. Among these, 43 (66.2%; 95% CI: 61.2–70.6) presented with varied anemia in which 9 (20.9%) were moderately anemic, 6 (14.0%) had mild anemia and 1 (2.3%) was severely anemic. Among those aged 5 to 11 years, 30 children had anemia, with mild, moderate and severe forms occurring in 2 (6.7%), 14 (46.7%) and 2 (6.7%), respectively. There were 9 (75.0%; 95% CI: 72.2–78.6) anemic children among the 12–14 years age category, of which 3 (33.3%) were mildly anemic, 4 (44.4%) were moderately anemic and 2 (22.2%) were severely anemic. Among men who were >15 years of age, 50 (50.5%) participants were anemic, of which 27 (54.0%) had mild anemia, 16 (32.0%) had moderate anemia while 7 (14.0%) had severe anemia. The prevalence of anemia among women >15 years of age was 144 out of 184 (78.3%; 95% CI: 73.1–82.6). Of these, 28 (19.4%) had mild anemia, 89 (61.8%) had moderate anemia and 27 (18.8%) were severely anemic.
Diagnostic performance of the TrueHb® hemometer and Sysmex i3 analyzer
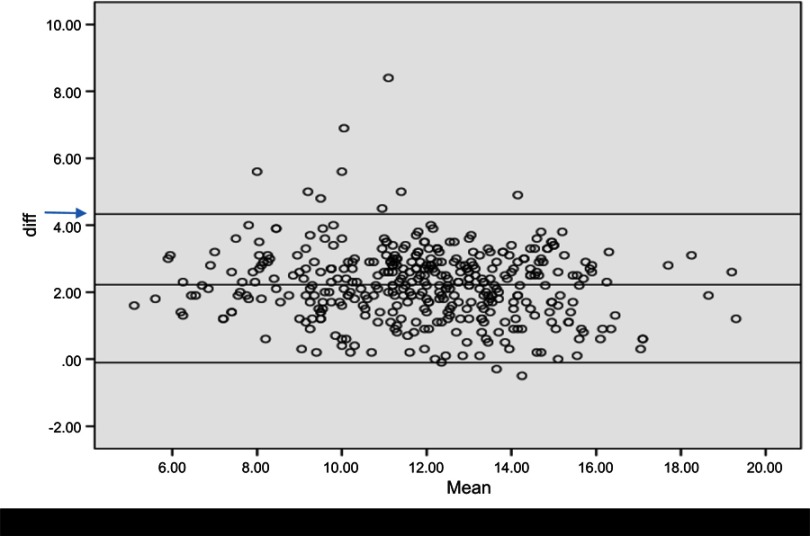
The mean difference of the two methods was 2.2219 (SD 1.07915); there was no difference in the measurements by the two analyzers (t=−2.407, p-value 0.017, 95% CI: −0.095–0.010) and a significant level of agreement (t=41.281, 95% CI: 2.1161–2.3277), as shown in Figure 1.
Figure 1.
The Bland–Altman plot showing the diagnostic performance of the TrueHb® hemometer and Sysmex i3 analyzer. From Figure 1, the mean difference of the two methods was 2.2219, indicated by the arrow in blue (→).
The average measure of accuracy of the two assays as measured by the ICC was 0.793, which indicates a moderate correlation between the two assays as shown in Table 3.
Table 3.
Intraclass correlation coefficient of the two analyzers
| Intraclass correlationb | 95% confidence interval | F test with true value 0 | |||||
|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | Value | df1 | df2 | Significance | ||
| Single measures | 0.657a | −0.072 | 0.889 | 21.081 | 401 | 401 | 0.000 |
| Average measures | 0.793c | −0.156 | 0.941 | 21.081 | 401 | 401 | 0.000 |
Notes: Cronbach’s alpha 0.953.
aThe estimator is the same, whether the interaction effect is present or not.
bType A intraclass correlation coefficients using an absolute agreement definition.
cThis estimate is computed assuming the interaction effect is absent, otherwise it is not estimable.
Data from the two assays are summarized in a 2 by 2 table (Table 4), to aid the computation of the diagnostic performance of the two methods.
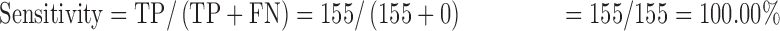 |
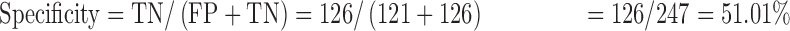 |
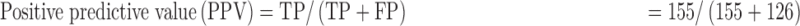 |
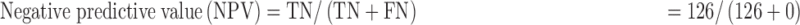 |
Table 4.
Diagnostic perfomance evaluation of the TrueHb® hemometer
| Disease | |||
|---|---|---|---|
| Present (n) | Absent (n) | ||
| Test | Positive | TP (155) | FP (121) |
| Negative | FN (0) | TN (126) | |
Abbreviations: FN, false negative; FP, false positive; TN, true negative; TP, true positive
The sensitivity, specificity, PPV and NPV of the TrueHb® were 100.00%, 51.01%, 55.16% and 100.00%, respectively.
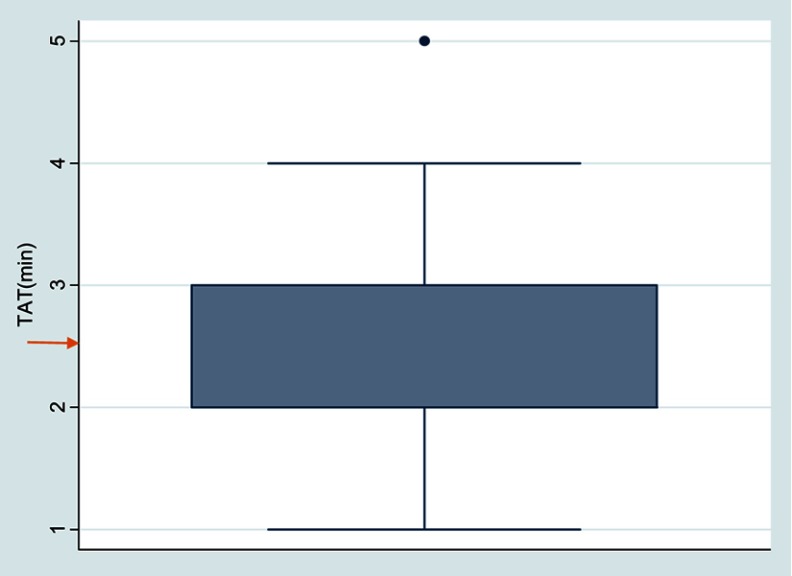
The average performance TAT for the TrueHb® hemometer was 2.46 mins (95% CI: 2.37–2.55) as indicated in Figure 2.
Figure 2.
A whisk plot showing the TAT of the TrueHb® hemometer. From Figure 2, the average performance TAT for the TrueHb® hemometer was 2.46 mins, indicated by an arrow in orange.
Abbreviation: TAT, turnaround time.
Discussion
Understanding the epidemiological burden of anemia is critical to the monitoring of the health status of the population. However, screening for anemia is not routinely done in our setting and is limited to pre-blood donation, antenatal care checkup, monitoring of individuals infected with HIV regarding disease progression and response to antiretroviral therapy. This makes Hb evaluation selective, which is mostly being performed among people who come to health facilities. Studies evaluating the burden of anemia in Uganda have focused on the vulnerable groups (pregnant women and children), and in all, the prevalence of anemia has been reported high (32.9–58.8%).19,20
In this study, the overall prevalence of anemia was 38.56% (95% CI: 35.17–40.38). Among children under 5 years of age, the prevalence was 24.6% (95% CI: 19.4–29.7), a value lower than 58.8% that was reported among children aged 6–59 months in Eastern Uganda.19 This is probably due to a different population studied. On the other hand, the prevalence is higher (34.4%) than that reported among pregnant women in Gulu (22.1%),20 in Hoima (32.9%)21 and in Kenya (28.8%).22 The higher prevalence in this group is due to the fact that our respondents comprised sick population with a clinical assessment of hematological dysfunction. Although our study population comprised sick participants, the prevalence of anemia is high, suggesting a high burden among all age categories. This underscores the need for intervention to be taken to minimize the burden of anemia and prevent the associated morbidity and mortality.
The mean difference in the Hb levels estimated by the two analyzers was 2.2219 (SD 1.07915), and a useful level of agreement was found (t=41.281, 95% CI: 2.1161–2.3277). Furthermore, there was no difference in the measurements obtained by the two machines (t=−2.407, p-value 0.017, 95% CI: −0.095–0.010), with ICC=0.793, which indicated a moderate correlation between the two analyzers. This finding is similar to what was reported in previous studies carried out in India,4,5,23 in which there was a linear distribution with positive correlations (r=0.99 and 0.80, respectively) and the mean difference in Bland–Altman plots of TrueHb® against the Sysmex values suggested good repeatability. This positive correlation makes TrueHb® hemometer an ideal device for use in resource-limited setting. Use of POC ought to gain more diagnostic value as it utilizes microvolumes of blood with minimal invasive procedure and rapid test results. In this way, the acceptable degree of agreement means that TrueHb® use would warrant home-based anemia screening to ensure timely care intervention.
The sensitivity, specificity, PPV and NPV of the TrueHb® hemometer were 100.00%, 51.01%, 55.16% and 100.00%, respectively. The sensitivity in this study is higher than 74.4%, while the specificity was lower than 86.9% obtained in a study carried out in India.5 The 100.00% sensitivity makes this device ideal to correctly screen for anemia. On the other hand, the low specificity (51.01%) from this assay limits its usefulness to correctly rule out anemia. This is in tandem with the previous studies that were conducted using TrueHb®.5 This study further reiterates the lower specificity of this device and is ascribed to the effect of venous blood samples.5 Although the results of suitability for use, portability, cost of device, recurring cost, efficiency in daylight, average TAT and skills for the operation of the device; this study has established that the TrueHb® hemometer is inexpensive, rapid and simple to use with good accuracy. The aptness for use of this device as a way to relay its use for community or primary health care use in a limited resource setting affirms findings from an earlier study that it is inexpensive, rapid and simple to use with good accuracy.4
The TrueHb® hemometer had an average TAT of 2.46 mins, lower than that reported from a previous study which showed an average TAT time of 5 mins.23 The shortened TAT will aid in quick decision-making in a health facility for the benefit of patients.
The results of this study are encouraging; however, they ought to be interpreted in light of the following: 1) although recommended to use capillary blood sample, this study used ethylenediaminetetraacetic acid anticoagulated venous sample and 2) this study did not elucidate the possible causes of anemia such as HIV, malaria, helminthiasis, hemoglobinopathies or lead intoxication. Since identification of the cause for anemia is crucial in providing effective management strategies, additional testing is necessary to determine the etiology of anemia and lessen its burden.
Conclusion and recommendations
The findings from this study have indicated that TrueHb® hemometer is reasonably an accurate POC screening assay for anemia. Further, it has a good performance agreement with the Sysmex i3 analyzer, a feature which coupled with its ease of use, portability, costs, TAT and operating skills offers it an irresistible diagnostic value. In a setting with high burden of anemia, it is valuable in timely provision of interventions and critical decisions like hospital admission, blood transfusion to a patient and posttransfusion assessment of Hb levels. This offers a diagnostic utility in lower-level health centers where a decision to refer patients to hospital for blood transfusion will be made easier. Also, this assay may be validated to extend its utility in donor testing and blood bank settings.
Acknowledgments
We are indebted to the laboratory staff of Lancet Laboratories, International Hospital Kampala branch for the assistance accorded during the time of data collection. We also acknowledge the logistic support from Wrig Nanosystems, India, who provided the evaluation products, and Reliable Diagnostic Supplies paid for the logistics. This study did not receive any funding for this study.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The principal investigator received a generous donation of the TruHb® hemometer and the test strips from the manufacturer (Wrig Nanosystems, India); however, this did not in any way interfere with the test method, results obtained, interpretation of the results as well as the decision to publish. The authors report no other conflicts of interest in this work.
References
- 1.McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information system, 1993–2005. Public Health Nutr. 2009;12(4):444–454. doi: 10.1017/S1368980008002401 [DOI] [PubMed] [Google Scholar]
- 2.Balarajan Y, Ramakrishnan U, Ozaltin E, Shankar AH, Subramanian SV. Anemia in low and middle income countries. Lancet. 2011;378:2123–2135. doi: 10.1016/S0140-6736(11)60984-7 [DOI] [PubMed] [Google Scholar]
- 3.Stevens GA, Finucane MM, De-Regil LM, et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. Lancet Global Health. 2013;1(1):e16–e25. doi: 10.1016/S2214-109X(13)70001-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anand H, Mir R, Saxena R. Hemoglobin color scale a diagnostic dilemma. Indian J Pathol Microbiol. 2009;52:360–362. doi: 10.4103/0377-4929.54994 [DOI] [PubMed] [Google Scholar]
- 5.Neogi SB, Negandhi H, Kar R, et al. Diagnostic accuracy of haemoglobin colour strip (HCS-HLL), a digital haemoglobinometer (TrueHb) and a non-invasive device (TouchHb) for screening patients with anaemia. J Clin Pathol. 2015. doi: 10.1136/jclinpath-2015-203135 [DOI] [PubMed] [Google Scholar]
- 6.Morris SS, Ruel MT, Cohen RJ, Dewey KG, de la Briere B, Hassan MN. Precision, accuracy, and reliability of hemoglobin assessment with use of capillary blood. Am J Clin Nutr. 1999;69(6):1243–1248. doi: 10.1093/ajcn/69.6.1243 [DOI] [PubMed] [Google Scholar]
- 7.Nkrumah B, Nguah SB, Sarpong N, et al. Hemoglobin estimation by the Hemocue portable haemoglobin photometer in a resource poor setting. BMC Clin Pathol. 2011;11:5. doi: 10.1186/1472-6890-11-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paddle JJ. Evaluation of the haemoglobin colour scale and comparison with the Hemocue haemoglobin assay. Bull World Health Organiz. 2002;80:813–816. [PMC free article] [PubMed] [Google Scholar]
- 9.Sari M, dePee S, Martini E, Herman S, Bloem MW, Yip R. Estimating the prevalence of anaemia:a comparison of three methods. Bull World Health Organiz. 2001;79:506–511. [PMC free article] [PubMed] [Google Scholar]
- 10.Singh A, Dubey A, Sonker A, Chaudhary R. Evaluation of various methods of point-of-care testing of haemoglobin concentration in blood donors. Blood Transfusion. 2015;13(2):233–239. doi: 10.2450/2014.0085-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mabey D, Peeling RW, Ustianowski A, Perkins MD. Tropical infectious diseases: diagnostics for the developing world. Nat Rev Microbiol. 2004;2:231–240. doi: 10.1038/nrmicro841 [DOI] [PubMed] [Google Scholar]
- 12.Ughasoro MD, Emodi IJ, Okafor HU, Ibe BC. Prevalence and risk factors of anaemia in paediatric patients in South-East Nigeria. S Afr J Child Health. 2015;9(1):14–17. doi: 10.7196/SAJCH.760 ISSN 1999-7671. [DOI] [Google Scholar]
- 13.Lee SJ, Stepniewska K, Anstey N, et al. The relationship between the haemoglobin concentration and haematocrit in plasmodium falciparum malaria. Malar J. 2008;7:149. doi: 10.1186/1475-2875-7-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou X, Yan H, Xing Y, Dang S, Zhuoma B, Wang D. Evaluation of portable haemoglobin photometer in pregnant women in a high altitude area: a pilot study. BMC Public Health. 2009;9(1):228. doi: 10.1186/1471-2458-9-253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.TrueHb® Hemometer System user manual.
- 16.WHO guidelines on drawing blood: best practices in phlebotomy. 2010. [PubMed]
- 17.WHO. Geneva; Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization; 2011. (WHO/NMH/NHD/MNM/11.1) Available from: http://www.who.int/vmnis/indicators/haemoglobin. [Google Scholar]
- 18.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. doi: 10.1016/S0140-6736(86)90837-8 [DOI] [PubMed] [Google Scholar]
- 19.Kuziga F, Adoke Y, Wanyenze RK. Prevalence and factors associated with anaemia among children aged 6 to 59 months in Namutumba district, Uganda: a cross-sectional study. BMC Pediatr. 2017;17(1):25. doi: 10.1186/s12887-017-0782-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Legason ID, Atiku A, Ssenyonga R, Olupot-Olupot P, Barugahare JB. Prevalence of anaemia and associated risk factors among children in North-western Uganda: a cross sectional study. BMC Hematol. 2017;17:10. doi: 10.1186/s12878-017-0081-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Obai G, Odongo P, Wanyama R. Prevalence of anaemia and associated risk factors among pregnant women attending antenatal care in Gulu and Hoima Regional Hospitals in Uganda: a cross sectional study. BMC Pregnancy Childbirth. 2016;16:76. doi: 10.1186/s12884-016-0982-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ngesa O, Mwambi H, Stoute JA. Prevalence and risk factors of anaemia among children aged between 6 months and 14 years in Kenya. PLoS One. 2014;9(11):e113756. doi: 10.1371/journal.pone.0113756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srivastava A, Koul V, Dwivedi SN, Upadhyaya AD, Ahuja A, Saxena R. Performance analysis of newly developed point-of-care hemoglobinometer (TrueHb) against an automated hematology analyzer (Sysmex XT 1800i) in terms of precision in hemoglobin measurement. Int J Lab Hematol. 2015;37(4):483–485. doi: 10.1111/ijlh.12314 [DOI] [PubMed] [Google Scholar]