Abstract
Objective: To investigate the expression of LncRNA MNX1-AS1 in NSCLC and its effect on NSCLC cell lines.
Methods: In this experiment, the expression of LncRNA MNX1-AS1 was detected by qRT-PCR in 116 NSCLC samples, and the correlation between MNX1-AS1 and NSCLC patients was further analyzed by chi-square test. The prognostic value of MNX1-AS1 was assessed by Kaplan-Meier survival curve. The expression of MNX1-AS1 in NSCLC cell line A549 was knocked down, and the effects of MNX1-AS1 on proliferation, apoptosis, migration and invasion of NSCLC cells were evaluated.
Results: Compared with normal lung tissue, the expression of MNX1-AS1 was significantly increased in lung cancer tissues (p<0.05). The expression level of MNX1-AS1 in NSCLC cell line A549 was significantly higher than that in human normal lung epithelial cell line Beas-2B (p<0.05). MNX1-AS1 expression was significantly associated with TNM stage and lymph node metastasis (p<0.05). Kaplan-Meier survival curve analysis showed that high expression of MNX1-AS1 was associated with a poor prognosis in NSCLC. In addition, knockdown of MNX1-AS1 inhibited proliferation, migration and invasion of the NSCLC cell line A549 and promoted apoptosis.
Conclusion: The up-regulation of LncRNA MNX1-AS1 is associated with the progression and prognosis of NSCLC. Knockdown of LncRNA MNX1-AS1 inhibits proliferation, migration and invasion of NSCLC cells and promotes apoptosis.
Keywords: non-small cell lung cancer, LncRNA MNX1-AS1, prognosis, A549 cell line
Introduction
Lung cancer is the most malignant tumor with the highest morbidity and mortality in the world. According to the International Agency for Research on Cancer(IARC) 2012, the incidence of lung cancer ranks first in the incidence of all malignant tumors.1 Non-small cell lung cancer (NSCLC) is the main type of lung cancer, accounting for about 80% of all lung cancer.2 NSCLC is mainly classified into squamous cell carcinoma, adenocarcinoma, adenosquamous carcinoma, large cell lung cancer, and sarcomatoid carcinoma. NSCLC lacks obvious clinical manifestations in the early stage, and most patients have discovered lymphatic metastasis and distant metastasis when they are found. Therefore, patients with NSCLC have a poor prognosis, and the 5-year survival rate is usually less than 20%,3 which is a serious threat to human life and health. Studying the mechanism of NSCLC occurrence and development is crucial for the diagnosis and treatment of NSCLC.
With the development of molecular biology technology, long-chain non-coding RNAs (lncRNAs) have gradually become a research hotspot, playing an important role in many biological activities such as tumorigenesis,4 cell growth5 and transcriptional regulation.6 LncRNA dysfunction is associated with a variety of diseases, including cancer,7 cardiovascular diseases8 and degenerative diseases.9 A large number of studies on NSCLC and LncRNA have shown that a variety of LncRNAs can play an important role in the formation and progression of NSCLC through different signaling pathways. LncRNA MNX1-AS1 (MNX1 antisense RNA1 (head to head)), also known as CCAT-5, is located on chromosome 7 and can be transcribed to generate 992 bp of non-coding RNA. There are currently few reports on MNX1-AS1. Wu10 found MNX1-AS1 abnormally high expressed in gallbladder carcinoma, and its co-expression network was associated with 23 lncRNAs and 39 mRNAs, which include tumor-associated protein-coding genes such as trim59, otx1, HOXB9, and Semac, suggesting its relationship with the pathogenesis of gallbladder cancer. Lv et al11 also confirmed that MNX1-AS1 was an oncogene in the pathogenesis of ovarian cancer. However, there was no relevant report on the relationship between LncRNA MNX1-AS1 and NSCLC.
In this study, we investigated the expression of LncRNA MNX1-AS1 in NSCLC and further analyzed the biological function of MNX1-AS1 in the progression of NSCLC.
Materials and methods
NSCLC tumor tissue and cell line
Tumor tissues and normal adjacent lung tissues of 116 patients with non-small cell lung cancer who were surgically resected from January 2010 to January 2013 were selected. The tumor tissues were confirmed to be NSCLC by the department of pathology of the No. 988 hospital of the joint logistic support force of the Chinese people’s liberation army.The clinical and follow-up data of all patients were complete, and no radiotherapy, chemotherapy or other tumor-related treatments were performed before surgery. Lymph node metastasis and TNM staging referred to the 7th International Union Against Cancer (2009). All lung cancer patients were reviewed regularly and the follow-up period was up to January 2018. Survival time refers to the initial hospitalization to death or the last follow-up. The study was approved by the Medical Ethics Committee of the No. 988 hospital of the joint logistic support force of the Chinese people’s liberation army and all patients signed informed consent. The non-small cell lung cancer cell line A549 and the human normal lung epithelial cell line Beas-2B were purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences.
The cells were cultured in RPMI-1640 medium (Thermo Fisher Scientific, Waltham, MA, USA) containing 10% fetal bovine serum (FBS, Life Technologies, USA), 100 U/ml penicillin and 100 mg/ml streptomycin (Gibco, NY, USA) in a humidified incubator at 37 °C in 5% CO2.
qRT-PCR
The expression level of MNX1-AS1 in tissues was detected using qRT-PCR. Total RNA was extracted from tissues using TRIzol reagent (Invitrogen). RNA was reverse transcribed using the SuperScript First Strand cDNA system (Invitrogen) according to the manufacturer’s instructions. PCR amplification was performed on an Applied Biosystems 7900HT (Applied Biosystems). The relative expression level of MNX1-AS1 was calculated by the 2−ΔΔCt method using GAPDH as an internal reference. qRT-PCR reaction conditions: 95 °C for 10 min, 95 °C for 15 s, 72°C for 15 s, a total of 40 cycles. Primer sequences were designed as follows: MNX1-AS1, 5ʹ-CCCGCATTTTCAGATTCAC-3ʹ(sense) and 5ʹ-GCTCTCAGCCTCGCCATA-3ʹ(antisense); GAPDH, 5ʹ-GTCAACGGATTTGGTCTGTATT-3ʹ(sense) and 5ʹ-AGTCTTCTGGGTGGCAGTGAT-3ʹ(antisense).
Transfection
The lentivirus knocking out MNX1-AS1 and the corresponding negative control lentivirus were purchased from GenePharma (Shanghai, China). Si-control and si-MNX1-AS1 lentiviruses were transfected into A549 cells respectively, using Lipofectamine 2000 transfer reagent according to the instructions. Cell lines stably transfected with lentivirus were harvested for subsequent assays.
Cell proliferation and colony formation experiments
Cell proliferation was measured using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell proliferation/viability assay kit (Sigma-Aldrich, USA). After 48 h of transfection, the cells were seeded at 1×105 cells/well into 96-well plates with 6 replicate wells per group. After further 48 h of incubation, 20 μL of MTT (5 g/L) was added to each well and incubated for 4 h. After discarding the MTT, 150 μL DMSO was added to shake and fully dissolved. The absorbance OD was measured at 490 nm by a microplate reader (Thermo, USA).
The transfected cells were seeded into the culture plate at 200 cells/well, and after 2 weeks of incubation, the cells were washed with PBS and stained with Giemsa solution. The number of colonies containing >50 cells were observed under the microscope.
Apoptosis experiment
Apoptosis was detected by flow cytometry (Becton Dickinson, USA) using Annexin-V and propidium iodide (PI) apoptosis detection kit (Sigma-Aldrich, USA). The cells transfected for 48 h were digested, resuspended in Binding Buffer, mixed and added with 5 μL of Annexin V, stained for 10 min in the dark, and then added with 50 mg/L PI staining at room temperature for 5 min. Flow cytometry was used to detect the proportion of Annexin V positive cells to determine the apoptotic rate. Three repetitions per group.
Cell migration and invasion experiments
The transfected cells were inoculated into the upper chamber of Transwell chamber (Corning) at 1×105 cells/mL, and the RPMI-1640 medium containing 30% FBS was added to the lower chamber, and cultured at 37 °C, 5% CO2, 100% humidity in constant temperature cells. Incubated in the box for 24 h. The chamber was removed, rinsed with PBS, and fixed in absolute ethanol for 40 min. After crystal violet staining, the cells that did not pass through the upper chamber were wiped off with a cotton swab, and the cells passing through the upper chamber were counted under an inverted microscope. Five fields of view were randomly selected to detect changes in migration ability. Matrigel (Corning) was diluted in RPMI-1640 medium, spread evenly in a chamber, and tested for cell invasion according to the procedure for detecting migration ability. The experiment was repeated three times.
Western blot analysis
The expression of intracellular related proteins was analyzed by Western Blot. The transfected cells were extracted from the lysate to extract total protein. The protein concentration was determined by the BCA method. The amount of protein loaded per well was 40 μg in SDS-PAGE electrophoresis. The separation gel concentration was 10%. The protein was transferred to the PVDF membrane after electrophoresis. Block overnight with TBST solution containing 5% skim milk powder. The primary antibodies (BD, USA) of Bax, Bcl-2, MMP-9, MMP-2, Vimentin, N-cadherin, and E-cadherin were incubated for 2 h at room temperature, washed with TBST, and then added with secondary antibodies. After incubation for 2 h at room temperature, ECL (Millipore) was used to luminesce in the dark room. Protein strip gray scale was quantified using β-actin as a reference.
Statistical analysis
All data in this study were analyzed using statistical software SPSS 21.0(SPSS Inc., Chicago, IL, USA). The measurement data was described by mean ± standard deviation (SD), and the t test was performed; The count data was described by “%”, and the chi squared test was performed. The survival curve was evaluated using the Kaplan-Meier method, and the comparison between groups was performed using the Log-rank method. When p<0.05, the difference was statistically significant.
Results
MNX1-AS1 expression is significantly elevated in NSCLC tissues and cell lines
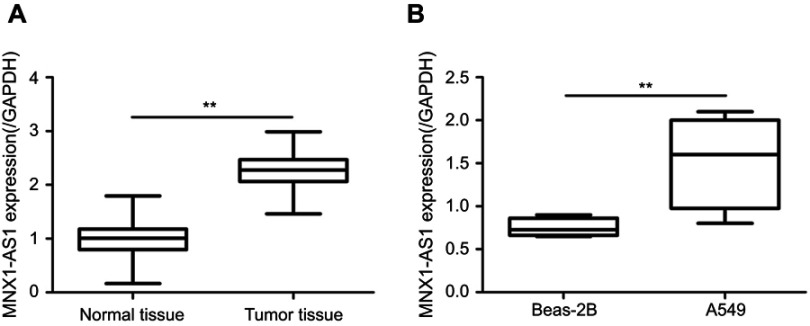
The expression of MNX1-AS1 in NSCLC tissues and cell lines were detected by qRT-PCR. As shown in Figure 1A, the expression of MNX1-AS1 was significantly increased in tumor tissues compared to normal lung tissue (t=35.52, p=0.000). In addition, the expression level of MNX1-AS1 in the NSCLC cell line A549 was significantly higher than that in the human normal lung epithelial cell line Beas-2B (t=2.795, p=0.031, Figure 1B). The results indicated that MNX1-AS1 is up-regulated in NSCLC tissues and cell lines.
Figure 1.
MNX1-AS1 expression was significantly elevated in NSCLC tissues and cell lines. (A) Expression of MNX1-AS1 in NSCLC tissues. (B) Expression of MNX1-AS1 in A549 cells. Values are means ± SD. **p<0.01. n=116.
Relationship between MNX1-AS1 and clinicopathological features of patients with NSCLC
The MNX1-AS1 expression level in the NSCLC tissues was classified according to the median value (M=2.28):≥2.28 was recorded as MNX1-AS1 high expression, and the <2.28 was recorded as MNX1-AS1 low expression. Univariate analysis showed that MNX1-AS1 was not associated with gender, age, tumor size, pathological type, and degree of differentiation in patients with NSCLC, but was significantly associated with TNM stage (p=0.006) and lymph node metastasis (p=0.000, Table 1).
Table 1.
Relationship between MNX1-AS1 expression and clinical features of patients with NSCLC
| Clinical features | n | MNX1-AS1 | p | ||
|---|---|---|---|---|---|
| Low expression | High expression | ||||
| Gender | Male | 70 | 38 | 32 | 0.255 |
| Female | 46 | 20 | 26 | ||
| Age | ≤50 | 41 | 21 | 20 | 0.846 |
| >50 | 75 | 37 | 38 | ||
| Tumor size | ≤3 cm | 54 | 22 | 32 | 0.063 |
| >3 cm | 62 | 36 | 26 | ||
| Pathological type | Squamous cell carcinoma | 53 | 29 | 24 | 0.351 |
| Adenocarcinoma | 63 | 29 | 34 | ||
| Differentiation | High | 22 | 12 | 10 | 0.641 |
| Medium | 27 | 15 | 12 | ||
| Low | 67 | 31 | 36 | ||
| TNM stage | Ⅰ+Ⅱ | 38 | 26 | 12 | 0.006 |
| Ⅲ+Ⅳ | 78 | 32 | 46 | ||
| Lymph node metastasis | No | 41 | 34 | 7 | 0.000 |
| Yes | 75 | 24 | 51 | ||
High expression of MNX1-AS1 is associated with poor prognosis of NSCLC
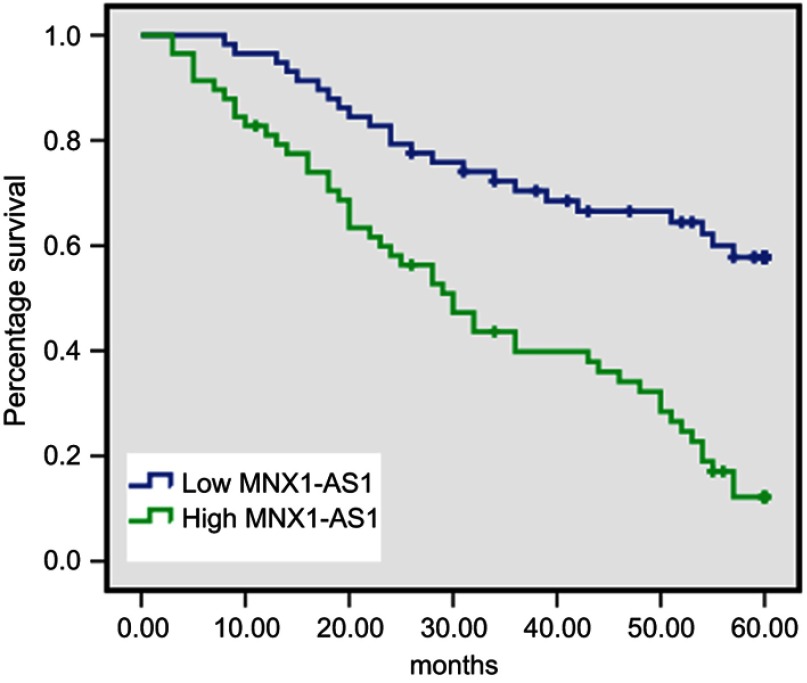
To analyze the relationship between MNX1-AS1 expression and prognosis in patients with NSCLC, univariate analysis was used to analyze the correlation between MNX1-AS1 expression and 5-year overall survival (Table 2). The Kaplan-Meier method confirmed that high expression of MNX1-AS1 was associated with a poor prognosis in NSCLC (p=0.000). In the MNX1-AS1 high expression group, the 5-year overall survival rate was 17.2%, and the mean survival time was 32.67 months. In the low expression group, the 5-year overall survival rate was 60.3%, and the average survival time was 47.26 months (Figure 2). In addition, lymph node metastasis was also associated with patient prognosis (p=0.000).
Table 2.
Univariate analysis of factors affecting the prognosis of patients with NSCLC
| Clinical features | n | Survival time (month) | 5-year survival rate | Log-rank p | |
|---|---|---|---|---|---|
| Gender | Male | 70 | 40.26 | 42.9% | 0.234 |
| Female | 46 | 39.20 | 32.6% | ||
| Age | ≤50 | 41 | 41.05 | 36.6% | 0.924 |
| >50 | 75 | 39.37 | 40.0% | ||
| Tumor size | ≤3cm | 54 | 40.31 | 40.6% | 0.592 |
| >3cm | 62 | 39.54 | 36.5% | ||
| Pathological type | Squamous cell carcinoma | 53 | 39.89 | 37.7% | 0.963 |
| Adenocarcinoma | 63 | 40.02 | 39.7% | ||
| Differentiation | High+medium | 49 | 43.40 | 49.0% | 0.059 |
| Low | 67 | 37.41 | 31.3% | ||
| TNM stage | Ⅰ+Ⅱ | 38 | 40.26 | 42.1% | 0.714 |
| Ⅲ+Ⅳ | 78 | 39.80 | 37.2% | ||
| Lymph node metastasis | No | 41 | 51.66 | 65.9% | 0.000 |
| Yes | 75 | 33.51 | 24.0% | ||
| MNX1-AS1 expression | Low | 58 | 32.67 | 17.2% | 0.000 |
| High | 58 | 47.26 | 60.3% | ||
Figure 2.
Correlation between MNX1-AS1 and overall survival in patients with NSCLC. Higher MNX1-AS1 expression can predict poor prognosis of NSCLC patients(Log-rank χ2=23.398,p=0.000).
Identification of transfection efficiency
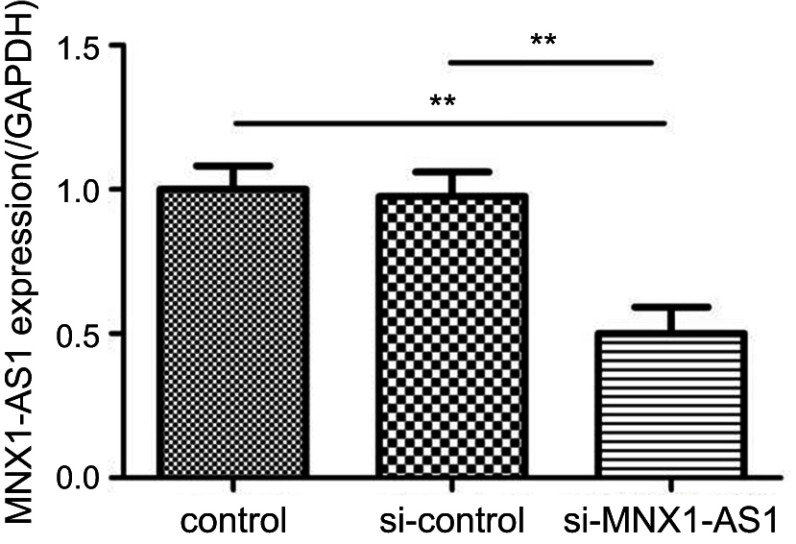
The constructed si-MNX1-AS1 and the corresponding negative control (si-control) were transfected into NSCLC cell line A549, respectively. Total RNA was extracted from the cells 48 h after transfection, and the transfection efficiency was examined by RT-PCR. As shown in Figure 3, MNX1-AS1 siRNA significantly inhibited the expression level of MNX1-AS1 in A549 cells (si-MNX1-AS1 group vs control group, t=4.082, p=0.007).
Figure 3.
Expression of MNX1-AS1 in NSCLC cell lines following transfection of siRNA. Values are means ± SD. **p<0.01.
Knockdown MNX1-AS1 inhibits proliferation of NSCLC cells
As shown in Figure 4A, MTT assay showed that the proliferation of A549 cells was significantly inhibited after knockdown of MNX1-AS1 (si-MNX1-AS1 group vs control group, t=7.877, p=0.000). Colony formation ability experiments were further employed to mimic the ability of cells to form multicellular clones from individual tumor cells. As shown in Figure 4B, after transfection of MNX1-AS1 siRNA, A549 cells formed smaller clones and fewer clones. Statistical analysis demonstrated that down-regulation of MNX1-AS1 significantly reduced the number of clones formed in A549 cells (si-MNX1-AS1 group vs control group, t=9.258, p=0.000). It was suggested that down-regulation of MNX1-AS1 can significantly inhibit the proliferation of NSCLC cell line A549 and attenuate the ability of colony formation.
Figure 4.
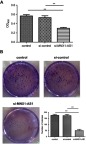
knockdown MNX1-AS1 inhibits proliferation of NSCLC cells. (A) MTT assay was used to detect NSCLC cell proliferation. (B) Clonal formation assay detects NSCLC cell proliferation. Values are means ± SD. **p<0.01.
Knockdown of MNX1-AS1 promotes apoptosis in NSCLC cells
The effect of knockdown MNX1-AS1 on apoptosis of NSCLC cells was detected by Annexin V/PI double staining flow cytometry. As shown in Figure 5A, after MNX1-AS1 knockdown, the apoptotic rate in the A549 cell line was significantly higher than that in the control group and the si-control group, while there was little change between the control group and the si-control group. Furthermore, the downstream marker proteins were detected. The results were shown in Figure 5B. Compared with the control group and the si-control group, the expression of the proapoptotic protein Bax was significantly up-regulated in the si-MNX1-AS1 group, and the anti-apoptotic protein Bcl-2 expression was significantly down-regulated. The results showed that knockdown of MNX1-AS1 promoted apoptosis of NSCLC, possibly by regulating Bcl-2 and Bax genes.
Figure 5.
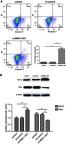
knockdown MNX1-AS1 promotes apoptosis in NSCLC cells. (A) Annexin V/PI double staining flow cytometry was used to detect apoptosis of NSCLC cells. (B) Western blot analysis of apoptosis-related protein expression. Values are means ± SD. **p<0.01.
Knockdown MNX1-AS1 inhibits migration and invasion of NSCLC cells
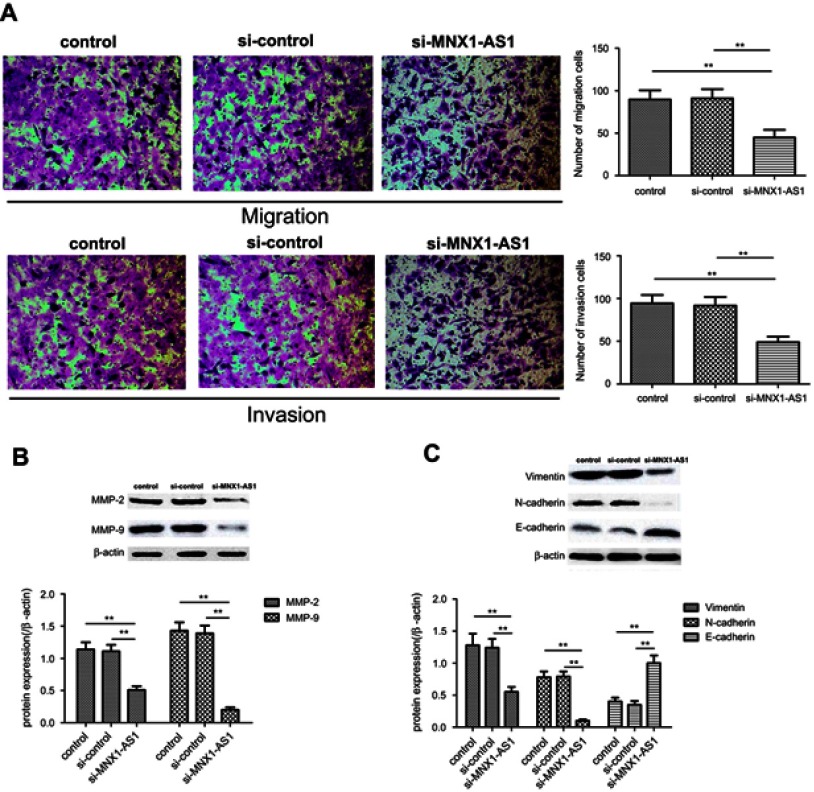
The effect of MNX1-AS1 on the migration and invasion of A549 cells was analyzed by Transwell assay. As shown in Figure 6A and B, the cell migration (si-MNX1-AS1 group vs control group, t=3.162, p=0.005) and invasion number (si-MNX1-AS1 group vs control group, t=3.875, p=0.001) of si-MNX1-AS1 group were significantly lower than those of the control group . In addition, Western Blot analysis of migration and invasion-associated marker protein expression showed that MMP-2 and MMP-9 expression levels were significantly down-regulated in the si-MNX1-AS1 group compared with the control (p<0.05). The results indicated that MNX1-AS1 may be involved in promoting the migration and invasion of NSCLC cells, thereby promoting tumor cell metastasis.
Figure 6.
Knockdown MNX1-AS1 inhibits migration and invasion of NSCLC cells. (A) Transwell assay analyzes migration and invasion of NSCLC cells. (B) Western blot analysis of migration and invasion-related protein expression. (C) Western blot analysis of EMT-related protein expression. Values are means ± SD. **p<0.01.
Epithelial-mesenchymal transition (EMT), as an invasive phenotype of cancer cells, plays an important role in tumor metastasis. During the EMT process, the expression of the interstitial marker proteins Vimentin and N-cadherin increased, and the expression of the epithelial marker protein E-cadherin decreased.12 Western blot analysis showed that (Figure 6C), compared with the control group, the expression level of E-cadherin in the si-MNX1-AS1 group significantly increased, and the expression levels of Vimentin and N-cadherin protein significantly decreased (p<0.05). The results suggested that MNX1-AS1 knockdown can inhibit the migration and invasion of NSCLC cell line A549 by regulating EMT.
Discussion
Lung cancer is one of the leading causes of cancer-related deaths worldwide. Finding new molecular targets for diagnosis, treatment, and prognosis assessment may improve clinical strategies and outcomes for this disease.13 With the rapid development and application of deep sequencing, gene chip, real-time quantitative PCR and in situ hybridization, the role of LncRNA in the development of lung cancer has also attracted much attention. According to reports, CCAT1,14 HOTAIR,15 PVT116 can induce malignant transformation of bronchial epithelial cells and form tumors. In addition, a variety of LncRNAs have been shown to be abnormally expressed in NSCLC tissues and blood, and can be used as markers for the diagnosis of NSCLC.17–19 Identification of tumor-associated LncRNA is not only helpful in understanding the role of LncRNA in tumorigenesis, but also important for exploring new therapeutic targets.20 This study was to investigate the expression and significance of LncRNA MNX1-AS1 in non-small cell lung cancer.
Wu10 used lncRNA chip to analyzed the differential expression of MNX1-AS1 in gallbladder carcinoma and adjacent tissues and found that the expression of MNX1-AS1 in gallbladder carcinoma tissues was 159 times that of adjacent tissues. Li et al21 revealed that the levels of MNX1-AS1 were higher in EOC tissue than in matched normal tissues, and MNX1-AS1 expression level was correlated with FIGO stage, grade and distant metastasis. Additionally, they also revealed that MNX1-AS1 may be a useful marker for predicting the outcome in patients with EOC. However, the clinical significance of MNX1-AS1 in patients with NSCLC is unclear. In the present study, we found that the expression of MNX1-AS1 was significantly higher in NSCLC tissues and cell lines than in corresponding normal lung tissues and human normal lung epithelial cell lines. Additionally, the expression of MNX1-AS1 was closely related to TNM stage and lymph node metastasis, and its high expression might be an important biological indicator for predicting the poor prognosis of patients with NSCLC. These results confirm that the involvement of MNX1-AS1 in the progression of NSCLC may be an independent prognostic factor in patients with NSCLC.
Additionally, Wu10 showed that MNX1-AS1 is associated with 39 mRNAs in the co-expression network, including otx1, trim59, MMP-12, HOXB9 and other tumor cell biological function-related encoded protein genes. Gao et al22 also found that knockdown of MNX1-AS1 can significantly inhibit the proliferation, migration and invasion of glioblastoma cells, and its carcinogenic mechanism may be related to its targeted regulation of miR-4443. Lv et al11 confirmed that as an oncogene, MNX1-AS1 knockdown can not only inhibit the proliferation and migration of ovarian cancer cell lines WVCA433 and SKOV-3, but also induce cell cycle arrest and promote cell apoptosis. These results suggest that MNX1-AS1 may have a role in regulating the biological function of gallbladder cancer cells. The clinical pathology data of this study also indicated that MNX1-AS1 may be involved in the regulation of NSCLC occurrence and metastasis. To investigate the functional role of MNX1-AS1 in the progression of NSCLC, we used a lentivirus to knockdown MNX1-AS1 in A549 cells. It was found that knockdown of MNX1-AS1 inhibited proliferation, colony formation, migration and invasion of NSCLC cells and promoted apoptosis. In addition, by analyzing the expression of apoptosis-related and metastasis-associated proteins, MNX1-AS1 knockdown could affect the expression of apoptosis-related proteins (Bcl-2 and Bax) and metastasis-associated proteins (MMP-2 and MMP-9). EMT is a key event in the ability of NSCLC cells to acquire metastasis.23 Studies have shown that in the event of EMT in NSCLC cells, their invasion and metastasis capacity is significantly enhanced.24 We found that MNX1-AS1 knockdown regulated the expression of EMT marker genes, suggesting that MNX1-AS1 may participate in the process of promoting the transfer of A549 cells by regulating the EMT process of NSCLC cells. These results confirm that MNX1-AS1 may play a carcinogenic role in NSCLC and may be a potential target for the disease.
Conclusion
In conclusion, this study demonstrated for the first time that up-regulation of LncRNA MNX1-AS1 was closely related to the progression and prognosis of NSCLC. We also found that LncRNA MNX1-AS1 played an important role in the proliferation, metastasis and apoptosis of NSCLC cells. These results suggest that MNX1-AS1 may be one of the new targets for NSCLC prognosis and treatment.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E86. doi: 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2.Pancewicz-Wojtkiewicz J, Bernatowicz PL. The effect of afatinib treatment in non-small cell lung cancer cells. Anticancer Res. 2017;37(7):3543–3546. doi: 10.21873/anticanres.11723 [DOI] [PubMed] [Google Scholar]
- 3.Spika D, Bannon F, Bonaventure A, et al. Life tables for global surveillance of cancer survival (the CONCORD programme): data sources and methods. BMC Cancer. 2017;17(1):159. doi: 10.1186/s12885-017-3117-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Z, Zhu Z, Watabe K, et al. Negative regulation of lncRNA GAS5 by miR-21. Cell Death Differ. 2013;20(11):1558–1568. doi: 10.1038/cdd.2013.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiyomaru T, Yamamura S, Fukuhara S, et al. Genistein inhibits prostate cancer cell growth by targeting miR-34a and oncogenic HOTAIR. PLoS One. 2013;8(8):e70372. doi: 10.1371/journal.pone.0070372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheik Mohamed J, Gaughwin PM, Lim B, Robson P, Lipovich L. Conserved long noncoding RNAs transcriptionally regulated by Oct4 and Nanog modulate pluripotency in mouse embryonic stem cells. RNA. 2010;16(2):324–337. doi: 10.1261/rna.1441510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Wan L, Lu K, et al. The long noncoding RNA MEG3 contributes to cisplatin resistance of human lung adenocarcinoma. PLoS One. 2015;10(5):e0114586. doi: 10.1371/journal.pone.0114586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma Y, Ma W, Huang L, Feng D, Cai B. Long non-coding RNAs, a new important regulator of cardiovascular physiology and pathology. Int J Cardiol. 2015;188:105–110. doi: 10.1016/j.ijcard.2015.04.021 [DOI] [PubMed] [Google Scholar]
- 9.Roberts TC, Morris KV, Wood MJ. The role of long non-coding RNAs in neurodevelopment, brain function and neurological disease. Philos Trans R Soc Lond B Biol Sci. 2014;369(1652):20130507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiangsong W. Screening and functional study of differentially expressed long-chain non-coding RNA in gallbladder carcinoma. 2014. Xinhua Hospital, Shanghai Jiao Tong University School of Medicine. [Google Scholar]
- 11.Lv Y, Li H, Li F, Liu P, Zhao X. Long noncoding RNA MNX1-AS1 knockdown inhibits cell proliferation and migration in ovarian cancer. Cancer Biother Radiopharm. 2017;32(3):91–99. doi: 10.1089/cbr.2017.2178 [DOI] [PubMed] [Google Scholar]
- 12.Sun M, Zhang N, Wang X, et al. Hedgehog pathway is involved in nitidine chloride induced inhibition of epithelial-mesenchymal transition and cancer stem cells-like properties in breast cancer cells. Cell Biosci. 2016;6:44. doi: 10.1186/s13578-016-0104-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yanaihara N, Caplen N, Bowman E, et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9(3):189–198. doi: 10.1016/j.ccr.2006.01.025 [DOI] [PubMed] [Google Scholar]
- 14.Hu B, Zhang H, Wang Z, Zhang F, Wei H, Li L. LncRNA CCAT1/miR-130a-3p axis increases cisplatin resistance in non-small-cell lung cancer cell line by targeting SOX4. Cancer Biol Ther. 2017;18(12):974–983. doi: 10.1080/15384047.2017.1385679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loewen G, Jayawickramarajah J, Zhuo Y, Shan B. Functions of lncRNA HOTAIR in lung cancer. J Hematol Oncol. 2014;7:90. doi: 10.1186/s13045-014-0090-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang YR, Zang SZ, Zhong CL, Li YX, Zhao SS, Feng XJ. Increased expression of the lncRNA PVT1 promotes tumorigenesis in non-small cell lung cancer. Int J Clin Exp Pathol. 2014;7(10):6929–6935. [PMC free article] [PubMed] [Google Scholar]
- 17.Yang J, Lin J, Liu T, et al. Analysis of lncRNA expression profiles in non-small cell lung cancers (NSCLC) and their clinical subtypes. Lung Cancer. 2014;85(2):110–115. doi: 10.1016/j.lungcan.2014.05.011 [DOI] [PubMed] [Google Scholar]
- 18.Yang Y, Li H, Hou S, Hu B, Liu J, Wang J. The noncoding RNA expression profile and the effect of lncRNA AK126698 on cisplatin resistance in non-small-cell lung cancer cell. PLoS One. 2013;8(5):e65309. doi: 10.1371/journal.pone.0065309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie X, Liu HT, Mei J, et al. LncRNA HMlincRNA717 is down-regulated in non-small cell lung cancer and associated with poor prognosis. Int J Clin Exp Pathol. 2014;7(12):8881–8886. [PMC free article] [PubMed] [Google Scholar]
- 20.Spizzo R, Almeida MI, Colombatti A, Calin GA. Long non-coding RNAs and cancer: a new frontier of translational research? Oncogene. 2012;31(43):4577–4587. doi: 10.1038/onc.2011.621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li AH, Zhang HH. Overexpression of lncRNA MNX1-AS1 is associated with poor clinical outcome in epithelial ovarian cancer. Eur Rev Med Pharmacol Sci. 2017;21(24):5618–5623. doi: 10.26355/eurrev_201712_14003 [DOI] [PubMed] [Google Scholar]
- 22.Gao Y, Xu Y, Wang J, Yang X, Wen L, Feng J. LncRNA MNX1-AS1 promotes glioblastoma progression through inhibition of miR-4443. Oncol Res. 2018;27(3):341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng J, Xu L, Ni S, et al. Involvement of FoxQ1 in NSCLC through regulating EMT and increasing chemosensitivity. Oncotarget. 2014;5(20):9689–9702. doi: 10.18632/oncotarget.2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soltermann A, Opitz I, Tischler V, et al. EMT in NSCLC and malignant pleural mesothelioma. Eur Med Oncol 2012;3(4):180–184. [Google Scholar]