Abstract
Context
Current guidelines recommend close monitoring of electrolytes in transgender patients using spironolactone given the risk of hyperkalemia from mineralocorticoid antagonism. In patients taking spironolactone for other conditions, the rate of hyperkalemia is low, and the utility of frequent monitoring has been questioned.
Objective
We hypothesized that the rate of hyperkalemia in gender-diverse adolescents taking spironolactone is low and, when present, clinically insignificant.
Design and Outcomes
A retrospective chart review of adolescents seen in a specialty gender clinic at a tertiary care pediatric hospital over 10 years identified patients prescribed spironolactone for gender transition. Study outcomes were the incidence of hyperkalemia, defined as serum potassium concentration >5.0 mmol/L, and the relationship between potassium levels and spironolactone dose and duration.
Results
Records were reviewed for 85 subjects with a mean ± SD age of 16.6 ± 1.7 years. There were a total of 269 potassium measurements (80 prior to spironolactone initiation and 189 during spironolactone treatment). Six potassium measurements in five subjects were >5.0 mmol/L, indicating a rate of hyperkalemia of 2.2%. None of the subjects had symptoms of hyperkalemia, and all elevated measurements were normal when repeated. Only one subject discontinued spironolactone after an elevated potassium measurement. There was no relationship between hyperkalemia and spironolactone dose. Potassium measurements decreased with increasing treatment duration.
Conclusions
Hyperkalemia in patients taking spironolactone for gender transition is rare and when present is transient and asymptomatic. In the absence of other medical comorbidities, routine electrolyte monitoring in this population may be unnecessary.
Keywords: transgender, gender diverse, gender dysphoria, spironolactone, hyperkalemia, antiandrogen
Medical treatment of gender-diverse adolescents includes GnRH analogs to suppress puberty, gender-affirming sex steroids, and adjunctive medications to suppress the unwanted effects of endogenous hormones. Spironolactone is an aldosterone antagonist routinely used for its antiandrogenic properties in the treatment of transgender female patients [1]. Specifically, spironolactone is thought to promote feminization via inhibition of testosterone synthesis by decreasing the activity of 17α-hydroxylase in the testes, competition at androgen receptors, and direct stimulation of the estrogen receptor [2, 3]. Spironolactone may be superior to other antiandrogen treatments, such as finasteride and cyproterone acetate, in treating hirsutism [4]. Because of its effectiveness in reducing unwanted male-pattern hair growth and its relatively low cost, spironolactone is a frequently used medication in medical gender transition.
Because of its mineralocorticoid antagonist activity, there is a concern that spironolactone may decrease renal potassium excretion and lead to hyperkalemia. Increased use of spironolactone in adults with heart failure has been linked to an increased incidence of hyperkalemia [5]. However, in healthy male volunteers, spironolactone did not cause a change in serum potassium [6]. In a large study of patients taking spironolactone for acne, only 13 (0.72%) of 1802 potassium measurements were >5.0 mmol/L. Six of the elevated potassium measurements were normal when remeasured. The remainder of the elevated measurements did not lead to repeat testing. There were no cases of symptomatic hyperkalemia [7, 8].
Multiple studies of transfeminine transgender adolescents and adults have noted no cases of hyperkalemia in otherwise healthy individuals taking spironolactone [9–11]. However, current guidelines recommend serial electrolyte monitoring for transgender patients on this medication [12, 13]. We hypothesized that the prevalence of hyperkalemia in gender-diverse adolescents taking spironolactone is low and that clinically significant hyperkalemia is rare.
1. Materials and Methods
A. Subjects and Protocol
We performed a retrospective chart review of gender-diverse youth of any age seen in the Gender Management Service Program at Boston Children’s Hospital from 2007 to 2017. Patients who were prescribed spironolactone for the purposes of gender transition were included in the analysis. Hyperkalemia was defined as a serum potassium concentration >5.0 mmol/L.
The study protocol was approved by the Institutional Review Board of Boston Children’s Hospital.
B. Statistical Analysis
Descriptive statistics were used to summarize patient demographics and clinical characteristics. Frequencies (%) were reported for categorical variables, and mean values ± SD were reported for continuous variables. Generalized estimating equations for a binary outcome with the log link function and exchangeable working correlation structure were used to assess the association between dose of spironolactone and risk of hyperkalemia. All analyses were performed using SAS 9.4. A P value <0.05 was considered statistically significant
2. Results
A. Study Cohort
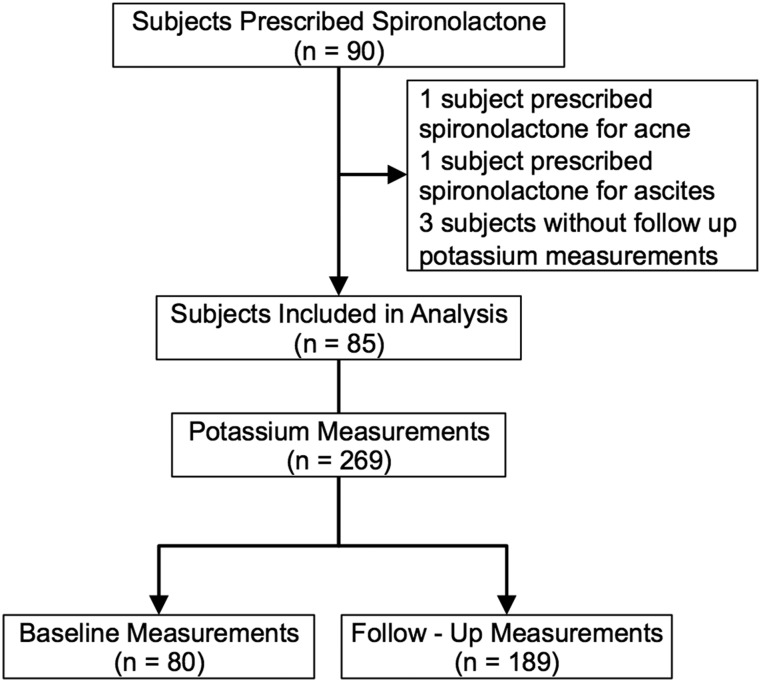
Ninety gender-diverse adolescents were prescribed spironolactone during the study period (Table 1). Two patients were prescribed spironolactone for indications other than gender transition and were excluded. Three patients were excluded because there were no potassium measurements recorded after spironolactone was initiated. The 85 subjects included in the analysis had a total of 269 potassium measurements. Of these, 80 measurements were performed before the initiation of spironolactone, and 189 measurements were performed during spironolactone treatment (Fig. 1). Most subjects (n = 70; 82%) had potassium measured before starting spironolactone. Thirty-six subjects (42%) had at least one potassium measurement within the first 3 months of spironolactone therapy, and 48 subjects (56%) had a measurement within the first 6 months. Potassium measurements were available for up to 7 years of spironolactone therapy. Doses of spironolactone ranged from 25 to 400 mg/d, with a mean ± SD daily dose of 105 ± 85 mg/d. The majority of subjects (n = 73; 86%) were using spironolactone as an adjunct to estrogen. Subjects had a mean ± SD of 3.2 ± 1.9 potassium measurements (Table 1).
Table 1.
Characteristics of Subjects
| Variable | All Subjects (n = 85) | Subjects Without Hyperkalemia (n = 79) | Subjects With Hyperkalemia (n = 6) |
|---|---|---|---|
| Age at spironolactone start, mean (SD), y | 16.6 (1.7) | 16.7 (1.7) | 16.0 (1.4) |
| Female gender identity, n (%) | 82 (96) | 76 (96) | 6 (100) |
| Nonbinary gender identity, n (%) | 3 (4) | 3 (4) | 0 (0) |
| Race | |||
| White, n (%) | 59 (69) | 54 (68) | 5 (83) |
| Black or African American, n (%) | 2 (2) | 2 (3) | 0 (0) |
| Asian, n (%) | 2 (2) | 2 (3) | 0 (0) |
| Other, n (%) | 8 (9) | 8 (10) | 0 (0) |
| Unknown, n (%) | 14 (16) | 13 (16) | 1 (17) |
| Hispanic or Latino, n (%) | 5 (6) | 5 (6) | 0 (0) |
| Using GnRH analog, n (%) | 19 (22) | 18 (23) | 1 (17) |
| Using estrogen, n (%) | 73 (86) | 68 (86) | 5 (83) |
| Number of potassium measurements per subject, median (range) | 3 (1–10) | 3 (1–10) | 4.5 (1–8) |
| Baseline potassium measurement available, n (%) | 70 (82) | 65 (82) | 5 (83) |
| Serum potassium, mean (SD), mmol/L | 4.25 (0.4) | 4.20 (0.3) | 4.65 (0.5) |
| Spironolactone dose, mean (SD), mg/d | 105 (42) | 105 (42) | 108 (49) |
Figure 1.
Flowchart of subject selection and potassium measurements. Five subjects were excluded from the analysis because they were prescribed spironolactone for indications other than gender transition or did not have sufficient follow-up measurements. Of the 85 subjects included in the analysis, 70 (82%) had a baseline potassium measurement.
B. Incidence of Hyperkalemia
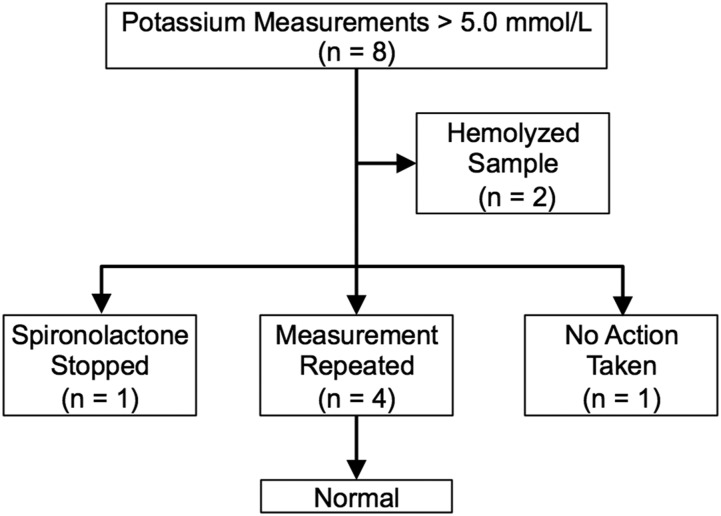
Eight potassium measurements in six subjects exceeded 5.0 mmol/L. Hemolysis was noted in two measurements in the same subject, including one measurement performed prior to spironolactone initiation. (Fig. 2). When these measurements were excluded, the rate of hyperkalemia was 2.2%. There were no cases of hyperkalemia in the baseline measurements. All cases of hyperkalemia occurred early in the treatment course (<6 months after starting spironolactone). There were no potassium measurements >6.0 mmol/L.
Figure 2.
Outcomes of cases of elevated potassium measurements. Eight potassium measurements in six subjects were >5.0 mmol/L. Two samples in the same subject were noted to be hemolyzed. In only one case was spironolactone discontinued. All potassium measurements in all cases were normal when repeated.
C. Features of Subjects With Hyperkalemia
Five subjects had potassium measurements >5.0 mmol/L in the absence of hemolysis (Fig. 2); all had normal baseline potassium concentrations. None of the subjects with hyperkalemia had symptoms of hyperkalemia. One subject had a potassium measurement >5.0 mmol/L 2 weeks after initiation of 50 mg/d of spironolactone. Spironolactone was continued, and the potassium measurement was normal when repeated 2 weeks later. This subject’s potassium was again elevated 3 months after spironolactone initiation and was normal 2 weeks later.
In two additional subjects, hyperkalemia was observed at 1 and 6 months after spironolactone initiation. Spironolactone was continued, and all repeat measurements were in the normal range.
One subject had an elevated potassium measurement 1 month after starting spironolactone. All subsequent potassium measurements were normal. This subject had a history of eosinophilic esophagitis and received gastrostomy-tube feeds overnight in addition to a proton pump inhibitor.
Spironolactone was discontinued in one subject who had hyperkalemia 5 months after spironolactone initiation. It was restarted after a repeat potassium measurement 2 weeks later was normal. All subsequent potassium measurements were normal in this subject.
D. Presence of Medical Comorbidities
In our study cohort there were eight subjects with medical comorbidities, which included asthma (three subjects), migraine headaches, narcolepsy, eosinophilic esophagitis, macrophage activation syndrome, hypertension, and chronic congestive heart failure secondary to hypoplastic left heart syndrome. The subject with underlying hypertension did not have hyperkalemia. The subject with congestive heart failure was started on a low dose of spironolactone (25 mg/d) and did not have any episodes of hyperkalemia despite concurrent use of an angiotension-converting enzyme inhibitor.
E. Predictors of Hyperkalemia
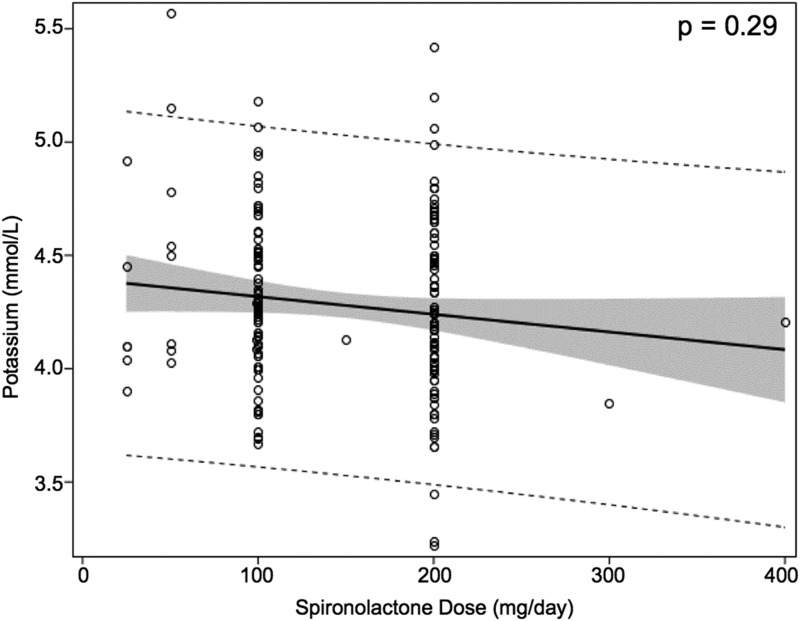
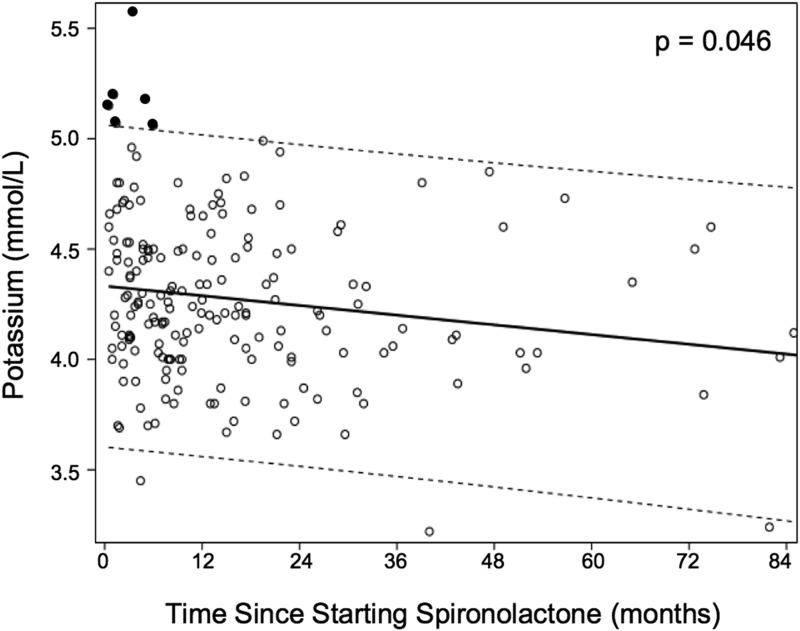
The most common starting spironolactone dose was 100 mg/d. Larger doses of spironolactone were not associated with a higher risk of hyperkalemia (Table 2). One subject had normal potassium measurements while taking 400 mg of spironolactone daily. There was no significant correlation between the dose of spironolactone and serum potassium concentration overall (Fig. 3). There was a significant trend toward lower serum potassium concentration with increasing duration of spironolactone treatment (Fig. 4). When the elevated potassium measurements, which all occurred early in the treatment course, were excluded, the correlation between treatment duration and potassium concentration was no longer significant.
Table 2.
Association Between Dose of Spironolactone and Hyperkalemia
| Dose of Spironolactone | Number of Potassium Measurements >5.0 mmol/L/Total Number of Potassium Measurements (%) | Relative Risk (95% CI) | P Value |
|---|---|---|---|
| <100 mg/d | 2/17 (11.8) | 1.0 | |
| 100–200 mg/d | 2/74 (2.7) | 0.23 (0.03–1.52) | 0.13 |
| >200 mg/d | 3/98 (3.1) | 0.26 (0.05–1.44) | 0.12 |
Baseline potassium measurements were excluded. A spironolactone dose of 100 mg/d was the most common starting dose. A dose of spironolactone <100 mg/dL was designated as the reference. There was no increased risk of hyperkalemia with higher spironolactone dose categories.
Figure 3.
Serum potassium concentration is not correlated with spironolactone dose. The solid black line indicates regression line. The shaded area indicates the 95% confidence intervals for the expected mean. Dashed lines indicate the 95% confidence interval for the individual predicted value.
Figure 4.
Correlation between duration of spironolactone treatment and serum potassium concentration. Baseline measurements and two measurements that were the result of a hemolyzed sample were excluded. Black circles indicate serum potassium concentrations >5.0 mmol/L. Dashed lines indicate 95% CI of potassium concentration normal range. There is a significant trend toward lower serum potassium concentration with longer duration of treatment. When the potassium measurements >5.0 mmol/L were excluded, there was no correlation between serum potassium concentration and duration of spironolactone exposure.
3. Discussion
Similar to other studies of otherwise healthy patients taking spironolactone for its anti-androgenic effects, we identified a low rate of hyperkalemia (2.2%) in our population of gender-diverse adolescents. Although this is higher than the rate of hyperkalemia reported in other healthy populations taking spironolactone, the cases of hyperkalemia we identified were likewise transient and not clinically significant [8].
Hyperkalemia is an important side effect in patients receiving spironolactone for heart failure. These patients are frequently also prescribed medications that affect renal potassium handling, such as angiotension-converting enzyme inhibitors [5]. In a large study of patients with congestive heart failure taking spironolactone, the risk of hyperkalemia increased with increasing doses of spironolactone from 13.5% in patients taking 25 mg/d to 41.4% in those taking 50 mg/d [14]. We did not find an increased risk of hyperkalemia with increasing doses of spironolactone despite routine use of larger doses (up to 400 mg/d) compared with those used in treating patients with congestive heart failure. It is the clinical context in which spironolactone is used, not the dose alone, that predicts risk of hyperkalemia.
All cases of hyperkalemia we identified occurred within the first 6 months of starting treatment. We monitored patients taking spironolactone for up to 7 years but did not identify additional cases of hyperkalemia. This observation suggests that if there is no evidence of hyperkalemia in the early treatment period, there is no increased risk with duration of spironolactone exposure.
Indeed, we noted a downward trend in serum potassium concentrations over the duration of spironolactone use. Although this association between potassium measurements and treatment duration may have been caused by the resolution of hyperkalemia in affected subjects, it also raises the possibility that there is renal accommodation to mineralocorticoid antagonism that leads to a slightly lower potassium set point and thus a lower risk for hyperkalemia over time. Renal accommodation may also explain why we did not see an effect of higher spironolactone doses on serum potassium concentrations and why all cases of hyperkalemia were early, transient, and self-resolved.
Beyond increasing the financial and time burden for patients, laboratory monitoring, if unneeded, may further increase barriers to gender-affirming care for an already vulnerable population [15]. Requiring frequent laboratory monitoring not only increases the complexity of caring for patients who desire gender transition but may also suggest that spironolactone, a commonly used gender-affirming medication, is risky and has concerning side effects.
The generalizability of this study is limited by the relatively small sample size and the use of only one study site. Its retrospective nature introduces potential selection bias; for example, clinicians may have avoided spironolactone use in patients with premorbid conditions or prior hyperkalemia. Further studies with a larger sample size are needed to confirm our findings.
We have shown that the risk of hyperkalemia in an otherwise healthy population taking spironolactone is low and that the risk of clinically important hyperkalemia is even lower. Additionally, when hyperkalemia occurs, it does so early in the treatment course and irrespective of spironolactone dose. We propose that close potassium monitoring beyond the first 6 months of therapy should be reserved for patients with medical comorbidities or for those who are taking medications that may impair renal potassium handling.
Acknowledgments
We thank Molly Plovanich and Alexandra Charrow of Brigham and Women’s Hospital for early guidance in this project and Joseph Wolfsdorf of Boston Children’s Hospital for thoughtful comments on the manuscript.
Financial Support: Y-M.C. was supported by National Institute of Health (NIH) Grant R01 HD082554 and K.M. was supported by NIH Grant T32 DK007699.
Disclosure Summary: The authors have nothing to disclose.
References and Notes
- 1. Prior JC, Vigna YM, Watson D. Spironolactone with physiological female steroids for presurgical therapy of male-to-female transsexualism. Arch Sex Behav. 1989;18(1):49–57. [DOI] [PubMed] [Google Scholar]
- 2. Corvol P, Michaud A, Menard J, Freifeld M, Mahoudeau J. Antiandrogenic effect of spirolactones: mechanism of action. Endocrinology. 1975;97(1):52–58. [DOI] [PubMed] [Google Scholar]
- 3. Sciarra F, Toscano V, Concolino G, Di Silverio F. Antiandrogens: clinical applications. J Steroid Biochem Mol Biol. 1990;37(3):349–362. [DOI] [PubMed] [Google Scholar]
- 4. Brown J, Farquhar C, Lee O, Toomath R, Jepson RG Spironolactone versus placebo or in combination with steroids for hirsutism and/or acne. Cochrane Database Syst. Rev.2009;April15(2):CD000194. [Google Scholar]
- 5. Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, Redelmeier DA. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004;351(6):543–551. [DOI] [PubMed] [Google Scholar]
- 6. Stripp B, Taylor AA, Bartter FC, Gillette JR, Loriaux DL, Easley R, Menard RH. Effect of spironolactone on sex hormones in man. J Clin Endocrinol Metab. 1975;41(4):777–781. [DOI] [PubMed] [Google Scholar]
- 7. Yemisci A, Gorgulu A, Piskin S. Effects and side-effects of spironolactone therapy in women with acne. J Eur Acad Dermatol Venereol. 2005;19(2):163–166. [DOI] [PubMed] [Google Scholar]
- 8. Plovanich M, YuWeng Q, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol 2015;151:941–944. [DOI] [PubMed] [Google Scholar]
- 9. Fernandez JD, Tannock LR. Metabolic effects of hormone therapy in transgender patients. Endocr Pract. 2016;22(4):383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jarin J, Pine-Twaddell E, Trotman G, Stevens J, Conard LA, Tefera E, Gomez-Lobo V. Cross-sex hormones and metabolic parameters in adolescents with gender dysphoria. Pediatrics. 2017;139(5):e20163173. [DOI] [PubMed] [Google Scholar]
- 11. Khatchadourian K, Amed S, Metzger DL. Clinical management of youth with gender dysphoria in Vancouver. J Pediatr. 2014;164(4):906–911. [DOI] [PubMed] [Google Scholar]
- 12. Coleman E, Bockting W, Botzer M, Cohen-Kettenis P, DeCuypere G, Feldman J, Fraser L, Green J, Knudson G, Meyer WJ, Monstrey S, Adler RK, Brown GR, Devor AH, Ehrbar R, Ettner R, Eyler E, Garofalo R, Karasic DH, Lev AI, Mayer G, Meyer-Bahlburg H, Hall BP, Pfaefflin F, Rachlin K, Robinson B, Schechter LS, Tangpricha V, van Trotsenburg M, Vitale A, Winter S, Whittle S, Wylie KR, Zucker K. Standards of care for the health of transsexual, transgender, and gender-nonconforming people, version 7. Int J Transgenderism. 2012;13(4):165–232. [Google Scholar]
- 13. Hembree WC, Cohen-Kettenis PT, Gooren L, Hannema SE, Meyer WJ, Murad MH, Rosenthal SM, Safer JD, Tangpricha V, T’Sjoen GG. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(11):3869–3903. [DOI] [PubMed] [Google Scholar]
- 14. Vardeny O, Claggett B, Anand I, Rossignol P, Desai AS, Zannad F, Pitt B, Solomon SD; Randomized Aldactone Evaluation Study (RALES) Investigators. Incidence, predictors, and outcomes related to hypo- and hyperkalemia in patients with severe heart failure treated with a mineralocorticoid receptor antagonist. Circ Heart Fail. 2014;7(4):573–579. [DOI] [PubMed] [Google Scholar]
- 15. Safer JD, Coleman E, Feldman J, Garofalo R, Hembree W, Radix A, Sevelius J. Barriers to healthcare for transgender individuals. Curr Opin Endocrinol Diabetes Obes. 2016;23(2):168–171. [DOI] [PMC free article] [PubMed] [Google Scholar]