Abstract
There is growing evidence that autonomous cortisol secretion (ACS), previously known as subclinical Cushing syndrome, is associated with greater prevalence of cardiovascular (CV) risk factors. However, it is unclear whether ACS is associated with greater prevalence of CV outcomes compared with nonfunctioning adrenal adenomas (NFAAs). The objective of this study is to evaluate CV outcomes and CV risk factors in patients with adrenal adenoma with ACS compared with NFAA. A literature review was performed in Embase, Medline, Cochrane Library, and reference lists within selected articles. The study protocol was registered with PROSPERO. A literature search yielded six studies that met the inclusion criteria. Studies varied in their definitions of ACS and CV outcomes. Two retrospective longitudinal studies further demonstrated higher incidence of new CV events (ACS 16.7% vs NFAA 6.7%, P = 0.04) and higher CV mortality in patients with ACS (ACS 22.6% vs 2.5%, P = 0.02). The prevalence of CV outcomes in ACS was more than three times greater than in patients with NFAA. Three of five studies found that ACS was associated with higher prevalence of diabetes and hypertension. There was no difference in dyslipidemia or body mass index demonstrated in any study. There is heterogeneity among the few studies evaluating the association between ACS and CV outcomes. Although these studies suggest a higher risk of CV outcomes in patients with ACS, many did not adjust for known confounders. Larger, high quality, prospective studies are needed to evaluate this association and to identify modifiable risk factors.
Keywords: Cushing syndrome, autonomous cortisol secretion, nonfunctional adrenal adenoma, cardiovascular outcomes
With radiological advancements, the frequency of incidentally discovered adrenal adenomas has increased, with studies quoting an incidence 3% in middle age and ≤10% in older adults [1–3]. Adrenal incidentalomas (AIs), although usually benign and nonfunctional, may be associated with cortisol excess. They are currently classified as Cushing syndrome, autonomous cortisol secretion (ACS), possible ACS, and nonfunctioning adrenal adenoma (NFAA). ACS, previously known as subclinical Cushing syndrome (SCS) or subclinical hypercortisolism, is a condition of biochemical cortisol excess in the absence of classic clinical features of Cushing syndrome [3–5]. The definition was modified in the 2016 European guidelines to distinguish ACS and overt Cushing syndrome as separate conditions with significantly different morbidity and mortality [6]. The prevalence of ACS is estimated to be 1% to 29% in those with AI [5, 6]. Serum cortisol levels after 1-mg overnight dexamethasone suppression test (DST) were used to categorize patients with AI ≤50 nmol/L (≤1.8 μg/dL) as NFAA, 51 to 138 nmol/L (1.9 to 5.0 μg/dL) as possible ACS (pACS), and >138 nmol/L (>5.0 μg/dL) without overt features of Cushing syndrome as ACS [6].
There is clear evidence that Cushing syndrome is associated with severe morbidity and elevated cardiovascular (CV) mortality [7–9]. Although most studies agree that ACS and NFAA rarely develop into overt Cushing syndrome [8, 10–14], there is evidence that ACS is associated with an elevated risk of CV outcomes and metabolic abnormalities [15–19], with studies supporting similar findings in patients with NFAA [20–22]. Although limited by cross-sectional data, these studies suggest that NFAAs secrete low levels of glucocorticoids and may not be truly “nonfunctional.” Although there have been several attempts to streamline the management of patients with AI, much remains controversial as a consequence of limited research, especially with regard to the role of adrenalectomy and follow-up in those who do not have overt signs and symptoms of Cushing syndrome. A better understanding of CV outcomes in ACS compared with NFAA will help guide the management of patients with AIs, including those that initially present as “nonfunctional.” Moreover, comparing the prevalence and incidence of traditional CV risk factors in relation to CV outcomes will help clarify the causal vs noncausal relationship between excess cortisol and the risk of CVD. This study aims to systematically review and summarize the literature on CV risk factors and outcomes in patients with NFAA and ACS.
1. Methods
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [23]. The study protocol was registered with PROSPERO (registration number CRD42017053153).
A. Literature Search
The search strategy was developed with the guidance of an information specialist. We used the Ovid Medline©, Embase, and Cochrane Reviews electronic databases. We searched the database for primary research studies evaluating CV outcomes in patients with adrenal adenomas, published between 1946 and February 2018. We limited the search to English-language publications. Additional publications were identified by a manual search through reference lists of articles identified by this search strategy.
B. Study Selection
We included all primary research studies (prospective and retrospective observational studies and randomized controlled trials) aimed at comparing CV risk factors and outcomes in NFAA and ACS. For this study, we included studies only if they reported at least one primary outcome (CV outcome). If the study reported only on CV risk factors (our secondary outcome), the study was not included. We included studies that enrolled participants with adrenal adenomas found incidentally on imaging (i.e., incidentalomas) and studies that grouped participants into NFAA and ACS based on definitions predetermined by the authors. The definitions of ACS and NFAA chosen for this systematic review were based on original definitions used in the included studies on the basis of morning cortisol after 1-mg DST: patients with ACS were defined as those with AI with morning cortisol after 1-mg DST >50 nmol/L or >138 nmol/L without any overt Cushing features. Patients with NFAA were defined as those with AI who did not meet the cortisol cutoff point for ACS. Suppressed ACTH, increased urinary free cortisol, and elevated midnight cortisol were also used in the studies as composite criteria for ACS. However, because these levels were not standardized between the studies, we opted to only include overnight 1-mg DST. Detailed description of the criteria for AI subcategories are available in Table 1.
Table 1.
Characteristics of Included Studies
| Cutoff Values for Subgroups | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Author | Study Design | Country | Population | Participants | ACS | pACS | NFAA | Follow-Up Duration | |
| Yener et al. (25) | Cross-sectional | Turkey | AI (2002–2009) | 273 | Overnight 1-mg DST >50 nmol/L | Overnight 1 mg DST ≤50 mmol/L | 24 mo only for NFAA group | ||
| At least 1 of: | At least 1 of: | ||||||||
| • ACTH <1.1 pmol/L (5 pg/mL) | • Morning DHEAS levels >40 μg/dL | ||||||||
| • Urinary free cortisol 110 μg/d | • Plasma ACTH >1.1 pmol/L (>5 pg/mL) | ||||||||
| • Midnight cortisol >207 nmol/L (>7.5 μg/dL) | • Urinary free cortisol <110 μg/d | ||||||||
| • Midnight cortisol <207 nmol/L (<7.5 μg/dL) | |||||||||
| Debono et al. (26) | Cohort | United Kingdom | AI (2005–2013) | 206 | Overnight 1-mg DST >138 nmol/L | Overnight 1-mg DST 50–137 nmol/L | Overnight 1-mg DST ≤0 mmol/L | Mean 4.2 y | |
| Di Dalmazi et al. (18) | Cohort | Italy | AI (1995–2010) | 198 | Overnight 1-mg DST >138 nmol/L | Overnight 1-mg DST 50–137 nmol/L | Overnight 1-mg DST ≤50 mmol/L | Mean 7.5 y (range 26 mo–5 y) | |
| Morelli et al. (19) | Cohort | Italy | AI (1996–2012) | 206 | Overnight 1-mg DST >138 nmol/L | Overnight 1-mg DST <137 nmol/L | Mean 83 mo (range 60–186 mo) | ||
| OR | |||||||||
| At least two: | |||||||||
| • ACTH 2.2 pmol/L (<10 pg/mL) | |||||||||
| • Elevated urinary free cortisol (above upper limit of normal of assay) | |||||||||
| • 1-mg DST >83 nmol/L (>3.0 μg/dL) | |||||||||
| Morelli et al. (28) | Case-control | Italy | AI (1996–2016) | 518 | Overnight 1-mg DST >50 nmol/L | Overnight 1-mg DST ≤50 mmol/L | Mean: 161.8 ± 45.1 (range 120–426 mo) | ||
| Patrova et al. (27) | Cohort | Sweden | AI (2003–2010) | 365 | Overnight 1-mg DST >138 nmol/L | Overnight 1-mg DST 50–137 nmol/L | Overnight 1-mg DST ≤50 mmol/L | Mean 5.2 ± 2.3 y (range 0.6–13.7 y) | |
We excluded nonoriginal studies and case reports. Titles and abstracts of potentially eligible studies were screened, and articles were rejected when the eligibility criteria were clearly not met. For the remaining articles, two investigators (J.P. and A.D.) independently assessed the full texts for eligibility. Differences in assessments were resolved by discussion to reach consensus.
C. Study Outcomes
The predefined primary outcome was the difference in CV outcome prevalence, incidence, and mortality between the two groups: NFAA and ACS. CV outcome was defined as any major CV event, including acute coronary syndrome, stable angina, coronary artery disease, stroke, and congestive heart failure. A broad definition was chosen because of potential heterogeneity regarding study outcome definition. Secondary outcomes included differences in prevalence and incidence of CV risk factors including body mass index (BMI), hypertension, type 2 diabetes mellitus (T2DM), and dyslipidemia.
D. Data Extraction and Validity Assessment
Two authors (J.P. and A.D.) independently extracted data from each included study. Discrepancies were resolved by discussion and adjudication with the other reviewers.
E. Data Synthesis and Analysis
The outcomes were organized into evidence tables for narrative synthesis. It was anticipated that the majority of included studies would be nonrandomized studies and that heterogeneity would exist between studies. For example, we anticipated that the included patient populations, definitions of NFAA and ACS, and definitions and type of CV outcomes and risk factors in each study would vary significantly. Based on this expectation, it was thought that a pooled analysis would be inappropriate. Instead, a detailed narrative synthesis of the included studies was planned.
F. Risk of Bias
Two investigators independently appraised each study for quality by using an assessment form based on the Newcastle Ottawa Scale (Appendix 2) [24]. The Newcastle Ottawa Scale assesses the risk of bias in nonrandomized studies (case-control and cohort studies) by using the following main categories: study group selection, comparative group selection, outcome assessment, and adequacy of follow-up.
For cohort studies, a maximum score of 9 stars was assigned, with a low risk of bias defined as a score of ≥8, moderate risk of bias 5 to 7, and high risk of bias ≤4. For cross-sectional studies, a maximum score of 7 stars was assigned, with a low risk of bias defined as a score of ≥6, moderate risk of bias 3 to 5, and high risk of bias ≤2. Discrepancies were resolved by discussion and adjudication with the other reviewers.
2. Results
A. Study Selection
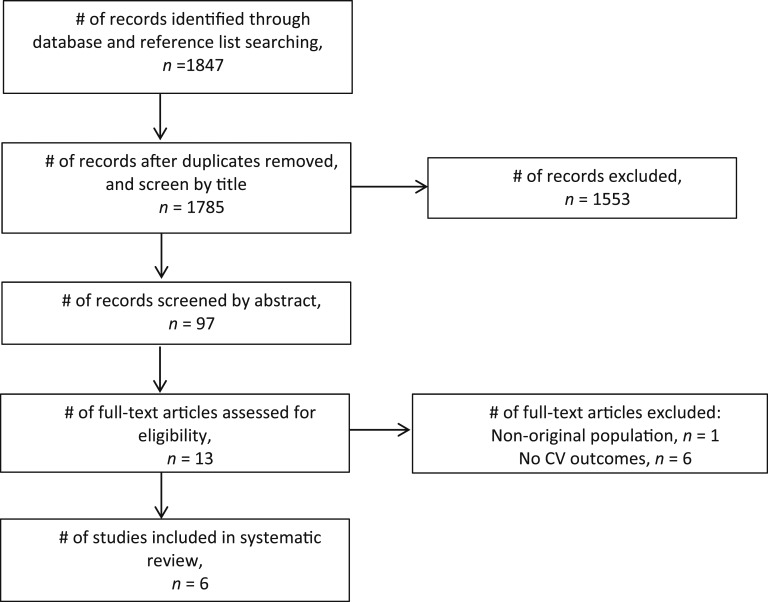
The search of the OVID Medline©, Embase, and Cochrane Reviews electronic databases, and review of the reference lists of included articles, provided total of 1847 citations. After we removed duplicates and screened titles, abstracts, and full text, a total of six studies met the eligibility criteria and were included in this systematic overview (Fig. 1).
Figure 1.
Study selection process.
B. Study Characteristics
Table 1 lists the characteristics of all six included studies [18, 19, 25–28]. Four studies were retrospective cohort studies [18, 19, 26, 27]. One study was a prospective design for one study group; however, only cross-sectional data were reported when the two reported study groups were compared [29]. One study used a case-control design [28]. The duration of the cohort studies ranged from 4 to 15 years. A total of 1766 participants were included in the six included studies, of which 363 were ACS, 279 were pACS, and 1124 were NFAA. There was an overlap of 95 subjects in Morelli’s 2012 and 2017 studies [19, 28], resulting in a total of 1671 unique individuals.
All studies included participants with adrenal lesions discovered incidentally by abdominal ultrasound, CT scan, or MRI and excluded patients with overt Cushing syndrome, suspected hormonally active lesion, or adrenal malignancy.
The definitions of ACS and NFAA varied between the studies, with differing cutoff values of serum cortisol after overnight 1-mg DST. These values are summarized in Table 1. Different criteria were used to define ACS, with the two most common criteria being secreted cortisol >138 nmol/L or >50 nmol/L after 1-mg DST. Three studies included an intermediate phenotype or possible ACS, defined as cortisol levels 50 to 138 nmol/L [18, 26, 27]. Two of the six studies also included suppressed ACTH, elevated 24-hour urinary free cortisol, and elevated midnight cortisol level as part of the composite criteria for ACS. The cutoff numbers for these tests were not standardized between the studies and therefore not used to determine participant categories [19, 25].
C. Risk of Bias
Of the six included studies, two studies were rated as having low risk of bias [18, 28] and four studies were rated as moderate risk of bias [18, 25–27] The results are summarized in Table 2.
Table 2.
Risk of Bias for Observational Studies Evaluating the Difference Between Patients With ACS and NFAA and CVEs Based on the Newcastle-Ottawa Scale for Nonrandomized Clinical Studies and Stratified by Type of Study
| Study Design | Selection | Comparability | Outcome | Risk of Bias | |
|---|---|---|---|---|---|
| Cohort studies | |||||
| Debono et al. (26) | RC | XXXX | X | XX | Moderate |
| Di Dalmazi et al. (15) | RC | XXXX | X | XXX | Low |
| Morelli et al. (19) | RC | XXX | XXX | Moderate | |
| Patrova et al. (27) | RC | XXX | X | XXX | Moderate |
| Cross-sectional and case-control studies | |||||
| Yener et al. (25) | CS | XXXX | X | Moderate | |
| Morelli et al. (28) | CC | XXXX | X | X | Low |
Each X represents a point awarded for each numbered item within the selection, comparability, and outcome or exposure.
Abbreviations: CC, case-control study; CS, cross-sectional study; RC, retrospective cohort study.
D. Cardiovascular Outcomes
The primary outcomes are summarized in Table 3. The difference in prevalence of CVD was reported in four of six studies [18, 19, 25, 28]. The prevalence was statistically greater by twofold to threefold in the ACS group compared with the NFAA group in all four studies, with all studies’ P values <0.05. Di Dalmazi et al. [18] reported the differences in coronary heart disease (CHD) prevalence and stroke prevalence independently, which revealed 11% more CHD (P < 0.03) and 9% more stroke (P = 0.03) in ACS than in NFAA.
Table 3.
Comparison of CV Outcomes in Patients With ACS Compared With Those With NFAA
| Author | Sample Size | Outcome | Cardiovascular Outcomes | ||
|---|---|---|---|---|---|
| ACSa | pACSb | NFAA | ACS vs NFAA | ||
| Yener et al. (25) | 42 | 231c | CVE (acute coronary syndrome, CAD, CABG, PAD, cerebrovascular disease, PCI, or stroke) | Prevalence of CVE: ACS 19.5% vs NFAA 6.7%, P < 0.016 | |
| Debono et al. (26) | 19 | 92 | 95d | All-cause mortality | All-cause mortality: ACS vs NFAA, HR 22.0 (95% CI, 2.6–188.3) |
| Mean survival | Survival ACS 6.9 y (95% CI, 5.6–8.3) vs pACS 7.3 (95% CI, 6.8–7.8) vs NFAA 8.4 (95% CI, 8.2–8.6), P < 0.001 | ||||
| Di Dalmazi et al. (18) | 10 | 59 | 129d | CHD | CHD prevalence: ACS 18% vs NFAA 7%, P < 0.03 |
| Stroke | Stroke prevalence: ACS 13% vs NFAA 4%, P = 0.03 | ||||
| MI | CVE incidence: ACS 16.7% vs NFAA 6.7%, P < 0.004 | ||||
| CVD-specific mortality | CVE incidence: ACS vs NFAA unadjusted HR 3.01 (95% CI, 1.04–8.72), P = 0.04 | ||||
| CVD-specific mortality at 15 y (unadjusted) ACS 22.6% vs NFAA 2.5%, P = 0.02 | |||||
| All-cause mortality (unadjusted) at 15 y: ACS 43.0% vs NFAA 8.8%, P = 0.005 | |||||
| Morelli et al. (19) | 39 | 167c | CVE (CHD, ischemic or hemorrhagic stroke) | CVE prevalence: ACS 20.5% vs NFAA 6.0%, P < 0.05 | |
| CVE incidence: ACS 20.5% vs NFAA 8.4%, P = 0.04 | |||||
| CVE incidence in ACS vs NFAA OR: 2.7 (95% CI, 1.0–7.1); P < 0.04 | |||||
| Morelli et al. (28) | 220e | 298d | CVE (MI, stroke, TIA, angina pectoris, PE, ICH, PAD) | CVE prevalence: ACS 26.8% vs NFAA 10.4%, P < 0.001 | |
| Patrova et al. (27) | 33 | 128 | 204d | All-cause mortality | All-cause mortality: ACS 18.2% vs pACS 11.7% vs 7.8% NFAA, P = 0.019; HR 1.27 (95% CI, 0.86–2.12) |
Abbreviations: CABG, coronary artery bypass graft; CAD, coronary artery disease; CHD, cardiovascular heart disease; HR, hazard ratio; ICH, intercranial hemorrhage; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; PE, pulmonary embolism; TIA, transient ischemic attack.
Serum cortisol levels after 1-mg DST >138 mmol/L.
Serum cortisol levels after 1-mg DST 51–138 mmol/L.
Serum cortisol levels after 1-mg DST ≤138 mmol/L.
Serum cortisol levels after 1-mg DST ≤50 mmol/L.
Serum cortisol levels after 1-mg DST >50 mmol/L.
Two studies compared the difference in incidence of CV events (CVEs) between ACS and NFAA [18, 19]. In one cohort, the incidence of CVEs in ACS was 20.5% compared with 8.4% in NFAA, with an odds ratio of 2.7 (95% CI, 1.0 to 7.1), P < 0.04 [19]. Likewise, the Di Dalmazi et al. [18] cohort demonstrated that there was a greater incidence of CVEs in ACS (16.7%) when compared with NFAA (6.7%), P = 0.04, with an unadjusted hazard ratio for new CVEs of 3.01 (95% CI, 1.04 to 8.72), P = 0.04. The definitions of CVEs varied between the two studies: Morelli et al. [19] defined CVE as CHD or ischemic or hemorrhagic stroke, whereas Di Dalmazi et al. [18] defined CVE as nonfatal acute myocardial infarction (MI), percutaneous transluminal coronary angioplasty and surgical bypass for ischemic heart disease, or ischemic stroke.
Two separate studies used multivariate regression analysis to test for incidence of CVEs and post–1-mg overnight DST serum cortisol level as a continuous variable [27, 28]. Both found that CVE occurrence was significantly associated with post–1-mg DST cortisol levels independently from other CV risk factors. Based on the regression analysis, Morelli et al. [28] determined that a serum cortisol level >50 nmol/L after overnight 1-mg DST predicted increased CVE risk by 2.5-fold.
Survival rates in patients with ACS were reduced (ACS 6.9 years vs NFAA 8.4 years, P < 0.01) [25], CV-specific mortality at 15 years was elevated (ACS 22.6% vs NFAA 2.5%, P < 0.02), and all-cause mortality at 15 years (ACS 43.0% vs NFAA 8.8%, P = 0.005) [18] and at 12 years were elevated (ACS 18.2% vs NFAA 7.8%) [27] in comparison with patients with NFAA [18, 26, 27].
Cause of death was reported in the three studies. In Debono et al.’s cohort [26], the main causes of death were CVD and respiratory infections. Similarly, Di Dalmazi et al. [18] also reported that their main cause of mortality was CVD. However, in Patrova et al.’s [27] cohort, the rate of death due to malignancies was higher than that of CVD.
E. CV Risk Factors
The secondary outcomes are summarized in Table 4. Five studies looked at the prevalence of CV risk factors at baseline. In three of five studies, there was significantly more baseline hypertension [25, 26, 28] and T2DM [18–20] documented in subjects with ACS compared with NFAA. One of the four studies found dyslipidemia to be more prevalent in the ACS group [28].
Table 4.
Comparison of CV Risk Factors in ACS vs NFAA in Patients With AI
| Diabetes (%) | Dyslipidemia or on Treatment (%) | Hypertension (%) | BMI (Mean) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACS | NFAA | ACS | NFAA | ACS | NFAA | ACS | NFAA | |||||||||
| Author | BL | FU | BL | FU | BL | FU | BL | FU | BL | FU | BL | FU | BL | FU | BL | FU |
| Yener et al. (25) | 16.6 | 18.7 | — | — | — | — | 68.2a | — | 51.7 | 29.5 | — | 28.6 | — | |||
| Di Dalmazi et al. (18) | 40a | 38a (ACS + pACS) | 18 | 19 | 30 | 25 (ACS + pACS) | 14 | 28 | 90 | 77 (ACS + pACS) | 73 | 73 | 29.2 | 27.5a (ACS + pACS) | 28.5 | 28.9 |
| Morelli et al. (19) | 33.3a | 43.6a | 16.8 | 22.2 | 53.8 | 69.2 | 41.9 | 53.9 | 66.7 | 87.2a | 53.9 | 62.9 | 28.3 | 29.2 | 27.9 | 28.2 |
| Patrova et al. (27) | 9.1 | — | 12.3 | — | 12.1 | — | 17.2a | — | 57.6a | — | 39.2 | — | 25.7 | — | 28.7 | — |
| Morelli et al. (28) | 25.9a | — | 18.5 | — | 41.4a | — | 32.9 | — | 74.5a | — | 60.7 | — | 27.4 | — | 27.8 | — |
Abbreviations: BL, baseline; FU, follow-up.
Statistically significant difference between ACS and NFAA, P < 0.05.
Of the five studies, two examined the incidence of CV risk factors at follow-up interval [18, 19]. One of two studies also reported an increased risk of hypertension at follow-up in those with ACS compared with NFAA [19]. Both studies reported higher T2DM rates at follow-up. None of the studies showed differences in dyslipidemia at follow-up [18, 19]. None of the studies demonstrated a difference in BMI between the ACS and NFAA groups at baseline or at follow-up [18, 19, 25, 26, 28].
F. Subjects With Progression or Normalization of Disease
Our search identified three studies that described a subgroup of patients who progressed in their disease, with rising cortisol levels after 1-mg DST [18, 19, 27]. In these studies, progression of NFAA to pACS or pACS to ACS was noted in 3% to 12% of participants. Di Dalmazi et al. [18] also demonstrated in its subgroup analysis that participants with progression of disease had the worst mortality and CVD outcomes compared with the other groups. Moreover, Morelli et al. [19] demonstrated that tumor size >2.4 cm was associated with the development of ACS from NFAA (OR 2.97; 95% CI, 1.37 to 6.44; P < 0.006). In contrast, one study reported normalization of disease to pACS or NFAA in 12% to 15% [27]. This finding of normalization was not reported in the other two studies. Progression to overt Cushing syndrome was not reported in any of the studies [18, 19, 25–28].
G. Other Related Outcomes
Several studies have suggested a possible correlation between tumor size and the degree of alterations in adrenal function [19, 25, 26, 28]. However, there were inconsistent data regarding a direct correlation between the tumor size and cortisol secretion [18, 27]. Moreover, one study that looked at adenoma size and its effect on CVEs found no significant differences [28].
3. Discussion
With advances in imaging techniques, the incidental discovery of adrenal adenoma is rising, leading to increased detection of ACS. Recent evidence suggests greater morbidity and mortality associated with ACS when compared with the general population. The management of this patient group remains largely controversial, however [30]. There have been multiple clinical practice guidelines from various societies in the past 10 years, including the National Institutes of Health in 2003, Endocrine Society in 2008, French Society of Endocrinology in 2008, American Association of Clinical Endocrinologists in 2009, Italian Association of Clinical Endocrinologists in 2011, and most recently, European Society of Endocrinology in 2016 [6, 31–36]. There has been nonconformity between the clinical practice guidelines especially with regard to classification of AI based on 1-mg DST morning cortisol cutoffpoints, as well as the role of adrenalectomy and follow-up in ACS and NFAA.
The current European guidelines from 2016 suggest an individualized approach for surgical management for those with ACS based on comorbidities, age, and degree of cortisol excess. Surgery and repeat hormonal workup are not recommended for those with NFAA and benign features on imaging [6]. In the Italian Association of Clinical Endocrinologists 2015 guidelines, adrenalectomy is suggested for those for whom adequate medical therapy does not achieve treatment goals associated with comorbidities possibly linked to cortisol excess [36]. Similarly, the American Association of Clinical Endocrinologists 2009 guidelines recommend surgery in ACS with worsening hypertension, abnormal glucose tolerance, dyslipidemia, or osteoporosis [35]. By contrast, the National Institutes of Health (2003) and French Society of Endocrinology (2008) clinical practice guidelines did not recommend adrenalectomy [24, 31].
This systematic review suggests that that there is greater CVE prevalence, mortality, and reduced survival in patients with ACS when compared with those with NFAA. These findings, although limited by heterogeneity of data, suggest that despite both ACS and NFAA groups having more CVD risks and outcomes compared with the general population, the degree of cortisol secretion plays a role in the risk of developing CVD outcomes. This finding is further supported by two studies that showed a direct correlation between the incidence of CVEs and post–1-mg overnight DST serum cortisol level as a continuous variable. This correlation supports the notion that serum cortisol levels after 1-mg overnight DST should be a continuous variable rather than categorical entities. This notion is in agreement with the European guidelines from 2016 [6]. Moreover, our results indicate that there is a greater relative indication for surgery in those with ACS when compared with NFAA. However, the absolute indication for adrenalectomy in both ACS and NFAA hinges on future prospective and randomized controlled studies comparing adrenalectomy with conservative management on CV outcomes.
European guidelines from 2016 also suggest using the cutoff point value of cortisol ≤50 nmol/L on post–1-mg DST to exclude the diagnosis of autonomous cortisol secretion [6]. This guideline was based on two studies that showed greater mortality and morbidity in ACS when compared with NFAA [18, 26]. Although our review agrees that ACS has greater risk of CVEs than NFAA, there are limitations to both the Debono et al. [26] and Di Dalmazi et al. [18,] categorical subgroups: NFAA and ACS. The groups were preselected based on existing definitions of AI: ≤50 nmol/L cortisol after 1-mg DST as NFAA. Both of these studies did not account for other cortisol cutoff values in grouping NFAA and ACS. Furthermore, in another study, post–1-mg DST cortisol of >41 nmol/L was the most sensitive to determine when CVE prevalence correlated with the level of excess cortisol (measured by 1-mg DST). Therefore, whether cortisol of 50 nmol/L after 1-mg DST should be the cutoff for “nonfunctioning” AI risk remains unclear.
Our systematic review also shows that ≤12% of patients progressed to worsened categories of AI, although none converted to overt Cushing syndrome. Although this progression has not been demonstrated in all studies, it supports the hypothesis that ACS is part of a biochemical spectrum. With the exception of one study that demonstrated an association between tumor size and disease progression, other predictors of progression vs normalization of adrenal function have not yet been described [19]. Based on Di Dalmazi et al.’s [18] subgroup analysis, which shows increased mortality in the group of patients who worsened in their cortisol secretion, it is possible that this group possesses phenotypical differences despite being on a biochemical continuum. This finding has not been confirmed in other studies.
Interestingly, despite all studies demonstrating more CVEs in the ACS group, not all studies showed a statistical difference in prevalence or incidence of traditional CV risk factors (diabetes, dyslipidemia, and hypertension). Thus, the mechanism of hypercortisolemia causing greater CV mortality may not work solely via traditional CV risk factors. Some studies have suggested that elevated post–1-mg DST cortisol itself is an independent risk factor for CVEs when adjusted for CV risk factors [18, 27, 28]. Other studies have demonstrated that patients with ACS had a higher prevalence of diastolic dysfunction, higher arterial stiffness, and greater left ventricular mass when compared with NFAA independent of other cardiac risk factors [37]. Additional work investigating the mechanism of cortisol on CV outcomes in patients with ACS and NFAA is warranted.
A. Limitations
Our systematic review includes only retrospective and cross-sectional studies, limiting the opportunity for assessing causality and introducing risks of bias and confounders. Based on the results, it is difficult to know whether ACS and CV risk factors or events are simply correlated or whether there is a causal relationship. Very few studies adjusted for known confounders, further limiting our ability to accurately interpret the data. Moreover, four of the six included studies had moderate risk of bias. Most studies had limitations that were related to patient selection, adequacy of control group, follow-up, and retrospective nature of the study.
Based on current European guidelines, patients with NFAA do not need any additional follow-up or screening for CV outcomes and metabolic complications [6]. Patients with pACS and ACS probably had more follow-up, more investigations, and more contact with the health care system. Given the retrospective design of the included studies, it is possible that CV events and outcomes were more likely to be reported for these patients than for patients with NFAA.
Heterogeneity in existing studies also made it challenging to interpret outcomes and synthesize conclusions. First, the definition of ACS varied between studies. Hormonal assays mostly involved serum cortisol after 1-mg DST but sometimes in combination with other assays. Moreover, the definitions used for both ACS and CV outcomes differed between studies. The method of determining the cause of mortality also differed between studies. Some studies used the reports of general practitioners, cardiologists, or endocrinologists, whereas others used electronic medical records. One study used communication via telephone to family members to determine the cause of death, which could have resulted in inaccurate outcome information. These differences in methods and definitions of outcome measures limited our ability to pool data and analyze data. Qualitative analysis was also limited by studies differing in their definitions and measurement of CVEs.
B. Review Implications
Our review supports the current European guidelines regarding the biochemical continuous definition and diagnosis of adrenal incidentaloma using serum cortisol after 1-mg DST. It also indicates that until more definitive studies are performed, adrenalectomy should be offered only to those with established cortisol excess, on the basis of individualized risk-benefit assessment.
This review highlights the importance of future research in this area. Additional studies are needed to evaluate the use of cortisol after 1-mg DST as a continuous variable rather than distinct categories of AI. Large prospective studies are also warranted to better understand the progression in cortisol secretion, changes in tumor size, and the mechanisms by which excess cortisol may increase CVE risk independent of traditional CV risk factors.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ACS
autonomous cortisol secretion
- AI
adrenal incidentaloma
- BMI
body mass index
- CHD
coronary heart disease
- CV
cardiovascular
- CVE
cardiovascular event
- DST
dexamethasone suppression test
- MI
myocardial infarction
- NFAA
nonfunctioning adrenal adenoma
- pACS
possible autonomous cortisol secretion
- SCS
subclinical Cushing syndrome
- T2DM
type 2 diabetes mellitus
References and Notes
- 1. Bovio S, Cataldi A, Reimondo G, Sperone P, Novello S, Berruti A, Borasio P, Fava C, Dogliotti L, Scagliotti GV, Angeli A, Terzolo M. Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J Endocrinol Invest. 2006;29(4):298–302. [DOI] [PubMed] [Google Scholar]
- 2. Kloos RT, Gross MD, Francis IR, Korobkin M, Shapiro B. Incidentally discovered adrenal masses. Endocr Rev. 1995;16(4):460–484. [DOI] [PubMed] [Google Scholar]
- 3. Mantero F, Terzolo M, Arnaldi G, Osella G, Masini AM, Alì A, Giovagnetti M, Opocher G, Angeli A; Study Group on Adrenal Tumors of the Italian Society of Endocrinology. A survey on adrenal incidentaloma in Italy. J Clin Endocrinol Metab. 2000;85(2):637–644. [DOI] [PubMed] [Google Scholar]
- 4. Rossi R, Tauchmanova L, Luciano A, Di Martino M, Battista C, Del Viscovo L, Nuzzo V, Lombardi G. Subclinical Cushing’s syndrome in patients with adrenal incidentaloma: clinical and biochemical features. J Clin Endocrinol Metab. 2000;85(4):1440–1448. [DOI] [PubMed] [Google Scholar]
- 5. Chiodini I. Clinical review: diagnosis and treatment of subclinical hypercortisolism. J Clin Endocrinol Metab. 2011;96(5):1223–1236. [DOI] [PubMed] [Google Scholar]
- 6. Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, Tabarin A, Terzolo M, Tsagarakis S, Dekkers OM. Management of adrenal incidentalomas: European society of endocrinology clinical practice guideline in collaboration with the European network for the study of adrenal tumors. Eur J Endocrinol. 2016;175(2):G1–G34. [DOI] [PubMed] [Google Scholar]
- 7. Dekkers OM, Horváth-Puhó E, Jørgensen JO, Cannegieter SC, Ehrenstein V, Vandenbroucke JP, Pereira AM, Sørensen HT. Multisystem morbidity and mortality in Cushing’s syndrome: a cohort study. J Clin Endocrinol Metab. 2013;98(6):2277–2284. [DOI] [PubMed] [Google Scholar]
- 8. Nieman LK. Cushing’s syndrome: update on signs, symptoms and biochemical screening. Eur J Endocrinol. 2015;173(4):M33–M38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lacroix A, Feelders RA, Stratakis CA, Nieman LK. Cushing’s syndrome. Lancet. 2015;386(9996):913–927. [DOI] [PubMed] [Google Scholar]
- 10. Barzon L, Sonino N, Fallo F, Palu G, Boscaro M. Prevalence and natural history of adrenal incidentalomas. Eur J Endocrinol. 2003;149(4):273–285. [DOI] [PubMed] [Google Scholar]
- 11. Barzon L, Fallo F, Sonino N, Boscaro M. Development of overt Cushing’s syndrome in patients with adrenal incidentaloma. Eur J Endocrinol. 2002;146(1):61–66. [DOI] [PubMed] [Google Scholar]
- 12. Libe R, Dall'Asta C, Barbetta L, Baccarelli A, Beck-Peccoz P, Ambrosi B. Long-term follow-up study of patients with adrenal incidentalomas. Eur J Endocrinol. 2002;147(4):489–494. [DOI] [PubMed] [Google Scholar]
- 13. Terzolo M, Osella G, Alì A, Borretta G, Cesario F, Paccotti P, Angeli A. Subclinical Cushing’s syndrome in adrenal incidentaloma. Clin Endocrinol (Oxf). 1998;48(1):89–97. [DOI] [PubMed] [Google Scholar]
- 14. Bernini GP, Moretti A, Oriandini C, Bardini M, Taurino C, Salvetti A. Long-term morphological and hormonal follow-up in a single unit on 115 patients with adrenal incidentalomas. Br J Cancer. 2005;92(6):1104–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Di Dalmazi G, Vicennati V, Rinaldi E, Morselli-Labate AM, Giampalma E, Mosconi C, Pagotto U, Pasquali R. Progressively increased patterns of subclinical cortisol hypersecretion in adrenal incidentalomas differently predict major metabolic and cardiovascular outcomes: a large cross-sectional study. Eur J Endocrinol. 2012;166(4):669–677. [DOI] [PubMed] [Google Scholar]
- 16. Tauchmanovà L, Rossi R, Biondi B, Pulcrano M, Nuzzo V, Palmieri EA, Fazio S, Lombardi G. Patients with subclinical Cushing’s syndrome due to adrenal adenoma have increased cardiovascular risk. J Clin Endocrinol Metab. 2002;87(11):4872–4878. [DOI] [PubMed] [Google Scholar]
- 17. Tsuiki M, Tanabe A, Takagi S, Naruse M, Takano K. Cardiovascular risks and their long-term clinical outcome in patients with subclinical Cushing’s syndrome. Endocr J. 2008;55(4):737–745. [DOI] [PubMed] [Google Scholar]
- 18. Di Dalmazi G, Vicennati V, Garelli S, Casadio E, Rinaldi E, Giampalma E, Mosconi C, Golfieri R, Paccapelo A, Pagotto U, Pasquali R. Cardiovascular events and mortality in patients with adrenal incidentalomas that are either non-secreting or associated with intermediate phenotype or subclinical Cushing’s syndrome: a 15-year retrospective study. Lancet Diabetes Endocrinol. 2014;2(5):396–405. [DOI] [PubMed] [Google Scholar]
- 19. Morelli V, Reimondo G, Giordano R, Della Casa S, Policola C, Palmieri S, Salcuni AS, Dolci A, Mendola M, Arosio M, Ambrosi B, Scillitani A, Ghigo E, Beck-Peccoz P, Terzolo M, Chiodini I. Long-term follow-up in adrenal incidentalomas: an Italian multicenter study. J Clin Endocrinol Metab. 2014;99(3):827–834. [DOI] [PubMed] [Google Scholar]
- 20. Peppa M, Boutati E, Koliaki C, Papaefstathiou N, Garoflos E, Economopoulos T, Hadjidakis D, Raptis SA. Insulin resistance and metabolic syndrome in patients with nonfunctioning adrenal incidentalomas: a cause-effect relationship? Metabolism. 2010;59(10):1435–1441. [DOI] [PubMed] [Google Scholar]
- 21. Lopez D, Luque-Fernandez MA, Steele A, Adler GK, Turchin A, Vaidya A. “Nonfunctional” adrenal tumors and the risk for incident diabetes and cardiovascular outcomes: a cohort study. Ann Intern Med. 2016;165(8):533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Androulakis II, Kaltsas GA, Kollias GE, Markou AC, Gouli AK, Thomas DA, Alexandraki KI, Papamichael CM, Hadjidakis DJ, Piaditis GP. Patients with apparently nonfunctioning adrenal incidentalomas may be at increased cardiovascular risk due to excessive cortisol secretion. J Clin Endocrinol Metab. 2014;99(8):2754–2762. [DOI] [PubMed] [Google Scholar]
- 23. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. Newcastle-Ottawa Quality Assessment Scale, cohort studies. 2014.www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 25. Yener S, Ertilav S, Secil M, Akinci B, Demir T, Kebapcilar L, Yesil S.. Increased risk of unfavorable metabolic outcomes during short-term follow-up in subjects with nonfunctioning adrenal adenomas. Med Princ Pract. 2012;21(5):429–434. [DOI] [PubMed] [Google Scholar]
- 26. Debono M, Bradburn M, Bull M, Harrison B, Ross RJ, Newell-Price J. Cortisol as a marker for increased mortality in patients with incidental adrenocortical adenomas. J Clin Endocrinol Metab. 2014;99(12):4462–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Patrova J, Kjellman M, Wahrenberg H, Falhammar H. Increased mortality in patients with adrenal incidentalomas and autonomous cortisol secretion: a 13-year retrospective study from one center. Endocrine. 2017;58(2):267–275. [DOI] [PubMed] [Google Scholar]
- 28. Morelli V, Palmieri S, Lania A, Tresoldi A, Corbetta S, Cairoli E, Eller-Vainicher C, Arosio M, Copetti M, Grossi E, Chiodini I. Cardiovascular events in patients with mild autonomous cortisol secretion: analysis with artificial neural networks. Eur J Endocrinol. 2017;177(1):73–83. [DOI] [PubMed] [Google Scholar]
- 29. Yener S, Ertilav S, Secil M, Demir T, Akinci B, Kebapcilar L, Comlekci A, Bayraktar F, Yesil S. Prospective evaluation of tumor size and hormonal status in adrenal incidentalomas. J Endocrinol Invest. 2010;33(1):32–36. [DOI] [PubMed] [Google Scholar]
- 30. Bancos I, Alahdab F, Crowley RK, Chortis V, Delivanis DA, Erickson D, Natt N, Terzolo M, Arlt W, Young WF Jr, Murad MH. Therapy of endocrine disease: improvement of cardiovascular risk factors after adrenalectomy in patients with adrenal tumors and subclinical Cushing’s syndrome: a systematic review and meta-analysis. Eur J Endocrinol. 2016;175(6):R283–R295. [DOI] [PubMed] [Google Scholar]
- 31. Grumbach MM, Biller BM, Braunstein GD, Campbell KK, Carney JA, Godley PA, Harris EL, Lee JK, Oertel YC, Posner MC, Schlechte JA, Wieand HS. Management of the clinically inapparent adrenal mass (“incidentaloma”). Ann Intern Med. 2003;138(5):424–429. [DOI] [PubMed] [Google Scholar]
- 32. Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM. The diagnosis of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(5):1526–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nieman LK, Biller BM, Findling JW, Murad MH, Newell-Price J, Savage MO, Tabarin A; Endocrine Society. Treatment of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(8):2807–2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tabarin A, Bardet S, Bertherat J, Dupas B, Chabre O, Hamoir E, Laurent F, Tenenbaum F, Cazalda M, Lefebvre H, Valli N, Rohmer V. Exploration and management of adrenal incidentalomas. French Society of Endocrinology consensus. Ann Endocrinol (Paris). 2008;69(6):487–500. [DOI] [PubMed] [Google Scholar]
- 35. Zeiger MA, Thompson GB, Duh QY, Hamrahian AH, Angelos P, Elaraj D, Fishman E, Kharlip J;. American Association of Clinical Endocrinologists; American Association of Endocrine Surgeons. The American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas. Endocr Pract. 2009;15(suppl 1):1–20. [DOI] [PubMed] [Google Scholar]
- 36. Terzolo M, Stigliano A, Chiodini I, Loli P, Furlani L, Arnaldi G, Reimondo G, Pia A, Toscano V, Zini M, Borretta G, Papini E, Garofalo P, Allolio B, Dupas B, Mantero F, Tabarin A; Italian Association of Clinical Endocrinologists. AME position statement on adrenal incidentaloma. Eur J Endocrinol. 2011;164(6):851–870. [DOI] [PubMed] [Google Scholar]
- 37. Sbardella E, Minnetti M, D’Aluisio D, Rizza L, Di Giorgio MR, Vinci F, Pofi R, Giannetta E, Venneri MA, Vestri A, Morelli S, Lenzi A, Isidori AM. Cardiovascular features of possible autonomous cortisol secretion in patients with adrenal incidentalomas. Eur J Endocrinol. 2018;178(5):501–511. [DOI] [PubMed] [Google Scholar]