Abstract
The exocyst is a highly conserved protein complex found in most eukaryotic cells and is associated with many functions, including protein translocation in the endoplasmic reticulum, vesicular basolateral targeting, and ciliogenesis in the kidney. To investigate the exocyst functions, here we exchanged proline for alanine in the highly conserved VXPX ciliary targeting motif of EXOC5 (exocyst complex component 5), a central exocyst gene/protein, and generated stable EXOC5 ciliary targeting sequence–mutated (EXOC5CTS-m) Madin–Darby canine kidney (MDCK) cells. The EXOC5CTS-m protein was stable and could bind other members of the exocyst complex. Culturing stable control, EXOC5-overexpressing (OE), Exoc5-knockdown (KD), and EXOC5CTS-m MDCK cells on Transwell filters, we found that primary ciliogenesis is increased in EXOC5 OE cells and inhibited in Exoc5-KD and EXOC5CTS-m cells. Growing cells in collagen gels until the cyst stage, we noted that EXOC5-OE cells form mature cysts with single lumens more rapidly than control cysts, whereas Exoc5-KD and EXOC5CTS-m MDCK cells failed to form mature cysts. Adding hepatocyte growth factor to induce tubulogenesis, we observed that EXOC5-OE cell cysts form tubules more efficiently than control MDCK cell cysts, EXOC5CTS-m MDCK cell cysts form significantly fewer tubules than control cell cysts, and Exoc5-KD cysts did not undergo tubulogenesis. Finally, we show that EXOC5 mRNA almost completely rescues the ciliary phenotypes in exoc5-mutant zebrafish, unlike the EXOC5CTS-m mRNA, which could not efficiently rescue the phenotypes. Taken together, these results indicate that the exocyst, acting through the primary cilium, is necessary for renal ciliogenesis, cystogenesis, and tubulogenesis.
Keywords: cilia, trafficking, development, exocytosis, cell biology, ciliary targeting sequence, exocyst, primary cilia, protein trafficking, vesicle
Introduction
The exocyst is a highly conserved eight-protein complex that was originally identified in a secretory screen in yeast by Novick et al. in 1980 (1). The eight homologous mammalian exocyst proteins were first identified in 1996 from rat brain (2). The exocyst is found in most cell types and has been linked by us and others to a wide variety of cellular processes, including: vesicular transport to the basolateral membrane (3, 4), primary ciliogenesis in the kidney and eye (5–7), protein synthesis in the endoplasmic reticulum (8, 9), and postendocytic recycling (10).
Until recently, relatively little was known about the exocyst structure; therefore, it has been difficult to tease out the various functions of the exocyst. We previously showed that Exoc5 (also called Sec10) is a central component of the exocyst, linking Exoc6, which binds Rab8 (11), found on the surface of vesicles targeted by the exocyst, to the rest of the exocyst at the plasma membrane. In the absence of Exoc5, the exocyst complex disintegrates and is degraded, most likely via the proteasome (7). In 2017, the crystal structure of EXOC5 (12) was solved, and an in vivo 3D integrative approach to the exocyst was performed (13). More recently, cryo-EM detailing the exocyst structure was reported (14). With the exocyst structure available, our goal here was to determine the role of the exocyst, and especially the central Exoc5 component, in renal primary ciliogenesis, and then, by extension, the role of the exocyst, and ciliogenesis, in cystogenesis and tubulogenesis.
Results
Site-directed mutagenesis of the human EXOC5 ciliary targeting sequence leads to a stable protein that can bind other exocyst complex proteins
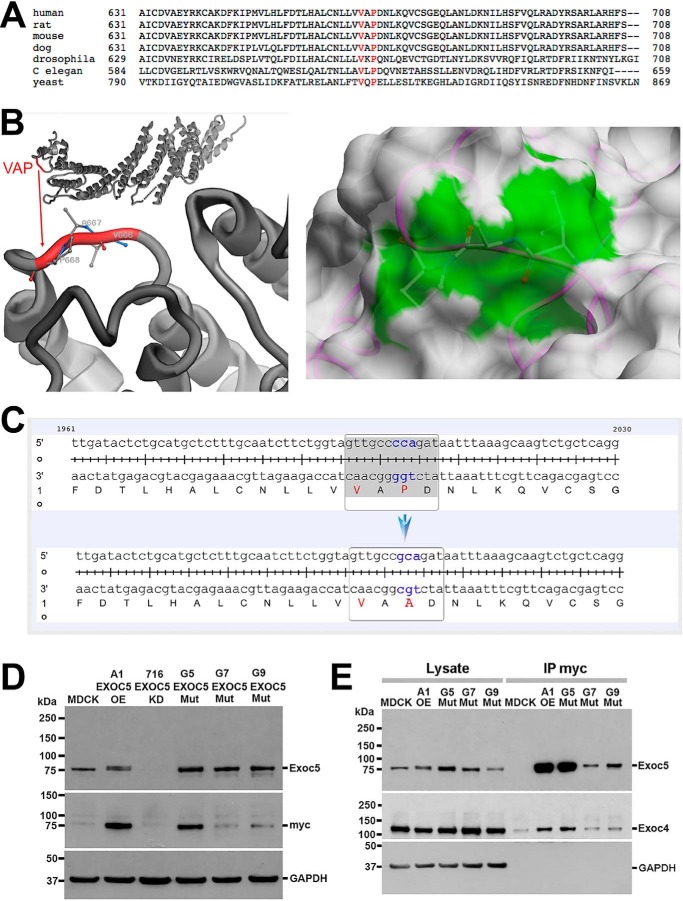
EXOC5 contains a VXPX ciliary targeting sequence that is highly conserved from yeast to humans (Fig. 1A). We analyzed solvent accessibility of the Val666, Ala667, and Pro668 residues of EXOC5 represented by the 5h11 structure (12). Solvent-accessible surface areas were 37, 49, and 52 Å2 and relative accessibilities were 32, 73, and 50%, respectively. Thus, all three residues are exposed to solvent and are available for binding, although proline to a greater degree than valine (Fig. 1B). We therefore performed site-directed mutagenesis of EXOC5-myc cDNA in a pcDNA3 vector, mutating the cytosine at position 2002 to a guanine (cca to gca), leading to alanine being translated instead of proline. Successful site-directed mutagenesis was confirmed by sequencing the full cDNA transcript (Fig. 1C). The pcDNA3 vector containing the human EXOC5-myc mutated ciliary targeting sequence (EXOC5CTS-m) was transfected into MDCK2 (type II) cells, and stable cell lines were generated. Three clonal cell lines expressing human EXOC5CTS-m were identified (G5, G7, and G9) using an antibody we made against human EXOC5 (7), and an antibody against the myc epitope tag (Fig. 1D). Using our anti-EXOC5 antibody, we show that the A1 MDCK cells have ∼2-fold higher expression of Exoc5 than untransfected MDCK cells (transfected EXOC5-myc is the top band in Fig. 1D and is of equal intensity to the bottom native Exoc5 band, and to the band in the untransfected MDCK cells). Based on the intensity of the bands stained with the anti-myc antibody, clone G5 expressed the human EXOC5CTS-m protein to a similar degree as A1 MDCK cells stably expressing WT human EXOC5-myc that we previously generated and used in multiple studies (4, 7, 15, 16) (Fig. 1D). We then performed co-immunoprecipitation using antibody against the myc epitope tag and found that human EXOC5CTS-m protein was able to bind another exocyst component, Exoc4 (Fig. 1E).
Figure 1.
Site-directed mutagenesis of the ciliary targeting sequence in human EXOC5 cDNA results in a stable protein that can bind other exocyst components. A, the EXOC5 VXPX ciliary targeting sequence is highly conserved from yeast to humans. B, the VXPX ciliary targeting sequence in the EXOC5 3D protein model shows that proline (and to a lesser degree valine) are on the outside of the EXOC5 protein and hence are available for binding. The right panel demonstrates the solvent-accessible surface of EXOC5 in the 5h11 structure. The protein is shown as backbone trace (magenta) and the molecular surface obtained with a spherical water probe with a radius of 1.4 Å (white). Three residues Val666, Ala667, and Pro668 (VAP) are shown by balls and sticks. The contribution of these residues to the molecular surface is marked in green. C, site-directed mutagenesis of cytosine at position 2002 results in a guanine substitution (cca to gca), which leads to alanine being translated instead of a proline. D, EXOC5CTS-m protein is stable as determined by Western blotting. Indeed, lysates from the three stable clonal EXOC5CTS-m cell lines (G5, G7, G9) show more mutated EXOC5 protein than endogenous Exoc5 protein found in control MDCK cells. The amount of mutated EXOC5 protein, especially in clone G5, is similar to the amount of EXOC5 protein that we found in EXOC5 OE cells that we previously generated (clone A1). The mutated and control EXOC5 proteins likely run slower on the gel because of the additional amino acids found in the myc epitope tag. Confirmation of the presence of human EXOC5 protein is demonstrated by staining using 9E10 antibody against the myc epitope tag. E, immunoprecipitation using antibody against the myc epitope tag of the EXOC5CTS-m protein shows that EXOC5CTS-m co-immunoprecipitates endogenous Exoc4. IP, immunoprecipitation; Mut, mutant.
Mutation of the EXOC5 ciliary targeting sequence inhibits ciliogenesis
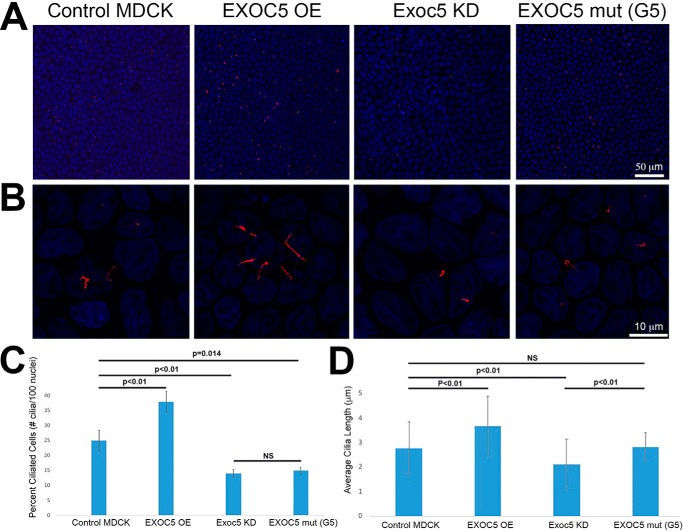
c2002g mutagenesis of the ciliary targeting sequence did not inhibit cell growth or the time it took cells to reach confluence (Fig. 2A; data not shown); however, ciliogenesis was inhibited. We previously reported that EXOC5 OE MDCK cells (A1) have longer cilia, and Exoc5 KD cells have shorter, or absent, cilia, compared with control MDCK cells (7). We also show here that EXOC5-myc OE cells have longer (Fig. 2, B and D) and more abundant cilia (Fig. 2, B and C), compared with the usual 25% ciliation of MDCK cells. EXOC5CTS-m cells (G5 clone) have fewer ciliated cells compared with control MDCK cells. The decrease in ciliogenesis in EXOC5CTS-m cells was, in fact, very similar to what we found in Exoc5 KD cells (Fig. 2, B and C), although the length of the primary cilia were not significantly different from control MDCK cell cilia (Fig. 2, B and D).
Figure 2.
Mutation of the EXOC5 ciliary targeting sequence inhibits ciliogenesis. A, control, EXOC5-OE, Exoc5 KD, and EXOC5CTS-m (mut) stable cell lines were grown on Transwell filters to confluency. All cell lines reached confluency at the same time, as determined by a fluid maintenance test (36). The Transwell filters were fixed, and the cells were stained with nuclear DAPI (blue) and acetylated α-tubulin antibody against ciliary axonemes (red). B, higher magnification image of control, EXOC5 OE, Exoc5 KD, and EXOC5CTS-m MDCK cells stained with DAPI (blue) and acetylated α-tubulin antibody (red). C, quantitation of the percent of cells that have primary cilia shows that EXOC5 OE cells are more ciliated, whereas Exoc5 KD and EXOC5CTS-m are less ciliated, than control MDCK cells. D, quantitation of ciliary length shows longer cilia in EXOC5 OE, compared with control, MDCK cells. n = 700 cells counted for each cell line, with the experiment repeated three times.
Mutation of the EXOC5 ciliary targeting sequence inhibits cystogenesis and tubulogenesis
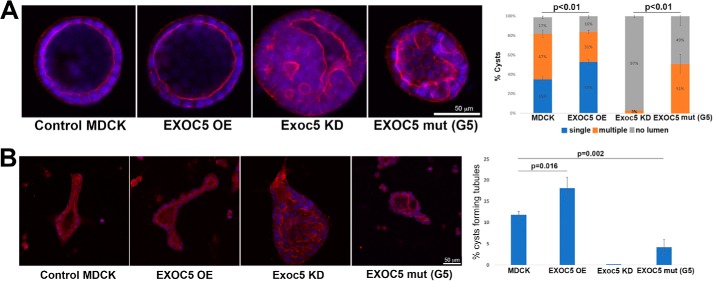
We previously showed in 3D collagen gel culture that EXOC5 OE MDCK cells formed mature single lumen cysts more rapidly and that Exoc5 KD MDCK cells formed cysts more slowly and were unable to form a proper lumen (7), compared with control MDCK cells. Here, we show that overexpression of the mutated EXOC5 ciliary targeting sequence in MDCK cells also prevents cystogenesis, similar to what we found in Exoc5 KD cells (Fig. 3A). These data support the idea that the inhibition of cystogenesis in MDCK cells following Exoc5 KD is due to the exocyst failing to act at the primary cilium.
Figure 3.
Mutation of the EXOC5 ciliary targeting sequence inhibits cystogenesis and tubulogenesis. A, control, EXOC5-OE, Exoc5 KD, and EXOC5CTS-m (mut) cysts were grown from single cells in 3D collagen gels until the cyst stage. At day 10 the collagen gels were fixed and stained with nuclear DAPI (blue) and Alexa Fluor 555 phalloidin that stains F-actin (red). More mature single lumen cysts were found in EXOC5 OE cell cysts, compared with control cysts. In Exoc5 KD and EXOC5CTS-m cell cysts, no mature single lumen cysts were seen, and many “cysts” had no lumen at all. B, following addition of HGF, MDCK cell cysts are induced to form tubules. Three days following the addition of HGF, more mature tubules (containing lumens) were seen in EXOC5 OE, compared with control, MDCK cell cysts. No tubules were seen in Exoc5 KD cell cysts, and fewer tubules were seen in EXOC5CTS-m, compared with control, MDCK cell cysts. For both A and B, n = 100 cysts (or aggregations of cells) were counted for each cell line, with the experiment repeated three times.
We also previously showed that EXOC5 OE cell cysts experience enhanced tubulogenesis when exposed to hepatocyte growth factor (HGF) (4). We therefore added 10 ng/ml HGF to the media of control, EXOC5 OE, Exoc5 KD, and EXOC5CTS-m MDCK cell cysts and found that EXOC5 OE cell cysts had enhanced tubulogenesis, whereas Exoc5 KD and EXOC5CTS-m MDCK cell cysts exhibited impaired tubulogenesis following induction with HGF. Specifically, mature tubules containing lumens projecting from the cysts were not observed in Exoc5 KD cell cysts, and significantly fewer mature tubules were seen in EXOC5CTS-m MDCK cell cysts, compared with control MDCK cell cysts, induced with HGF (Fig. 3B). These data support the idea that Exoc5, acting at the primary cilium, is also centrally involved in renal tubulogenesis.
Mutation of the EXOC5 ciliary targeting sequence prevents rescue of exoc5 mutant zebrafish
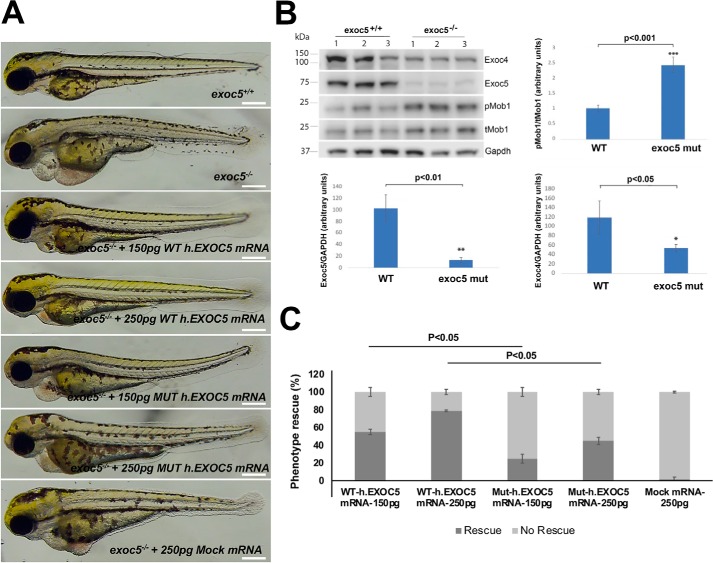
We recently showed multiple ciliary phenotypes, including in the kidney and heart, in exoc5 morphant (5, 17) and mutant (6) zebrafish (Fig. 4A). We also found decreased levels of Exoc4 protein in exoc5 morphant and mutant zebrafish. Furthermore, phosphorylated (active) Mob1 (pMob1) of the Hippo pathway was found in exoc5 mutant zebrafish. This suggests that the Hippo pathway, involved in organogenesis, is activated following loss of the exocyst (Fig. 4B). Injection of WT EXOC5 mRNA rescued exoc5 mutant zebrafish in a dose-dependent manner; however, EXOC5CTS-m mRNA was unable to efficiently rescue the ciliary phenotypes (Fig. 4C), and there was no rescue with mRNA for retinol-binding protein receptor 2 (Rbpr2), a gene not known to interact with the exocyst or primary cilia (18). We also injected EXOC5 mRNA and EXOC5CTS-m mRNA into WT embryos, and there was no phenotypic effect (n = 82 embryos; data not shown).
Figure 4.
Exoc5 mutant zebrafish display ciliopathy phenotypes and cannot be efficiently rescued by EXOC5 ciliary targeting sequence–mutated mRNA. A, lateral view of representative WT (exoc5+/+) and exoc5 homozygous mutant (exoc5−/−) zebrafish at 3.5 dpf. Exoc5 mutants showed cilia defects, including pericardial edema, small eyes, and a curved tail. Scale bar, 0.276 mm for all the zebrafish images. B, by Western blotting analysis, Exoc5 protein, normalized to glyceraldehyde-3-phosphate dehydrogenase (Gapdh) (a housekeeping protein), was almost undetectable in exoc5 mutant (mut) larvae, and Exoc4 protein, normalized to Gapdh, was also significantly decreased, when compared with WT siblings. The ratio of phosphorylated (active) Mob1 (pMob1) to total Mob1 (tMob1) was increased in the exoc5 mutant zebrafish. Quantification is shown below and next to the Western blotting. The y axis shows arbitrary units. C, injection of WT human EXOC5 mRNA rescued the exoc5 mutant phenotypes in zebrafish in a dose-dependent manner, whereas human EXOC5 mRNA with a mutated ciliary targeting sequence (VXPX to VXAX) was unable to efficiently rescue the phenotypes, and mRNA for Rbpr2 (retinol-binding protein receptor 2), a gene not known to interact with the exocyst or primary cilia, showed no rescue of exoc5−/− zebrafish (designated Mock mRNA). n = 50 zebrafish for WT.EXOC5 mRNA injections, 60 zebrafish for the ciliary targeting sequence mutant EXOC5 human (Mut-h.EXOC5) mRNA, and 60 zebrafish for the Rbpr2 mRNA. All of the zebrafish were genotyped.
Discussion
We report two principle findings here, both of which are novel and important for our understanding of ciliogenesis, cystogenesis, and tubulogenesis. First, we show that the EXOC5 VXPX motif is necessary for the generation of primary cilia, although not for Exoc5 protein stability or the ability to bind other exocyst complex members. This is supported by the structural data showing that proline (and valine) are on the outer surface of EXOC5, available for binding (12–14). Interestingly, although several other exocyst complex members also have VXPX motifs (Exoc1, Exoc3, Exoc6, Exoc7, and Exoc8), mutation of the VXPX ciliary targeting sequence in EXOC5 alone was sufficient to prevent ciliogenesis. This is consistent with our previous data showing that knockdown of Exoc4 and Exoc7 did not result in loss of other members of the exocyst complex or changes in ciliogenesis, cystogenesis, or tubulogenesis, whereas loss of Exoc5 did result in loss of other exocyst proteins and changes in ciliogenesis, cystogenesis, and tubulogenesis (Ref. 7 and Fig. 4B). These data demonstrate the centrality of Exoc5 to the function of the exocyst complex, with the idea that without Exoc5, the complex disintegrates and is degraded by the proteasome. How mutation of the VXPX motif prevents ciliogenesis is not totally clear, although it is likely that when EXOC5 is absent from the nascent primary cilium, the rest of the exocyst complex cannot form at the cilium (or gets degraded), thereby preventing vesicles originating from the trans Golgi network and carrying ciliary proteins from being targeted and docked at the primary cilium. In stable cell lines containing the mutated EXOC5 ciliary targeting sequence, normal Exoc5 protein was present, so a dominant-negative mode of action of the EXOC5 ciliary targeting sequence–mutated protein is a strong possibility.
The second finding is that cilia are necessary for cystogenesis and tubulogenesis. Cysts and tubules are two basic “building blocks” involved in the development of many mammalian epithelial organs, including the kidney, lungs, mammary glands, salivary glands, and liver (19). We had previously linked the exocyst and primary cilia to cystogenesis and tubulogenesis by showing that Exoc5 KD inhibited ciliogenesis and then cystogenesis and tubulogenesis, whereas EXCO5 OE enhanced these processes (4, 7). Nevertheless, the possibility that the effect of Exoc5 on cystogenesis/tubulogenesis was unrelated to the cilia defect, i.e. because of the exocyst acting at a different location (perhaps via basolateral transport), could not be excluded. Here, we have begun to tease out the role of Exoc5 and the exocyst by mutating only the EXOC5 ciliary targeting sequence and leaving the other functions of Exoc5 and the exocyst intact. The specificity of the mutation is supported by the fact that the EXOC5CTS-m protein is stable and binds other exocyst complex members. Our work is the first to directly link both the exocyst and primary ciliogenesis and the role of primary cilia in cystogenesis and tubulogenesis. How cilia are involved in cystogenesis and tubulogenesis is not known and is an area ripe for further study. We also showed, similar to what we previously reported (6), that the Hippo pathway was activated in exoc5 mutant zebrafish. We and others have linked alterations in the Hippo pathway to abnormal ciliogenesis and cystogenesis (6, 17, 20), and activation of the Hippo pathway has been shown to control organ development (20, 21). Activation of Mob1, of the Hippo pathway, could help explain the ciliary phenotypes that we reported in exoc5 mutant zebrafish and might be an important area for therapeutic investigation, because small-molecule modulators of the Hippo pathway have already been approved by the Food and Drug Administration (22).
Finally, there is the question of how the exocyst can be involved in so many different cellular processes. We and others have shown that small GTPases from the Rab (23), Arf (10, 17), Rho (24–26), and Ral (27–30) families regulate the exocyst. We hypothesize that the different small GTPases, found at different locations in the cell, give the exocyst specificity of function. We have shown using cell culture, zebrafish, and kidney-specific knockout mice that Cdc42, a Rho family member, is found at the primary cilium and regulates the exocyst (25). Likewise, Tuba, a ciliary Cdc42 guanine nucleotide exchange factor, regulates the exocyst and is also necessary for proper ciliogenesis, cystogenesis, and tubulogenesis (31, 32). We have similarly shown that Arl13b, an Arf family member, in its GTP form regulates the exocyst, arl13b and cdc42 genetically interact in zebrafish, and knockout of Arl13b in mice leads to renal cystogenesis, which phenocopies mice surviving for 30 days after kidney-specific knockout of Exoc5 (17). The fact that multiple small GTPases seem to regulate the exocyst at the primary cilium suggests that the exocyst, in addition to trafficking vesicles to the primary cilium, may have other function(s) in the primary cilium (e.g. secretion of small extracellular vesicles).
In summary, we show here for the first time that that the exocyst acting through the primary cilium is necessary for renal ciliogenesis, cystogenesis, and tubulogenesis (Fig. 5). Given our studies showing that the Exoc5 is necessary, in both zebrafish and mice, for renal (7) and photoreceptor (6) ciliogenesis, these results may be applicable to a wide variety of organs and species.
Figure 5.
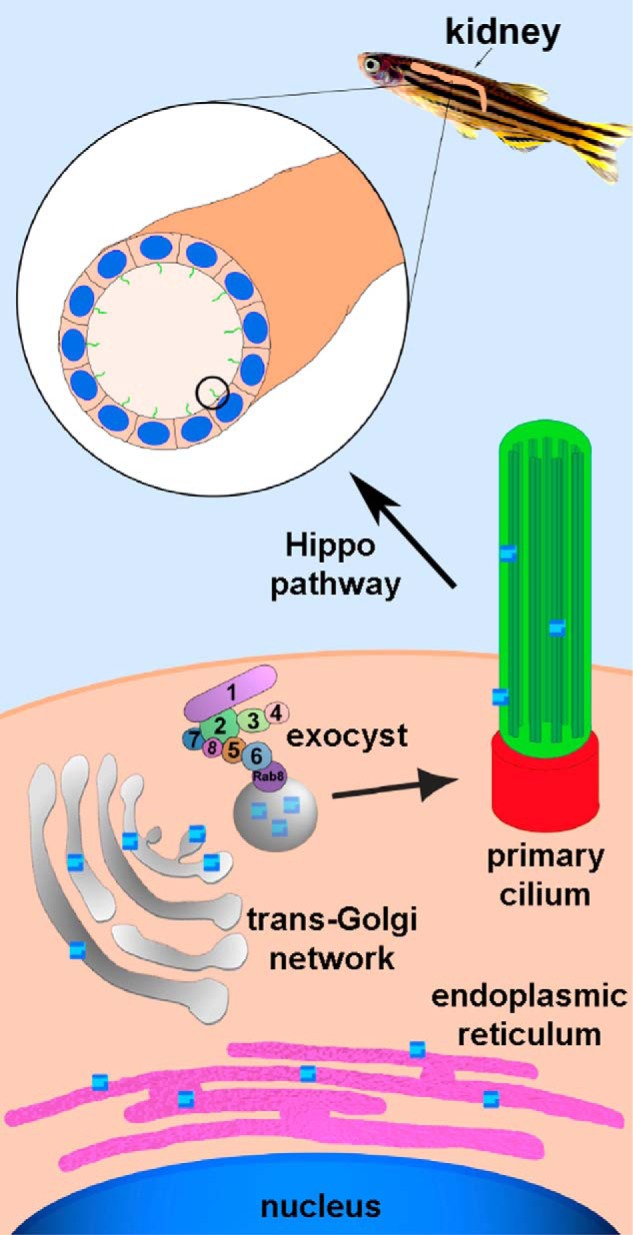
Model for how the exocyst is involved in ciliogenesis and subsequently cystogenesis, tubulogenesis, and organogenesis. Genes are transcribed into mRNA in the nucleus, and mRNA is translated into proteins in the endoplasmic reticulum. Proteins destined for the primary cilium are packaged in vesicles in the trans-Golgi network and trafficked to the primary cilium by the exocyst complex. Exoc5 is a central exocyst member because it links Exoc6 (bound to the vesicle via Rab8) and the rest of the exocyst complex. Primary cilia are necessary for generating cysts and tubules, which in turn are necessary for generating the kidney, and involve the Hippo pathway.
Experimental procedures
Materials
All chemicals, unless stated otherwise, were cell culture grade and purchased from Sigma–Aldrich.
Animal approval
All experiments on zebrafish were approved by the Institutional Animal Care and Use Committee of the Medical University of South Carolina and/or the Ralph H. Johnson Veterans Affairs Medical Center.
Zebrafish husbandry
Adult zebrafish were maintained and raised in an Aquatic Habitats recirculating water system (Tecniplast) in a 14:10-h light–dark cycle. The exoc5 mutant line was purchased from the Zebrafish International Resource Center (exoc5-sa23168). The exoc5 C377T point-nonsense mutation was verified by PCR and direct sequencing of both strands in heterozygote adults and mutant larvae progeny. Genomic DNA from clipped fins or whole 3.5 dpf zebrafish was extracted in 50 μl of 1× lysis buffer (10 mm Tris-HCl, pH 8.0, 50 mm KCl, 0.3% Tween 20, 0.3% Nonidet P-40), denatured at 98 °C for 10 min, and digested at 55 °C for 6 h with 10 μg/ml proteinase K, and the reaction was stopped at 98 °C for 10 min. The PCR primers were 5′-CTATATAGACATGGAGCGGCAAT-3′ (forward primer) and 5′-CCAACAATTCCTCACCTTCC-3′ (reverse primer). Sequencing was performed by Genewiz (South Plainfield, NJ) with the forward PCR primer.
Immunofluorescence confocal microscopy
Cells were grown on Transwell filters and fixed with 4% paraformaldehyde for 15 min at room temperature, permeabilized for 15 min at 37 °C with 0.025% saponin in PBS containing 0.7% fish skin gelatin (PFS buffer), and incubated with primary antibodies overnight at 4 °C and secondary antibodies for 1 h at room temperature.
Cysts grown in collagen gel were fixed with 4% paraformaldehyde for 30 min at room temperature after digesting in collagenase (100 units/ml; Sigma) for 10 min at 37 °C as previously described (33). The cysts were blocked and permeabilized with PFS buffer for 30 min at room temperature and stained with DAPI and Alexa Fluor 555 phalloidin for 10–20 h at 4 °C. The cells or cysts were postfixed with 4% paraformaldehyde and mounted with mounting medium (Kirkegaard & Perry Laboratories). 100 cysts or aggregations of cells were assessed to determine lumen formation for each cell line, and the experiment was repeated three times.
To induce tubulogenesis, HGF (a gift from Genentech) was added to the medium bathing the cysts on days 11, 12, and 13 at 10 ng/ml concentration. 100 cysts or aggregations of cells were assessed at the area of greatest diameter to determine tubule formation for each cell line, and the experiment was repeated three times. Images were acquired on a confocal microscope (Leica TCS SP5) with the accompanying software (both from Leica, Inc.), using an HCX PL APO 63×/1.4–0.6 oil objective to detect fluorochromes of cilia and an HCX PL APO 20×/0.70 dry CS objective to detect fluorochromes of cysts and tubules.
Co-immunoprecipitation and Western blotting analysis
MDCK type II cells grown on 10-cm dishes were collected on ice in a lysis buffer containing 20 mm HEPES, pH 7.4, 120 mm NaCl, 1 mm EDTA, 1% Igepal CA-630, 0.02% NaN3, 0.2% Trasylol, and proteinase inhibitor mixture (1:1000) and then centrifuged at 14,000 rpm for 20 min at 4 °C. The soluble supernatants were incubated overnight at 4 °C with the anti-myc antibody (Cell Signaling Technology, Inc.) at a concentration of 1 μl/ml. Immunocomplexes were then precipitated with protein A/G-agarose (Pierce). The immunocomplexes were washed five times with lysis buffer, eluted by boiling in SDS-PAGE sample buffer, and then subjected to immunoblot analysis. The immunocomplex was blotted with a rabbit polyclonal anti-EXOC5 antibody that we generated (7) and a mouse anti-EXOC4 antibody (Enzo Life Sciences). The blots were developed by enhanced chemiluminescence (Thermo Scientific).
To measure the expression level of the targeted protein in the zebrafish, dechorionated zebrafish embryos at 3.5 or 4 dpf were homogenized in radioimmune precipitation assay buffer containing protease inhibitor mixture (Sigma) and phosphatase inhibitor (Thermo Scientific) to perform Western blotting analysis. Five zebrafish embryos were pooled per genotype. The homogenized lysates were boiled for 5 min at 95 °C followed by centrifugation at 13,500 rpm for 20 min at 4 °C, and the supernatants were collected and mixed with 5× Laemmli sample buffer for the protein electrophoresis. The protein samples were separated on Bolt 4–12% Bis-Tris gels (Invitrogen) and then transferred to a nitrocellulose membrane (Novex). The antibodies included: rabbit polyclonal anti-EXOC5 that we generated (7), mouse anti-EXOC4 (Enzo), rabbit anti-MOB1 and phospho-MOB1 (Thr35) (Cell Signaling Technology, Inc.), and mouse anti-GAPDH mAb (Sigma), all at 1:1000 dilution. Secondary antibodies were from Jackson ImmunoResearch Laboratories and Thermo Fisher Scientific.
Exoc5 mutant mRNA rescue experiments
For rescue experiments of zebrafish exoc5 mutants, capped and polyadenylated mRNA of WT EXOC5, ciliary targeting sequence–mutated human EXOC5, and Rbpr2 (retinol-binding protein receptor 2) (18), mRNA was synthesized in vitro using the mMESSAGE mMACHINE kit (Ambion). Two doses of EXOC5 WT and mutant (low, 150 pg; or high, 250 pg) or 250 pg of Rbpr2, mRNA were injected, using a Sutter Instruments microinjector, into 100 embryos at the one-cell stage. At 3.5 dpf, 12 randomly selected larvae, individually genotyped by direct sequencing, were imaged as outlined above.
Analysis of amino acid accessibility in the EXOC5
The protein structure was loaded from the Protein Data Bank (code 5h11) (12) and protonated with the ICM-Browser (34). Solvent-accessible surface areas of side chains of Val666, Ala667, and Pro668 residues were calculated using solvent probe radius 1.4 Å as implemented in the ICM-Browser. Relative accessibility was calculated using maximal values derived from Gly-Xaa-Gly tripeptides (35). An amino acid residue was considered as solvent-accessible if its accessibility was more than 30%, which is the mean accessibility in proteins (35).
Site-directed mutagenesis
To inhibit the ciliary targeting sequence VXPX in human EXOC5, the QuikChange site-directed mutagenesis kit (Stratagene, catalog no. 200518) was used for in vitro site-directed mutagenesis of proline 668 to alanine. The primers were 5′-CTTCTGGTAGTTGCCGCAGATAATTTAAAGCAAGTCTGC-3′ and 5′-GCAGACTTGCTTTAAATTATCTGCGGCAACTACCAGAAG-3′. Plasmid pcDNA3-hEXOC5 was used as template. Sequencing confirmed the specific amino acid change
Statistics
For cilia length, z-series confocal images were used to reconstruct a 3D cilia image using IMARIS software (V7.2, Bitplane), and the cilia number and individual cilia length were then quantified. For cysts, 100 cysts were randomly identified for each cell line, and the number of cysts with single lumens, multiple lumens, and no lumens were determined. For tubules, 100 cysts treated with 10 ng/ml HGF were randomly identified for each cell line, and the number of tubules with lumens were counted at the area of greatest cyst diameter. The Student's t test was applied to determine the difference in ciliogenesis, cystogenesis, and tubulogenesis between control and EXOC5-perturbed MDCK cell lines. All statistical tests were two-sided and unpaired and are expressed as the means and standard deviations. Statistical significance was defined as p < 0.05. Data analysis was performed using Microsoft Excel software.
Author contributions
X. Z., G. L., D. F., Y. D., Y. S., D. V. I., and D. N. data curation; X. Z. and J. H. L. investigation; X. Z., G. L., D. F., L.G., D. V. I., D. N., B. R., S. C. B., R. A. N., and J. H. L. writing-review and editing; J. H. L. conceptualization; J. H. L. formal analysis; J. H. L. supervision; J. H. L. funding acquisition; J. H. L. visualization; J. H. L. writing-original draft; J. H. L. project administration.
This work was supported in part by Veterans Affairs Merit Award I01 BX000820 (to J. H. L.) and Grants RX000444 and BX003050 (to B. R.); National Institutes of Health Grants P30DK074038 (to J. H. L.), R21EY025034 (to G. P. L.), R01HL131546 to (R. A. N.), P20GM103444 (to R. A. N.), R01HL127692 (to R. A. N.), R01DK087956 (to D. N.), R01EY019320 (to B. R.), R00DK105160 (to D. V. I.), and T32HL007260 and F31HL142159 (to D. B. F.); Polycystic Kidney Disease Foundation Grant 221G18a (to D. V. I.); a grant from the SC SmartState Centers of Excellence Endowment (to B. R., and D. N.); and American Heart Association AWRP Winter 2017 Collaborative Sciences Award (to R. A. N., J. H. L., and S. B.) and Grant 18PRE34080172 (to L. G.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- MDCK
- Madin–Darby canine kidney
- OE
- overexpressing
- KD
- knockdown
- HGF
- hepatocyte growth factor
- dpf
- days postfertilization
- DAPI
- 4′,6′-diamino-2-phenylindole.
References
- 1. Novick P., Field C., and Schekman R. (1980) Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell 21, 205–215 10.1016/0092-8674(80)90128-2 [DOI] [PubMed] [Google Scholar]
- 2. Hsu S. C., Ting A. E., Hazuka C. D., Davanger S., Kenny J. W., Kee Y., and Scheller R. H. (1996) The mammalian brain rsec6/8 complex. Neuron 17, 1209–1219 10.1016/S0896-6273(00)80251-2 [DOI] [PubMed] [Google Scholar]
- 3. Grindstaff K. K., Yeaman C., Anandasabapathy N., Hsu S. C., Rodriguez-Boulan E., Scheller R. H., and Nelson W. J. (1998) Sec6/8 complex is recruited to cell–cell contacts and specifies transport vesicle delivery to the basal–lateral membrane in epithelial cells. Cell 93, 731–740 10.1016/S0092-8674(00)81435-X [DOI] [PubMed] [Google Scholar]
- 4. Lipschutz J. H., Guo W., O'Brien L. E., Nguyen Y. H., Novick P., and Mostov K. E. (2000) Exocyst is involved in cystogenesis and tubulogenesis and acts by modulating synthesis and delivery of basolateral plasma membrane and secretory proteins. Mol. Biol. Cell 11, 4259–4275 10.1091/mbc.11.12.4259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fogelgren B., Lin S. Y., Zuo X., Jaffe K. M., Park K. M., Reichert R. J., Bell P. D., Burdine R. D., and Lipschutz J. H. (2011) The exocyst protein Sec10 interacts with Polycystin-2 and knockdown causes PKD-phenotypes. PLoS Genet. 7, e1001361 10.1371/journal.pgen.1001361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lobo G. P., Fulmer D., Guo L., Zuo X., Dang Y., Kim S. H., Su Y., George K., Obert E., Fogelgren B., Nihalani D., Norris R. A., Rohrer B., and Lipschutz J. H. (2017) The exocyst is required for photoreceptor ciliogenesis and retinal development. J. Biol. Chem. 292, 14814–14826 10.1074/jbc.M117.795674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zuo X., Guo W., and Lipschutz J. H. (2009) The exocyst protein Sec10 is necessary for primary ciliogenesis and cystogenesis in vitro. Mol. Biol. Cell 20, 2522–2529 10.1091/mbc.e08-07-0772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lipschutz J. H., Lingappa V. R., and Mostov K. E. (2003) The exocyst affects protein synthesis by acting on the translocation machinery of the endoplasmic reticulum. J. Biol. Chem. 278, 20954–20960 10.1074/jbc.M213210200 [DOI] [PubMed] [Google Scholar]
- 9. Toikkanen J. H., Miller K. J., Söderlund H., Jäntti J., and Keränen S. (2003) The β subunit of the Sec61p ER translocon interacts with the exocyst complex in Saccharomyces cerevisiae. J. Biol. Chem. 278, 20946–20953 10.1074/jbc.M213111200 [DOI] [PubMed] [Google Scholar]
- 10. Prigent M., Dubois T., Raposo G., Derrien V., Tenza D., Rossé C., Camonis J., and Chavrier P. (2003) ARF6 controls post-endocytic recycling through its downstream exocyst complex effector. J. Cell Biol. 163, 1111–1121 10.1083/jcb.200305029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Knödler A., Feng S., Zhang J., Zhang X., Das A., Peränen J., and Guo W. (2010) Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc. Natl. Acad. Sci. U.S.A. 107, 6346–6351 10.1073/pnas.1002401107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen J., Yamagata A., Kubota K., Sato Y., Goto-Ito S., and Fukai S. (2017) Crystal structure of Sec10, a subunit of the exocyst complex. Sci. Rep. 7, 40909 10.1038/srep40909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Picco A., Irastorza-Azcarate I., Specht T., Böke D., Pazos I., Rivier-Cordey A. S., Devos D. P., Kaksonen M., and Gallego O. (2017) The in vivo architecture of the exocyst provides structural basis for exocytosis. Cell 168, 400–412.e18 10.1016/j.cell.2017.01.004 [DOI] [PubMed] [Google Scholar]
- 14. Mei K., Li Y., Wang S., Shao G., Wang J., Ding Y., Luo G., Yue P., Liu J. J., Wang X., Dong M. Q., Wang H. W., and Guo W. (2018) Cryo-EM structure of the exocyst complex. Nat. Struct. Mol. Biol. 25, 139–146 10.1038/s41594-017-0016-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fogelgren B., Zuo X., Buonato J. M., Vasilyev A., Baek J. I., Choi S. Y., Chacon-Heszele M. F., Palmyre A., Polgar N., Drummond I., Park K. M., Lazzara M. J., and Lipschutz J. H. (2014) Exocyst Sec10 protects renal tubule cells from injury by EGFR/MAPK activation and effects on endocytosis. Am. J. Physiol. Renal Physiol. 307, F1334–F1341 10.1152/ajprenal.00032.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Park K. M., Fogelgren B., Zuo X., Kim J., Chung D. C., and Lipschutz J. H. (2010) Exocyst Sec10 protects epithelial barrier integrity and enhances recovery following oxidative stress, by activation of the MAPK pathway. Am. J. Physiol. Renal Physiol. 298, F818–F826 10.1152/ajprenal.00596.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seixas C., Choi S. Y., Polgar N., Umberger N. L., East M. P., Zuo X., Moreiras H., Ghossoub R., Benmerah A., Kahn R. A., Fogelgren B., Caspary T., Lipschutz J. H., and Barral D. C. (2016) Arl13b and the exocyst interact synergistically in ciliogenesis. Mol. Biol. Cell 27, 308–320 10.1091/mbc.e15-02-0061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shi Y., Obert E., Rahman B., Rohrer B., and Lobo G. P. (2017) The retinol binding protein receptor 2 (Rbpr2) is required for photoreceptor outer segment morphogenesis and visual function in zebrafish. Sci. Rep. 7, 16207 10.1038/s41598-017-16498-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O'Brien L. E., Tang K., Kats E. S., Schutz-Geschwender A., Lipschutz J. H., and Mostov K. E. (2004) ERK and MMPs sequentially regulate distinct stages of epithelial tubule development. Dev. Cell 7, 21–32 10.1016/j.devcel.2004.06.001 [DOI] [PubMed] [Google Scholar]
- 20. Happé H., van der Wal A. M., Leonhard W. N., Kunnen S. J., Breuning M. H., de Heer E., and Peters D. J. (2011) Altered Hippo signalling in polycystic kidney disease. J. Pathol. 224, 133–142 10.1002/path.2856 [DOI] [PubMed] [Google Scholar]
- 21. Badouel C., Garg A., and McNeill H. (2009) Herding Hippos: regulating growth in flies and man. Curr. Opin. Cell Biol. 21, 837–843 10.1016/j.ceb.2009.09.010 [DOI] [PubMed] [Google Scholar]
- 22. Johnson R., and Halder G. (2014) The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat. Rev. Drug. Discov. 13, 63–79 10.1038/nrd4161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guo W., Roth D., Walch-Solimena C., and Novick P. (1999) The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J. 18, 1071–1080 10.1093/emboj/18.4.1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Choi S. Y., Baek J. I., Zuo X., Kim S. H., Dunaief J. L., and Lipschutz J. H. (2015) Cdc42 and sec10 are required for normal retinal development in zebrafish. Invest. Ophthalmol. Vis. Sci. 56, 3361–3370 10.1167/iovs.14-15692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Choi S. Y., Chacon-Heszele M. F., Huang L., McKenna S., Wilson F. P., Zuo X., and Lipschutz J. H. (2013) Cdc42 deficiency causes ciliary abnormalities and cystic kidneys. J. Am. Soc. Nephrol. 24, 1435–1450 10.1681/ASN.2012121236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang X., Bi E., Novick P., Du L., Kozminski K. G., Lipschutz J. H., and Guo W. (2001) Cdc42 interacts with the exocyst and regulates polarized secretion. J. Biol. Chem. 276, 46745–46750 10.1074/jbc.M107464200 [DOI] [PubMed] [Google Scholar]
- 27. Brymora A., Valova V. A., Larsen M. R., Roufogalis B. D., and Robinson P. J. (2001) The brain exocyst complex interacts with RalA in a GTP-dependent manner: identification of a novel mammalian Sec3 gene and a second Sec15 gene. J. Biol. Chem. 276, 29792–29797 10.1074/jbc.C100320200 [DOI] [PubMed] [Google Scholar]
- 28. Moskalenko S., Henry D. O., Rosse C., Mirey G., Camonis J. H., and White M. A. (2002) The exocyst is a Ral effector complex. Nat. Cell Biol. 4, 66–72 10.1038/ncb728 [DOI] [PubMed] [Google Scholar]
- 29. Polzin A., Shipitsin M., Goi T., Feig L. A., and Turner T. J. (2002) Ral-GTPase influences the regulation of the readily releasable pool of synaptic vesicles. Mol. Cell Biol. 22, 1714–1722 10.1128/MCB.22.6.1714-1722.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sugihara K., Asano S., Tanaka K., Iwamatsu A., Okawa K., and Ohta Y. (2002) The exocyst complex binds the small GTPase RalA to mediate filopodia formation. Nat. Cell Biol. 4, 73–78 10.1038/ncb720 [DOI] [PubMed] [Google Scholar]
- 31. Baek J. I., Kwon S. H., Zuo X., Choi S. Y., Kim S. H., and Lipschutz J. H. (2016) Dynamin binding protein (Tuba) deficiency inhibits ciliogenesis and nephrogenesis in vitro and in vivo. J. Biol. Chem. 291, 8632–8643 10.1074/jbc.M115.688663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zuo X., Fogelgren B., and Lipschutz J. H. (2011) The small GTPase Cdc42 is necessary for primary ciliogenesis in renal tubular epithelial cells. J. Biol. Chem. 286, 22469–22477 10.1074/jbc.M111.238469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rogers K. K., Jou T. S., Guo W., and Lipschutz J. H. (2003) The Rho family of small GTPases is involved in epithelial cystogenesis and tubulogenesis. Kidney Int. 63, 1632–1644 10.1046/j.1523-1755.2003.00902.x [DOI] [PubMed] [Google Scholar]
- 34. Abagyan R., Totrov M., and Kuznetsov D. (1994) ICM: A new method for protein modeling and design: Applications to docking and structure prediction from the distorted native conformation. J. Comput. Chem. 15, 488–506 10.1002/jcc.540150503 [DOI] [Google Scholar]
- 35. Miller S., Janin J., Lesk A. M., and Chothia C. (1987) Interior and surface of monomeric proteins. J. Mol. Biol. 196, 641–656 10.1016/0022-2836(87)90038-6 [DOI] [PubMed] [Google Scholar]
- 36. Lipschutz J. H., O'Brien L. E., Altschuler Y., Avrahami D., Nguyen Y., Tang K., and Mostov K. E. (2001) Analysis of membrane traffic in polarized epithelial cells. Curr. Protoc. Cell Biol. Chapter 15, Unit 15.5 10.1002/0471143030.cb1505s12 [DOI] [PubMed] [Google Scholar]