Abstract
Dynamic regulation of the actin cytoskeleton is an essential feature of cell motility. Action of Enabled (Ena)/vasodilator-stimulated phosphoprotein (VASP), a family of conserved actin-elongating proteins, is an important aspect of regulation of the actin cytoskeletal architecture at the leading edge that controls membrane protrusion and cell motility. In this study, we performed mutagenesis experiments in overexpression and knockdown–rescue settings to provide, for the first time, direct evidence of the role of the actin-binding protein profilin1 (Pfn1) in VASP-mediated regulation of cell motility. We found that VASP's interaction with Pfn1 is promoted by cell–substrate adhesion and requires down-regulation of PKA activity. Our experimental data further suggest that PKA-mediated Ser137 phosphorylation of Pfn1 potentially negatively regulates the Pfn1–VASP interaction. Finally, Pfn1's ability to be phosphorylated on Ser137 was partly responsible for the anti-migratory action elicited by exposing cells to a cAMP/PKA agonist. On the basis of these findings, we propose a mechanism of adhesion–protrusion coupling in cell motility that involves dynamic regulation of Pfn1 by PKA activity.
Keywords: profilin, PKA, cell adhesion, cell motility, phosphorylation, cytoskeleton, kinase signaling, Mena, vasodilator-stimulated phosphoprotein (VASP)
Introduction
Cell motility plays an important role in both physiological and pathological processes ranging from embryonic development to angiogenesis and tumor metastasis (1). It is a highly orchestrated event that can be summarized as a cycle of four fundamental steps: membrane protrusion at the leading edge driven by actin polymerization, stabilization of protrusion through integrin-mediated cell matrix adhesion, cell body translocation driven by actomyosin contractile force, and finally rear detachment as a result of the mechanical action of contractile force and/or proteolysis of cell matrix adhesion components (2). Many of these processes involve dynamic remodeling of the actin cytoskeleton, relying on both transcriptional and functional regulation of important structural and regulatory components of the actin cytoskeletal system (3).
Actin nucleators and elongators are major molecular workforces in shaping the branched actin filament network at the leading edge that leads to lamellipodial protrusion and initiation of cell migration (4). Enabled (Ena)/vasodilator-stimulated phosphoprotein (VASP)4 belong to one such family of actin-binding proteins that consists of three members: Mena (mammalian homolog of Ena), VASP, and Ena/VASP-like (Evl). These proteins associate with the barbed ends of actin filaments and elongate Arp2/3 complex–nucleated actin filaments to drive membrane protrusion (5). Ena/VASP proteins also cooperate with the molecular component of the WASP-family verprolin-homologous protein (WAVE) complex to promote Rac-dependent activation of the Arp2/3 complex and actin polymerization (6). These proteins localize at sites of dynamic actin reorganization (e.g. edges of lamellipodia, filopodial tips) and focal adhesions in motile cells (7–9). All members of Ena/VASP proteins share conserved domain structures. The N-terminal Ena/VASP homology 1 (EVH1) domain binds to focal adhesion (e.g. vinculin, zyxin) (10) and membrane-associated proteins (e.g. lamellipodin) (11), allowing Ena/VASP to be recruited to specific cellular locations. The central polyproline (PLP) domain enables Ena/VASP to interact with certain SH3 domain–bearing proteins (Src, Abl) and profilin (Pfn), a family of G-actin–binding proteins and a prominent nucleotide exchange factor of actin that inhibits spontaneous nucleation of actin but promotes barbed end–directed actin polymerization (7, 12). The C-terminal EVH2 domain has a G-actin–binding site, an F-actin–binding region (these interactions are essential for Ena/VASP-driven actin polymerization), and a coiled-coil region that mediates tetramerization of Ena/VASP and, in turn, allows bundling of actin filaments (13–15).
Loss of Ena/VASP function inhibits multiple actin-dependent processes, including axonal guidance (16–18) and intracellular propulsion of bacterial pathogens (a molecular mimicry of membrane protrusion) (19), and higher Ena/VASP activity at the leading edge positively correlates with the speed of membrane protrusion of motile cells (20, 21). Although Ena/VASP proteins promote 3D invasive migration of breast cancer cells (22, 23) (an exception is Evl, which inhibits invasiveness of breast cancer cells (24, 25)), the effect of Ena/VASP perturbation on 2D cell motility is context-specific. Knockout and knockdown of VASP inhibit 2D migration of murine cardiac fibroblasts (26) and MCF7 breast cancer cells (27), respectively. In contrast, the random 2D motility of mouse embryonic fibroblast (MEFs) was found to be enhanced in the absence of Ena/VASP activity (28). The apparent paradox of faster 2D motility of MEFs under Ena/VASP-devoid conditions was attributed to Ena/VASP's anti-capping action. Specifically, by displacing capping protein from the barbed end of actin filaments, Ena/VASP activity results in longer actin filaments and faster membrane protrusion, but these protrusions tend to be unstable (as longer actin filaments are prone to bucking), leading to low persistence of protrusion and unproductive global cell motility (29, 30). Relevant to protrusion, an intact PLP domain of VASP is necessary for efficient actin polymerization–driven intracellular motility of bacterial pathogens (19). In fact, the rate of actin assembly by VASP is dramatically enhanced by its PLP interaction with Pfn1 (the major isoform of Pfn and a key promoter of membrane protrusion) in vitro (29, 31). These findings are also consistent with enriched Pfn1-VASP interaction at the leading edge of motile cells (32). Surprisingly however, PLP interaction of VASP was found to be dispensable for whole-cell motility, at least in the case of MEFs (33). Specifically, this study showed that re-expression of VASP in Ena/VASP-null fibroblasts reduced the overall speed of cell motility, and this effect required an intact EVH2 but not the PLP domain of VASP (33). Although the underlying reasons for this discrepancy are not clear, a simple explanation could be that whole-cell motility is more complex than membrane protrusion alone. Alternatively, the dispensable nature of PLP interaction of VASP in cell motility could be cell type–specific. Another potential issue could be that, because VASP also interacts with multiple SH3 and WW domain proteins using its PLP domain, deletion of the entire PLP domain of VASP is not specific for selectively interfering with its interaction with Pfn1. Therefore, the significance of the VASP–Pfn1 interaction in cell motility has yet to be conclusively resolved. In this study, we directly demonstrate, for the first time, that VASP regulates cell motility through its interaction with Pfn1 and that this interaction is regulated by cell adhesion in a PKA-dependent manner that likely involves phosphorylation of Pfn1 on its Ser137 residue.
Results
Ena/VASP modulates cell motility through its interaction with Pfn1
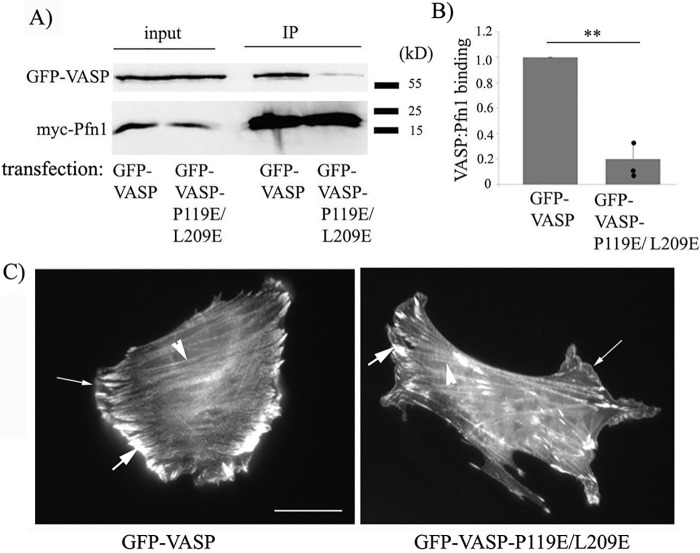
VASP contains three distinct PLP regions: a single GPPPPP (GP5) site within amino acids (aa) 116–135, a repeat of three GP5 sites within aa 160–194, and a 202GPPPAPPLP210 site (the aa numbers correspond to the human VASP sequence). A previous X-ray crystallography study of VASP suggested that the last GPPPAPPLP segment of VASP has a nearly 10-fold higher binding affinity for Pfn1 compared with GP5 sites and that Leu209 in this segment makes a critical hydrophobic interaction with the Tyr6 residue of Pfn1 (34). To selectively impair the VASP–Pfn1 interaction without causing any major structural perturbation of VASP, we set out an initial strategy to introduce a glutamic acid substitution on the third proline residue in each of the first four GP5 clusters and Leu209 in the last PLP segment in a cumulative manner. By co-immunoprecipitation assay in HEK293 cells, we confirmed that P119E mutation in the first GP5 region superimposed with the L209E mutation in the loading region was sufficient to drastically reduce (by ∼80%) VASP's binding to Pfn1 (Fig. 1, A and B) and therefore did not need to introduce any additional mutation in the other GP5 regions. Because SH3 domain proteins typically bind to the XPPXP motif, we predicted that our mutation strategy would selectively reduce VASP's interaction with Pfn1 without affecting its binding to SH3 domain–bearing proteins. As a proof of concept, we confirmed that the P119E/L209E mutation did not reduce VASP's interaction with Abl, a known SH3 ligand of VASP (Fig. S1). Because the EVH1 domain is responsible for VASP's targeting to membrane and focal adhesions, and because the EVH2 domain of VASP contains binding sites for G-actin, F-actin, and coiled-coiled regions (allowing VASP to bundle actin filaments), we predicted that the P119E/L209E mutations in the PLP regions should not interfere with VASP's ability to localize at the leading edge, focal adhesions, and actin stress fibers. Immunofluorescence images of murine NR6 fibroblasts (we chose these fibroblasts because these cells form robust actin stress fibers and focal adhesions) following transient transfection of either GFP-VASP or GFP-VASP-P119E/L209E confirmed localization of both forms of GFP-VASP at all of those sites (Fig. 1C).
Figure 1.
Effect of the P119E/L209E substitution on Pfn1 interaction and cellular localization of VASP. A and B, total lysates and myc-Pfn1 immunoprecipitates (IP) of HEK293 cells co-transfected with myc-Pfn1 and either GFP-VASP or GFP-VASP-L119E/L209E immunoblotted with GFP and myc antibodies (A). The bar graph in B summarizes the relative binding of GFP-VASP and GFP-VASP-P119E/L209E to myc-Pfn1 immunoprecipitates (n = 3 experiments; **, p < 0.01). C, representative fluorescence images of NR6 fibroblasts transfected with GFP-VASP (left panel) or GFP-VASP-P119E/L209E (right panel) (scale bar = 25 μm). Thin arrows, thick arrows, and arrowheads indicate VASP localization to the leading edge, focal adhesion, and stress fibers, respectively.
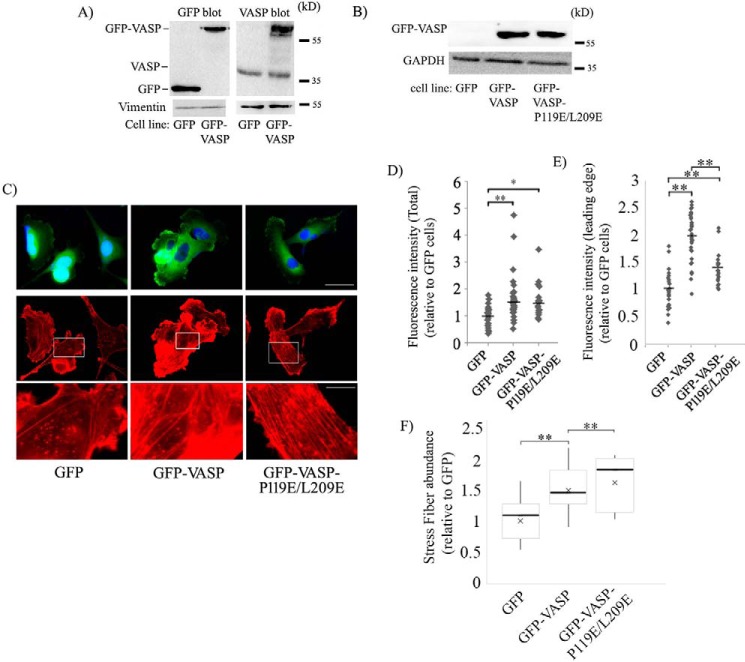
Next, to examine whether interfering with Pfn1's interaction has any effect on VASP's ability to regulate the actin cytoskeleton, focal adhesions, and cell motility, we generated stable clones of MDA-MB-231 cells (MDA-231 is a highly motile metastatic human breast cancer cell line) overexpressing either GFP-VASP or GFP-VASP-P119E/L209E (stable GFP expressers served as controls). We estimated ∼3.5-fold-overexpression of VASP in the GFP-VASP cell line compared with control GFP cells and comparable expression levels of GFP-VASP and GFP-VASP-P119E/L209E in the respective cell lines (Figs. 2, A and B). Note that the expression of endogenous VASP was not affected by GFP-VASP overexpression (Fig. 2A). Rhodamine–phalloidin staining of these cells revealed that overexpression of both forms of GFP-VASP increased the total F-actin content and the abundance of actin stress fibers and F-actin at the leading edge relative to control cells (Fig. 2, C–F), and this is consistent with the F-actin elongating and bundling functions of VASP. Interference with Pfn1's interaction did not affect the VASP-induced increase in total F-actin content. However, F-actin at the leading edge was more prominent in GFP-VASP (∼2-fold increase over the control) than in GFP-VASP-P119E/L209E expressers (∼1.5-fold increase over the control), suggesting that VASP–Pfn1 interaction promotes actin polymerization at the leading edge. There was only a marginal difference in actin stress fiber density between the two VASP cell lines. Immunostaining for vinculin (a marker for focal adhesions) also revealed an increase in both abundance and the average area of focal adhesions upon overexpression of either VASP or P119E/L209E-VASP relative to control cells, but there was no statistically significant difference in either of those focal adhesion parameters between the two VASP cell lines (Fig. S2). Therefore, VASP–Pfn1 interaction does not appear to play a role in focal adhesion formation.
Figure 2.
Effect of stable overexpression of GFP-VASP and GFP-VASP-L119E/L209E on the actin cytoskeleton in MDA-231 cells. A, GFP (left) and VASP (right) immunoblot of lysates of GFP and GFP-VASP expressers of MDA-231 cells (vimentin blot, loading control). B, representative GFP immunoblot of lysates of MDA-231 cells stably expressing GFP, GFP-VASP, and GFP-VASP-P119E/L209E constructs (GAPDH blot, loading control). C, representative fluorescence images (acquired with a ×60 objective) of MDA-231 cells stably expressing GFP, GFP-VASP, or GFP-VASP-P119E/L209E and stained with DAPI (nucleus, blue) and rhodamine–phallodin (F-actin, red). The bottom panels are the magnified insets from the center panels to reveal actin stress fibers. Scale bars = 50 μm (top) and 16 μm (bottom). D–F, scatterplots of fluorescence intensities of rhodamine–phalloidin (D and E; D, total; E, leading edge; bars indicates the mean) and box-and-whisker plot summarizing stress fiber abundance (F) of various groups of cells relative to GFP expressers of MDA-231 cells (data summarized from 25–32 cells from two experiments. *, p < 0.05; **, p < 0.01).
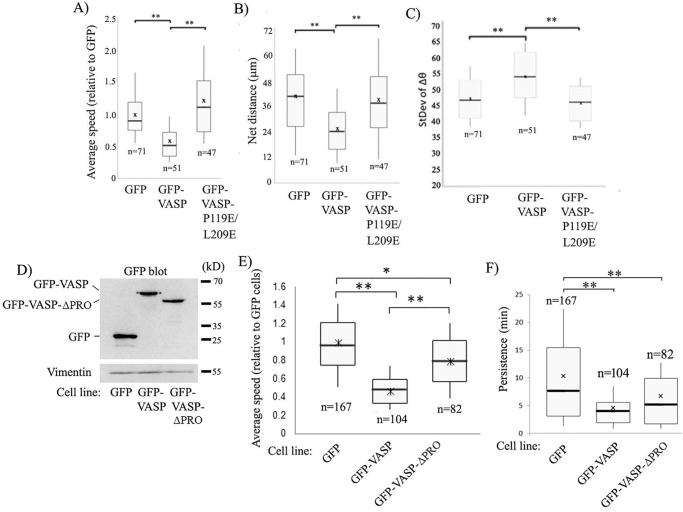
We next performed random 2D cell motility assays with MDA-231 cells in both stable and transient overexpression settings of GFP-VASP and GFP-VASP-P119E/L209E using GFP transfectants as a control. First, in stable transfection settings, overexpression of GFP-VASP resulted in a 50% reduction in the average speed of MDA-231 cells (in terms of both average speed and the net distance traveled), and this effect was completely mitigated upon P119E/L209E substitution (Fig. 3, A and B; Movies M1–M3 show representative random motility of GFP, GFP-VASP, and GFP-VASP-P119E/L209E expressers). From the time-lapse images, we also calculated the change in the direction of centroid movement (Δθ) as a function of time and further computed the standard deviation of Δθ (a higher value indicates a larger fluctuation in the directionality of movement), as we had done before (35). VASP overexpression resulted in a larger standard deviation of Δθ (consistent with the frequent turning movement of these cells, a feature that is counterproductive for efficient cell motility) compared with control cells, and this effect was abolished upon P119E/L209E substitution, suggesting that VASP's negative effect on directional persistence depends on its interaction with Pfn1 (Fig. 3C). Our overall motility results obtained with stable overexpressers of VASP were further confirmed in transient overexpression settings (representing a mixed pool of cells with varying degrees of VASP overexpression), which also revealed slower migration of MDA-231 cells upon overexpression of VASP and reversal of this effect upon P119E/L209E substitution (Fig. S3A). Based on these overexpression results, we suspected that inhibition of cell motility upon VASP overexpression would be less pronounced in a background of Pfn1 that is unable to interact with PLP ligands. To test this, we transiently silenced endogenous Pfn1 in stable GFP and GFP-VASP expressers by siRNA treatment, resulting in a mean knockdown efficiency of Pfn1 of ∼90% (Fig. S3, B and C), and then rescuing with siRNA-resistant forms of either the WT or PLP binding–deficient H133S mutant of Pfn1 (36) as cyan fluorescent protein (CFP)-tagged proteins. Motility analyses of CFP-positive cells (which displayed a range of CFP-Pfn1 expression in transfected cells, as judged by their fluorescence intensity) revealed that, in the WT-Pfn1–rescued background, stable GFP-VASP overexpression led to ∼50% reduction in the average cell speed of MDA-231 cells (Fig. S3D). However, in a PLP binding–deficient Pfn1 background (H133S-Pfn1), VASP-induced suppression of cell motility (now by only ∼20%) is significantly reduced. Note that the slight 20% reduction in the speed of GFP-VASP expressers in the H133S-Pfn1 background compared with the WT-Pfn1 background is not surprising because H133S substitution disrupts Pfn1 binding to all PLP domain–containing proteins, including other important actin cytoskeleton regulators (e.g. formins). Finally, to compare the effects of deletion of the entire PLP domain versus selective interference with Pfn1's interaction of VASP-induced changes in cell motility, we analyzed MDA-231 cell motility in stable overexpression settings of either the GFP-VASP or PLP-deleted form of GFP-VASP (denoted GFP-VASP-ΔPRO, as described previously (19)) or GFP (control). By immunoblot analyses, we confirmed comparable expression levels of GFP-VASP and GFP-VASP-ΔPRO (by ∼3.5-fold) in the respective cell lines (Fig. 3D). Conforming with our earlier results, these experiments also showed the general feature of partial reversal of VASP-induced inhibition of cell motility in the absence of its PLP domain (50% versus 20% reduction in speed in GFP-VASP versus GFP-VASP-ΔPRO overexpression settings, respectively) (Fig. 3E). Persistence analyses also revealed that control GFP expressers had better directional persistence (whether calculated based on time or standard deviation of Δθ; only time-based data are shown here) than the other two cell lines (Fig. 3F). Although GFP-VASP-ΔPRO overexpressers showed a trend of better directional persistence than GFP-VASP overexpressers, the difference was not statistically significant (unlike the difference between GFP-VASP and GFP-VASP-P119E/L209E expressers). These minor differences between our results in the ΔPRO and P119E/L209E settings of VASP were not surprising, given that ΔPRO-VASP is not only deficient in its interaction with Pfn1 but also other SH3 domain proteins, some of which might play a role in influencing the directional persistence of motility. In summary, our results from all of these overexpression studies demonstrate that VASP elevation inhibits cell motility by utilizing its PLP domain interaction with Pfn1.
Figure 3.
VASP overexpression inhibits MDA-231 cell motility through its PLP interaction of Pfn1. A–C, box-and-whisker plots summarizing the average speed (A), net distance (B), and standard deviation of Δθ (C) analyzed from time-lapse motility of the indicated sublines of MDA-231 cells. n indicates the number of cells analyzed for each group, pooled from three independent experiments. *, p < 0.05; **, p < 0.01. D, representative GFP immunoblot of lysates of MDA-231 cells stably expressing GFP, GFP-VASP, and GFP-VASP-ΔPRO constructs (vimentin blot, loading control). Note that the immunoblot represents the same experimental data shown in the left panel of Fig. 2A, with an additional lane of GFP-VASP-ΔPRO cell lysate shown alongside. E, box-and-whisker plot showing the relative speed of migration of GFP-, GFP-VASP–, and GFP-VASP-ΔPRO–expressing MDA-231 cells. F, box-and-whisker plot summarizing the angular persistence of the motility of GFP, GFP-VASP, and GFP-VASP-ΔPRO expressers. n indicates the number of cells analyzed for each group, pooled from three independent experiments. *, p < 0.05; **, p < 0.01).
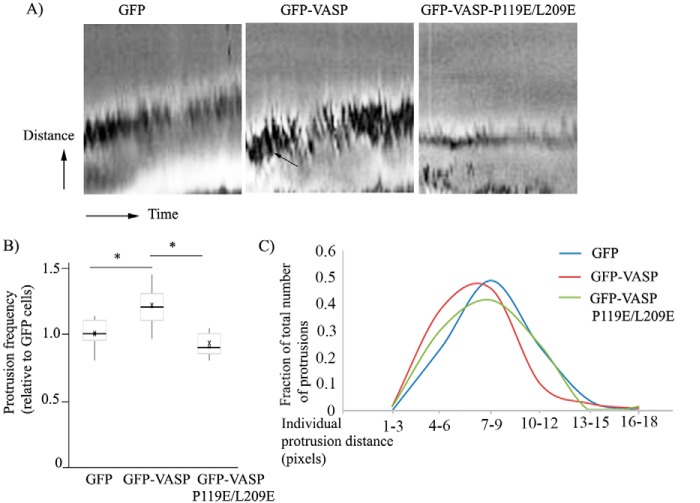
To further determine whether VASP–Pfn1 interaction plays a role in regulating membrane protrusion dynamics, we performed kymography analyses of membrane protrusions of GFP, GFP-VASP, and GFP-VASP-P119E/L209E overexpressers of MDA-231 cells (Fig. 4A shows representative kymography scans of these different groups of cells). The most prominent changes induced by overexpression of fully functional VASP were increased membrane ruffling (a feature that is counterproductive for cell motility and represented by dark lines in kymography scans (Fig. 4A)) and ∼20% higher protrusion frequency (Fig. 4B) than the other two cell lines. Because of the natural heterogeneity of membrane protrusions, we performed a histogram analysis of protrusion distance, which revealed that, compared with either the GFP or GFP-VASP-P119E/L209E expresser, GFP-VASP expressers displayed a somewhat higher percentage of protrusion events biased toward lower range of protrusion distance (Fig. 4C). Collectively, these data demonstrate that hyperactivity of VASP increases the propensity but decreases the stability of membrane protrusions (as judged by prominent ruffling) and that VASP regulates protrusion dynamics through its interaction with Pfn1.
Figure 4.
VASP regulates protrusion dynamics through its interaction of Pfn1. A, representative kymograph images revealing the protrusion dynamics of MDA-231 cells stably expressing either GFP, GFP-VASP, or GFP-VASP-P119E/L209E (horizontal axis, time (total duration, 10 min); vertical axis, distance (the sawtooth pattern indicates individual protrusion and retraction events of the membrane; ruffles are indicated by the arrow). B, box-and-whisker plot summarizing the protrusion frequency (number of protrusions recorded within a 10-min time period) of different groups of cells relative to GFP expressers. C, relative occurrence of protrusion events for different ranges of protrusion distance (as indicated in pixels) of various groups of cells. These data are based on analyses of all protrusions detected at approximately three different locations at the leading edge per cell from at least 15 cells/group (*, p < 0.05).
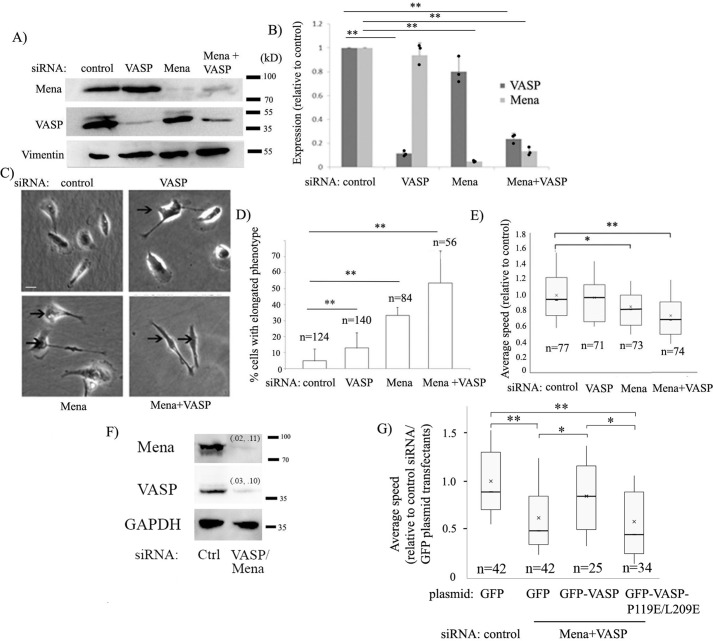
As a complementary strategy to our overexpression experiments, we also evaluated the effect of down-regulation of Ena/VASP activity on MDA-231 cell motility by selective knockdown of either VASP or Mena or both (as control, cells were transfected with nontargeting control siRNA). We achieved an ∼90% mean reduction in Mena and VASP expression in MDA-231 cells 72 h after the respective siRNA transfection (when both siRNAs were used, the mean knockdown efficiency of Mena and VASP was 80% or more) (Fig. 5, A and B). Phenotypic analyses revealed that a significant fraction (∼50%) of MDA-231 cells subjected to dual knockdown of Mena and VASP exhibited a highly elongated morphology lacking the characteristic well-spread lamellipodial structure. This phenotype was also seen in cells subjected to either VASP or Mena knockdown (more prominently in the latter) but was not as pronounced as seen in cells where expression of both Mena and VASP was silenced (Fig. 5, C and D). These observations are consistent with Ena/VASP's critical importance in lamellipodial protrusion, as revealed previously in other cell types (11, 21, 30, 38). When analyzed for random motility, VASP knockdown alone had no statistically significant effect on the average speed of randomly migrating MDA-231 cells (Fig. 5E). Silencing Mena alone reduced the average speed by about 15% (p < 0.05). Although dual knockdown of Mena and VASP induced a strong morphological phenotype, surprisingly, the average speed decreased by only 25% (p < 0.01), suggesting a rather modest inhibitory effect of Mena/VASP knockdown on the overall speed of MDA-231 cells (the possible reasons are discussed later). Next, we performed rescue experiments where we co-silenced endogenous Mena and VASP expression in MDA-231 cells (because VASP knockdown alone had no discernible effect on overall cell motility) and then rescued by transient transfection of either GFP-VASP– or GFP-VASP-P119E/L209E–encoding plasmids. Use of an siRNA that targets the 3′ UTR of VASP avoided siRNA-mediated targeting of exogenous VASP constructs. We achieved a 90% or higher efficiency of Mena and VASP knockdown (Fig. 5F). As additional groups, cells transfected with either control siRNA or Mena/VASP knockdown cells were transfected with the GFP plasmid. We observed an ∼40% slower migration of MDA-231 cells when Mena and VASP expression was down-regulated simultaneously (Movies M4–M5), and this overall motility phenotype was rescued by ectopically expressed GFP-VASP but not GFP-VASP-P119E/L209E (Fig. 5G). These data provide further evidence that VASP regulates cell migration through its interaction with Pfn1. Furthermore, the qualitative similarity between the overall effects of Mena/VASP knockdown and VASP overexpression on MDA-231 cell motility suggests that an optimum level of Ena/VASP activity promotes migration of MDA-231 cells.
Figure 5.
Effect of Ena/VASP knockdown on MDA-231 cell motility. A and B, representative Mena, VASP, and vimentin (loading control) immunoblots of lysates of MDA-231 cells transfected with the indicated siRNAs (A). The bar graph in B shows the relative expression levels (mean ± S.D.) of Mena and VASP for these transfection conditions (n = 3 experiments). C and D, phase-contrast images comparing the morphology of MDA-231 cells for different transfection conditions (C, cells with an elongated phenotype are indicated by arrows; scale bar = 20 μm). The bar graph in D summarizes the percentage of cells with an elongated phenotype for each of the transfection conditions. E, box-and-whisker plot representing the average speed of MDA-231 cells for the various transfection conditions. In D and E, n indicates the number of cells analyzed for each group pooled from three independent experiments. *, p < 0.05; **, p < 0.01. F and G, representative Mena, VASP, and GAPDH (loading control) immunoblots (F) of lysates of MDA-231 cells transfected with the indicated siRNAs in the rescue experiments (numbers in parentheses indicate the knockdown efficiencies of Mena and VASP from each of the two experiments). G, box-and-whisker plot representing the average speed of MDA-231 cells for the indicated siRNA and plasmid transfection conditions. n indicates the number of cells analyzed for each group, pooled from two independent experiments. *, p < 0.05; **, p < 0.01.
VASP–Pfn1 interaction is regulated by cell–cell adhesion in a PKA-dependent manner
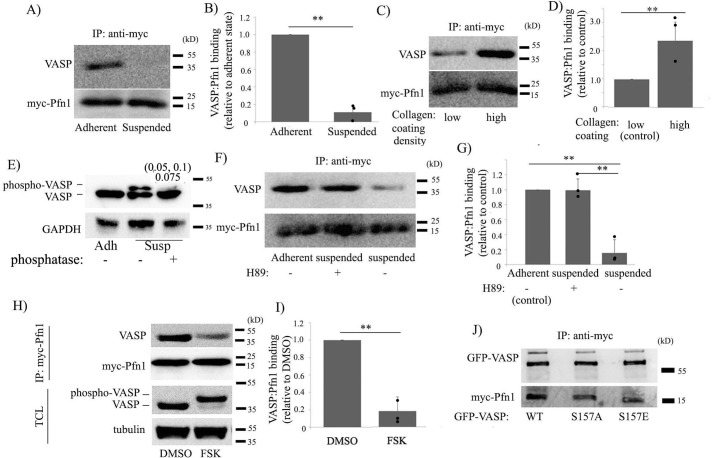
Ena/VASP proteins are prominently regulated by serine/threonine phosphorylation at multiple sites (e.g. Ser157, Ser239, and Thr278 for VASP) by the actions of cAMP- and cGMP-dependent protein kinases, including PKA and PKG (29, 39, 40). Phosphorylation of VASP on Ser239 and Thr278 in its EVH2 domain reduces its affinity for G-actin, whereas phosphorylation of Ser157 in the PLP domain of VASP abrogates its interactions with various SH3 domain–bearing proteins (Abl, Src) (29, 41). Interestingly, Ser157 phosphorylation does not affect VASP's intrinsic binding to Pfn1, at least in a purified protein mixture setting (39). Therefore, whether and how VASP's interaction with Pfn1 is regulated in cells is unclear. We next performed a series of biochemical experiments to obtain insight into which cellular pathway could potentially regulate VASP–Pfn1 interaction in cells. We chose HEK293 cells for these biochemical experiments, mainly because of the high efficiency of transfection of this cell line. An important insight came from experiments where we examined VASP's interaction with ectopically expressed myc-Pfn1 in HEK293 cells in attached versus suspended states. Specifically, co-immunoprecipitation experiments revealed that VASP's interaction with myc-Pfn1 is dramatically reduced when cells were switched from adherent to suspended conditions (Fig. 6, A and B). Conversely, VASP's interaction with myc-Pfn1 was prominently up-regulated when cell culture conditions were changed from low-adhesive to high-adhesive (created by increasing the coating density of collagen I on the tissue culture plate before seeding the cells) (Fig. 6, C and D).
Figure 6.
VASP–Pfn1 interaction is regulated by cell adhesion involving the action of PKA. A–D, Myc-Pfn1 immunoprecipitates (IP) from the lysates of HEK293 cells expressing myc-Pfn1 in the adherent versus suspended state (A) and low-adhesive versus high-adhesive culture conditions (C) probed with VASP and myc antibodies (collagen coating concentration, 10 μg/ml (low adhesive) and 50 μg/ml (high adhesive)). B and D quantify the relative binding of VASP to myc-Pfn1 for the indicated experimental conditions (n = 3 experiments). E, total lysates of HEK-293 cells in attached versus detached states (with or without phosphatase treatment) were run on 10% SDS-PAGE and probed with the VASP antibody to demonstrate detachment-induced VASP phosphorylation. The numbers in parentheses indicate the individual phospho-VASP band intensity under phosphatase-treated relative to untreated conditions from each of the two experiments, with the average value indicated below. F and G, Myc-Pfn1 immunoprecipitates from HEK293 cells expressing myc-Pfn1 adherent versus suspended (with or without pretreatment of H89), immunoblotted with VASP and myc antibodies (F); the relative binding of VASP to myc-Pfn1 for these different conditions is summarized in G (n = 3 experiments). H and I, total cell lysates (TCL, run on 10% SDS-PAGE to reveal VASP phosphorylation) and myc-Pfn1 immunoprecipitates (run on 15% SDS-PAGE) of DMSO-treated versus 50 μm FSK–treated HEK293 cells expressing myc-Pfn1, probed with the indicated antibodies (H; these treatments were done for 15 min). Relative binding of VASP to myc-Pfn1 in DMSO- versus FSK-treated conditions is summarized in I. J, Myc-Pfn1 immunoprecipitates of HEK293 cells that were co-transfected with myc-Pfn1 and the various indicated VASP constructs (GFP-tagged) probed with GFP and myc antibodies. **, p < 0.01.
PKA activity is modulated by cell–substrate adhesion (specifically, cell detachment promotes PKA activity) (41). VASP phosphorylation (marked by a phosphatase-sensitive electrophoretic mobility shift) is a characteristic signature of PKA activation that we also observed in HEK293 cells upon their detachment from the underlying substrate (Fig. 6E). Furthermore, cell detachment–induced down-regulation of VASP–Pfn1 interaction was completely blocked when cells were pretreated with H89, a pharmacological inhibitor of PKA (Fig. 6, F and G), suggesting PKA's involvement in cell adhesion–dependent modulation of VASP–Pfn1 interaction. Consistent with these findings, acute treatment of cells with forskolin (FSK; a cAMP agonist and potent activator of PKA) (42) also dramatically reduced VASP–Pfn1 interaction in cells (Fig. 6, H and I). PKA activation was confirmed by the characteristic electrophoretic mobility shift of VASP associated with its phosphorylation in response to FSK treatment (Fig. 6H). Although Ser157 phosphorylation has no effect on the intrinsic VASP–Pfn1 binding in vitro (39) because Ser157 phosphorylation promotes membrane localization of VASP (43), one cannot totally exclude the possibility of PKA-induced changes in the localization of VASP indirectly affecting its interaction with Pfn1. Therefore, we performed co-immunoprecipitation experiments to assess myc-Pfn1 binding to various forms (WT, phosphodead (S157A), or phosphomimetic (S157E)) of GFP-VASP in HEK293 cells. Consistent with previous findings of in vitro binding studies (39), we also found no effect of S157E substitution of VASP on its interaction with Pfn1 (Fig. 6J). Collectively, these results demonstrate that VASP–Pfn1 interaction can be negatively regulated by loss of cell–substrate adhesion through PKA activation but at least not involving Ser157 phosphorylation of VASP.
Ser137 phosphorylation of Pfn1 plays a role in PKA-dependent regulation of VASP–Pfn1 interaction and cell motility
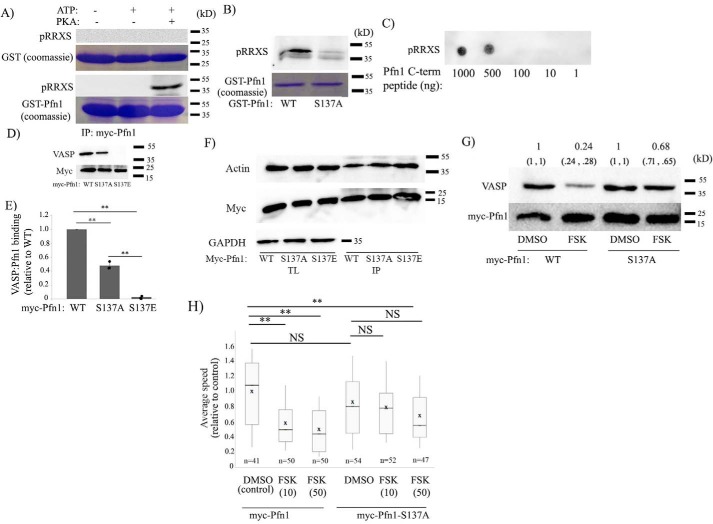
We and others previously demonstrated increased phosphorylation of Pfn1 in cells in response to FSK treatment in a PKA-dependent manner (44, 45). We further showed that Pfn1 is a direct phosphorylation substrate of PKA and identified several PKA phosphorylation sites of Pfn1 by MS (Ser56/Ser57, Thr89, Ser91/Thr92) (44). Phosphorylation of Ser57 or Ser91/Thr92 does not have any effect on Pfn1's ability to interact with either actin or PLP ligands (such as VASP), whereas Thr89 phosphorylation elicits changes in Pfn1 that are hallmarks of proteins folded into alternative 3D conformations, including detergent insolubility, protein aggregation, and accelerated proteolysis. In silico analyses predicted that Thr89 phosphorylation is likely to enhance Pfn1's interaction with actin. However, because protein coverage was incomplete in our previous study, we could not rule out the possibility of additional phosphorylation sites of Pfn1 by PKA. Two bioinformatics programs (NetphosK and Kinasephos) predicted that PKA can potentially phosphorylate Pfn1 on Ser137, a residue that can be also phosphorylated by other AGC kinases (e.g. PKC and rho-associated protein kinase (ROCK)), leading to reduced Pfn1–PLP interaction (46, 47). In fact, among all predicted phosphorylation sites, Ser137 has the maximum likelihood of phosphorylation of Pfn1 by PKA. Therefore, we further explored whether PKA-mediated Ser137 phosphorylation of Pfn1 could be a potential mechanism of negative regulation of Pfn1–VASP interaction. Although Pfn1 does not have the consensus PKA phosphorylation RRXS/T motif, it has an RRS motif involving the Ser137 residue. First, to test whether PKA can phosphorylate Pfn1 on Ser137, we performed an in vitro kinase assay where recombinant GST-tagged Pfn1 or GST (control) was treated with the catalytic subunit of PKA, and the kinase assay products were analyzed by immunoblotting with a phospho-PKA substrate antibody (which recognizes the RRX(S/T)p motif). Immunoreactivity of the kinase assay product of GST-Pfn1, but not GST, with the phospho-PKA substrate antibody confirmed that PKA is capable of phosphorylating GST-Pfn1 (Fig. 7A). Substitution of the Ser137 residue of Pfn1 with nonphosphorylatable alanine (S137A) dramatically abrogated PKA's ability to phosphorylate Pfn1 in vitro, as demonstrated by loss of immunoreactivity of the phospho-RRXS antibody of the kinase assay product (Fig. 7B). We also performed a similar in vitro PKA phosphorylation assay with the membrane-immobilized 13-mer C-terminal peptide of Pfn1 (CYEMASHLRRSQY; this peptide is restricted to only one consensus site (S137) of PKA phosphorylation), which also showed evidence of PKA-induced phosphorylation of the peptide (Fig. 7C). These data are consistent with the notion that Ser137 is a potential PKA phosphorylation site of Pfn1. Unfortunately, we were unable to confirm Ser137 as an in vivo PKA phosphorylation site by MS of immunoprecipitated Pfn1 from FSK-treated cell lysate, possibly because of the low abundance of the Ser137-phosphorylated form of Pfn1 (note that a direct in vivo demonstration of Ser137 phosphorylation of Pfn1 by MS is lacking in the literature to date).
Figure 7.
Ser137 phosphorylation of Pfn1 is involved in PKA-dependent regulation of VASP–Pfn1 interaction and cell motility. A, in vitro PKA assay products of GST (control) and GST-Pfn1 probed with pRRXS antibody (reactions without ATP or PKA served as additional controls). Coomassie staining in parallel confirmed comparable loading of GST and GST-Pfn1 under the various experimental conditions. B, PKA assay products of GST-Pfn1 (WT) and GST-Pfn1-S137A probed with pRRXS antibody (Coomassie staining confirmed comparable loading of the WT and S137 mutant forms of GST-Pfn1). C, dot blot of various amounts of membrane-immobilized C-terminal Pfn1 peptide treated with PKA and ATP and probed with pRRXS antibody. D–F, Myc-Pfn1 immunoprecipitates (IP) of HEK293 cells expressing the indicated various myc-tagged Pfn1 constructs probed with VASP (D), actin (F), and myc (D and F) antibodies. E quantifies the relative binding of VASP to the various myc-Pfn1 constructs (n = 3 experiments). G, Myc-Pfn1 immunoprecipitates from the lysates of myc-Pfn1– and myc-Pfn1-S137A–expressing HEK293 cells following treatment with either DMSO or FSK (50 μm, 15 min), probed with VASP and myc antibodies. The numbers in parentheses indicate relative VASP binding to the WT and S137A mutant form of myc-Pfn1 in DMSO- versus FSK-treated conditions from two individual experiments, with the mean of the two numbers indicated at the top (for any given transfection, the VASP band intensity for the corresponding DMSO treatment was used for normalization purposes). H, box-and-whisker plot showing the speed of migration of MDA-231 cells following transient transfection of either myc-Pfn1 or myc-Pfn1-S137A constructs and treated with either DMSO or FSK (endogenous Pfn1 expression was silenced in these cells by siRNA treatment). n, number of cells from three experiments; *, p < 0.05; **, p < 0.01; NS, not significant.
Next, we performed immunoprecipitation experiments in HEK293 cells to examine VASP's binding to either WT, S137A (phosphodead), or S137E (phosphomimetic) variants of myc-Pfn1, which showed that Pfn1–VASP interaction is almost abolished upon S137E substitution of Pfn1 (Fig. 7, D and E). Interestingly, S137A substitution of Pfn1 (which prevents Pfn1's ability to be phosphorylated on the Ser137 residue) also reduced the basal Pfn1–VASP interaction in these cells to some extent, but the effect was not as dramatic as seen for the S137E mutant of Pfn1 (possible reasons and the implication of these results are discussed later). However, actin binding of Pfn1 was not affected by any of these mutations (Fig. 7F), suggesting that the Ser137 residue is not involved in the actin interaction of Pfn1. Next, when we compared FSK-induced changes in VASP's interaction with WT- versus S137A-Pfn1, we found that FSK-induced attenuation of VASP–Pfn1 interaction was much more pronounced for WT-Pfn1 (∼76%) than S137A-Pfn1 (∼32%) (these numbers are based on an average of two experiments) (Fig. 7G). Collectively, these data are consistent with a scenario where Ser137 phosphorylation of Pfn1 is at least partially involved in PKA-dependent negative regulation of Pfn1–VASP interaction.
Finally, to determine whether Ser137 phosphorylation of Pfn1 has any relevance in the cell motility response to a PKA agonist, we assessed MDA-231 cell motility following acute stimulation with FSK (DMSO-treated cells served as controls). In these experiments, cells were subjected to endogenous Pfn1 knockdown and rescued with either the WT or nonphosphorylatable S137A form of myc-Pfn1 by transient transfection (these constructs also contained an internal ribosome entry site (IRES)-GFP–coding region). Analyses of time-lapse images of GFP-positive cells revealed that FSK stimulation caused a prominent 40% reduction in the speed of cells expressing WT-Pfn1. In the basal state, S137A-Pfn1 expressers exhibited a trend of slightly slower speed compared with the WT-Pfn1 expressers, although the difference was not statistically significant. Importantly, however, FSK stimulation did not reduce the speed of S137A-Pfn1 expressers any further (Fig. 7H). These data imply that Ser137 phosphorylation of Pfn1 may play a role in PKA-dependent modulation of cell motility.
Discussion
Ena/VASP proteins are major players in regulating actin cytoskeletal geometry, membrane protrusion, and cell motility (10, 18, 19, 21, 28, 30). Based on evidence that Pfn1 profoundly enhances the F-actin elongation capability of Ena/VASP (29) and that both Pfn1 and the PLP region of VASP are required for efficient actin-driven intracellular motility of bacterial pathogens (19, 29), it has been widely postulated that Ena/VASP's interaction with Pfn1 plays a role in membrane protrusion and cell motility. However, direct experimental proof of this postulation is still lacking in the literature. In fact, a previous study of fibroblasts that utilized a deletion mutant of VASP lacking the entire PLP domain indicated that PLP domain interactions of VASP may be dispensable for cell motility (33). As PLP domain deletion also abrogates VASP's interaction with SH3 and WW domain proteins, we introduced minimal point mutations in the PLP region of VASP to query the effect of specific down-regulation of VASP–Pfn1 interactions on VASP's ability to modulate the actin cytoskeleton, focal adhesions, membrane protrusion dynamics, and cell motility using either overexpression or knockdown or knockdown/rescue strategies. Our studies in overexpression settings showed that Pfn1's interaction plays a role in VASP-stimulated actin polymerization at the leading edge of migrating cells. Clearly, these data provide a functional relevance of the previously reported enriched Pfn1–VASP interaction at the leading edge of motile cells, including MDA-231 cells (32, 48). We further showed that VASP stimulates focal adhesion formation, but this does not require Pfn1's interaction. This is not surprising given that VASP's targeting to focal adhesions depends on its EVH1 rather than PLP domain. Our kymography studies also provide, for the first time, direct evidence of VASP's ability to modulate membrane protrusion dynamics utilizing its interaction with Pfn1. We showed that VASP overexpression stimulated protrusion frequency and ruffling (indicators of unstable protrusions, a feature that has been previously related to the anti-capping activity of Ena/VASP (29, 30)) and that these effects are mitigated upon interference of its interaction with Pfn1. These protrusion features are consistent with reduced directional persistence of protrusions and slower overall speeds of migration of MDA-231 breast cancer cells upon VASP overexpression (similar to the anti-migratory effect of VASP in fibroblasts (28)) and reversal of motility-related phenotypes when the Pfn1 binding capability of VASP was down-regulated. Similar results were also obtained when the general PLP–ligand interaction capability of Pfn1 was abrogated, although the phenotypic reversion in this case was not complete; this was not unexpected, as Pfn1 also interacts with many other PLP domain proteins besides VASP.
Our motility results in three different settings of Ena/VASP perturbation, including VASP overexpression (which represents artificially induced highest Ena/VASP activity), Mena/VASP knockdown (lowest Ena/VASP activity), and Mena/VASP knockdown with VASP rescue (modest Ena/VASP activity) suggest that too high or too low Ena/VASP activity can be counterproductive for cell migration. A few points are worth discussing in this context. First, our finding that Pfn1's interaction was critical for VASP's ability to rescue the motility defect of Mena/VASP-depleted MDA-231 cells clearly suggests that there is likely a concentration range of Ena/VASP for which Ena/VASP promotes cell migration through its interaction with Pfn1. Second, slower MDA-231 cell migration upon Mena/VASP depletion, as shown, here contrasts the previous finding of increased motility of MEFs under Mena/VASP-depleted conditions (28). This suggests that the effect of loss of function of Mena/VASP on cell motility can be cell type–specific. Third, although the effect of Mena/VASP knockdown on MDA-231 cell morphology was striking, the overall motility phenotype was much less severe (resulting in a 25–40% reduction in overall speed). There could be several possible explanations. For example, the effect of Mena/VASP knockdown could be partly mitigated by a compensatory action of Evl, the third member of the Ena/VASP protein family. We think it is unlikely because Evl has an anti-migratory effect on breast cancer cell motility (this action is related to Evl-induced stimulation of contractility and suppression of membrane protrusion utilizing its interaction with Pfn2, the minor isoform of Pfn) (25), and we showed previously that inhibiting the action of all Ena/VASP proteins by mitochondrial sequestration had no effect on the overall speed of MDA-231 cells (which may also indirectly suggest opposite effects of Mena/VASP and Evl on breast cancer cell motility) (35). Second, because various Ena/VASP proteins have shared ligands but with different affinities (13, 25), it is possible that, in the near absence of Mena/VASP, Evl might promote cell motility through its interactions with certain ligands that are otherwise preferred for Mena and/or VASP when all three members are present. Although membrane protrusion is an obligatory step for cell migration, random protrusions in all directions are counterproductive for cell migration. Therefore, another simple explanation could be that, by reducing the overall flare of protrusions (including lateral protrusions), Mena/VASP depletion could enhance the polarized feature of cells (a feature that promotes cell motility) and partly offset the protrusion-related motility defect.
Although Pfn1 is an important ligand for VASP, which biochemical pathways regulate Ena/VASP–Pfn1 interaction in cells is not known. In this study, we demonstrate for the first time that VASP–Pfn1 interaction is negatively regulated by PKA, most likely through PKA-mediated Ser137 phosphorylation of Pfn1. Our kinase assay data showing PKA's ability to phosphorylate Pfn1 as well as the C-terminal peptide of Pfn1 that only contains a single PKA consensus site of phosphorylation (Ser137) and, importantly, abrogation of PKA-mediated Pfn1 phosphorylation upon S137A substitution provide reasonable in vitro evidence of PKA-mediated Ser137 phosphorylation of Pfn1. However, because we do not have direct S-based evidence of Ser137 phosphorylation of Pfn1 in FSK-treated cells, we acknowledge that our results are suggestive of but not definitive regarding Ser137 phosphorylation of Pfn1 being responsible for PKA-dependent negative regulation of Pfn1–VASP interaction. In fact, our experiments showed that the basal VASP–Pfn1 interaction is somewhat lower for S137A-Pfn1 compared with WT Pfn1 and, furthermore, that S137A substitution does not completely prevent FSK-induced down-regulation of Pfn1–VASP interaction in cells. One possibility is that the S137A substitution may open Pfn1 to phosphorylation of some other neighboring residues that can also reduce Pfn1's affinity for PLP ligands. Future studies are needed to address these issues.
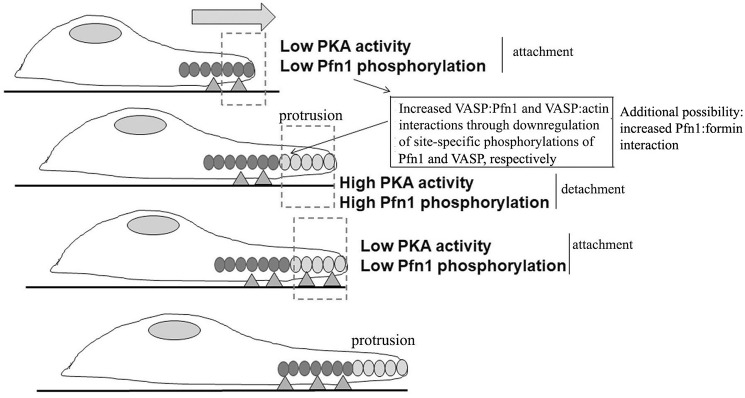
Another key finding of this study is the regulation of Pfn1–VASP interaction by cell–substrate adhesion through PKA. These results open the possibility of a new mechanistic model of adhesion–protrusion coupling during cell migration involving cycles of PKA activity, as schematized in Fig. 8. Specifically, we propose that stabilization of membrane protrusions through adhesions to the underlying substrate triggers down-regulation of PKA activity and reduced phosphorylation of Pfn1 (on Ser137) and VASP. Because Ser137 phosphorylation of Pfn1 inhibits its PLP interaction with VASP, and phosphorylation of VASP reduces its actin binding (29, 39), down-regulation of these phosphorylation events as a consequence of cell attachment could potentially stimulate VASP's interactions with both actin and Pfn1, driving actin polymerization and a new round of membrane protrusion. Given that S137D substitution inhibits Pfn1's interaction with huntingtin (a PLP ligand of Pfn1) (47), Pfn1's interaction with other PLP domain–bearing actin cytoskeleton regulators besides VASP might be also sensitive to Ser137 phosphorylation. In fact, we have preliminary indications of reduced mDia1 (a formin family protein and a known ligand of Pfn1) binding to S137E-Pfn1 compared with S137A-Pfn1 (Fig. S4). Therefore, in our model, we further propose that, although the protrusion remains unattached, a local rise in PKA activity could act as a brake on VASP–Pfn1– (and/or formin–Pfn1)- mediated actin assembly, limiting the protrusion until new adhesions are formed.
Figure 8.
A proposed schematic model of adhesion–protrusion coupling during cell migration. Cycles of localized changes in PKA activity in response to deadhesion and adhesion (marked by triangles) regulates Pfn1–VASP–mediated actin (marked by ovals) polymerization and membrane protrusion through modulating Pfn1 and VASP phosphorylation (see “Discussion” for further description). The hypothetical model also incorporates a possible scenario of Pfn1's interaction with formin proteins being modulated by phosphorylation of Pfn1.
Finally, PKA influences different facets of actin cytoskeleton–regulatory processes, including modulation of the activities of Rho family GTPases (Rho, Rac, and Cdc42), actin-binding proteins (e.g. VASP and myosin), and kinases that indirectly control the function of actin-binding proteins (e.g. p21-activated kinase) (49). PKA's role in cell migration is complex and context-specific. There is pharmacological and genetic evidence of PKA's negative regulation of cell motility, and in other cellular contexts, cAMP/PKA signaling has a pro-migratory role (49–52). This study showed that the cAMP agonist FSK suppresses breast cancer cell motility and that FSK-induced inhibition of cell motility is partly dependent on Pfn1's ability to be phosphorylated on Ser137. As a key consequence of Ser137 phosphorylation of Pfn1 is down-regulation of its PLP interactions (as seen with VASP and formins), our data imply that negative regulation of Pfn1's PLP interaction could be an important mechanism of the anti-migratory action of PKA. Although this study is specifically focused on VASP, it will be interesting to determine in future studies whether Pfn1's interaction with other PLP ligands that are relevant in the context of cell motility are also affected by PKA or other kinases activated downstream of PKA through phosphorylation of Pfn1.
Experimental procedures
Plasmid and siRNAs
WT-Pfn1 and H133S-Pfn1 were subcloned into the ECFP-vector at Hind3 and BamH1 sites. The S137A and S137E mutants of myc-Pfn1 (on an IRES-GFP backbone vector) were created by site-directed mutagenesis using the following primers: S137A (sense, 5′-CTCACCTGCGGCGTGCCCAGTACTG-3′) and S137E (sense, 5′-CTCACCTGCGGCGTGAACAGTACTG-3′). Pfn1 constructs were made Pfn1 siRNA–resistant by placing a silent mutation in the siRNA targeting region without changing peptide encoding (the targeting region of Pfn1 siRNA has been described previously (53)). VASP and Mena siRNAs, targeted to the UTR (GAGUGAAUCUGCGCGGAGA) and the ORF (GAGAGAGAGCGCAGAAUAU), respectively, were purchased from Dharmacon (Lafayette, CO). Sequential point mutations of EGFP-VASP were performed on the regulatory GP5 site Pro120 and loading site Leu210 using PCR-based site-directed mutagenesis. The forward and reverse primers for the P120E mutant were 5′-GGA GGT GGG CCC CCT GAA CCC CCA GCA CTT CCC-3′ and 5′-GGG AAG TGC TGG GGG TTC AGG GGG CCC ACC TCC-3′, respectively. The forward and reverse primers for the L210E mutant were 5′-CCC CCT GCA CCC CCT GAA CCG GCA GCA CAG GGC-3′ and 5′-GCC CTG TGC TGC CGG TTC AGG GGG TGC AGG GGG-3′, respectively. EGFP-VASP-ΔPRO (which lacks the entire PLP domain of VASP) was a generous gift from Dr. Frank Gertler (Massachusetts Institute of Technology).
Cell culture and transfection
HEK293 cells were cultured in DMEM–F12 medium supplemented with 10% FBS and antibiotics (Invitrogen). MDA-231 breast cancer cells were cultured in Eagle's minimum essential medium (EMEM) medium supplemented with 10% FBS, sodium pyruvate, and antibiotics. NR6 mouse fibroblasts were cultured in α-minimum Eagle's medium supplemented with 7.5% FBS, 1× nonessential amino acids, 1× l-glutamine, 1× sodium pyruvate, and antibiotics. MDA-231 cells stably transfected with EGFP or various VASP constructs using Lipofectamine 2000 reagent (Invitrogen) were cultured in the medium described above, with an additional supplement of 500 μg/ml G418. siRNAs were transfected at a working concentration of 100 nm using a transfection reagent commercially available from Dharmacon, following the manufacturer's protocol. All silencing-based experiments were performed 72 h after transfection.
Immunoblotting
Total lysates were prepared by extracting cells with modified radioimmune precipitation assay buffer (25 mm Tris-HCl (pH 7.5), 150 mm NaCl, 1% (v/v) NP-40, 5% (v/v) glycerol, 1 mm EDTA, 50 mm NaF, 1 mm sodium pervanadate, and protease inhibitors). The lysates were clarified at 13,000 rpm for 10 min at 4 °C, and the protein concentration was determined using a Bradford protein assay kit (Pierce). For protein electrophoresis, equal amounts of protein samples (ranging from 10 μg for HEK293 cells to 20 μg for MDA-231 cells) were loaded for SDS-PAGE and transferred onto a nitrocellulose membrane. After blocking the membrane with 5% nonfat dry milk in Tris-buffered saline with Tween 20 (TBST) for 1 h at room temperature, immunoblotting was performed overnight with the appropriate antibodies. After extensive washing with TBST, the blot was incubated with the appropriate secondary antibody (1:1000 dilution, Pharmingen, San Diego, CA) and washed three times with TBST before performing chemiluminescence for visualization of protein bands.
Immunostaining/phalloidin staining
For immunostaining, cells were washed with PBS, fixed with 3.7% formaldehyde for 15 min, permeabilized with 0.5% Triton X-100 for 5 min, and then blocked with 10% goat serum for 1 h at room temperature. After incubation with primary antibodies (vinculin at 1:100 dilution) for 1 h at room temperature, cells were washed twice with PBS containing 0.02% Tween and twice with PBS and then incubated with secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA). Vinculin-stained cells were imaged with a ×60 objective on a Nikon A1 confocal microscope. For rhodamine–phalloidin staining, cells were washed with PBS, fixed with 3.7% formaldehyde for 15 min, permeabilized with 0.5% Triton X-100 for 5 min, and then stained with rhodamine–phalloidin following the manufacturer's protocol. Stained cells were washed twice with PBS containing 0.02% Tween, twice with PBS, and then once with distilled water before mounting on slides for imaging using a ×60 oil immersion objective on an Olympus IX71 inverted microscope. Quantitative fluorescence analyses of images were performed by Metamorph software.
Immunoprecipitation
Cell extracts (500–100 μg) were precleared by constant mixing with Protein G Plus/protein A–conjugated agarose beads (Calbiochem) at 4 °C for 1 h. Precleared lysates were incubated with the appropriate antibody overnight, followed by Protein G Plus/protein A–conjugated agarose beads for 2 h at 4 °C on a rotor. The beads were washed with lysis buffer before eluting the immunoprecipitated protein complex by boiling the beads in the presence of 2-mercaptoethanol. The eluate was directly loaded for SDS-PAGE for immunoblotting.
In vitro kinase assay
Bacterial expression and purification of GST and GST-Pfn1 and the PKA assay were performed as described previously (44). Kinase assay products were run on SDS-PAGE and probed with phospho-RRXS antibody. For the peptide kinase assay, different quantities of C-terminal Pfn1 peptide (CYEMASHLRRSQY, synthesized by the peptide synthesis facility at the University of Pittsburgh) was spotted on a nitrocellulose membrane presoaked in 5% BSA–TBST solution and allowed to dry briefly before subjecting the membrane to a PKA assay as described previously (44). The membrane was washed thoroughly with TBST before probing with the phospho-RRXS antibody.
Phosphatase assay
After total lysate was prepared, λ phosphatase (New England Biolabs) was added to the lysate and incubated according to the manufacturer's protocol for 30 min at 30 °C.
Antibodies
The monoclonal GFP, VASP, and vimentin antibodies were obtained from Pharmingen (San Diego, CA). The polyclonal GFP antibody was obtained from Abcam (Cambridge, MA). The polyclonal Pfn1 and monoclonal Mena antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). The monoclonal GAPDH antibody was obtained from Abd Serotec (Raleigh, NC). The polyclonal myc (1:2000) antibody was obtained from Sigma-Aldrich (St. Louis, MO). The phospho-RRXS antibody was a product of Cell Signaling Technology (Denver, CO). The vinculin and mDia1 antibodies were obtained from Cell Signaling Technology. The monoclonal tubulin antibody was purchased through Sigma-Aldrich. The Abl antibody was kindly provided by Dr. Alan Howe (University of Vermont). The concentrations of different antibodies for immunoblotting were as follows: GFP, 1:1000; VASP, 1:1000; Pfn1, 1:1000; Mena, 1:200; GAPDH, 1:2000; myc 1:2000; tubulin, 1:3000; Abl, 1:1000; mDia1, 1:1000; and phospho-RRXS, 1:1000.
Single-cell migration assay
MDA-231 cells were sparsely plated on a 35-mm plastic tissue culture dish coated with collagen I. After overnight incubation, time-lapse videomicroscopy of randomly migrating cells was performed simultaneously at multiple fields of observations with a ×10 objective at an interval of 1 min for a total duration of 120 min. The cell trajectory was built via frame-by-frame analyses of the centroid positions (x and y) of cell nuclei. Protrusion direction was determined by creating a vector from the centroid of a cell nucleus and the farthest point of a protrusion before protrusion was retracted. Persistence was calculated using a nonoverlapping interval random walk model as described previously (37). The acquired images were analyzed using the NIH ImageJ software.
Kymography
Short (10 min) time-lapse images of cells were recorded at a 10-s time interval. Kymographs marking the beginning to the end of protrusion were constructed based on 1-pixel-wide (0.3 μm) lines drawn at multiple locations (3, 4) across the protruding membrane. All images were acquired and analyzed using Metamorph and NIH ImageJ software, respectively, as described previously by us (35).
Statistics and data representation
All statistical tests were performed with analysis of variance followed by Tukey–Kramer post hoc test analysis. A p value of less than 0.05 was considered to be statistically significant. In box-and-whisker plots, crosses represent the mean, the middle lines of the box indicate the median, the top of the box indicates the 75th percentile, the bottom of the box measures the 25th percentile, and the two whiskers indicate the 10th and 90th percentiles, respectively. In bar graphs, the data are represented as mean ± S.D.
Author contributions
D. G., W. V., and P. R. conceptualization; D. G. and W. V. data curation; D. G. and W. V. formal analysis; D. G. validation; D. G., W. V., and P. R. investigation; D. G., W. V., and P. R. writing-original draft; S. G. S. and P. R. supervision; S. G. S. and P. R. funding acquisition; P. R. project administration; P. R. writing-review and editing.
Supplementary Material
Acknowledgment
We thank the Center for Biological Imaging at the University of Pittsburgh for confocal imaging support.
This work was supported by NCI, National Institutes of Health Grant 2R01CA108607 (to P. R.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Figs. S1–S4 and Movies S1–S5.
- Ena
- Enabled
- VASP
- vasodilator-stimulated phosphoprotein
- PLP
- polyproline
- SH
- Src homology
- MEF
- mouse embryonic fibroblast
- aa
- amino acids
- FSK
- forskolin
- EGFP
- enhanced GFP.
References
- 1. Le Clainche C., and Carlier M. F. (2008) Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol. Rev. 88, 489–513 10.1152/physrev.00021.2007 [DOI] [PubMed] [Google Scholar]
- 2. Sheetz M. P., Felsenfeld D., Galbraith C. G., and Choquet D. (1999) Cell migration as a five-step cycle. Biochem. Soc. Symp. 65, 233–243 [PubMed] [Google Scholar]
- 3. Small J. V., Stradal T., Vignal E., and Rottner K. (2002) The lamellipodium: where motility begins. Trends Cell Biol. 12, 112–120 10.1016/S0962-8924(01)02237-1 [DOI] [PubMed] [Google Scholar]
- 4. Krause M., and Gautreau A. (2014) Steering cell migration: lamellipodium dynamics and the regulation of directional persistence. Nat. Rev. Mol. Cell Biol. 15, 577–590 10.1038/nrm3861 [DOI] [PubMed] [Google Scholar]
- 5. Stradal T. E., and Scita G. (2006) Protein complexes regulating Arp2/3-mediated actin assembly. Curr. Opin. Cell Biol. 18, 4–10 10.1016/j.ceb.2005.12.003 [DOI] [PubMed] [Google Scholar]
- 6. Chen X. J., Squarr A. J., Stephan R., Chen B., Higgins T. E., Barry D. J., Martin M. C., Rosen M. K., Bogdan S., and Way M. (2014) Ena/VASP proteins cooperate with the WAVE complex to regulate the actin cytoskeleton. Dev. Cell 30, 569–584 10.1016/j.devcel.2014.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bear J. E., and Gertler F. B. (2009) Ena/VASP: towards resolving a pointed controversy at the barbed end. J. Cell Sci. 122, 1947–1953 10.1242/jcs.038125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reinhard M., Giehl K., Abel K., Haffner C., Jarchau T., Hoppe V., Jockusch B. M., and Walter U. (1995) The proline-rich focal adhesion and microfilament protein VASP is a ligand for profilins. EBMO J. 14, 1583–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reinhard M., Jouvenal K., Tripier D., and Walter U. (1995) Identification, purification, and characterization of a zyxin-related protein that binds the focal adhesion and microfilament protein VASP (vasodilator-stimulated phosphoprotein). Proc. Natl. Acad. Sci. U.S.A. 92, 7956–7960 10.1073/pnas.92.17.7956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krause M., Dent E. W., Bear J. E., Loureiro J. J., and Gertler F. B. (2003) Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration. Annu. Rev. Cell Dev. Biol. 19, 541–564 10.1146/annurev.cellbio.19.050103.103356 [DOI] [PubMed] [Google Scholar]
- 11. Krause M., Leslie J. D., Stewart M., Lafuente E. M., Valderrama F., Jagannathan R., Strasser G. A., Rubinson D. A., Liu H., Way M., Yaffe M. B., Boussiotis V. A., and Gertler F. B. (2004) Lamellipodin, an Ena/VASP ligand, is implicated in the regulation of lamellipodial dynamics. Dev. Cell 7, 571–583 10.1016/j.devcel.2004.07.024 [DOI] [PubMed] [Google Scholar]
- 12. Pernier J., Shekhar S., Jegou A., Guichard B., and Carlier M. F. (2016) Profilin interaction with actin filament barbed end controls dynamic instability, capping, branching, and motility. Dev. Cell 36, 201–214 10.1016/j.devcel.2015.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gertler F. B., Niebuhr K., Reinhard M., Wehland J., and Soriano P. (1996) Mena, a relative of VASP and Drosophila Enabled, is implicated in the control of microfilament dynamics. Cell 87, 227–239 10.1016/S0092-8674(00)81341-0 [DOI] [PubMed] [Google Scholar]
- 14. Bachmann C., Fischer L., Walter U., and Reinhard M. (1999) The EVH2 domain of the vasodilator-stimulated phosphoprotein mediates tetramerization, F-actin binding, and actin bundle formation. J. Biol. Chem. 274, 23549–23557 10.1074/jbc.274.33.23549 [DOI] [PubMed] [Google Scholar]
- 15. Walders-Harbeck B., Khaitlina S. Y., Hinssen H., Jockusch B. M., and Illenberger S. (2002) The vasodilator-stimulated phosphoprotein promotes actin polymerisation through direct binding to monomeric actin. FEBS Lett. 529, 275–280 10.1016/S0014-5793(02)03356-2 [DOI] [PubMed] [Google Scholar]
- 16. Goh K. L., Cai L., Cepko C. L., and Gertler F. B. (2002) Ena/VASP proteins regulate cortical neuronal positioning. Curr. Biol. 12, 565–569 [DOI] [PubMed] [Google Scholar]
- 17. Forrester W. C., and Garriga G. (1997) Genes necessary for C. elegans cell and growth cone migrations. Development 124, 1831–1843 [DOI] [PubMed] [Google Scholar]
- 18. Kwiatkowski A. V., Rubinson D. A., Dent E. W., Edward van Veen J., Leslie J. D., Zhang J., Mebane L. M., Philippar U., Pinheiro E. M., Burds A. A., Bronson R. T., Mori S., Fässler R., and Gertler F. B. (2007) Ena/VASP is required for neuritogenesis in the developing cortex. Neuron 56, 441–455 10.1016/j.neuron.2007.09.008 [DOI] [PubMed] [Google Scholar]
- 19. Geese M., Loureiro J. J., Bear J. E., Wehland J., Gertler F. B., and Sechi A. S. (2002) Contribution of Ena/VASP proteins to intracellular motility of listeria requires phosphorylation and proline-rich core but not F-actin binding or multimerization. Mol. Biol. Cell 13, 2383–2396 10.1091/mbc.e02-01-0058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rottner K., Behrendt B., Small J. V., and Wehland J. (1999) VASP dynamics during lamellipodia protrusion. Nat. Cell Biol. 1, 321–322 10.1038/13040 [DOI] [PubMed] [Google Scholar]
- 21. Lacayo C. I., Pincus Z., VanDuijn M. M., Wilson C. A., Fletcher D. A., Gertler F. B., Mogilner A., and Theriot J. A. (2007) Emergence of large-scale cell morphology and movement from local actin filament growth dynamics. PLoS Biol. 5, e233 10.1371/journal.pbio.0050233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carmona G., Perera U., Gillett C., Naba A., Law A. L., Sharma V. P., Wang J., Wyckoff J., Balsamo M., Mosis F., De Piano M., Monypenny J., Woodman N., McConnell R. E., Mouneimne G., et al. (2016) Lamellipodin promotes invasive 3D cancer cell migration via regulated interactions with Ena/VASP and SCAR/WAVE. Oncogene 35, 5155–5169 10.1038/onc.2016.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Philippar U., Roussos E. T., Oser M., Yamaguchi H., Kim H. D., Giampieri S., Wang Y., Goswami S., Wyckoff J. B., Lauffenburger D. A., Sahai E., Condeelis J. S., and Gertler F. B. (2008) A Mena invasion isoform potentiates EGF-induced carcinoma cell invasion and metastasis. Dev. Cell 15, 813–828 10.1016/j.devcel.2008.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Padilla-Rodriguez M., Parker S. S., Adams D. G., Westerling T., Puleo J. I., Watson A. W., Hill S. M., Noon M., Gaudin R., Aaron J., Tong D., Roe D. J., Knudsen B., and Mouneimne G. (2018) The actin cytoskeletal architecture of estrogen receptor positive breast cancer cells suppresses invasion. Nat. Commun. 9, 2980 10.1038/s41467-018-05367-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mouneimne G., Hansen S. D., Selfors L. M., Petrak L., Hickey M. M., Gallegos L. L., Simpson K. J., Lim J., Gertler F. B., Hartwig J. H., Mullins R. D., and Brugge J. S. (2012) Differential remodeling of actin cytoskeleton architecture by profilin isoforms leads to distinct effects on cell migration and invasion. Cancer Cell 22, 615–630 10.1016/j.ccr.2012.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. García Arguinzonis M. I., Galler A. B., Walter U., Reinhard M., and Simm A. (2002) Increased spreading, Rac/p21-activated kinase (PAK) activity, and compromised cell motility in cells deficient in vasodilator-stimulated phosphoprotein (VASP). J. Biol. Chem. 277, 45604–45610 10.1074/jbc.M202873200 [DOI] [PubMed] [Google Scholar]
- 27. Tian Y., Xu L., He Y., Xu X., Li K., Ma Y., Gao Y., Wei D., and Wei L. (2018) Knockdown of RAC1 and VASP gene expression inhibits breast cancer cell migration. Oncol. Lett. 16, 2151–2160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bear J. E., Loureiro J. J., Libova I., Fässler R., Wehland J., and Gertler F. B. (2000) Negative regulation of fibroblast motility by Ena/VASP proteins. Cell 101, 717–728 10.1016/S0092-8674(00)80884-3 [DOI] [PubMed] [Google Scholar]
- 29. Barzik M., Kotova T. I., Higgs H. N., Hazelwood L., Hanein D., Gertler F. B., and Schafer D. A. (2005) Ena/VASP proteins enhance actin polymerization in the presence of barbed end capping proteins. J. Biol. Chem. 280, 28653–28662 10.1074/jbc.M503957200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bear J. E., Svitkina T. M., Krause M., Schafer D. A., Loureiro J. J., Strasser G. A., Maly I. V., Chaga O. Y., Cooper J. A., Borisy G. G., and Gertler F. B. (2002) Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell 109, 509–521 10.1016/S0092-8674(02)00731-6 [DOI] [PubMed] [Google Scholar]
- 31. Hansen S. D., and Mullins R. D. (2010) VASP is a processive actin polymerase that requires monomeric actin for barbed end association. J. Cell Biol. 191, 571–584 10.1083/jcb.201003014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li Y., Grenklo S., Higgins T., and Karlsson R. (2008) The profilin:actin complex localizes to sites of dynamic actin polymerization at the leading edge of migrating cells and pathogen-induced actin tails. Eur. J. Cell Biol. 87, 893–904 10.1016/j.ejcb.2008.06.003 [DOI] [PubMed] [Google Scholar]
- 33. Loureiro J. J., Rubinson D. A., Bear J. E., Baltus G. A., Kwiatkowski A. V., and Gertler F. B. (2002) Critical roles of phosphorylation and actin binding motifs, but not the central proline-rich region, for Ena/vasodilator-stimulated phosphoprotein (VASP) function during cell migration. Mol. Biol. Cell 13, 2533–2546 10.1091/mbc.e01-10-0102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ferron F., Rebowski G., Lee S. H., and Dominguez R. (2007) Structural basis for the recruitment of profilin-actin complexes during filament elongation by Ena/VASP. EMBO J. 26, 4597–4606 10.1038/sj.emboj.7601874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bae Y. H., Ding Z., Zou L., Wells A., Gertler F., and Roy P. (2009) Loss of profilin-1 expression enhances breast cancer cell motility by Ena/VASP proteins. J. Cell. Physiol. 219, 354–364 10.1002/jcp.21677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zou L., Jaramillo M., Whaley D., Wells A., Panchapakesa V., Das T., and Roy P. (2007) Profilin-1 is a negative regulator of mammary carcinoma aggressiveness. Br. J. Cancer 97, 1361–1371 10.1038/sj.bjc.6604038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dickinson R. B., and Tranquillo R. T. (1993) Optimal estimation of cell-movement indexes from the statistical-analysis of cell tracking data. AIChE J. 39, 1995–2010 10.1002/aic.690391210 [DOI] [Google Scholar]
- 38. Scott J. A., Shewan A. M., den Elzen N. R., Loureiro J. J., Gertler F. B., and Yap A. S. (2006) Ena/VASP proteins can regulate distinct modes of actin organization at cadherin-adhesive contacts. Mol. Biol. Cell 17, 1085–1095 10.1091/mbc.e05-07-0644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Harbeck B., Hüttelmaier S., Schluter K., Jockusch B. M., and Illenberger S. (2000) Phosphorylation of the vasodilator-stimulated phosphoprotein regulates its interaction with actin. J. Biol. Chem. 275, 30817–30825 10.1074/jbc.M005066200 [DOI] [PubMed] [Google Scholar]
- 40. Lindsay S. L., Ramsey S., Aitchison M., Renné T., and Evans T. J. (2007) Modulation of lamellipodial structure and dynamics by NO-dependent phosphorylation of VASP Ser239. J. Cell Sci. 120, 3011–3021 10.1242/jcs.003061 [DOI] [PubMed] [Google Scholar]
- 41. Howe A. K., Hogan B. P., and Juliano R. L. (2002) Regulation of vasodilator-stimulated phosphoprotein phosphorylation and interaction with Abl by protein kinase A and cell adhesion. J. Biol. Chem. 277, 38121–38126 10.1074/jbc.M205379200 [DOI] [PubMed] [Google Scholar]
- 42. Alasbahi R. H., and Melzig M. F. (2012) Forskolin and derivatives as tools for studying the role of cAMP. Die Pharmazie 67, 5–13 [PubMed] [Google Scholar]
- 43. Benz P. M., Blume C., Seifert S., Wilhelm S., Waschke J., Schuh K., Gertler F., Münzel T., and Renné T. (2009) Differential VASP phosphorylation controls remodeling of the actin cytoskeleton. J. Cell Sci. 122, 3954–3965 10.1242/jcs.044537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gau D., Veon W., Zeng X., Yates N., Shroff S. G., Koes D. R., and Roy P. (2016) Threonine 89 is an important residue of profilin-1 that is phosphorylatable by protein kinase A. PLoS ONE 11, e0156313 10.1371/journal.pone.0156313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schweinhuber S. K., Meßerschmidt T., Hänsch R., Korte M., and Rothkegel M. (2015) Profilin isoforms modulate astrocytic morphology and the motility of astrocytic processes. PLoS ONE 10, e0117244 10.1371/journal.pone.0117244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sathish K., Padma B., Munugalavadla V., Bhargavi V., Radhika K. V., Wasia R., Sairam M., and Singh S. S. (2004) Phosphorylation of profilin regulates its interaction with actin and poly (L-proline). Cell Signal. 16, 589–596 10.1016/j.cellsig.2003.10.001 [DOI] [PubMed] [Google Scholar]
- 47. Shao J., Welch W. J., Diprospero N. A., and Diamond M. I. (2008) Phosphorylation of profilin by ROCK1 regulates polyglutamine aggregation. Mol. Cell. Biol. 28, 5196–5208 10.1128/MCB.00079-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gau D., Ding Z., Baty C., and Roy P. (2011) Fluorescence resonance energy transfer (FRET)-based detection of Profilin-VASP interaction. Cell. Mol. Bioeng. 4, 1–8 10.1007/s12195-010-0133-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Howe A. K. (2004) Regulation of actin-based cell migration by cAMP/PKA. Biochim. Biophys. Acta 1692, 159–174 10.1016/j.bbamcr.2004.03.005 [DOI] [PubMed] [Google Scholar]
- 50. Edin M. L., Howe A. K., and Juliano R. L. (2001) Inhibition of PKA blocks fibroblast migration in response to growth factors. Exp. Cell Res. 270, 214–222 10.1006/excr.2001.5345 [DOI] [PubMed] [Google Scholar]
- 51. Lim C. J., Kain K. H., Tkachenko E., Goldfinger L. E., Gutierrez E., Allen M. D., Groisman A., Zhang J., and Ginsberg M. H. (2008) Integrin-mediated protein kinase A activation at the leading edge of migrating cells. Mol. Biol. Cell 19, 4930–4941 10.1091/mbc.e08-06-0564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chen L., Zhang J. J., and Huang X. Y. (2008) cAMP inhibits cell migration by interfering with Rac-induced lamellipodium formation. J. Biol. Chem. 283, 13799–13805 10.1074/jbc.M800555200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ding Z., Gau D., Deasy B., Wells A., and Roy P. (2009) Both actin and polyproline interactions of profilin-1 are required for migration, invasion and capillary morphogenesis of vascular endothelial cells. Exp. Cell Res. 315, 2963–2973 10.1016/j.yexcr.2009.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.