Abstract
Background
There have been few studies to evaluate the prognostic implications of guideline-directed therapy according to the temporal course of heart failure. This study assessed the relationship between adherence to guideline-directed therapy at discharge and 60-day clinical outcomes in de novo acute heart failure (AHF) and acute decompensated chronic heart failure (ADCHF) separately.
Methods
Among 5,625 AHF patients who were recruited from a multicenter cohort registry of Korean Acute Heart Failure, 2,769 patients with reduced ejection fraction were analyzed. Guideline-directed therapies were defined as the use of angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor II blocker (ARB), β-blocker, and mineralocorticoid receptor antagonist.
Results
In de novo AHF, ACEI or ARB reduced re-hospitalization (hazard ratio [HR], 0.57; 95% confidence interval [CI], 0.34–0.95), mortality (HR, 0.41; 95% CI, 0.24–0.69) and composite endpoint (HR, 0.52; 95% CI, 0.36–0.77) rates. Beta-blockers reduced re-hospitalization (HR, 0.62; 95% CI, 0.41–0.95) and composite endpoint (HR, 0.65; 95% CI, 0.47–0.90) rates. In ADCHF, adherence to ACEI or ARB was associated with only mortality and β-blockers with composite endpoint.
Conclusion
The prognostic implications of adherence to guideline-directed therapy at discharge were more pronounced in de novo heart failure. We recommend that guideline-directed therapy be started as early as possible in the course of heart failure with reduced ejection fraction.
Keywords: De Novo Acute Heart Failure, Acute Decompensated Heart Failure, Guideline-Directed Therapy
Graphical Abstract
INTRODUCTION
The American College of Cardiology (ACC)/American Heart Association (AHA) and the European Society of Cardiology (ESC) have developed evidence-based guidelines for the treatment of heart failure (HF) to assist clinicians in clinical decision-making by describing acceptable approaches to the diagnosis, management, and prevention of specific diseases or conditions.1,2 In chronic HF with reduced ejection fraction (HFrEF), evidence-based benefit on outcome is documented for angiotensin-converting enzyme inhibitors (ACEI), angiotensin-receptor II blockers (ARB), β-blockers, mineralocorticoid receptor antagonists (MRA), angiotensin receptor neprilysin inhibitors (ARNI), and ivabradine. However, acute heart failure (AHF) is characterized by rapid worsening of symptoms and signs of HF. Although survival rates have improved, mortality is still high, typically greater than 4%. However, most morbidity and mortality of hospitalized AHF occurs early after index hospital discharge.3,4 Hospitalized HF patients have 30-day readmission rates from 20% to 27%, with mortality rate reaching up to 12.2% at 30-days.5,6 Once the patient is stabilized, the priority should transition to initiation of chronic medical therapy. Modalities initiated in the hospital engender increased outpatient adherence and improved outcomes. Therefore, comprehensive strategies must focus on factors during hospitalization and during the early recovery period soon after discharge to target stressors that contribute to patient vulnerability. The guideline-directed therapy in HF inpatient is associated with post-discharge mortality or re-hospitalization.7,8,9 AHF has two forms according to the time course of heart failure: newly arisen (“de novo”) AHF and acutely decompensated chronic heart failure (ADCHF).1,2 Acute and chronic HF differ both in their temporal course and treatment.3,10 However, there are limited data regarding the prognostic implications of guideline-directed therapy according to the temporal course of HF. We assessed the relationship between guideline-directed therapy at discharge and 60-day relevant patient clinical outcomes, including all-cause mortality, re-hospitalization because of aggravated HF, and composite endpoint of mortality or HF hospitalization in de novo AHF and ADCHF separately.
METHODS
Study population
We used the registry of Korean Acute Heart Failure (KorAHF), which is a multicenter prospective cohort study. Between March 2011 and February 2014, the registry prospectively enrolled 5,625 consecutive patients admitted for treatment of AHF from 10 tertiary university hospitals. Patients were followed-up until 2018. The registry included patients with signs or symptoms of HF who met at least one of the following inclusion criteria: 1) lung congestion or 2) objective findings of left ventricular systolic dysfunction (LVSD) or structural heart disease. Detailed information on the study design and results of the KorAHF registry have been described previously.11
Adherence to guideline-directed therapy
Guideline-directed therapy was defined by ACC/AHA and ECS guidelines.1,2 Numerators were defined as HF patients who were prescribed each medication and denominator as HF patients with LVSD and without contraindication for medication. The adherence to guideline-directed therapy was assessed by the ratio of the numerator to the dominator.12,13 Of these guideline-directed therapies, we excluded ARNI and ivabradine because this therapy was not available in Korea during the study period.
The adherence to guideline-directed therapy was defined as follows: 1) β-blocker therapy for LVSD: percentage of patients who were prescribed β-blocker therapy with bisoprolol, carvedilol, sustained-release metoprolol succinate, or nebivolol at hospital discharge. Because the 2016 ESC guidelines for HF recommend β-blockers, including nebivolol, for the treatment of HFrEF, patients prescribed nebivolol were defined as numerators.14 Patients not eligible for β-blocker therapy were those with systolic blood pressure < 90 mmHg or resting heart rate < 60 bpm at discharge.2 An equivalent dose of carvedilol was calculated for bisoprolol- and nebivolol-treated subjects (dose × 5), and for metoprolol-treated subjects (dose/4), again taking into account several possible confounders15; 2) ACEI or ARB therapy for LVSD: percentage of patients who were prescribed ACEI or ARB therapy at hospital discharge. Patients not eligible for ACEI or ARB therapy were those with systolic blood pressure < 90 mmHg or serum creatinine > 2.5 mg/dL or serum K ≥ 5.0 mmol/L at discharge.2 Equivalent doses of ramipril were calculated for ACEI, and equivalent doses of candesartan were calculated for ARB.16
An additional performance measure for MRA was developed, excluding patients with documented MRA contraindications or intolerance (serum K ≥ 5.0 mmol/L or creatinine > 2.5 mg/dL at discharge).2
Clinical outcomes
The follow-up data were collected from the patients by the attending physician and stored in a web-based case report form. The outcome data of subjects who had not been followed-up were ascertained by telephone interview. In addition, the outcome data of patients lost to follow-up were collected from the National Death Records. All clinical events, such as death and re-hospitalization were monitored and verified by a Clinical Event Committee comprising independent experts in HF who did not participate in patient enrolment for the study.11 The outcomes were 60-day all-cause mortality, re-hospitalization because of aggravated HF, and composite endpoint of mortality or HF hospitalization.
Statistical analysis
Statistical analyses were conducted by the Center of Biomedical Data Science, Yonsei University, Wonju College of Medicine. Continuous variables are expressed as mean ± SD and categorical variables as percentages. For continuous variables, the independent t-test was used, and for dichotomous variables, the χ2 test was adopted, as appropriate. The Kaplan-Meier method was used to report survival curves and estimate the mean survival, and the 95% confidence interval (CI) and the log rank test were applied. Cox proportional hazards regression models were used to estimate hazard ratios (HRs) and their 95% CIs. Models were adjusted for gender; age; history of hypertension, diabetes mellitus, ischemic heart disease, and chronic obstructive pulmonary disease; New York Heart Association functional class; systolic blood pressure; heart rate; creatinine; presence of atrial fibrillation at admission; and LVEF. Model discrimination was assessed using Harrell's C-statistic. In all cases, a P value < 0.05 was considered statistically significant. Statistical analyses were performed using SAS 9.4 (SAS Inc., Cary, NC, USA) and R software version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria).
Ethics statement
The study protocol was approved by the ethics committee at each hospital and the Wonju Christian Hospital, Wonju College of Medicine, Yonsei University (Wonju, Korea; Approval No. CR311003), and written informed consent was obtained from each patient or their relative or legal representative.
RESULTS
Baseline characteristics of the study population and clinical outcomes
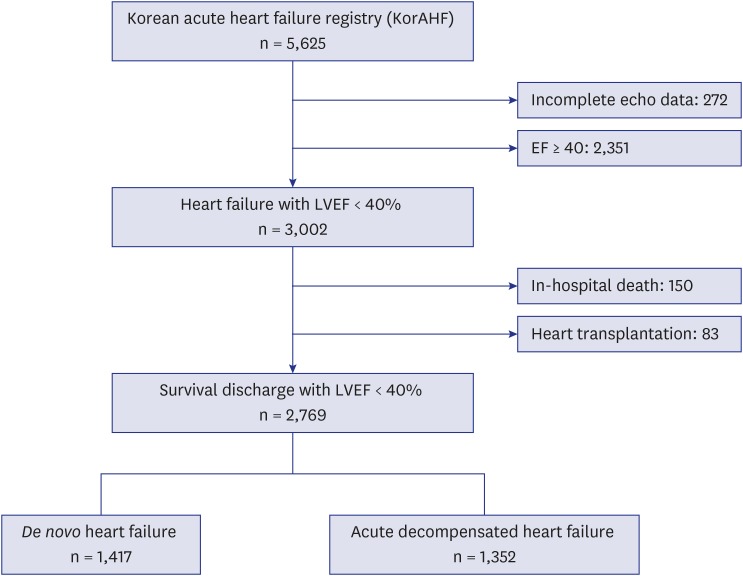
Of 5,625 patients, we selected patients with LVSD, which was defined as LVEF < 40% using echocardiography. After excluding 272 patients without quantitative LVEF data and 83 heart transplantation candidates, 2,769 patients were analyzed (Fig. 1). Patients were classified by the attending physician according to the contemporary guidelines on AHF, based on the clinical presentation at admission. ADCHF was defined as worsening of HF in patients with a previous diagnosis or hospitalization for HF. De novo AHF, defined as AHF in patients with no prior history of HF14 included 1,417 patients. There were 1,352 patients with ADCHF. Demographic characteristics were significantly different between the 2 groups. Patients with ADCHF were older and had lower body weight, blood pressure, and heart rate than did patients with de novo AHF. ADCHF patients had higher rates of comorbid disease. Electrocardiographically, atrial fibrillation and left bundle branch block were more prevalent in ADCHF. At admission, sodium and hemoglobin levels were lower and creatinine and N-terminal pro-brain natriuretic peptide (NT-proBNP) levels were higher in patients with ADCHF. On echocardiography, patients with ADCHF had a more deteriorated heart function (Table 1). In patients with ADCHF, 60-day rehospitalization (14.3% vs. 6.8%; P < 0.001), mortality (8.2% vs. 5.9%; P = 0.02), and composite endpoint (20.6% vs. 11.8%; P < 0.001) rates were higher compared with those in patients with de novo HF (Supplementary Table 1).
Fig. 1. Flow diagram of patients included.
LVEF = left ventricular ejection fraction.
Table 1. Baseline characteristics of the study population.
| Characteristics | De novo AHF (n = 1,417) | ADCHF (n = 1,352) | P value | ||
|---|---|---|---|---|---|
| Demographic characteristic at admission | |||||
| Gender, men | 873 (61.6) | 816 (60.4) | 0.50 | ||
| Age, yr | 63.9 ± 15.6 | 69.0 ± 13.2 | < 0.001 | ||
| Height, cm | 162.6 ± 9.2 | 161.2 ± 9.3 | < 0.001 | ||
| Weight, cm | 62.5 ± 14.1 | 59.9 ± 12.6 | < 0.001 | ||
| Body mass index, kg/m2 | 23.5 ± 4.0 | 22.9 ± 3.7 | < 0.001 | ||
| Systolic blood pressure, mmHg | 133.9 ± 30.1 | 125.7 ± 28.2 | < 0.001 | ||
| Diastolic blood pressure, mmHg | 83.9 ± 20.0 | 76.8 ± 17.1 | < 0.001 | ||
| Heart rate, beat/min | 99.3 ± 25.1 | 92.5 ± 24.5 | < 0.001 | ||
| NYHA functional class | 0.13 | ||||
| Class II | 206 (14.5) | 182 (13.5) | |||
| Class III | 503 (35.5) | 530 (39.2) | |||
| Class IV | 708 (50.0) | 640 (47.3) | |||
| Comorbidity | |||||
| Hypertension | 725 (51.2) | 817 (60.4) | < 0.001 | ||
| Diabetes mellitus | 460 (32.5) | 566 (41.9) | < 0.001 | ||
| Ischemic heart disease | 227 (16.0) | 604 (44.7) | < 0.001 | ||
| Chronic kidney disease | 137 (9.7) | 271 (20.0) | < 0.001 | ||
| Chronic obstructive pulmonary disease | 123 (9.0) | 165 (12.2) | 0.01 | ||
| Medication at admission | |||||
| ACEI | 96 (26.0) | 273 (74.0) | < 0.001 | ||
| ARB | 243 (17.0) | 494 (36.5) | < 0.001 | ||
| β-blocker | 188 (13.3) | 561 (41.5) | < 0.001 | ||
| MRA | 110 (7.8) | 437 (32.3) | < 0.001 | ||
| Etiology of heart failure | < 0.001 | ||||
| Ischemic heart disease | 538 (38.0) | 598 (44.2) | |||
| Valvular heart disease | 63 (4.4) | 137 (10.1) | |||
| Cardiomyopathy | 447 (31.5) | 402 (29.7) | |||
| ECG characteristics at admission | |||||
| Atrial fibrillation at admission | 362 (25.5) | 454 (33.6) | < 0.001 | ||
| Left bundle branch block | 90 (6.4) | 128 (9.5) | < 0.001 | ||
| Right bundle branch block | 64 (4.5) | 93 (6.9) | 0.01 | ||
| Laboratory characteristics at admission | |||||
| Na, mmol/L | 138.2 ± 4.2 | 136.9 ± 4.8 | < 0.001 | ||
| K, mmol/L | 4.3 ± 0.7 | 4.5 ± 0.7 | < 0.001 | ||
| Albumin, g/dL | 3.7 ± 0.5 | 3.7 ± 0.5 | 0.91 | ||
| Hemoglobin, g/dL | 13.1 ± 2.4 | 12.4 ± 2.2 | < 0.001 | ||
| Creatinine, mg/dL | 1.4 ± 1.6 | 1.6 ± 1.5 | < 0.001 | ||
| hs-CRP, mg/dL | 2.1 ± 3.5 | 2.1 ± 4.1 | 0.96 | ||
| NT-proBNP, pg/mL | 9,308.9 ± 11,845.1 | 11,506.2 ± 11,171.0 | < 0.001 | ||
| BNP, pg/mL | 1,597.9 ± 1,466.3 | 1,636.0 ± 1,381.7 | 0.67 | ||
| CK-MB, ng/mL | 9.4 ± 27.8 | 5.2 ± 12.0 | < 0.001 | ||
| Troponin I, mg/mL | 3.2 ± 22.2 | 0.6 ± 2.8 | < 0.001 | ||
| Echocardiographic characteristics | |||||
| LVEF, % | 27.3 ± 7.9 | 26.2 ± 7.9 | < 0.001 | ||
| LVEDV, mL | 166.5 ± 68.2 | 188.0 ± 76.5 | < 0.001 | ||
| LVESV, mL | 119.6 ± 55.6 | 138.2 ± 64.1 | < 0.001 | ||
| LA dimension, mm | 46.1 ± 8.0 | 49.9 ± 9.2 | < 0.001 | ||
Values are presented as number (%) or mean ± standard deviation, unless otherwise indicated.
AHF = acute heart failure, ADCHF = acutely decompensated chronic heart failure, NYHA = New York Heart Association, ACEI = angiotensin-converting enzyme inhibitor, ARB = angiotensin-receptor II blocker, MRA = mineralocorticoid receptor antagonists, ECG = electrocardiography, hs-CRP = high-sensitivity C-reactive protein, NT-proBNP = N-terminal-proBNP, BNP = B-type natriuretic peptide, CK-MB = creatinine kinase-MB, LVEF = left ventricular ejection fraction, LVEDV = left ventricular end diastolic volume, LVESV = left ventricular end systolic volume, LA = left atrium.
Discharge medication and guideline adherence
Compliance rates for performance measures ranged from high for ACEI or ARB (84.5%) to low for β-blocker (64.5%) and MRA therapy (58.1%) in patients with de novo AHF. Compliance with ACEI or ARB (75.2%), β-blocker (52.9%), and MRA (56.1%) decreased in patients with ADCHF (Supplementary Table 2). With regard to medication at discharge, the calculated equivalent doses for ACEI, ARB, β-blockers, and MRA were compared, and they did not differ between the 2 groups (Table 2).
Table 2. Medical therapy at discharge.
| Variables | De novo AHF (n = 1,417) | ADCHF (n = 1,352) | P value | ||
|---|---|---|---|---|---|
| ACEI or ARB | |||||
| ACEI at discharge | |||||
| Ramipril equivalent dose, mg | 2.9 ± 2.3 | 3.0 ± 2.3 | 0.65 | ||
| Titration to target dose, No. (%) | 32 (2.3) | 22 (1.6) | 0.23 | ||
| ARB at discharge | |||||
| Candesartan equivalent dose, mg | 11.3 ± 8.3 | 10.5 ± 7.1 | 0.09 | ||
| Titration to target dose, No. (%) | 44 (3.1) | 25 (1.8) | 0.03 | ||
| β-blocker at discharge | |||||
| Carvedilol equivalent dose, mg | 16.2 ± 15.9 | 16.0 ± 16.9 | 0.82 | ||
| Titration to target dose, No. (%) | 66 (4.7) | 51 (3.8) | 0.25 | ||
| MRA at discharge | |||||
| MRA dose, mg | 23.8 ± 13.3 | 24.2 ± 14.5 | 0.56 | ||
AHF = acute heart failure, ADCHF = acutely decompensated chronic heart failure, ACEI = angiotensin-converting enzyme inhibitor, ARB = angiotensin-receptor II blocker, MRA = mineralocorticoid receptor antagonists.
Adherence to guideline-directed therapy-outcome link in de novo AHF
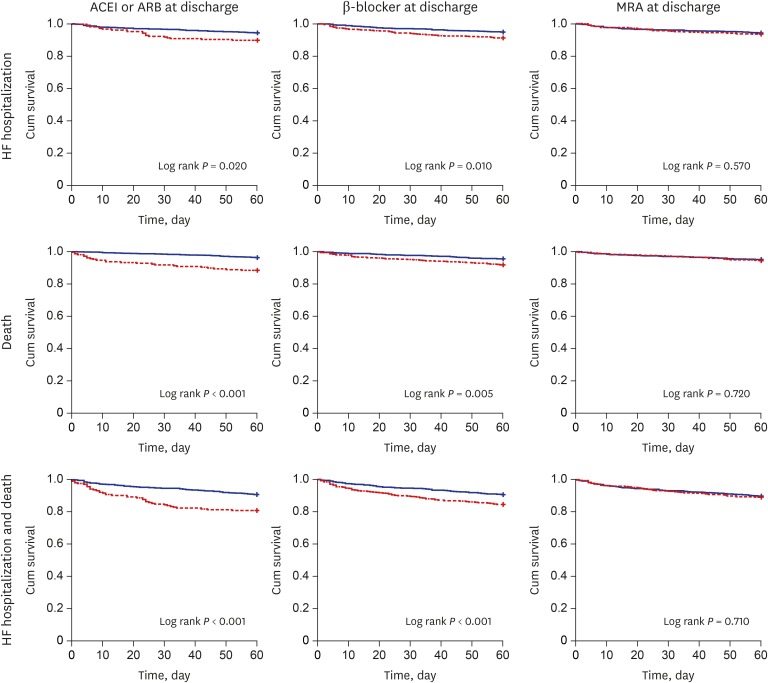
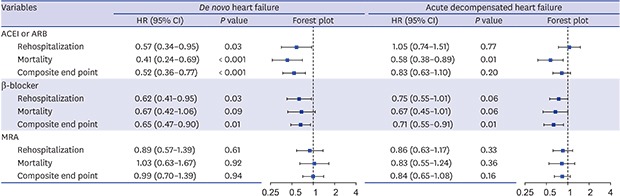
The 60-day event free survival curves for each guideline-directed therapy are illustrated in Fig. 2. After adjustment for clinical risk factors, ACEI or ARB at discharge significantly reduced re-hospitalization (HR, 0.57; 95% CI, 0.34–0.95), mortality (HR, 0.41; 95% CI, 0.24–0.69), and composite endpoint (HR, 0.52; 95% CI, 0.36–0.77) rates during the 6-month follow-up period. Beta-blockers had a protective effect against re-hospitalization (HR, 0.62; 95% CI, 0.41–0.95) and composite endpoint (HR, 0.65; 95% CI, 0.47–0.90), but the effect on mortality was not significant (HR, 0.67; 95% CI, 0.42–1.06). After multivariate adjustment, MRA was not associated with any of the endpoints (Table 3).
Fig. 2. 60-day event free survival according to the adherence of performance measures at discharge in de novo HF (blue line: with adherence, red line: without adherence).
ACEI = angiotensin-converting enzyme inhibitor, ARB = angiotensin receptor II blocker, MRA = mineralocorticoid receptor antagonists, HF = heart failure.
Table 3. Risk-adjusted performance measures-outcome link.
| Variables | De novo heart failure | Acute decompensated heart failure | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| ACEI or ARB | |||||
| Rehospitalization | 0.57 (0.34–0.95) | 0.03 | 1.05 (0.74–1.51) | 0.77 | |
| Mortality | 0.41 (0.24–0.69) | < 0.001 | 0.58 (0.38–0.89) | 0.01 | |
| Composite end point | 0.52 (0.36–0.77) | < 0.001 | 0.83 (0.63–1.10) | 0.20 | |
| β-blocker | |||||
| Rehospitalization | 0.62 (0.41–0.95) | 0.03 | 0.75 (0.55–1.01) | 0.06 | |
| Mortality | 0.67 (0.42–1.06) | 0.09 | 0.67 (0.45–1.01) | 0.06 | |
| Composite end point | 0.65 (0.47–0.90) | 0.01 | 0.71 (0.55–0.91) | 0.01 | |
| MRA | |||||
| Rehospitalization | 0.89 (0.57–1.39) | 0.61 | 0.86 (0.63–1.17) | 0.33 | |
| Mortality | 1.03 (0.63–1.67) | 0.92 | 0.83 (0.55–1.24) | 0.36 | |
| Composite end point | 0.99 (0.70–1.39) | 0.94 | 0.84 (0.65–1.08) | 0.16 | |
Multivariable Cox regression adjusted for gender, age, history of hypertension, diabetes mellitus, ischemic heart disease, chronic obstructive pulmonary disease, New York Heart Association functional class, systolic blood pressure, heart rate, creatinine, atrial fibrillation at admission and left ventricular ejection fraction.
HR = hazard ratio, CI = confidence interval, ACEI = angiotensin-converting enzyme inhibitor, ARB = angiotensin-receptor II blocker, MRA = mineralocorticoid receptor antagonists.
Adherence to guideline-directed therapy-outcome link in ADCHF
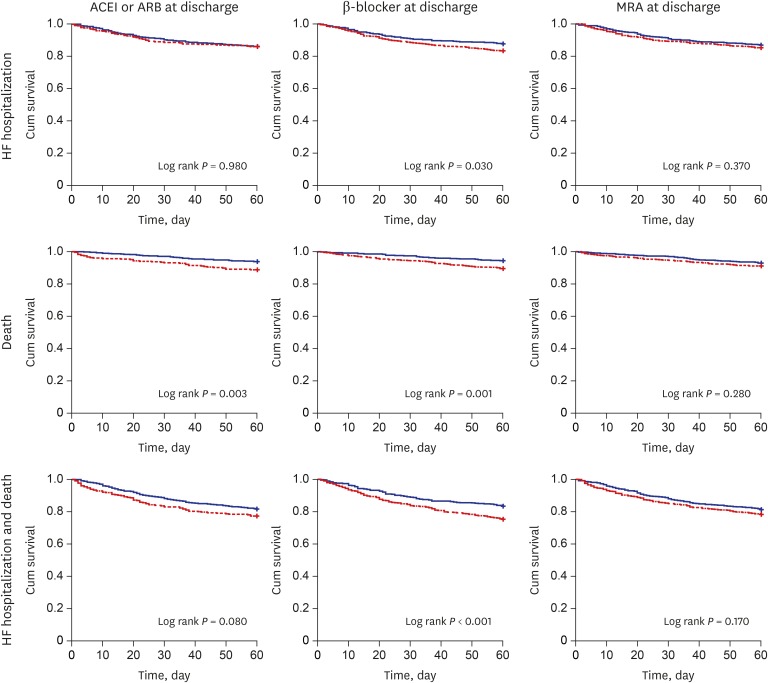
Fig. 3 shows the Kaplan-Meier curves for each outcome according to each guideline-directed therapy for 60 days after discharge in ADCHF. After multivariable adjustment, the use of ACEI or ARB at discharge was significantly associated with mortality (HR, 0.58; 95% CI, 0.38–0.89). β-blockers reduced the risk of 60-day composite endpoint (HR, 0.71; 95% CI, 0.55–0.91), but their protective effect on re-hospitalization (HR, 0.75; 95% CI, 0.55–1.01) and mortality (HR, 0.67; 95% CI, 0.45–1.01) did not reach statistical significance. There was no association between MRA and any of the endpoints (Table 3).
Fig. 3. 60-day event free survival according to the adherence of performance measures at discharge in ADCHF (blue line: with adherence, red line: without adherence).
ACEI = angiotensin-converting enzyme inhibitor, ARB = angiotensin receptor II blocker, MRA = mineralocorticoid receptor antagonists, ADCHF = acute decompensated chronic heart failure.
DISCUSSION
In this analysis, baseline characteristics of patients admitted due to AHF were significantly different according to the temporal course of heart failure. The guideline-recommended therapy at discharge, including ACEI/ARB or β-blockers, was associated with improvements in 60-day prognosis in patients with de novo AHF, except that β-blockers did not improve the 60-day mortality. In patients with ADCHF, ACEI/ARB was associated with mortality and β-blockers with composite endpoint. MRA had no effect on the prognosis in both types of HF.
De novo HF is different from ADCHF
AHF has two forms: newly arisen (“de novo”) acute HF and ADCHF according to the temporal course. The differences in baseline characteristics and prognoses in de novo AHF and ADCHF are well-established.17 In the EuroHeart Failure Survey (EHFS) II, patients with a previous history of HF had worse long-term prognoses than those with de novo AHF, and concomitant diseases are more common in patients with ADCHF.3 In concordance with a previous study, our study identified important differences in prognoses for different types of HF. Patients with a previous history of HF had worse long-term prognosis than those with de novo AHF. Importantly, concomitant diseases are more common in patients with ADCHF than those in patients with de novo AHF. Thus, ADCHF is associated with more severe symptoms, LV dysfunction, and worse prognosis than de novo HF, and these 2 conditions should be kept distinct.
Guideline-directed therapy in the time course of heart failure
It is well established that cardiac dysfunction is generally progressive even when there are no signs and symptoms of HF. The progression of heart failure is associated with left ventricular remodeling, which manifests as gradual increases in left ventricular end-diastolic and end-systolic volumes, wall thinning, and a change in chamber geometry. Due to continuous maladaptive remodeling, myocardial dysfunction is usually a progressive condition, where even mild initial dysfunction may develop into severe HF over a time course of months to years.18 Neurohormonal activation including the sympathetic nervous system and renin angiotensin system in HF is known to be a major mediator of the remodeling process.19 Therapeutic manipulation of these pathways with β-blockers, ACEIs, ARBs, and MRA has become the cornerstone of the management of HF. Consequently, early initiation of these neurohumoral pathway modulators may prevent or slow ventricular remodeling.20,21 In acute myocardial infarction, the mortality reduction effect of ACEIs early in the course of treatment is proved in a previous study. In a systematic overview of individual data from 96,712 acute myocardial infarction patients, 30-day mortality was 7.1% among patients allocated to early initiation of ACE Is and 7.6% among control subjects, corresponding to a 7% (SD, 2%) proportional reduction (95% CI, 2%–11%; P = 0.004).22 In the Carvedilol Post Infarction Survival Control in left ventricular dysfunction (CAPRICORN) study that enrolled patients with myocardial infarction occurring 3–21 days before randomization; all-cause mortality alone was lower in the carvedilol group than that in the placebo group (HR, 0.77; 95% CI, 0.60–0.98; P = 0·03).23 At 6 months, left ventricular end systolic volume was 9.2 mL less in the carvedilol group than that in the placebo group (P = 0.023), and left ventricular ejection fraction was 3.9% higher (P = 0.015).24
In HF, there were some data that support the efficacy, safety, and tolerability of beginning β blockers early in patients presenting with clinical signs and symptoms of HF.25,26,27 But those studies included patients with a diagnosis of chronic HF at least 3 months prior and did not include de novo HF. There have been few studies to evaluate the prognostic implications of guideline-directed therapy according to the temporal course of HF. In this study, the beneficial effect of guideline directed therapy was more pronounced in de novo HF. However, we could not identify the pathophysiologic explanation for the difference in prognoses according to the time course of HF. There are possible assumptions for the explanation. First, because early initiation of neurohumoral pathway modulators may prevent or slow ventricular remodeling, guideline-directed therapy in the early course of the disease has more noticeable prognostic implication. Second, the beneficial effect of neurohumoral modulators in ADCHF may simply reflect that the efficacy of therapy is modest once a patient undergoes decompensation while on chronic therapy.
The usefulness of aldosterone receptor antagonists in the setting of acute decompensated heart failure has not been determined. Current evidence from randomized aldactone evaluation study (RALES) supporting the use of aldosterone receptor antagonists is based on long-term clinical outcome data, but the acute effects of these agents are less established.28 In contrast to data from the RALES, there was conflicting results of the prognostic implications of MRA.8,9 ACCF/AHA/AMA-PCPI 2011 performance measures for adults with heart failure did not adapt MRA as performance measures.29 MRA was not associated with prognosis in both heart failure groups in this study.
Some limitations of our study merit emphasis. First, treatment options are entirely dependent on the attending physician in the KorAHF registry; so, selection bias may exist. Risk-treatment mismatch is present in the guideline-directed therapy. ADCHF is associated with increased comorbidity and deteriorated cardiac function; hence, patients with ADCHF were at increased risk of death. However, rates of treatment with guideline-directed therapy were low in high-risk patients. Second, the influence of background therapy and dose of each guideline-directed therapy were not analyzed because of the limited number of subjects. Third, because adherence to guidelines was defined irrespective of whether therapy was prescribed at discharge, the doses of therapy may not be reflected in this analysis. Fourth, HF duration may have influenced prognosis; however, we have no data regarding HF duration in patients with ADCHF. Finally, drug adherence during follow up period also may have an impact on the prognosis; however, this was not considered in the present study.
In conclusion, the 2 forms of HF are distinct with regard to baseline characteristics, comorbidity, and prognosis. The prognostic implications of adherence to guideline-directed therapy at discharge were more pronounced in de novo HF. We recommend that guideline-directed therapy be started as early as possible in the course of HFrEF.
Footnotes
Funding: This work was supported by Research of Korea Centers for Disease Control and Prevention (grant No. 2010-E63003-00, 2011-E63002-00, 2012-E63005-00, 2013-E63003-00, 2013-E63003-01, 2013-E63003-02, and 2016-ER6303-00).
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Yoo BS.
- Data curation: Youn YJ, Lee JW, Son JW.
- Formal analysis: Kim HS, Kang DR.
- Investigation: Lee SE, Cho HJ, Lee HY, Jeon ES, Kang SM, Choi DJ, Cho MC.
- Methodology: Kim JY, Ahn SG.
- Supervision: Yoon J, Lee SH.
- Writing - original draft: Ahn MS.
- Writing - review & editing: Yoo BS.
SUPPLEMENTARY MATERIALS
Clinical outcomes
Compliance rates for performance measures
References
- 1.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70(6):776–803. doi: 10.1016/j.jacc.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 2.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 3.Harjola VP, Follath F, Nieminen MS, Brutsaert D, Dickstein K, Drexler H, et al. Characteristics, outcomes, and predictors of mortality at 3 months and 1 year in patients hospitalized for acute heart failure. Eur J Heart Fail. 2010;12(3):239–248. doi: 10.1093/eurjhf/hfq002. [DOI] [PubMed] [Google Scholar]
- 4.Aranda JM, Jr, Johnson JW, Conti JB. Current trends in heart failure readmission rates: analysis of Medicare data. Clin Cardiol. 2009;32(1):47–52. doi: 10.1002/clc.20453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dharmarajan K, Hsieh AF, Lin Z, Bueno H, Ross JS, Horwitz LI, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309(4):355–363. doi: 10.1001/jama.2012.216476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kociol RD, Hammill BG, Fonarow GC, Klaskala W, Mills RM, Hernandez AF, et al. Generalizability and longitudinal outcomes of a national heart failure clinical registry: Comparison of Acute Decompensated Heart Failure National Registry (ADHERE) and non-ADHERE Medicare beneficiaries. Am Heart J. 2010;160(5):885–892. doi: 10.1016/j.ahj.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, et al. Association between performance measures and clinical outcomes for patients hospitalized with heart failure. JAMA. 2007;297(1):61–70. doi: 10.1001/jama.297.1.61. [DOI] [PubMed] [Google Scholar]
- 8.Youn YJ, Yoo BS, Lee JW, Kim JY, Han SW, Jeon ES, et al. Treatment performance measures affect clinical outcomes in patients with acute systolic heart failure: report from the Korean Heart Failure Registry. Circ J. 2012;76(5):1151–1158. doi: 10.1253/circj.cj-11-1093. [DOI] [PubMed] [Google Scholar]
- 9.Yoo BS, Oh J, Hong BK, Shin DH, Bae JH, Yang DH, et al. Survey of guideline adherence for treatment of systolic heart failure in real world (SUGAR): a multi-center, retrospective, observational study. PLoS One. 2014;9(1):e86596. doi: 10.1371/journal.pone.0086596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tavazzi L, Senni M, Metra M, Gorini M, Cacciatore G, Chinaglia A, et al. Multicenter prospective observational study on acute and chronic heart failure: one-year follow-up results of IN-HF (Italian Network on Heart Failure) outcome registry. Circ Heart Fail. 2013;6(3):473–481. doi: 10.1161/CIRCHEARTFAILURE.112.000161. [DOI] [PubMed] [Google Scholar]
- 11.Lee SE, Cho HJ, Lee HY, Yang HM, Choi JO, Jeon ES, et al. A multicentre cohort study of acute heart failure syndromes in Korea: rationale, design, and interim observations of the Korean Acute Heart Failure (KorAHF) registry. Eur J Heart Fail. 2014;16(6):700–708. doi: 10.1002/ejhf.91. [DOI] [PubMed] [Google Scholar]
- 12.Bonow RO, Bennett S, Casey DE, Jr, Ganiats TG, Hlatky MA, Konstam MA, et al. ACC/AHA clinical performance measures for adults with chronic heart failure: a report of the American College of Cardiology/American Heart Association Task Force on performance measures (writing committee to develop heart failure clinical performance measures): endorsed by the Heart Failure Society of America. Circulation. 2005;112(12):1853–1887. doi: 10.1161/CIRCULATIONAHA.105.170072. [DOI] [PubMed] [Google Scholar]
- 13.Bonow RO, Ganiats TG, Beam CT, Blake K, Casey DE, Jr, Goodlin SJ, et al. ACCF/AHA/AMA-PCPI 2011 performance measures for adults with heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on performance measures and the American Medical Association-Physician Consortium for performance improvement. Circulation. 2012;125(19):2382–2401. doi: 10.1161/CIR.0b013e3182507bec. [DOI] [PubMed] [Google Scholar]
- 14.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Rev Esp Cardiol (Engl Ed) 2016;69(12):1167. doi: 10.1016/j.rec.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Swedberg K, Cleland J, Dargie H, Drexler H, Follath F, Komajda M, et al. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): the Task Force for the diagnosis and treatment of chronic heart failure of the European Society of Cardiology. Eur Heart J. 2005;26(11):1115–1140. doi: 10.1093/eurheartj/ehi204. [DOI] [PubMed] [Google Scholar]
- 16.Belz GG. Angiotensin II dose-effect curves and Schild regression plots for characterization of different angiotensin II AT1 receptor antagonists in clinical pharmacology. Br J Clin Pharmacol. 2003;56(1):3–10. doi: 10.1046/j.1365-2125.2003.01880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greene SJ, Hernandez AF, Dunning A, Ambrosy AP, Armstrong PW, Butler J, et al. Hospitalization for recently diagnosed versus worsening chronic heart failure: from the ASCEND-HF trial. J Am Coll Cardiol. 2017;69(25):3029–3039. doi: 10.1016/j.jacc.2017.04.043. [DOI] [PubMed] [Google Scholar]
- 18.Katz AM. Cardiomyopathy of overload. A major determinant of prognosis in congestive heart failure. N Engl J Med. 1990;322(2):100–110. doi: 10.1056/NEJM199001113220206. [DOI] [PubMed] [Google Scholar]
- 19.Packer M. The neurohormonal hypothesis: a theory to explain the mechanism of disease progression in heart failure. J Am Coll Cardiol. 1992;20(1):248–254. doi: 10.1016/0735-1097(92)90167-l. [DOI] [PubMed] [Google Scholar]
- 20.ACE Inhibitor Myocardial Infarction Collaborative Group. Indications for ACE inhibitors in the early treatment of acute myocardial infarction: systematic overview of individual data from 100,000 patients in randomized trials. Circulation. 1998;97(22):2202–2212. doi: 10.1161/01.cir.97.22.2202. [DOI] [PubMed] [Google Scholar]
- 21.Doughty RN, Whalley GA, Walsh HA, Gamble GD, López-Sendón J, Sharpe N, et al. Effects of carvedilol on left ventricular remodeling after acute myocardial infarction: the CAPRICORN Echo Substudy. Circulation. 2004;109(2):201–206. doi: 10.1161/01.CIR.0000108928.25690.94. [DOI] [PubMed] [Google Scholar]
- 22.Latini R, Tognoni G, Maggioni AP, Baigent C, Braunwald E, Chen ZM, et al. Clinical effects of early angiotensin-converting enzyme inhibitor treatment for acute myocardial infarction are similar in the presence and absence of aspirin: systematic overview of individual data from 96,712 randomized patients. J Am Coll Cardiol. 2000;35(7):1801–1807. doi: 10.1016/s0735-1097(00)00638-0. [DOI] [PubMed] [Google Scholar]
- 23.Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet. 2001;357(9266):1385–1390. doi: 10.1016/s0140-6736(00)04560-8. [DOI] [PubMed] [Google Scholar]
- 24.Yaoita H, Sakabe A, Maehara K, Maruyama Y. Different effects of carvedilol, metoprolol, and propranolol on left ventricular remodeling after coronary stenosis or after permanent coronary occlusion in rats. Circulation. 2002;105(8):975–980. doi: 10.1161/hc0802.104503. [DOI] [PubMed] [Google Scholar]
- 25.Hjalmarson A, Goldstein S, Fagerberg B, Wedel H, Waagstein F, Kjekshus J, et al. Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF) JAMA. 2000;283(10):1295–1302. doi: 10.1001/jama.283.10.1295. [DOI] [PubMed] [Google Scholar]
- 26.CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353(9146):9–13. [PubMed] [Google Scholar]
- 27.Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med. 1996;334(21):1349–1355. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 28.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341(10):709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 29.Bonow RO, Ganiats TG, Beam CT, Blake K, Casey DE, Jr, Goodlin SJ, et al. ACCF/AHA/AMA-PCPI 2011 performance measures for adults with heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on performance measures and the American Medical Association-Physician Consortium for performance improvement. J Am Coll Cardiol. 2012;59(20):1812–1832. doi: 10.1016/j.jacc.2012.03.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical outcomes
Compliance rates for performance measures