Abstract
Background
Although guidelines to prevent surgical site infections (SSIs) were published more than a decade ago, prophylactic antibiotics are still used subjectively in clinical practice. In this study, we evaluated the safety of single-dose preoperative intravenous antibiotics without postoperative antibiotics in the field of clean wound surgery performed under local anesthesia. We also surveyed the present clinical conditions for prophylactic antibiotic use in the plastic surgery departments of training hospitals in Korea.
Methods
A total of 360 consecutive patients who underwent clean wound surgery under local anesthesia in an outpatient clinic from March 2018 to October 2018 were reviewed. In the study group, a single surgeon administered first-generation cephalosporins intravenously within 1 hour of skin incision and did not prescribe additional antibiotics. In the control group, 2 other surgeons prescribed oral first-generation cephalosporins postoperatively for 2 to 3 days without preoperative antibiotics. A telephone survey about perioperative antibiotic regimens was conducted at the departments of plastic surgery in training hospitals.
Results
There were 128 patients in the study group and 232 patients in the control group. There were no significant differences between the 2 groups regarding SSIs and other surgical complications. A total of 41 training hospitals answered the survey and every hospital had protocols of prescribing postoperative oral antibiotics routinely at the time of discharge with a mean duration of 3.9 days. Only 11 hospitals (26.8%) prescribed parenteral antibiotics before surgery as well as postoperative oral antibiotics.
Conclusion
Intravenous injection of single-dose first-generation cephalosporins 1 hour before surgery without postoperative antibiotics did not increase the incidence of SSIs compared with the usual practice of giving only postoperative antibiotics prescription for 2 to 3 days in cases of clean wound surgery performed under local anesthesia. Proper antibiotic prophylaxis should be performed by surgeons in training hospitals without hesitation.
Keywords: Antibiotics, Prophylaxis, Surgical Wound, Surgical Site Infection
Graphical Abstract
INTRODUCTION
Surgical site infection (SSI) refers to infections occurring in the incision site or deep tissues where surgery was performed within 30 days of surgery.1,2 According to the United States Centers for Disease Control National Nosocomial Infections Surveillance (CDC NNIS), SSI is the third most common cause of nosocomial infections, occurring in 14% to 16% of hospitalized patients and 38% of patients undergoing surgery.2,3 This is a burden on healthcare resources as well as a cause of mortality and morbidity. To prevent SSIs, the CDC published guidelines regarding the use of antimicrobial prophylaxis, which is as important as patient preparation and aseptic practice. These guidelines are as follows: first, administration should be started within the hour preceding skin incision; second, the use of first-generation cephalosporins alone is recommended; and third, antibiotic use should be discontinued within 24 hours after surgery.4 The goal of antimicrobial prophylaxis is not to sterilize tissues, but to reduce intraoperative contamination to a level that does not overwhelm the patient's defenses.2
However, in clinical practice, prophylactic antibiotics are still used subjectively by physicians. Although the rate of prophylactic antibiotic administration 1 hour before skin incision increased to 88.2% according to the 7th Evaluation of Prophylactic Antibiotic Adequacy of Surgery Conducted by the Korean Health Insurance Review and Assessment Agency (analysis of prophylactic antibiotic use from 768 institutions between September 2015 and November 2015), the antibiotic prescription rate at the time of discharge was 16.7% and the total duration of prophylactic antibiotic use was 4.1 days after surgery.
In our institution, we demonstrated the safety of reducing the duration of postoperative oral medication from 5 to 2 days for outpatients undergoing clean wound surgery under local anesthesia in 2010.5 Since then, continuous efforts have been made to follow the appropriate guidelines for adequate prophylactic antibiotic use, and some surgeons have discontinued the use of postoperative antibiotics in clean wound surgery. In this study, we reviewed patients who were treated with injection of preoperative antibiotics an hour before surgery and who did not receive postoperative antibiotic treatment. We also surveyed the present clinical conditions for prophylactic antibiotic use in the plastic surgery departments of training hospitals in Korea.
METHODS
Subjects
We reviewed consecutive outpatients who underwent surgery under local anesthesia from March 2018 to October 2018. Surgery was performed by 3 surgeons. The types of surgery included resection of benign or malignant skin tumors across the whole body, eye cosmetic surgery, secondary procedures performed after breast reconstruction, nipple reconstruction, and scar revision. Laser and filler procedures, which are generally not subject to antibiotic treatment, were excluded. Patients were limited to those aged ≥ 18 years, and patients with underlying chronic diseases were included in the study. Exclusion criteria were: 1) local and systemic infections, 2) patients currently receiving antibiotics, 3) patients with exposed implants, and 4) patients allergic to certain antibiotics.
Antibiotics
Patients were treated with different antibiotic regimens determined by surgeons and were divided into 2 groups. In the study group, patients were administered a first-generation cephalosporin (cefazolin, 1 g) intravenously within 1 hour before skin incision and did not receive additional postoperative antibiotics. Patients in the control group underwent clean wound surgery performed by 2 other surgeons. Preoperative intravenous antibiotics were not administered, and a first-generation cephalosporin (cephradine, 500 mg) was prescribed for 2 to 3 days as a postoperative oral antibiotic.
Skin preparation and postoperative management
Chlorhexidine gluconate was used for antiseptic skin preparation in all surgeries. After surgery, the patients were evaluated for presence of SSIs in outpatient clinics 3 days after surgery, at the time of stitch removal, which differed according to the surgical site, and 1 month after surgery.
Outcomes
A retrospective chart review was performed to collect patient demographic information and data associated with the operation, including surgical site, type of operation, and duration of operation. The results were evaluated and other data were recorded, including the occurrence of infection during the 30-day follow up period, complications, and antibiotic-associated side effects.
Surveys
Surveys were conducted through telephonic interviews with the resident or clinical instructor of each plastic surgery department at training hospitals in Korea. Questions concerned: 1) the use of prophylactic antibiotics before surgery, and if used, the type of antibiotic prescribed, and 2) the use of oral antibiotics after surgery, and if used, the type and duration of antibiotic prescribed.
Statistical analysis
Statistical analyses were performed using IBM SPSS version 21.0 (IBM Corp., Armonk, NY, USA). An independent-sample Student's t-test was used to compare patient characteristics between the study and control groups and a Fisher's exact test was used to compare postoperative complications. Statistical significance was defined as P < 0.05.
Ethics statement
The present study protocol was reviewed and approved by the Institutional Review Board of Asan Medical Center (2019-0353). The study was conducted after obtaining a waiver of informed consent from individual patients.
RESULTS
A total of 360 patients underwent surgery during the review period, with 128 patients in the study group and 232 patients in the control group. The mean age of the patients was 44.2 years in the study group and 49.8 years in the control group. There was no significant difference in body mass index between the 2 groups, and the number of patients with other comorbidities including hypertension, diabetes, and current smokers is listed in Table 1. The patients were classified according to surgical site, and the types of surgery included in this study were mass excision, upper and lower blepharoplasty, nipple-areola complex reconstruction, inverted nipple correction, skin banking excision after deep inferior epigastric artery perforator flap reconstruction, local flap coverage, scar revision, debridement, and secondary free fat injection (Table 2). The average operation time was 37.3 minutes in the study group and 46.3 minutes in the control group (P < 0.05).
Table 1. Patient demographics of patients who underwent clean wound surgery under local anesthesia.
| Variables | Study group (n = 128)a | Control group (n = 232)b | P value | |
|---|---|---|---|---|
| Age, yr | 44.2 ± 14.9 | 49.8 ± 13.2 | < 0.050 | |
| BMI | 23.0 ± 2.51 | 24.1 ± 2.48 | 0.104 | |
| Gender | 0.162 | |||
| Men | 28 (21.9) | 37 (15.9) | ||
| Women | 100 (78.1) | 195 (84.1) | ||
| Comorbidities | ||||
| Hypertension | 9 (7.0) | 18 (7.8) | 0.802 | |
| Diabetes | 7 (5.5) | 14 (6.0) | 0.826 | |
| Smoker, active | 3 (2.3) | 8 (3.4) | 0.753 | |
Data are presented as mean ± standard deviation or number (%).
BMI = body mass index.
aStudy group: first-generation cephalosporin was administered intravenously within 1 hour before skin incision; bControl group: first-generation cephalosporin was prescribed for 2 to 3 days as a postoperative oral antibiotic.
Table 2. Sites and type of the clean wound surgery in two groups.
| Variables | No. (%) | ||
|---|---|---|---|
| Study groupa | Control groupb | ||
| Sites | |||
| Face, ear, scalp | 36 (28.1) | 55 (23.7) | |
| Breast, NAC | 57 (44.5) | 136 (58.6) | |
| Abdomen, groin | 10 (7.8) | 15 (6.5) | |
| Back, hip | 12 (9.4) | 11 (4.7) | |
| Upper extremity | 10 (7.8) | 11 (4.7) | |
| Lower extremity | 3 (2.3) | 4 (1.7) | |
| Type | |||
| Excision | 40 (31.3) | 45 (19.4) | |
| Upper blepharoplasty | 12 (9.4) | 30 (12.9) | |
| Lower blepharoplasty | 3 (2.3) | 11 (4.7) | |
| NAC reconstruction | 47 (36.7) | 73 (31.5) | |
| Inverted nipple correction | 1 (0.8) | 2 (0.9) | |
| Skin banking excision | 0 (0.0) | 28 (12.1) | |
| Local flap coverage | 2 (1.6) | 7 (3.0) | |
| Scar revision | 6 (4.7) | 14 (6.0) | |
| Debridement | 13 (10.2) | 18 (7.8) | |
| Fat injection | 4 (3.1) | 4 (1.7) | |
| Total | 128 | 232 | |
NAC = nipple-areola complex.
aStudy group: first-generation cephalosporin was administered intravenously within 1 hour before skin incision; bControl group: first-generation cephalosporin was prescribed for 2 to 3 days as a postoperative oral antibiotic.
There were 2 patients with infection-related complications in the study group and 1 patient in the control group. Other complications unrelated to infections were wound dehiscence, partial skin necrosis, hematoma, and seroma. Complications related to antibiotics were not reported in the study group, but 4 patients complained of diarrhea after postoperative antibiotic use in the control group (Table 3). Diarrhea disappeared after discontinuation of antibiotics.
Table 3. Surgical and antibiotic-related complications after clean wound surgery in two groups.
| Variables | Study groupa | Control groupb | P value | |
|---|---|---|---|---|
| Surgical site infections | 2 | 1 | 0.289 | |
| Other surgical complications | ||||
| Wound dehiscence | 0 | 3 | 0.555 | |
| Partial skin necrosis | 4 | 1 | 0.056 | |
| Hematoma or seroma | 1 | 0 | 0.356 | |
| Antibiotic-related complications | ||||
| Diarrhea | 0 | 4 | 0.301 | |
| Nausea or vomiting | 0 | 0 | - | |
| Allergic reaction (skin rash, pruritus) | 0 | 0 | - | |
| Headache or dizziness | 0 | 0 | - | |
aStudy group: first-generation cephalosporin was administered intravenously within 1 hour before skin incision; bControl group: first-generation cephalosporin was prescribed for 2 to 3 days as a postoperative oral antibiotic.
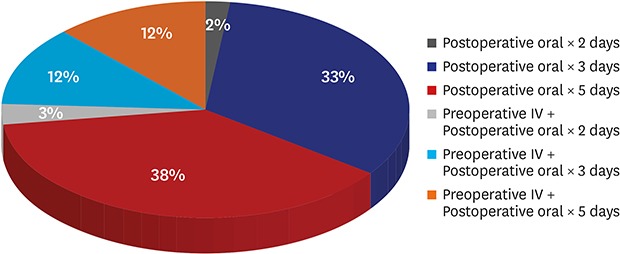
Results of the survey conducted on prophylactic antibiotic use in outpatient surgery under local anesthesia are shown in Fig. 1. Out of a total of 67 training hospitals, 41 were investigated. Preoperative parenteral antibiotics were administered in 11 hospitals (26.8%), of which 10 used first- and second-generation cephalosporins. Regardless of preoperative antibiotic use, all 41 hospitals prescribed postoperative oral antibiotics routinely at the time of discharge with a mean duration of 3.9 days (range, 2–5 days).
Fig. 1. Result of the survey concerning the use of perioperative antibiotics in clean wound surgery.
DISCUSSION
Although efforts have been made to decrease postoperative infectious morbidities, including improvement of operating room ventilation, sterilization methods, surgical technique, and availability of antibiotics since the mid-19th century, SSIs remain some of the most common nosocomial infections. SSIs are significant as they not only increase hospitalization frequency and costs but are also a cause of death, resulting in approximately 1 additional week of hospitalization; they also increase the risk of death by 2- to 11-fold compared to that in uninfected surgical patients.6 As a result, perioperative administration of antimicrobial agents is routinely performed after almost every surgery in hopes of preventing SSIs. However, the excessive use of antimicrobial agents has resulted in the emergence of multidrug-resistant pathogens, increased opportunistic infections, and other side effects.7,8,9 Many organizations and experts in infectious diseases have defined and recommended the appropriate use of antimicrobials in efforts to limit overuse.10,11 For the prevention of SSIs, the 2017 CDC Guidelines recommend the following: “In clean and clean-contaminated procedures, do not administer additional prophylactic antimicrobial agent doses after the surgical incision is closed in the operating room, even in the presence of a drain”.4 Similarly, according to the consensus of the Korean surgical infection prevention project, it is recommended that antibiotics should be discontinued within 24 hours after surgery.
Surgical wounds are classified into 4 categories: 1) clean, 2) clean-contaminated, 3) contaminated, and 4) dirty-infected wound. A clean wound refers to an uninfected operative wound in which no inflammation is encountered and no respiratory, alimentary, genital, or urinary tract is entered. A clean-contaminated wound is defined as an operative wound in which the respiratory, alimentary, genital, or urinary tracts are entered under controlled conditions and without unusual contamination. There are many reports regarding the use of prophylactic antibiotics in clean-contaminated wounds. In colorectal cancer surgery, discontinuation of prophylactic antibiotics within 24 hours after surgery had no significant influence on the incidence of SSIs.12 Similarly, in a study of donor nephrectomy patients, patients receiving single-dose antibiotics did not show significantly different SSI occurrence from that of patients receiving prophylactic antibiotics for 5 days after surgery.13 In addition, studies of intraoral bone graft surgery, urinary surgery, and hysterectomy have all confirmed the safety of single-dose antibiotics without additional medication.14,15,16
Most surgeries performed in the fields of plastic surgery, such as aesthetic surgery, tumor excision, and scar revision, are all clean wound operations. However, few studies have investigated the use of antimicrobial prophylaxis in these clean wound surgeries and despite the guidelines published for the prevention of SSIs, disagreements persist in clinical settings in the field of plastic surgery. According to a 6-year study by Baran et al.,17 there was no significant difference in infection rates between a group of patients treated with antibiotics and a group not treated with antibiotics. Anigian et al.18 also demonstrated that postoperative administration of antibiotics did not result in lower morbidity rates than preoperative administration alone, and Hunter et al.19 recommended the discontinuation of prophylactic antibiotics within 24 hours after surgery as part of the recommendations for prophylactic antibiotic use in plastic surgery. Conversely, there are several studies demonstrating the benefits of postoperative antibiotic use. A retrospective study of complication rates in inferior pedicle reduction mammaplasty revealed that the use of postoperative antibiotics for at least 5 days reduced wound dehiscence and improved breast scars.20 Another study in 2010 demonstrated a reduction in SSIs with the use of preoperative and postoperative antibiotics, although the respective roles of antibiotics administered postoperatively versus preoperatively could not be determined.21
Since 2008, our institution has tried to reduce the duration of postoperative antibiotic use. First, we reduced the duration of postoperative antibiotic prescription from 5 to 2 days in clean wound surgery rather than discontinuing entirely, considering the difficulty of wound monitoring in outpatients. Consequently, there were no significant differences in systemic or infection-related complications after this prescription change and no other infection-related complications have been reported in the last 10 years after clean wound surgeries performed under local anesthesia. Finally, we decided to use only intravenous first-generation cephalosporin antibiotics 1 hour before surgery without prescribing postoperative medications. Out of a total of 128 cases of clean wound surgery in this protocol, only 2 cases of infection-related complications were reported, including a case of excision of a mass on the left buttock of a 45-year-old man patient and a case of excision of epidermal cyst on the cheek of a 50-year-old man patient. The symptoms were relieved after oral administration of antibiotics for 5 days in both cases. There was also a case of SSIs in the control group and consequently there was no significant difference in SSI rates between the two groups. Other complications unrelated to infection did not show any differences between the 2 groups.
One may be concerned that the two groups in this study are too different to conclude which one is superior to the other. However, this study was not designed to determine the superior efficacy of single-dose preoperative intravenous antibiotic injection over postoperative oral administration but it was designed to emphasize the safety and effectiveness of established guidelines, which many surgeons are unconvinced of and are still hesitating to apply in practice, and to indicate the adverse effect resulting from the misuse of antibiotics. In result, 4 patients receiving oral antibiotics after surgery in the control group complained of diarrhea. Because of the limitation that this is a retrospective study, we expect that other patients also experienced gastrointestinal symptoms but did not report these, as such symptoms are common after antibiotic use and may have been expected by these patients. The incidence of gastrointestinal adverse events by oral cephalosporin agents are reported up to 21.0% for diarrhea and 5.0% for nausea/vomiting22 and these high incidences reflect the tendency of taking the symptoms for granted by the patients. Furthermore, the increasing incidence of Clostridium difficile colitis during the last decade has contributed to increased healthcare costs.23 One study even reported that C. difficile replaced methicillin-resistant Staphylococcus aureus as the most common cause of healthcare-associated infection.24 The reduction of antibiotic overuse is necessary to lower the incidence of C. difficile colitis and discontinuing prescription of oral antibiotics after clean wound surgery is one of the easiest ways of achieving this.
To evaluate the present conditions of perioperative antibiotic use in clean wound surgery in Korea, we questioned plastic surgeons in training hospitals nationwide regarding their perioperative antibiotic use protocols in cases of surgery under local anesthesia in outpatients. Only 11 (26.8%) of 41 hospitals prescribed preoperative antibiotic injections, although all of these 11 hospitals also prescribed postoperative antibiotic use for 2 to 5 days. The remaining 30 hospitals (73.2%) prescribed only postoperative antibiotics for an average of 3.9 days. Similarly, in the United States, a 2008 survey revealed that 74% of a total of 5,112 plastic surgeons, who were members of the American Society of Plastic Surgeons, prescribed postoperative antibiotics after breast reduction.25 The numbers from both surveys show the extent of perioperative antibiotic misuse by surgeons and the disregard of evidence-based guidelines. Furthermore, improper perioperative antibiotic usage is expected to be even more problematic in private clinics as a result of systemic and human factors.
The main reason for the overuse of prophylactic antibiotics is the human factor. Certain surgeons are not up to date with the latest knowledge and continue to follow outdated guidelines for antibiotic use. However, even surgeons who are aware of recent guidelines hesitate to change the discharge medication protocols because of their stability and reliability and a lack of experience of severe complications. Particularly in the field of plastic surgery, most patients are discharged on the same day after surgery under local anesthesia, and surgeons have difficulties monitoring the postoperative status of the surgical wound. As a result, they prefer to prescribe postoperative antibiotics without firm evidence in the hope of preventing SSIs, and there are some residents who hesitate to reduce the duration of postoperative antibiotic use even in our hospital. However, it is not only the surgeons but also the patients who are responsible for antibiotic overuse. Korean patients show a level of psychological dependence on antibiotics as revealed through complaints and requests for postoperative antibiotics if they are not readily prescribed. Therefore, efforts should be made not only with regard to surgeons but also to enlighten patients. Another reason is related to conditions and facilities in private clinics. Outdated air conditioning or positive pressure systems in operating rooms promote anxiety and insecurity among surgeons and render them dependent on antibiotics. Furthermore, insufficient manpower in hospitals leads to inadequate wound preparation and management before and after surgery. It is true that countermeasures against side effects and complications are more difficult than those of advanced 3rd general hospitals. As a result, surgeons in smaller-sized hospitals treat patients more conservatively.
To date, there is no single definite guideline for antibiotic prophylaxis especially in plastic surgery, and most plastic surgeons prescribe antibiotics according to their own standards. However, except for the cases involving implants, most surgeries performed under local anesthesia in outpatients are clean wound surgeries. Therefore, guidelines regarding clean wound surgery should be followed without further hesitation. There are only a few principles to remember in antimicrobial prophylaxis. First, the appropriate antimicrobial agent must be selected based on the knowledge of the expected flora. Second, the antibiotic should be administered before surgery to achieve effective concentrations at the time of incision in the target tissue. Third, adequate concentrations should be maintained until the end of the operation according to drug pharmacokinetics.4 According to Mangram et al.,2 S. aureus and coagulase-negative staphylococci are common causative strains of infection in the breast, whereas S. aureus, streptococci, and oropharyngeal anaerobes are more common in the head and neck. To neutralize these strains, first- or second-generation cephalosporins with high anti-staphylococcal activity, such as cefazolin, are the most suitable drugs for antibiotic prophylaxis. Consequently, preoperative intravenous injection of first- or second-generation cephalosporins 1 hour before surgery without additional postoperative antibiotics would be the correct prescription to achieve effective concentrations at the time of incision and maintain these until the end of the operation in clean wound surgery.
In conclusion, intravenous injection of a single-dose first-generation cephalosporin 1 hour before surgery without postoperative antibiotics did not increase the incidence of SSIs in cases of clean wound surgery performed under local anesthesia. However, most plastic surgery departments in Korean training hospitals are still hesitant to change their antibiotic prophylaxis protocols and discontinue prescribing postoperative oral antibiotics after clean wound surgery. Proper antibiotic prophylaxis must be applied, starting in training hospitals, to not only prevent SSIs and antibiotic-related complications but also to disseminate the proper guidelines to every other hospital and clinic.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Cha HG, Kim EK.
- Data curation: Eom JS, Han HH, Kim EK.
- Formal analysis: Cha HG, Kwon JG, Kim EK.
- Investigation: Cha HG, Kwon JG.
- Methodology: Cha HG, Eom JS, Han HH, Kim EK.
- Software: Cha HG, Kwon JG.
- Validation: Cha HG, Eom JS, Han HH, Kim EK.
- Writing - original draft: Cha HG, Kwon JG.
- Writing - review & editing: Cha HG, Kim EK.
References
- 1.Young PY, Khadaroo RG. Surgical site infections. Surg Clin North Am. 2014;94(6):1245–1264. doi: 10.1016/j.suc.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Guideline for prevention of surgical site infection, 1999. Am J Infect Control. 1999;27(2):97–132. [PubMed] [Google Scholar]
- 3.Emori TG, Gaynes RP. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin Microbiol Rev. 1993;6(4):428–442. doi: 10.1128/cmr.6.4.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berríos-Torres SI, Umscheid CA, Bratzler DW, Leas B, Stone EC, Kelz RR, et al. Centers for Disease Control and Prevention Guideline for the prevention of surgical site infection, 2017. JAMA Surg. 2017;152(8):784–791. doi: 10.1001/jamasurg.2017.0904. [DOI] [PubMed] [Google Scholar]
- 5.Kim EK, Jung IU, Choi JW, Eom JS, Hong JP, Lee TJ, et al. Appropriate administration of prophylactic antibiotics in clean operations: a preliminary report. J Korean Soc Aesthetic Plast Surg. 2010;16(1):41–44. [Google Scholar]
- 6.Kirkland KB, Briggs JP, Trivette SL, Wilkinson WE, Sexton DJ. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20(11):725–730. doi: 10.1086/501572. [DOI] [PubMed] [Google Scholar]
- 7.Harbarth S, Samore MH, Lichtenberg D, Carmeli Y. Prolonged antibiotic prophylaxis after cardiovascular surgery and its effect on surgical site infections and antimicrobial resistance. Circulation. 2000;101(25):2916–2921. doi: 10.1161/01.cir.101.25.2916. [DOI] [PubMed] [Google Scholar]
- 8.Burke JP. Maximizing appropriate antibiotic prophylaxis for surgical patients: an update from LDS Hospital, Salt Lake City. Clin Infect Dis. 2001;33(Suppl 2):S78–S83. doi: 10.1086/321861. [DOI] [PubMed] [Google Scholar]
- 9.Hecker MT, Aron DC, Patel NP, Lehmann MK, Donskey CJ. Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med. 2003;163(8):972–978. doi: 10.1001/archinte.163.8.972. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz B, Bell DM, Hughes JM. Preventing the emergence of antimicrobial resistance. A call for action by clinicians, public health officials, and patients. JAMA. 1997;278(11):944–945. doi: 10.1001/jama.278.11.944. [DOI] [PubMed] [Google Scholar]
- 11.Goldmann DA, Weinstein RA, Wenzel RP, Tablan OC, Duma RJ, Gaynes RP, et al. Strategies to prevent and control the emergence and spread of antimicrobial-resistant microorganisms in hospitals. A challenge to hospital leadership. JAMA. 1996;275(3):234–240. [PubMed] [Google Scholar]
- 12.Park YY, Kim CW, Park SJ, Lee KY, Lee JJ, Lee HO, et al. Influence of shorter duration of prophylactic antibiotic use on the incidence of surgical site infection following colorectal cancer surgery. Ann Coloproctol. 2015;31(6):235–242. doi: 10.3393/ac.2015.31.6.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jang HS, Choi KH, Yang SC, Han WK. A prospective study of single-dose antibiotic prophylaxis in live donor nephrectomy. Korean J Urol. 2011;52(2):115–118. doi: 10.4111/kju.2011.52.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindeboom JA, van den Akker HP. A prospective placebo-controlled double-blind trial of antibiotic prophylaxis in intraoral bone grafting procedures: a pilot study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96(6):669–672. doi: 10.1016/j.tripleo.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 15.Wolf JS, Jr, Bennett CJ, Dmochowski RR, Hollenbeck BK, Pearle MS, Schaeffer AJ, et al. Best practice policy statement on urologic surgery antimicrobial prophylaxis. J Urol. 2008;179(4):1379–1390. doi: 10.1016/j.juro.2008.01.068. [DOI] [PubMed] [Google Scholar]
- 16.Van Eyk N, van Schalkwyk J Infectious Diseases Committee. Antibiotic prophylaxis in gynaecologic procedures. J Obstet Gynaecol Can. 2012;34(4):382–391. doi: 10.1016/S1701-2163(16)35222-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baran CN, Sensöz O, Ulusoy MG. Prophylactic antibiotics in plastic and reconstructive surgery. Plast Reconstr Surg. 1999;103(6):1561–1566. doi: 10.1097/00006534-199905060-00002. [DOI] [PubMed] [Google Scholar]
- 18.Anigian KT, Miller T, Constantine RS, Farkas J, Cortez R, Hein R, et al. Effectiveness of prophylactic antibiotics in outpatient plastic surgery. Aesthet Surg J. 2014;34(8):1252–1258. doi: 10.1177/1090820X14545984. [DOI] [PubMed] [Google Scholar]
- 19.Hunter JG. Appropriate prophylactic antibiotic use in plastic surgery: the time has come. Plast Reconstr Surg. 2007;120(6):1732–1734. doi: 10.1097/01.prs.0000280567.18162.12. [DOI] [PubMed] [Google Scholar]
- 20.O'Grady KF, Thoma A, Dal Cin A. A comparison of complication rates in large and small inferior pedicle reduction mammaplasty. Plast Reconstr Surg. 2005;115(3):736–742. doi: 10.1097/01.prs.0000152428.43300.19. [DOI] [PubMed] [Google Scholar]
- 21.Veiga-Filho J, Veiga DF, Sabino-Neto M, Amorim MC, Novo NF, Ferreira LM. The role of antibiotics in reduction mammaplasty. Ann Plast Surg. 2010;65(2):144–146. doi: 10.1097/SAP.0b013e3181c47d88. [DOI] [PubMed] [Google Scholar]
- 22.Mitropoulos IF, Rotschafer JC, Rodvold KA. Adverse events associated with the use of oral cephalosporins/cephems. Diagn Microbiol Infect Dis. 2007;57(3) Suppl:67S–76S. doi: 10.1016/j.diagmicrobio.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Lessa FC, Gould CV, McDonald LC. Current status of Clostridium difficile infection epidemiology. Clin Infect Dis. 2012;55(Suppl 2):S65–S70. doi: 10.1093/cid/cis319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller BA, Chen LF, Sexton DJ, Anderson DJ. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect Control Hosp Epidemiol. 2011;32(4):387–390. doi: 10.1086/659156. [DOI] [PubMed] [Google Scholar]
- 25.Okoro SA, Barone C, Bohnenblust M, Wang HT. Breast reduction trend among plastic surgeons: a national survey. Plast Reconstr Surg. 2008;122(5):1312–1320. doi: 10.1097/PRS.0b013e3181881dd7. [DOI] [PubMed] [Google Scholar]