A mass spectrometry (MS) method that detects a serum disaccharide (DS) (MS-DS) was recently described for the diagnosis of invasive fungal infections (IFI). We carried out a European collaborative study to evaluate this assay.
KEYWORDS: invasive aspergillosis, invasive candidiasis, invasive fungal infection, mass spectrometry, mucormycosis, serological diagnosis
ABSTRACT
A mass spectrometry (MS) method that detects a serum disaccharide (DS) (MS-DS) was recently described for the diagnosis of invasive fungal infections (IFI). We carried out a European collaborative study to evaluate this assay. Patients with the following IFI were selected according to the availability of sera obtained at about the time that IFI was documented: invasive candidiasis (IC; n = 26 patients), invasive aspergillosis (IA; n = 19), and mucormycosis (MM; n = 23). Control sera originated from 20 neutropenic patients and 20 patients with bacteremia. MS-DS was carried out in blind manner for the diagnosis of IFI. A diagnosis of IC or IA was confirmed by detection of mannan (Man) or galactomannan (GM), respectively, associated with detection of (1,3)-β-d-glucan (BDG) in both infections. MM was detected by quantitative real-time PCR (qPCR). All tests discriminated sera from patients with IC from sera from control subjects with bacteremia (P ≤ 0.0009). For IC, the MS-DS sensitivity and specificity were 51% and 87%, respectively. MS-DS complemented the high specificity of Man monitoring. All tests discriminated sera from IA patients from sera from neutropenic controls (P ≤ 0.0009). For IA, MS-DS sensitivity and specificity were 64% and 95%, respectively. Only 13/36 serum samples from patients with MM were concordant by MS-DS and qPCR (6 were positive, and 7 were negative); 14 were positive by MS-DS alone. qPCR and MS-DS made a similar contribution to the diagnosis of MM. In patients undergoing long-term monitoring, the persistent circulation of serum disaccharide was observed, whereas DNA was detected only for a short period after initiation of treatment. MS-DS has an important role to play in the early diagnosis of IFI. Its panfungal nature and complementarity with other tests may justify its use in the management of IFI.
INTRODUCTION
Numerous reports have described the increasing problem of nosocomial invasive fungal infections (IFI) in immunocompromised patients (1, 2). This is due to the increased number of at-risk patients in parallel with progress in intensive care and/or hematology, leading to deeper or longer immunosuppression (3). In parallel, the spectrum of isolated fungi has shifted from well-known opportunistic pathogens with characterized virulence factors (4, 5) to other species rarely reported to be a cause of human infection (6–8). Disruptions in the microbial balance induced by antibacterial antibiotics favor fungal fitness (9). Antifungal therapy is generally prescribed in patients with persistent fever despite 3 days of antibiotic treatment (10). Diagnostic strategies involve conventional methods of isolation and identification of fungi from blood or sterile sites, imaging, and a panel of biological tests whose nature depends on the fungal agent suspected (11, 12). The few biological tests currently considered by physicians to be of diagnostic help do not involve changes in the host response related to fungal infections (e.g., antifungal antibodies [13] or cytokine profiling [14]) but consist of the detection of circulating fungal molecules, either glycans or DNA, in the patients' sera using tests developed with the advent of hybridoma technology (15, 16), discoveries in biochemical cascades (17), or PCR (18). Due to the poor sensitivity of conventional mycological methods (12, 19), infectious disease societies have produced different levels of recommendations for immunological tests for the diagnosis of invasive candidiasis (IC) and invasive aspergillosis (IA) (20, 21). These tests, which are considered specific but which sometimes lack sensitivity, were later complemented by recommendations for the more sensitive Fungitell test, which detects both IC and IA (22). Despite years of extensive research into the detection of fungal DNA, a consensus has been reached only for IA, while standardization is still in progress for IC and mucormycosis (MM) (23). For MM, a standardized PCR method would be of primary interest since, in contrast to IC, glycan detection tests are not available (24). Recently, the T2MR technology, combining DNA amplification and detection by magnetic resonance, has provided significant progress in terms of reducing the delay in the time to diagnosis in comparison with the time to diagnosis by blood cultures for IC (25). More recently, matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry (MS) has become essential in clinical mycology laboratories, providing a more rapid and accurate means of identification of fungal species isolated from patients (26).
We developed an approach based on the ability of MS to detect fungal molecules in patients’ sera (27). Subsequent work showed that this method, which allows the detection and relative quantification of a panfungal serum disaccharide (DS), gave results that were comparable to those of methods recommended for the diagnosis of IFI (28). Here, we describe the results of a European collaborative study carried out in a blind manner to validate the use of MS for DS (MS-DS) for the diagnosis of IFI.
(This study was presented in part at the Russian Scientific Conference on Medical Microbiology and Clinical Mycology, Saint Petersburg, Russia, 14 to 16 June 2016; at the 13th Annual Fungal Update meeting, St Bartholomew's Hospital, London, United Kingdom, 2 to 3 March 2018; and as an e-poster at the 20th Congress of the International Society for Human and Animal Mycology, Amsterdam, Netherlands, 30 June to 4 July 2018 [29].)
MATERIALS AND METHODS
Study design and participants.
Different centers provided sera from patients with IFI classified as proven or probable according to the European Organization for the Research and Treatment of Cancer (EORTC)/Mycoses Study Group (MSG) criteria (30). Patients with IC were selected at the University Hospital of Genoa and Catholic University of Sacred Heart, Rome, Italy. Controls for IC patients consisted of febrile patients who were admitted to the Emergency Department of the University Hospital of Lausanne, Lausanne, Switzerland, and in whom bacteremia was subsequently documented. Two cases of nocardiosis from Lille University Hospital were also included among the controls. Patients with IA were selected at the University Hospital of Strasbourg, Strasbourg, France. Controls consisted of neutropenic patients hospitalized at the University Hospital of Genoa. Patients with MM were selected at Saint Louis University Hospital, Paris, France, and Lille University Hospital, Lille, France.
Altogether 95 patients and 189 serum and plasma samples were selected. They were distributed as follows: 17 patients with IC (27 serum samples), 19 patients with IA (53 serum samples), 16 patients with MM (36 serum samples), 10 control neutropenic patients (20 serum samples), 20 patients (21 plasma samples) with bacterial infections, and 2 patients with Actinomycete infections (2 serum samples).
MS-DS detection.
Serum and plasma samples were frozen and sent on dry ice to the Laboratory of Clinical Mycology, Lille University Hospital, for processing as described previously (27). After a preanalytical step for the extraction and purification of oligosaccharides, spectra were recorded and analyzed using a 4800 MALDI-TOF/TOF analyzer (Applied Biosystems/MDS Sciex) at a fixed laser intensity within a 300- to 800-m/z range (UMR CNRS 8576, University of Lille).
Detection of circulating poly- and oligosaccharides and DNA in serum or plasma.
For the diagnosis of IC and IA, detection of mannan (Man) or galactomannan (GM) and (1,3)-β-d-glucan (BDG) was performed in the participating centers during routine patient screening or in the Lille Clinical Mycology Laboratory if they had not been tested previously.
BDG was measured using a Fungitell kit (Associates of Cape Cod Inc., Falmouth, MA, USA) following the manufacturer’s instructions. The recommended cutoff of 80 pg/ml was used to determine clinical relevance.
Measurement of serum Man and GM was performed using a Platelia Candida Ag Plus test and Platelia Aspergillus Ag test (Bio-Rad, Marnes la Coquette, France), respectively, according to the manufacturer's instructions. The recommended cutoffs of a concentration of 62.5 pg/ml and an index of ≥0.5, respectively, were used.
For the diagnosis of MM, quantitative real-time PCR (qPCR) was performed as described previously (24, 31).
Ethics statement.
No additional sampling was necessary in any center due to the retrospective nature of the study. In Lille, agreement for the establishment of a biological collection of IFI samples was obtained from the French Ministry of Education and Research under reference number DC2008-642. Institutional review board approval was granted by the Comité de Protection des Personnes Nord-Ouest IV, the ethical committee of the university hospital of Lille.
Statistical analysis.
GraphPad Prism (version 6) software was used to compare the distribution of biomarkers in the different groups with the Mann-Whitney two-tailed test and to generate receiver operating characteristic (ROC) curves, derive cutoffs, and construct graphs. A P value of <0.05 was considered statistically significant.
RESULTS
Invasive candidiasis.
(i) Study population. The origin of the IC patients, the delay to serum sampling in relation to the time of the first positive blood culture, and the Candida species isolated are shown in Table 1. The Candida species were representative of the usual epidemiology encountered in southern Europe, with a higher prevalence of Candida parapsilosis complex isolates. The control patients with bacteremia are listed in Table 2. These included the usual panel of patients with community-acquired bacterial infections and two cases of Nocardia infection.
TABLE 1.
Origin of sera from patients with IC, delay between serum sampling and time of first positive blood culture, and Candida species isolateda
| Patient no. (site) | Species isolated | Delay vs BC (days) | Man concn (pg/ml) | BDG concn (pg/ml) | MS-DS index |
|---|---|---|---|---|---|
| I1 (G) | Candida albicans | 2 | >500 | 688 | 2,000 |
| C. albicans | 5 | >500 | 1,528 | 550 | |
| C. albicans | 7 | >500 | 861 | 625 | |
| I2 (G) | C. albicans | 2 | 81 | 943 | 79 |
| C. albicans | 5 | 192 | 405 | 133 | |
| C. albicans | 7 | 79 | 286 | 200 | |
| I3 (G) | C. albicans | 2 | 69 | 155 | 400 |
| C. albicans | 7 | 30 | 108 | 526 | |
| C. albicans | 13 | 12 | 50 | 300 | |
| I4 (G) | C. albicans | 0 | >500 | 5,000 | 1,000 |
| C. albicans | 5 | >500 | 5,000 | 333 | |
| C. albicans | 7 | >500 | 5,000 | 285 | |
| I5 (G) | C. albicans | 1 | 441 | 407 | 133 |
| C. albicans | 6 | 22 | 222 | 110 | |
| C. albicans | 10 | 38 | 336 | 300 | |
| I6 (G) | C. albicans | −5 | 108 | 403 | 70 |
| C. albicans | 0 | 150 | 283 | 85 | |
| C. albicans | 4 | 232 | 2,528 | 500 | |
| I7 (G) | C. albicans | 2 | >500 | 850 | 350 |
| C. albicans | 10 | >500 | 367 | 667 | |
| I8 (G) | C. albicans | 1 | 41 | 158 | 238 |
| C. albicans | 7 | 39 | 58 | 151 | |
| I9 (R) | C. albicans | 1 | 4 | >500 | 1,000 |
| I10 (R) | C. albicans | 2 | 0 | >500 | 385 |
| I11 (R) | C. albicans | 1 | 3 | >500 | 175 |
| C. albicans | 0 | 3 | >500 | 435 | |
| I12 (R) | C. albicans | 1 | >500 | >500 | 159 |
| I13 (R) | C. albicans | 1 | 0 | >500 | 122 |
| I14 (R) | C. glabrata | 0 | 64 | >500 | 78 |
| I15 (G) | C. tropicalis | 3 | >500 | 159 | 110 |
| C. tropicalis | 7 | 18 | 189 | 357 | |
| C. tropicalis | 14 | 31 | 42 | 345 | |
| I16 (R) | C. tropicalis | 3 | >500 | >500 | 714 |
| I17 (G) | C. parapsilosis | 2 | 5 | 61 | 53 |
| C. parapsilosis | 5 | 0 | 245 | 172 | |
| C. parapsilosis | 6 | 82 | 7 | 238 | |
| I18 (G) | C. parapsilosis | 1 | 16 | 125 | 122 |
| C. parapsilosis | 4 | 14 | 159 | 400 | |
| C. parapsilosis | 14 | 34 | 70 | 667 | |
| I19 (G) | C. parapsilosis | 0 | 21 | 26 | 159 |
| C. parapsilosis | 4 | 18 | 77 | 65 | |
| I20 (G) | C. parapsilosis | −1 | 110 | 39 | 833 |
| C. parapsilosis | 3 | 6 | 20 | 450 | |
| I21 (R) | C. parapsilosis | 1 | 2 | >500 | 1,000 |
| I22 (R) | C. parapsilosis | 1 | NA | >500 | 109 |
| C. parapsilosis | 1 | NA | >500 | 106 | |
| I23 (R) | C. parapsilosis | 7 | 256 | >500 | 333 |
| I24 (R) | C. parapsilosis | 0 | 0 | >500 | 96 |
| I25 (R) | C. orthopsilosis | 1 | >500 | >500 | 769 |
| C. orthopsilosis | 1 | >500 | >500 | 588 | |
| C. orthopsilosis | 1 | >500 | >500 | 1,000 | |
| C. orthopsilosis | 1 | >500 | >500 | 625 | |
| I26 (G) | C. krusei | 3 | 12 | 327 | 357 |
| C. krusei | 7 | 10 | 385 | 344 | |
| C. krusei | 10 | 8 | 191 | 400 | |
| I27 (G) | C. krusei | 2 | 12 | 7 | 300 |
| C. krusei | 4 | 16 | 14 | 167 |
BC, time of first positive blood culture; BDG, (1,3)-β-d-glucan; G, Genoa, Italy; Man, mannan; MS-DS, mass spectrometry method for detection of a serum disaccharide; NA, not available; R, Rome, Italy. Bold characters correspond to positive values.
TABLE 2.
Origin of the control sera used in the MS-DS test for invasive candidiasisa
| Patient no. (site) | Reason(s) for hospitalization | Risk factor(s) for fungal infection | Species isolated | Delay vs BC (days) | Man concn (pg/ml) | BDG concn (pg/ml) | MS-DS index |
|---|---|---|---|---|---|---|---|
| S1 | Septic shock, infected kidney stone | Corticosteroids, panhypopituitarism (substitution) | Escherichia coli | −1 | 2 | 97 | 230 |
| S2 | Septic shock, urinary infection | MGUS, CRF, corticosteroids (unconfirmed Horton’s disease) | E. coli | 0 | 3 | 83 | 286 |
| S3 | Cholangitis | Corticosteroids, polymyalgia rheumatica | E. coli | 0 | 4 | 7 | 70 |
| S4 | Urosepsis | IDDM, PPI | E. coli | 0 | 1 | 7 | 65 |
| S5 | Urosepsis | CRF, chronic ulcers, gout | E. coli | 0 | 9 | 169 | 400 |
| S6 | Cholecystitis | PPI | E. coli | 0 | 3 | 29 | 122 |
| S7 | Urosepsis | CRF, corticosteroids, polymyalgia rheumatica, PPI | E. coli | 0 | 0 | 7 | 667 |
| S8 | Urosepsis | Indwelling urinary catheter | E. coli | 0 | 0 | 122 | 43 |
| S9 | Skin infection | CRF | Streptococcus agalactiae | 0 | 0 | 7 | 120 |
| S10 | Skin infection | None | Streptococcus dysgalactiae | 0 | 2 | 7 | 154 |
| S11 | Skin infection | None | S. dysgalactiae | 0 | 1 | 22 | 76 |
| S12 | Pneumococcal pneumonia | NIDDM | Streptococcus pneumoniae | 0 | 3 | 7 | 55 |
| S13 | Pneumonia | None | Streptococcus pyogenes | 0 | 4 | 7 | 500 |
| S14 | Plantar abscess | IDDM, metabolic syndrome | S. pyogenes | 0 | 0 | 7 | 115 |
| S15 | Wound infection | IDDM, diffuse vascular disease, PPI | Staphylococcus aureus | 0 | 4 | 280 | 238 |
| S16 | Endocarditis/spondylodiscitis | PPI | S. aureus | 4 | 2 | 7 | 53 |
| S17 | Pyelonephritis | IDDM | Klebsiella pneumoniae | 1 | 7 | 7 | 54 |
| S18 | Urosepsis | Indwelling urinary catheter | Proteus mirabilis | 2 | 3 | 77 | 65 |
| S19 | Stroke, dental abscess | Diffuse cerebrovascular disease, PPI | Parvimonas micra | 0 | 1 | 7 | 106 |
| S20 | Skin infection | IDDM, diffuse vascular disease | Corynebacterium spp. | 0 | 4 | 7 | 104 |
| S21 | Cholangitis | None | Enterococcus faecalis, Enterobacter cloacae, Pseudomonas aeruginosa | 0 | 2 | 7 | 115 |
| L1 | Pneumonia | Renal graft and leukemia | Nocardia nova | −2 | 0 | 35 | 72 |
| L2 | Skin infection and adenitis | Renal graft | N. nova | −5 | 0 | 2,336 | 131 |
BC, time of first positive blood culture; BDG, (1,3)-β-d-glucan; CRF, chronic renal failure; IDDM, insulin-dependent diabetes mellitus; L, Lille, France; Man, mannan; MGUS, monoclonal gammopathy of undetermined significance; MS-DS, mass spectrometry method for detection of a serum disaccharide; NNIDDM, non-insulin-dependent diabetes mellitus; PPI, proton pump inhibitor; S, Switzerland. Bold characters correspond to positive values.
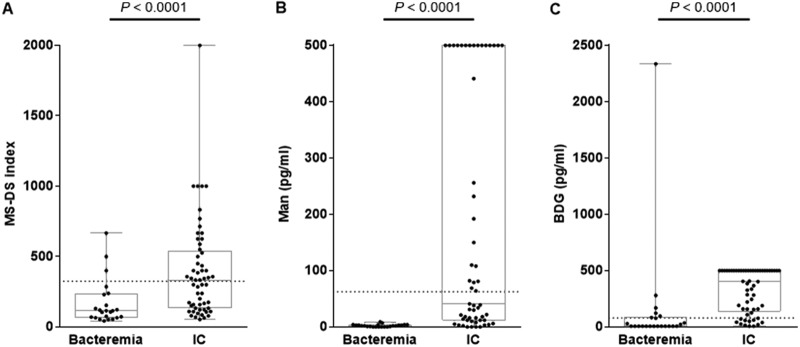
(ii) MS-DS diagnosis of IC in comparison to BDG and Man detection. The distribution of BDG and Man concentrations and MS-DS index values is shown in Fig. 1. All tests significantly discriminated IC patients from bacteremic controls (P < 0.0001).
FIG 1.
Distribution of MS-DS indexes (A), Man concentrations (B), and BDG concentrations (C) in patients with invasive candidiasis (IC) versus the controls. The results for each biomarker for the patient and control groups were compared using the Mann-Whitney test (significant at P ≤ 0.05). The dotted lines represent the cutoff values for each biomarker.
Among the 27 IC patients, 9 were positive by all three tests, 12 were positive by two tests (6 by BDG detection and MS-DS; 5 by BDG and Man detection; 1 by Man detection and MS-DS), and 4 were positive by BDG detection alone. Only two patients (patients I19 and I27) infected by C. parapsilosis and C. krusei were negative by all tests. When considering control patients with bacteremia, three were positive by MS-DS, whereas six were positive for BDG, including the patient with Nocardia infection who displayed very high glucan levels. Only one control (patient S5) was positive for two biomarkers (BDG and DS).
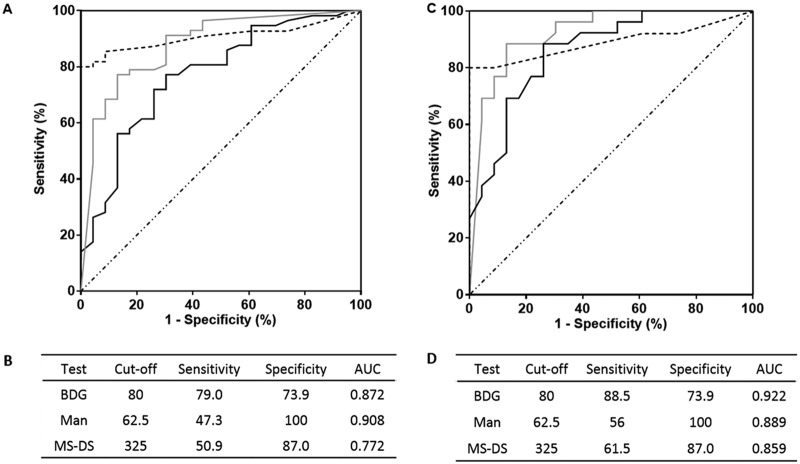
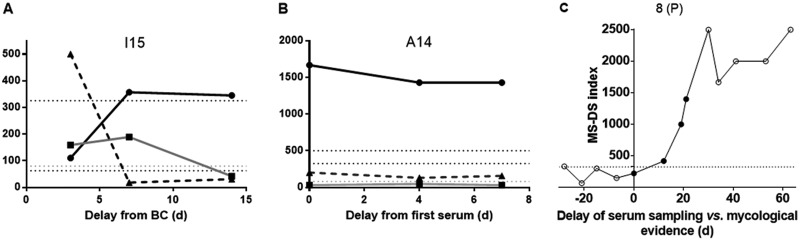
Figure 2 shows the ROC curves and corresponding sensitivities and specificities for the MS-DS, BDG, and Man detection tests. When considering the results for serum samples (Fig. 2A and B), application of the cutoff value of 325 for MS-DS showed a sensitivity of 51% and a specificity of 87%, which were intermediate values compared to those obtained by the BDG and Man tests. Analysis of the MS-DS index values for IC diagnosis was then performed for patients (Fig. 2C and D), and the sensitivity reached 67% without altering the high specificity estimated for serum. Comparison of ROC curves established for the MS-DS and the BDG and Man tests revealed that the diagnostic value of MS-DS was similar to that of the BDG test and positively complemented the high specificity of Man monitoring (revealed by the asymptotic curve). A lack of concordance between MS-DS and the BDG and Man tests was observed for serial serum samples from a given patient. This appeared to be more moderate when considering the global biomarker patterns per patient, since most patients (22/26) aggregated into two groups: those that displayed three positive test and those that displayed two positive tests. Figure 3A shows an example of biomarker kinetics during the time course of IC in one patient. Tests for BDG and Man were positive on day 1 and decreased on day 7, whereas MS-DS became positive at the end of monitoring. Due to the retrospective nature of the study, few serum samples were available per patient, but analysis of the whole IC patient population confirmed the transient nature of Man detection, in contrast to a slower decrease in glucanemia.
FIG 2.
(A, C) ROC curves for serum (A) and patients (C) for invasive candidiasis. Gray, dashed, and black lines, results for BDG detection, Man detection, and MS-DS, respectively. (B, D) Sensitivity/specificity values according to preestablished cutoff values for each biomarker for analysis of serum (B) and patients (D). AUC, area under the concentration-time curve.
FIG 3.
Examples of kinetics of serum biomarkers in patients with invasive candidiasis (IC) (A), invasive aspergillosis (IA) (B), and mucormycosis (MM) (C). Day 0 indicates the date of a positive fungal blood culture (BC) for IC or the first available serum sample for IA and the date of mycological evidence for MM. (A, B) Black circles and black solid line, MS-DS; black triangles and dashed line, Man or GM detection; black squares and gray line, BDG detection. Biomarker levels are indicated on the y axis with reference to BDG values (pg/ml), MS-DS index, and GM (index × 1,000). (C) Empty and full circles, negative and positive Mucorales qPCR results, respectively, for MM patients. Horizontal dotted lines indicate the cutoff values for BDG (80 pg/ml), Man (62.5 pg/ml), GM (index value, 0.5 · 1,000), and MS-DS (index value, 325). d, day.
Invasive aspergillosis.
(i) Study population. The characteristics of the IA patients, the level of certainty of a diagnosis of IA according to EORTC criteria, and the Aspergillus species isolated are shown in Table 3. Except for one patient with invasive sinusitis, all patients presented with invasive pulmonary aspergillosis. “Day 0” indicates the date of the first serum available in the collection relative to the episodes of IA defined according to clinical and radiological arguments.
TABLE 3.
Origins of sera from patients with invasive aspergillosis, Aspergillus species isolated, and level of evidence of Aspergillus infectiona
| Patient no. | Age (yr) | Sex | Underlying condition(s) | Species isolated | EORTC classification | Treatment | Outcome | GM in BAL fluid | Delay between serum sample collections (days) | BDG concn (pg/ml) | GM index in serum | MS-DS index |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | 44 | M | Heart transplant | Aspergillus fumigatus | Proven IPA | VCZ | Complete remission, alive at wk 12 | NA | 0 | 4,279 | >6 | 350 |
| 7 | 5,000 | 4.06 | 333 | |||||||||
| 17 | 4,362 | 2.63 | 555 | |||||||||
| 24 | 4,045 | 2.12 | 333 | |||||||||
| A2 | 56 | M | CLL with Richter transformation | NA | Probable IPA | VCZ | Progression, death on day 28 | Pos | 0 | 134 | 1.09 | 123 |
| 11 | 42 | 0.17 | 400 | |||||||||
| 16 | 98 | 0.16 | 714 | |||||||||
| A3 | 90 | M | AML | A. fumigatus | Probable IPA | VCZ | Progression, death on day 44 | NA | 0 | 7 | 0.09 | 113 |
| A4 | 63 | M | COPD, corticosteroids, diabetes mellitus | A. fumigatus | Probable IPA | VCZ | Complete remission, alive at wk 12 | NA | 0 | 1,643 | 1.59 | 58 |
| A5 | 35 | M | Lymphoma, MAS | NA | Probable IPA | VCZ | Progression, death on day 66 | NA | 0 | 60 | 0.97 | 90 |
| 3 | 79 | 0.55 | 76 | |||||||||
| A6 | 56 | F | Liver transplant | A. fumigatus | Probable IPA | VCZ | Complete remission, alive at wk 12 | NA | 0 | 489 | 2.30 | 3,333 |
| 8 | 235 | 0.87 | 1,250 | |||||||||
| 15 | 340 | 0.57 | 2,000 | |||||||||
| A7 | 59 | M | Liver transplant | Aspergillus nidulans | Probable IPA | VCZ and then VCZ + CAS | Progression, death on day 84 | Pos | 0 | 40 | 1.02 | 526 |
| 10 | 78 | 0.54 | 400 | |||||||||
| 21 | 64 | 0.34 | 526 | |||||||||
| A8 | 56 | M | ALL | NA | Probable IPA | VCZ | Progression, alive at wk 12 | Pos | 0 | 7 | 0.06 | 105 |
| 10 | 7 | 0.05 | 135 | |||||||||
| 19 | 35 | 0.05 | 72 | |||||||||
| A9 | 32 | M | Testicular cancer | NA | Probable IPA | VCZ and then L-AMB | Progression, death on day 71 | Pos | 0 | 73 | 1.37 | 1,250 |
| 4 | 102 | 0.67 | 1,667 | |||||||||
| 10 | 66 | 0.35 | 3,333 | |||||||||
| A10 | 53 | F | AML, Allo-HSCT | A. fumigatus | Proven IA sinusitis | L-AMB | Partial response, death on day 61 | NA | 0 | 7 | 0.55 | 87 |
| 3 | 36 | 0.98 | 625 | |||||||||
| 9 | 7 | 0.22 | 1,000 | |||||||||
| A11 | 54 | M | Heart and liver transplant | A. fumigatus | Probable IPA | VCZ and then L-AMB | Progression, death on day 82 | Pos | 0 | 949 | 0.44 | 714 |
| 14 | 1,999 | 0.51 | 333 | |||||||||
| 21 | 1,776 | 0.31 | 833 | |||||||||
| A12 | 26 | M | Lymphoma, HTLV-1, Allo-HSCT | NA | Probable IPA | VCZ | Stable, alive at wk 12 | NA | 0 | 208 | 3.32 | 1,429 |
| 3 | 454 | 0.56 | 5,000 | |||||||||
| 14 | 312 | 0.31 | 1,429 | |||||||||
| A13 | 62 | M | CMML, COPD | A. fumigatus | Probable IPA | VCZ and then VCZ + CAS | Partial response, alive at wk 12 | Pos | 0 | 238 | 0.13 | 127 |
| 11 | 195 | 0.07 | 78 | |||||||||
| 15 | 217 | 0.06 | 83 | |||||||||
| A14 | 47 | M | Posthepatitis liver fibrosis | A. fumigatus | Probable IPA | L-AMB | Progression, death on day 27 | Pos | 0 | 31 | 0.20 | 1,667 |
| 4 | 48 | 0.13 | 1,429 | |||||||||
| 7 | 30 | 0.16 | 1,429 | |||||||||
| A15 | 71 | M | Multiple myeloma | NA | Probable IPA | VCZ | Progression, death on day 54 | Pos | 0 | 7 | 0.08 | 333 |
| 3 | 7 | 0.08 | 400 | |||||||||
| 9 | 7 | 0.07 | 300 | |||||||||
| A16 | 60 | M | AML | NA | Probable IPA | VCZ | Progression, death on day 21 | Pos | 0 | 27 | 2.39 | 149 |
| 7 | 7 | 1.89 | 52 | |||||||||
| A17 | 51 | M | Lymphoma | NA | Probable IPA | VCZ and then L-AMB | Complete response, alive at wk 12 | NA | 0 | 12 | 0.73 | 61 |
| 4 | 7 | 0.11 | 109 | |||||||||
| 6 | 20 | 0.07 | 238 | |||||||||
| A18 | 73 | M | CLL | NA | Probable IPA | VCZ | Progression, death on day 31 | NA | 0 | 73 | 0.82 | 109 |
| 11 | 292 | >6 | 588 | |||||||||
| 24 | 111 | 3.08 | 625 | |||||||||
| A19 | 39 | F | Lymphoma | NA | Probable IPA | VCZ and then L-AMB | Progression, death on day 61 | NA | 0 | 3,030 | >6 | 714 |
| 8 | 517 | 3.34 | 2,000 | |||||||||
| 15 | 479 | 1.22 | 3,333 |
ALL, acute lymphoblastic leukemia; Allo-HSCT, allogeneic hematopoietic stem cell transplantation; AML, acute myeloid leukemia; BAL, bronchoalveolar lavage; BDG, (1,3)-β-d-glucan; CAS, caspofungin; CLL, chronic lymphoblastic leukemia; CMML, chronic myelomonocytic leukemia; COPD, chronic obstructive pulmonary disease; EORTC, European Organization for Research and Treatment of Cancer; F, female; GM, galactomannan; HTLV-1, human T cell leukemia virus type 1; IPA, invasive pulmonary aspergillosis; L-AMB, liposomal amphotericin B; M, male; MAS, macrophage activation syndrome; MS-DS, mass spectrometry method for detection of a serum disaccharide; NA, not available; Pos, positive; VCZ, voriconazole. Bold characters correspond to positive values.
The characteristics of the controls, consisting of neutropenic patients, are summarized in Table 4. Retrospective analysis of the clinical evolution of IA revealed that control patients 3, 6, and 7 developed probable IA 1, 2, and 6 months after serum sampling, respectively.
TABLE 4.
Origin of control sera used in MS-DS test for invasive aspergillosisa
| Patient no. | Age (yr) | Sex | Underlying condition(s) | Antifungal prophylaxis | Delay between serum sample collections (days) | BDG concn (pg/ml) | GM index in serum | MS-DS index |
|---|---|---|---|---|---|---|---|---|
| 1 | 50 | M | Neutropenia, myelofibrosis | Fluconazole | 0 | 7 | 0.07 | 68 |
| 3 | 7 | 0.08 | 86 | |||||
| 2 | 40 | M | Neutropenia, Hodgkin’s disease | Fluconazole | 0 | 7 | 0.09 | 147 |
| 4 | 7 | 0.06 | 143 | |||||
| 3 | 48 | F | Neutropenia, Allo-HSCT (ALL) | No | 0 | 7 | 0.18 | 200 |
| 5 | 7 | 0.21 | 81 | |||||
| 4 | 44 | F | Neutropenia/GVHD, Allo-HSCT (myelofibrosis) | Posaconazole | 0 | 7 | 0.04 | 161 |
| 1 | 7 | 0.04 | 120 | |||||
| 5 | 21 | F | Neutropenia, Allo-HSCT (AML) | Posaconazole | 0 | 7 | 0.05 | 66 |
| 7 | 7 | 0.05 | 39 | |||||
| 6 | 49 | M | Neutropenia, Allo-HSCT (myeloma) | Fluconazole | 0 | 7 | 0.10 | 400 |
| 3 | 7 | 0.06 | 200 | |||||
| 7 | 57 | F | Neutropenia/GVHD, Allo-HSCT (AML) | Fluconazole | 0 | 7 | 0.03 | 200 |
| 7 | 7 | 0.04 | 300 | |||||
| 8 | 75 | F | Neutropenia, AML | Fluconazole | 0 | 41 | 0.03 | 85 |
| 3 | 7 | 0.03 | 49 | |||||
| 9 | 36 | M | Neutropenia, AML | Posaconazole | 0 | 7 | 0.05 | 58 |
| 3 | 7 | 0.03 | 86 | |||||
| 10 | 52 | F | Neutropenia, AML | Posaconazole | 0 | 7 | 0.05 | 50 |
| 11 | 7 | 0.05 | 51 |
ALL, acute lymphoblastic leukemia; Allo-HSCT, allogeneic hematopoietic stem cell transplantation; AML, acute myeloid leukemia; BDG, (1,3)-β-d-glucan; F, female; GM, galactomannan; GVHD, graft-versus-host disease; M, male; MS-DS, mass spectrometry method for detection of a serum disaccharide. Bold characters correspond to positive values.
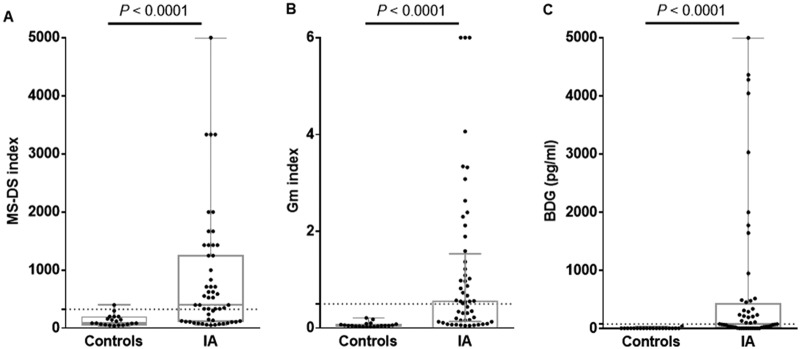
(ii) MS-DS diagnosis of IA in comparison to BDG and GM detection. The distribution of BDG concentrations and MS-DS index values is shown in Fig. 4. The values for the biomarkers were significantly higher in IA patients than in the controls (P ≤ 0.0001).
FIG 4.
Distribution of MS-DS indexes (A), GM indexes (B), and BDG concentrations (C) in patients with invasive aspergillosis (IA) and controls. The patient and control groups were compared using the Mann-Whitney test (significant at P ≤ 0.05). The dotted lines represent the cutoff values for each biomarker.
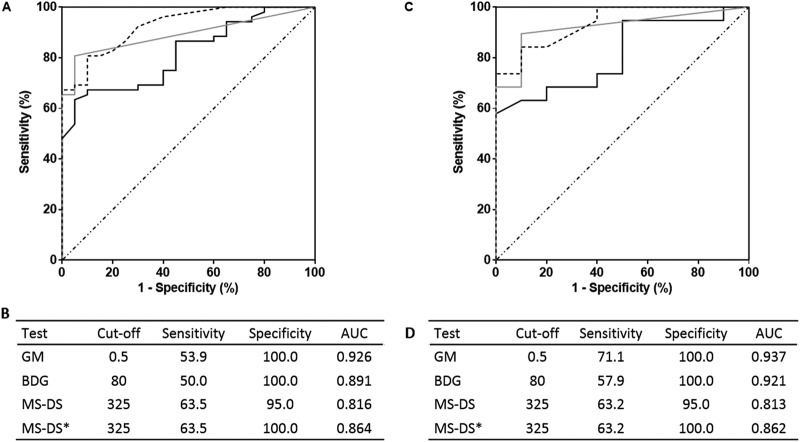
The sensitivity, specificity, and cutoff values for MS-DS and BDG detection were assessed by establishing ROC curves for serum, as shown in Fig. 5A and B. With a cutoff value of 325 for the MS-DS index, the sensitivity and specificity were 64% and 95%, respectively. The sensitivity values were intermediate between those for GM and BDG detection, which had 100% specificity. Interestingly, the 95% specificity of MS-DS was due to the high MS-DS index observed for control patient 6, who subsequently developed IA. In Fig. 5C and D, an analysis of MS-DS index values for IA diagnosis was performed for the patients, and no difference in terms of sensitivity and specificity was revealed with the data obtained for serum. A comparison of MS-DS and BDG detection ROC curves (according to the manufacturer’s recommended threshold) showed that the diagnostic value of MS-DS was better than that of BDG detection, with a maximum sensitivity of 64% for MS-DS and 50% for BDG detection.
FIG 5.
(A, C) ROC curves for serum (A) and patients (C) for invasive aspergillosis (IA). (B, D) Sensitivity/specificity values according to preestablished cutoff values for each biomarker for analysis of serum (B) and patients (D). MS-DS*, results obtained by exclusion of the control who developed IA 2 months later. Gray, dashed, and black lines, results for BDG detection, GM detection, and MS-DS, respectively.
Figure 3B shows an example of MS-DS and BDG detection kinetic evolution during GM monitoring. For this patient, only one serum sample was positive at the GM cutoff 14 days after the beginning of monitoring, while BDG levels were already positive on day 0, before increasing at unusually high levels. MS-DS was constantly positive during the whole survey, although it decreased at the time when BDG levels and GM index values were maximum.
Mucormycosis.
(i) Study population. The characteristics of patients and the level of certainty of a diagnosis of MM according to EORTC criteria are shown in Table 5. The genera/species involved were representative of the usual spectra of Mucorales isolated, as were the risk factors, infection sites, and high mortality.
TABLE 5.
Origin of the sera used in the MS-DS test for detection of mucormycosisa
| Patient no. (site) | Age (yr) | Sex | Underlying condition(s) | Site(s) of MM | Imaging result | Diagnostic sample | Histology/DE | Species | MM level of certainty, EORTC | Treatment | Outcome | Delay vs time of mycological evidence (days) | Cq value by PCR for Mucorales | BDG concn (pg/ml) | GM index in serum | MS-DS index |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (P) | 27 | M | Lymphoma | Lung | Bronchiectasis, ground glass | Sputum | NA | Syncephalastrum sp. culture | Probable | L-AMB | Death | −15 | Neg | NA | <0.5 | 3,333 |
| 0 | Neg | NA | <0.5 | 123 | ||||||||||||
| 7 | Neg | NA | <0.5 | 278 | ||||||||||||
| 2 (P) | 23 | F | AML | Lung | Nodule | Biopsy | Hyphae | Lichtheimia corymbifera PCR | Proven | L-AMB | Death | −3 | 40 | NA | <0.5 | 37 |
| 3 (P) | 18 | F | ALL | Liver | Nodule | Biopsy | Hyphae | Mucor sp. culture | Proven | L-AMB | Alive | 45 | Neg | NA | <0.5 | 3,333 |
| 51 | Neg | NA | <0.5 | 3,333 | ||||||||||||
| 4 (P) | 75 | M | Myelodysplasia | Rhinocerebral | Sinus, eye, and brain invasion | Conjunctival swab | Hyphae | Lichtheimia sp. culture | Proven | No | Death | −18 | Neg | NA | <0.5 | 213 |
| −14 | 40 | NA | <0.5 | 122 | ||||||||||||
| −7 | 37 | NA | <0.5 | 139 | ||||||||||||
| 5 (P) | 72 | M | AML | Lung | Nodule | Serum | NA | Rhizomucor sp. PCR | Possible | L-AMB | Death | 0 | 31 | NA | <0.5 | 213 |
| 6 (P) | 77 | F | ALL | Lung | Nodule | Serum | NA | Mucor sp. PCR | Possible | L-AMB | Alive | 30 | 39 | NA | <0.5 | 100 |
| 7 (P) | 19 | F | Aplastic Fanconi anemia | Lung and kidney | Nodule | Kidney biopsy | Hyphae | Rhizomucor sp. PCR | Proven | L-AMB | Death | 45 | 39 | NA | <0.5 | 123 |
| 8 (P) | 57 | M | AML | Liver | Nodule | Biopsy | Hyphae | Lichtheimia sp. culture | Proven | L-AMB | Death | −28 | Neg | NA | <0.5 | 333 |
| −21 | Neg | NA | <0.5 | 66 | ||||||||||||
| −15 | Neg | NA | <0.5 | 300 | ||||||||||||
| −7 | Neg | NA | <0.5 | 149 | ||||||||||||
| 0 | 35 | NA | <0.5 | 222 | ||||||||||||
| 12 | 31 | NA | <0.5 | 417 | ||||||||||||
| 19 | 35 | NA | <0.5 | 1,000 | ||||||||||||
| 21 | 39 | NA | <0.5 | 1,400 | ||||||||||||
| 30 | Neg | NA | <0.5 | 2,500 | ||||||||||||
| 34 | Neg | NA | <0.5 | 1,667 | ||||||||||||
| 41 | Neg | NA | <0.5 | 2,000 | ||||||||||||
| 53 | Neg | NA | <0.5 | 2,000 | ||||||||||||
| 63 | Neg | NA | <0.5 | 2,500 | ||||||||||||
| 9 (L) | 61 | F | Allo-HSCT | Lung | Nodule | Biopsy | Hyphae | Rhizopus microsporus culture | Proven | No | Death | −4 | 35 | 0 | 0.07 | 132 |
| 10 (L) | 66 | F | Burns, CML | Skin | NA | Swab | Neg | Lichtheimia ramosa culture | Possible | NS | Alive | −2 | Neg | 31 | NA | 132 |
| 5 | 37 | 18 | NA | 159 | ||||||||||||
| 11 (L) | 45 | F | Allo-HSCT GVHD | Postoperative abscess, abdominal wall (biopsy) | NA | Biopsy | Hyphae | Rhizopus arrhizus culture | Proven | Surgery, hyperbaric oxygen | Death | −9 | 33 | 42 | 0.15 | 300 |
| 6 | 34 | 46 | NA | 2,000 | ||||||||||||
| 12 (L) | 42 | M | Burns | Skin | NA | Biopsy | Hyphae | L. corymbifera culture | Proven | L-AMB, surgery | Alive | 24 | Neg | 21 | NA | 1,429 |
| 13 (L) | 83 | F | Lymphoma, rituximab, diabetes | Lung | Nodule | BAL fluid | Neg | R. microsporus culture | Probable | No | Death | −6 | 35 | 18 | 0.06 | 455 |
| 0 | 35 | 0 | 0.04 | 400 | ||||||||||||
| 14 (L) | 76 | M | Trauma | Skin | NA | Biopsy | Hyphae | Mucor circinelloides culture | Proven | Switch from POSA to L-AMB, surgery, hyperbaric oxygen | Alive | 16 | Neg | 39 | NA | 3,333 |
| 15 (L) | 60 | M | Trauma | Skin | NA | Biopsy | Hyphae | M. circinelloides culture | Proven | L-AMB, surgery | Alive | 5 | Neg | 106 | 0.06 | 3,333 |
| 16 (L) | 3 | F | ALL | Disseminated | Disseminated | Vitreous humor | Hyphae | Lichtheimia sp. PCR | Proven | ISA/L-AMB | Alive | −1 | Neg | 18 | 0.05 | 333 |
ALL, acute lymphoblastic leukemia; Allo-HSCT, allogeneic hematopoietic stem cell transplantation; AML, acute myeloid leukemia; BAL, bronchoalveolar lavage; BDG, (1,3)-β-d-glucan; CML, chronic myelogenous leukemia; Cq, quantification cycle; DE, direct examination; EORTC, European Organization for Research and Treatment of Cancer; F, female; GM, galactomannan; GVHD, graft-versus-host disease; ISA, isavuconazole; L-AMB, liposomal amphotericin B; L, Lille, France; M, male; MM, mucormycosis; MS-DS, mass spectrometry method for detection of a serum disaccharide; NA, not available; Neg, negative; NS, not specified; P, Paris, France; POSA, posaconazole. Bold characters correspond to positive values.
(ii) MS-DS in comparison with qPCR for diagnosis of MM. In contrast to IC and IA, no biochemical or immunological assays for the detection of circulating fungal poly- or oligosaccharides are available for the diagnosis of MM. We therefore compared MS-DS with qPCR, which is the only test currently available for the diagnosis of MM. The qPCR method used detects species from the genera Mucor, Rhizomucor, Lichtheimia, and Rhizopus.
We tested 36 serum samples from 16 patients (1 to 13 serum samples/patient) by MS-DS and qPCR. The results for only 13 serum samples were concordant by both methods (positive or negative). Among the 23 serum samples with discordant results, 13 were positive by MS-DS only and 10 were positive by qPCR only. Only three patients were positive by both tests. Remarkably, all the patients in this series could be diagnosed with MM by at least one test.
The distribution of MS-DS index values in relation to the date of IFI diagnosis and in comparison to the qPCR results is shown in Fig. 6. Among the serum samples tested before a mycological diagnosis was obtained, three (from three patients) were positive by MS-DS and six (from five patients) were positive by qPCR. An example of the kinetic evolution of MS-DS and qPCR in a patient from whom numerous samples were available is shown in Fig. 3C. A steady increase in MS-DS index values was observed during the 2 months of follow-up, whereas DNA circulation could be detected only between day 0 and day 20.
FIG 6.
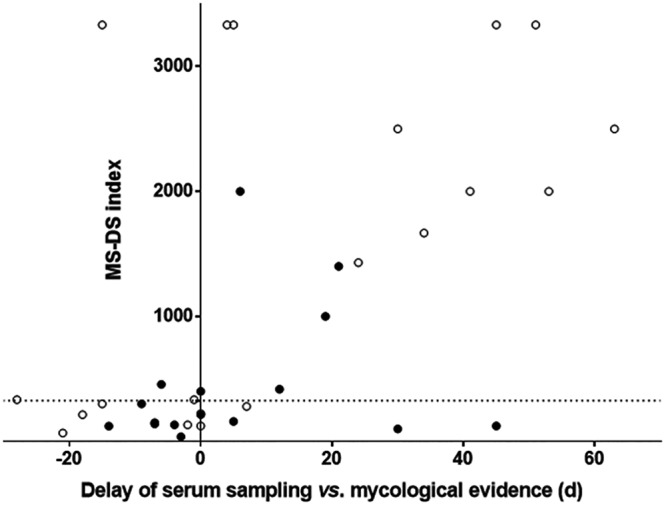
Distribution of DS in relation to the day of mycological diagnosis determined by MS-DS. Empty and full circles, negative and positive Mucorales qPCR results, respectively.
DISCUSSION
In contrast to diagnostic tests for obligate pathogens, whose detection is indicative of disease, diagnostic tests for opportunistic pathogens have to discriminate between the presence of microbes as endo- or exosaprophytes and their shift to a pathogen (19). This is a kinetic process where the assay has to present the best compromise between specificity and sensitivity over time from disease onset and its evolution to invasive infection. Currently, for IC and IA, only two types of tests have been shown to be clinically useful for disease management (6, 21, 32, 33). On the one hand, the Platelia Candida Ag Plus test and Platelia Aspergillus Ag test, which detect Man and GM, respectively, are considered specific but have a low sensitivity (especially for the detection of Man). On the other hand, the Fungitell test, which detects BDG, is considered more sensitive but less specific. The results of our previous study concerning MS-DS and those of the current study are in agreement with these conclusions. In both studies, MS-DS appeared to provide intermediate results, being more sensitive than the Platelia tests but more specific than the test for BDG (28). It is therefore suggested that MS-DS could be useful in the management of patients at risk of IC and IA.
For some other IFI, such as those caused by Mucorales, the lack of glucans in their cell wall and negativity for BDG in the context of host invasion have led to considerable efforts to develop alternative diagnostic methods; a specific PCR is now available (24). qPCR is of great help in the diagnosis of MM, whose emergence is worrying in terms of incidence and severity (34). MS-DS was previously shown to be positive during MM (28). In the present study, a comparison of the results of MS-DS with the results of qPCR showed a similar performance, confirming that a panfungal diagnostic assay is useful in daily practice when an IFI is suspected without mycological evidence. The reproducibility of the sensitivity values in mono- and multicenter studies confirms the robust character of the Platelia and BDG tests, in line with their extensive use worldwide for several decades. A similar reproducibility and, thus, robustness were observed for MS-DS. Additional information regarding MS-DS specificity concerned the absence of false positivity associated with Nocardia infection, in contrast to BDG detection, as reported previously (35) and as recently observed for one of the two patients included in this study. Conversely, among the three neutropenic patients who were included as IA controls and who were revealed to have subsequently developed IA, none were positive for either GM or BDG, while MS-DS index values were positive and above the limit of significance in two patients. Although these results could be considered a coincidence, the long delay before disease development should be considered with caution due to the possible subclinical character of DS circulation. Unpublished data described the structure and function of DS and showed that among the m/z 365 hex-disaccharide signals (27) is trehalose, an important fungal metabolite (36). What the present study, investigating four different IFI biomarkers in comparison with DS, makes particularly obvious is the different kinetics of their circulation, as shown in Fig. 3. From a pathophysiological point of view, the metabolite DS has kinetics of synthesis and release from fungal cells different from those of cell surface-associated Man and GM or BDG, which is thought to be deeply anchored in the cell wall. These fungal polysaccharides are synthesized in situ, and then different processes of degradation take place as a result of either fungal or host carbohydrate catabolism. In parallel, binding to host receptors, catabolism by host soluble enzymes, and circulation as immune complexes make the levels in the circulation completely different. The efficiency of human mannosidases, naturally present for degrading human glycoproteins, and the large amount of antimannan antibodies present in IC patients are responsible for the rapid clearance of mannan. In contrast, mammals are poorly equipped for degrading glucans, which are not self-components, and their poor immunogenicity does not help with their clearance, explaining their longer persistence than mannans (37, 38). Despite considerable efforts to solve the problems of DNA extraction and standardization, the lack of knowledge regarding the relationship between clinical outcome and Candida PCR results has prevented clinical recommendations (39). Recent progress has been made by the association of PCR with magnetic resonance detection in the T2MR system, leading to good specificity and increased sensitivity with regard to blood cultures for IC (25, 40). For MM and in the absence of other biomarkers, qPCR represents significant progress, as demonstrated by a large collaborative study showing its ability to confirm the diagnosis. Furthermore, the survival rate was significantly higher in patients with an initially positive PCR result that became negative after treatment initiation than in patients whose PCR result remained positive (41). In our study, Mucorales qPCR and MS-DS were complementary since all serum samples were positive by at least one test. Although the significance of DS persistence should be compared to a positive PCR result, these results emphasize the benefit of MS-DS in patient care, principally due to the combination of biomarkers whose kinetics of synthesis and release differ during the pathogenic processes. The panfungal characteristics of MS-DS adapted to the broad diversity of emerging fungal pathogens presents some advantages for first-line screening. This simple, robust physicochemically based technology is easily implementable in the majority of clinical mycology laboratories now equipped with MALDI-TOF MS and can be adapted to single tests or a large series (42). However, as the present collaborative study was retrospective, its promising results have to be confirmed through a prospective study. More generally, this method, which allows the early identification and quantification of fungal glycans, is in its infancy, and studies are in progress to explore its potential. In parallel, studies concerning the contribution of MS-DS complemented with currently recommended tests are exploring the possibility of improving antifungal stewardship based on a better knowledge of the diagnostic and prognostic significance of glycobiomarkers.
ACKNOWLEDGMENTS
We thank Val Hopwood for editing assistance.
This work was supported by internal funding from the Fonds d’Aide à l’Émergence et à l’Excellence du CHRU de Lille-Bonus H and by the Program Hospitalier de Recherche Clinique du Ministère des Affaires Sociales, de la Santé et de la Ville PHRC 1918, 2011, Candigène.
B.S. has received travel grants from Pfizer and MSD and a research grant from bioMérieux. M.M. has received speaker fees from Gilead, Pfizer, Biotest, Janssen, and MSD and a grant from Gilead, all of which are outside the present work. A.A. has received untied travel grants from MSD, Gilead, and Astellas and honoraria from Pathoquest and Gilead. C.V. has received research support to his institution from Pfizer and MSD and speaker and advisory board fees from Gilead, Pfizer, and MSD, all of which are outside the present work. R.H. reports personal fees from Astellas, Basilea, Gilead, and MSD and grants and personal fees from Pfizer, all of which are outside the present work. M.C., A.M., N.F., V.L.-B., E.D.C., L.D., M.T., P.-Y.B., M.S., Y.G., and D.P. declare no conflict of interest.
All authors have significantly contributed to this work. B.S., Y.G., and D.P. designed the MS-DS assay; M.C., B.S., and D.P. wrote the manuscript; A.M. and N.F. performed the assays; M.C. and A.M. performed the statistical analyses; M.C., M.M., V.L.-B., E.D.C., L.D., M.T., P.-Y.B., A.A., M.S., C.V., and R.H. collected the clinical and biological data; and all authors contributed to the data analyses. All authors approved the content of the final manuscript.
Footnotes
[This article was published on 26 April 2019 with Malgorzata Mikulska's surname incorrectly presented as "Mikulska Malgorzata" in the byline. The byline was updated in the current version, posted on 16 October 2019.]
REFERENCES
- 1.Colombo AL, de Almeida Junior JN, Slavin MA, Chen SC, Sorrell TC. 2017. Candida and invasive mould diseases in non-neutropenic critically ill patients and patients with haematological cancer. Lancet Infect Dis 17:e344–e356. doi: 10.1016/S1473-3099(17)30304-3. [DOI] [PubMed] [Google Scholar]
- 2.Kullberg BJ, Arendrup MC. 2015. Invasive candidiasis. N Engl J Med 373:1445–1456. doi: 10.1056/NEJMra1315399. [DOI] [PubMed] [Google Scholar]
- 3.Suleyman G, Alangaden GJ. 2016. Nosocomial fungal infections: epidemiology, infection control, and prevention. Infect Dis Clin North Am 30:1023–1052. doi: 10.1016/j.idc.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Poulain D. 2015. Candida albicans, plasticity and pathogenesis. Crit Rev Microbiol 41:208–217. doi: 10.3109/1040841X.2013.813904. [DOI] [PubMed] [Google Scholar]
- 5.van de Veerdonk FL, Gresnigt MS, Romani L, Netea MG, Latgé J-P. 2017. Aspergillus fumigatus morphology and dynamic host interactions. Nat Rev Microbiol 15:661–674. doi: 10.1038/nrmicro.2017.90. [DOI] [PubMed] [Google Scholar]
- 6.Arendrup MC, Boekhout T, Akova M, Meis JF, Cornely OA, Lortholary O, ESCMID EFISG Study Group, ECMM. 2014. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of rare invasive yeast infections. Clin Microbiol Infect 20:76–98. doi: 10.1111/1469-0691.12360. [DOI] [PubMed] [Google Scholar]
- 7.Caira M, Trecarichi EM, Mancinelli M, Leone G, Pagano L. 2011. Uncommon mold infections in hematological patients: epidemiology, diagnosis and treatment. Expert Rev Anti Infect Ther 9:881–892. doi: 10.1586/eri.11.66. [DOI] [PubMed] [Google Scholar]
- 8.Walsh TJ, Groll A, Hiemenz J, Fleming R, Roilides E, Anaissie E. 2004. Infections due to emerging and uncommon medically important fungal pathogens. Clin Microbiol Infect 10:48–66. doi: 10.1111/j.1470-9465.2004.00839.x. [DOI] [PubMed] [Google Scholar]
- 9.Romani L, Zelante T, Palmieri M, Napolioni V, Picciolini M, Velardi A, Aversa F, Puccetti P. 2015. The cross-talk between opportunistic fungi and the mammalian host via microbiota's metabolism. Semin Immunopathol 37:163–171. doi: 10.1007/s00281-014-0464-2. [DOI] [PubMed] [Google Scholar]
- 10.Cordonnier C, Pautas C, Maury S, Vekhoff A, Farhat H, Suarez F, Dhedin N, Isnard F, Ades L, Kuhnowski F, Foulet F, Kuentz M, Maison P, Bretagne S, Schwarzinger M. 2009. Empirical versus preemptive antifungal therapy for high-risk, febrile, neutropenic patients: a randomized, controlled trial. Clin Infect Dis 48:1042–1051. doi: 10.1086/597395. [DOI] [PubMed] [Google Scholar]
- 11.Clancy CJ, Nguyen MH. 2014. Undiagnosed invasive candidiasis: incorporating non-culture diagnostics into rational prophylactic and preemptive antifungal strategies. Expert Rev Anti Infect Ther 12:731–734. doi: 10.1586/14787210.2014.919853. [DOI] [PubMed] [Google Scholar]
- 12.Lamoth F, Calandra T. 2017. Early diagnosis of invasive mould infections and disease. J Antimicrob Chemother 72:i19–i28. doi: 10.1093/jac/dkx030. [DOI] [PubMed] [Google Scholar]
- 13.Clancy CJ, Nguyen ML, Cheng S, Huang H, Fan G, Jaber RA, Wingard JR, Cline C, Nguyen MH. 2008. Immunoglobulin G responses to a panel of Candida albicans antigens as accurate and early markers for the presence of systemic candidiasis. J Clin Microbiol 46:1647–1654. doi: 10.1128/JCM.02018-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krause R, Zollner-Schwetz I, Salzer HJ, Valentin T, Rabensteiner J, Pruller F, Raggam R, Meinitzer A, Prattes J, Rinner B, Strohmaier H, Quehenberger F, Strunk D, Heidrich K, Buzina W, Hoenigl M. 2015. Elevated levels of interleukin 17A and kynurenine in candidemic patients, compared with levels in noncandidemic patients in the intensive care unit and those in healthy controls. J Infect Dis 211:445–451. doi: 10.1093/infdis/jiu468. [DOI] [PubMed] [Google Scholar]
- 15.Fortier B, Hopwood V, Poulain D. 1988. Electric and chemical fusions for the production of monoclonal antibodies reacting with the in-vivo growth phase of Candida albicans. J Med Microbiol 27:239–245. doi: 10.1099/00222615-27-4-239. [DOI] [PubMed] [Google Scholar]
- 16.Stynen D, Sarfati J, Goris A, Prevost MC, Lesourd M, Kamphuis H, Darras V, Latge JP. 1992. Rat monoclonal antibodies against Aspergillus galactomannan. Infect Immun 60:2237–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagi N, Ohno N, Adachi Y, Aketagawa J, Tamura H, Shibata Y, Tanaka S, Yadomae T. 1993. Application of limulus test (G pathway) for the detection of different conformers of (1→3)-beta-d-glucans. Biol Pharm Bull 16:822–828. doi: 10.1248/bpb.16.822. [DOI] [PubMed] [Google Scholar]
- 18.Loffler J, Hebart H, Sepe S, Schumcher U, Klingebiel T, Einsele H. 1998. Detection of PCR-amplified fungal DNA by using a PCR-ELISA system. Med Mycol 36:275–279. doi: 10.1080/02681219880000441. [DOI] [PubMed] [Google Scholar]
- 19.Lass-Florl C. 2017. Current challenges in the diagnosis of fungal infections. Methods Mol Biol 1508:3–15. doi: 10.1007/978-1-4939-6515-1_1. [DOI] [PubMed] [Google Scholar]
- 20.Ullmann AJ, Akova M, Herbrecht R, Viscoli C, Arendrup MC, Arikan-Akdagli S, Bassetti M, Bille J, Calandra T, Castagnola E, Cornely OA, Donnelly JP, Garbino J, Groll AH, Hope WW, Jensen HE, Kullberg BJ, Lass-Florl C, Lortholary O, Meersseman W, Petrikkos G, Richardson MD, Roilides E, Verweij PE, Cuenca-Estrella M, ESCMID Fungal Infection Study Group (EFISG). 2012. ESCMID guideline for the diagnosis and management of Candida diseases 2012: adults with haematological malignancies and after haematopoietic stem cell transplantation (HCT). Clin Microbiol Infect 18:53–67. doi: 10.1111/1469-0691.12041. [DOI] [PubMed] [Google Scholar]
- 21.Ullmann AJ, Aguado JM, Arikan-Akdagli S, Denning DW, Groll AH, Lagrou K, Lass-Florl C, Lewis RE, Munoz P, Verweij PE, Warris A, Ader F, Akova M, Arendrup MC, Barnes RA, Beigelman-Aubry C, Blot S, Bouza E, Bruggemann RJM, Buchheidt D, Cadranel J, Castagnola E, Chakrabarti A, Cuenca-Estrella M, Dimopoulos G, Fortun J, Gangneux JP, Garbino J, Heinz WJ, Herbrecht R, Heussel CP, Kibbler CC, Klimko N, Kullberg BJ, Lange C, Lehrnbecher T, Loffler J, Lortholary O, Maertens J, Marchetti O, Meis JF, Pagano L, Ribaud P, Richardson M, Roilides E, Ruhnke M, Sanguinetti M, Sheppard DC, Sinko J, Skiada A. 2018. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect 24:e1–e38. doi: 10.1016/j.cmi.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Lamoth F, Cruciani M, Mengoli C, Castagnola E, Lortholary O, Richardson M, Marchetti O, Third European Conference on Infections in Leukemia (ECIL-3). 2012. β-Glucan antigenemia assay for the diagnosis of invasive fungal infections in patients with hematological malignancies: a systematic review and meta-analysis of cohort studies from the Third European Conference on Infections in Leukemia (ECIL-3). Clin Infect Dis 54:633–643. doi: 10.1093/cid/cir897. [DOI] [PubMed] [Google Scholar]
- 23.Barnes RA, White PL, Morton CO, Rogers TR, Cruciani M, Loeffler J, Donnelly JP. 2018. Diagnosis of aspergillosis by PCR: clinical considerations and technical tips. Med Mycol 56:60–72. doi: 10.1093/mmy/myx091. [DOI] [PubMed] [Google Scholar]
- 24.Millon L, Larosa F, Lepiller Q, Legrand F, Rocchi S, Daguindau E, Scherer E, Bellanger AP, Leroy J, Grenouillet F. 2013. Quantitative polymerase chain reaction detection of circulating DNA in serum for early diagnosis of mucormycosis in immunocompromised patients. Clin Infect Dis 56:e95–e101. doi: 10.1093/cid/cit094. [DOI] [PubMed] [Google Scholar]
- 25.Clancy CJ, Nguyen MH. 2018. T2 magnetic resonance for the diagnosis of bloodstream infections: charting a path forward. J Antimicrob Chemother 73:iv2–iv5. doi: 10.1093/jac/dky050. [DOI] [PubMed] [Google Scholar]
- 26.Wattal C, Oberoi JK, Goel N, Raveendran R, Khanna S. 2017. Matrix-assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF MS) for rapid identification of micro-organisms in the routine clinical microbiology laboratory. Eur J Clin Microbiol Infect Dis 36:807–812. doi: 10.1007/s10096-016-2864-9. [DOI] [PubMed] [Google Scholar]
- 27.Sendid B, Poissy J, Francois N, Mery A, Courtecuisse S, Krzewinski F, Jawhara S, Guerardel Y, Poulain D. 2015. Preliminary evidence for a serum disaccharide signature of invasive Candida albicans infection detected by MALDI mass spectrometry. Clin Microbiol Infect 21:88.e1–88.e6. doi: 10.1016/j.cmi.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 28.Mery A, Sendid B, Francois N, Cornu M, Poissy J, Guerardel Y, Poulain D. 2016. Application of mass spectrometry technology to early diagnosis of invasive fungal infections. J Clin Microbiol 54:2786–2797. doi: 10.1128/JCM.01655-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cornu M, Sendid B, Mery A, François N, Malgorzata M, Letscher-Bru V, De Carolis E, Damonti L, Titecat M, Bochud PY, Alanio A, Sanguinetti M, Viscoli C, Herbrecht R, Guerardel Y, Poulain D. 2018. Diagnosis of invasive fungal infections through detection of a circulating pan fungal disaccharide by mass spectrometry. A European multicentre study, poster PP2.171 Abstr 20th Congr Int Soc Hum Anim Mycol, Amsterdam, The Netherlands. [Google Scholar]
- 30.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Munoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE, European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group, National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Legrand M, Gits-Muselli M, Boutin L, Garcia-Hermoso D, Maurel V, Soussi S, Benyamina M, Ferry A, Chaussard M, Hamane S, Denis B, Touratier S, Guigue N, Frealle E, Jeanne M, Shaal JV, Soler C, Mimoun M, Chaouat M, Lafaurie M, Mebazaa A, Bretagne S, Alanio A. 2016. Detection of circulating Mucorales DNA in critically ill burn patients: preliminary report of a screening strategy for early diagnosis and treatment. Clin Infect Dis 63:1312–1317. doi: 10.1093/cid/ciw563. [DOI] [PubMed] [Google Scholar]
- 32.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. 2016. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 62:e1–e50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patterson TF, Thompson GR, Denning DW, Fishman JA, Hadley S, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Nguyen MH, Segal BH, Steinbach WJ, Stevens DA, Walsh TJ, Wingard JR, Young J-AH, Bennett JE. 2016. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 63:e1–e60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Danion F, Aguilar C, Catherinot E, Alanio A, DeWolf S, Lortholary O, Lanternier F. 2015. Mucormycosis: new developments into a persistently devastating infection. Semin Respir Crit Care Med 36:692–705. doi: 10.1055/s-0035-1562896. [DOI] [PubMed] [Google Scholar]
- 35.Sawai T, Nakao T, Yamaguchi S, Yoshioka S, Matsuo N, Suyama N, Yanagihara K, Mukae H. 2017. Detection of high serum levels of beta-d-glucan in disseminated nocardial infection: a case report. BMC Infect Dis 17:272. doi: 10.1186/s12879-017-2370-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perfect JR, Tenor JL, Miao Y, Brennan RG. 2017. Trehalose pathway as an antifungal target. Virulence 8:143–149. doi: 10.1080/21505594.2016.1195529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lefort A, Chartier L, Sendid B, Wolff M, Mainardi JL, Podglajen I, Desnos-Ollivier M, Fontanet A, Bretagne S, Lortholary O, French Mycosis Study Group. 2012. Diagnosis, management and outcome of Candida endocarditis. Clin Microbiol Infect 18:E99–E109. doi: 10.1111/j.1469-0691.2012.03764.x. [DOI] [PubMed] [Google Scholar]
- 38.Jaijakul S, Vazquez JA, Swanson RN, Ostrosky-Zeichner L. 2012. (1,3)-β-d-Glucan as a prognostic marker of treatment response in invasive candidiasis. Clin Infect Dis 55:521–526. doi: 10.1093/cid/cis456. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen MH, Clancy CJ. 2018. PCR-based methods for the diagnosis of invasive candidiasis: are they ready for use in the clinic? Curr Fungal Infect Rep 12:71–77. doi: 10.1007/s12281-018-0313-1. [DOI] [Google Scholar]
- 40.Mylonakis E, Zacharioudakis IM, Clancy CJ, Nguyen MH, Pappas PG. 2018. Efficacy of T2 magnetic resonance assay in monitoring candidemia after initiation of antifungal therapy: the Serial Therapeutic and Antifungal Monitoring Protocol (STAMP) trial. J Clin Microbiol 56:e01756-17. doi: 10.1128/JCM.01756-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Millon L, Herbrecht R, Grenouillet F, Morio F, Alanio A, Letscher-Bru V, Cassaing S, Chouaki T, Kauffmann-Lacroix C, Poirier P, Toubas D, Augereau O, Rocchi S, Garcia-Hermoso D, Bretagne S, French Mycosis Study Group. 2016. Early diagnosis and monitoring of mucormycosis by detection of circulating DNA in serum: retrospective analysis of 44 cases collected through the French Surveillance Network of Invasive Fungal Infections (RESSIF). Clin Microbiol Infect 22:810.e1–810.e8. doi: 10.1016/j.cmi.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 42.van Belkum A, Welker M, Pincus D, Charrier JP, Girard V. 2017. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry in clinical microbiology: what are the current issues? Ann Lab Med 37:475–483. doi: 10.3343/alm.2017.37.6.475. [DOI] [PMC free article] [PubMed] [Google Scholar]