Mayaro virus (MAYV) is a neglected arthropod-borne virus (arbovirus) antigenically clustered into the Semliki Forest complex group of Alphavirus genus (Togaviridae family), maintained in an unclear zoonotic cycle involving mosquitoes from Haemagogus genus as the main vector. The genome is composed of a positive single-stranded RNA of 11.5 kb in length, which contains two genes that encode four nonstructural (nsP1 to nsP4) and five structural (C, E3, E2, 6K, and E1) proteins.
KEYWORDS: envelope protein 2, MAYV, diagnostics, serology
ABSTRACT
Mayaro virus (MAYV) is a neglected arthropod-borne virus (arbovirus) antigenically clustered into the Semliki Forest complex group of Alphavirus genus (Togaviridae family), maintained in an unclear zoonotic cycle involving mosquitoes from Haemagogus genus as the main vector. The genome is composed of a positive single-stranded RNA of 11.5 kb in length, which contains two genes that encode four nonstructural (nsP1 to nsP4) and five structural (C, E3, E2, 6K, and E1) proteins. In the present study, we have developed an enzyme-linked immunosorbent assay (ELISA) using as antigen the recombinant envelope protein 2 of MAYV produced in an Escherichia coli system (rE2-MAYV ELISAs). A panel of 68 human serum samples from suspected arboviral cases was analyzed and titrated for anti-MAYV IgM and IgG antibody detection. The rE2-MAYV ELISA detected 33.8% (23/68) IgG-positive samples, demonstrating 100% sensitivity and 78.95% specificity compared to the MAYV-specific 50% plaque reduction neutralization assay. In addition, the positive MAYV-neutralizing samples showed high titers of detection by rE2-MAYV ELISA, suggesting a highly sensitive test. The rE2-MAYV ELISA also detected 42.5% (29/68) IgM-positive samples, of which 13.8% (4/29) presented high-avidity interactions with rE2-MAYV. Cross-reactivity was observed with Chikungunya virus (CHIKV)-specific murine antibody sample but not with CHIKV-specific human and other Alphavirus murine antibodies. In short, we have developed a rapid, simple, specific, and sensitive MAYV rE2-ELISA, and our preliminary results show its potential applicability to diagnosis of MAYV infections.
INTRODUCTION
Mayaro virus (MAYV) is a neglected arthropod-borne virus (arbovirus) initially isolated in 1954 in Trinidad and Tobago from blood samples of five rural workers with febrile disease (1). MAYV is classified into the Alphavirus genus (Togaviridae family) and, based on antigenic relationship, is included into the Semliki Forest group (2). MAYV genome is a positive single-stranded RNA of 11.5 kb that contains two genes that encode four nonstructural (nsP1 to nsP4) and five structural (C, E3, E2, 6K, and E1) proteins (3).
MAYV is maintained in nature in a poorly known zoonotic cycle involving mosquitoes of Haemagogus genus as main vectors and marsupials and primates as vertebrate hosts (4, 5). MAYV human infections may be asymptomatic or progress to an acute febrile illness similar to that of Chikungunya fever (6). Disease symptoms include fever, rash, myalgia, retro-orbital pain, headache, diarrhea lasting for 5 days, and, in some cases, severe arthralgia that can be recurrent and persist for months or even years (7–9).
MAYV outbreaks have been described in countries of South and Central America, including Bolivia, Brazil, Ecuador, French Guiana, Haiti, Mexico, Peru, Suriname, Trinidad and Tobago, and Venezuela (9–18). In Brazil, human infections by MAYV are incidental and associated mainly with rural or forestal areas of northern and west-central regions, including Amazonas, Goiás, Mato Grosso, Mato Grosso do Sul, and Pará states (8, 19–21). However, epidemiological data about Mayaro virus are limited due to misinterpreted clinical diagnosis with other arboviral infections that occur in the same geographic area, such as those caused by dengue virus and Chikungunya virus (CHIKV), as well as the lack of suitable and specific serological and molecular diagnosis assays (6, 22).
Furthermore, the short viremic phase of MAYV infections and the laborious laboratory techniques for identification hamper the implementation of routine diagnostic assays (23). Therefore, in this study, envelope protein 2 of MAYV (rE2-MAYV) was expressed in an Escherichia coli system, and after purification, it was used as an antigen in an indirect enzyme-linked immunosorbent assay (ELISA) for specific detection of IgG and IgM MAYV antibodies. Additionally, antibody detection of MAYV-infected serum samples was assessed, and cross-reactivity detection was evaluated using CHIKV-infected patient sera and murine specific antibodies of other alphaviruses by rE2-MAYV ELISA.
MATERIALS AND METHODS
This study was approved by the Human Research Ethics Committee of the Medical School of University of São Paulo, Ribeirão Preto, São Paulo, Brazil (no. 2.206.200). All procedures involving animals followed ethical principles of animal research, and the protocols were approved by the local Animal Ethical Committee of the Medical School of University of São Paulo, Ribeirão Preto, São Paulo, Brazil (no. 187/2016).
Recombinant envelope protein 2 of Mayaro virus.
We have produced a recombinant envelope protein 2 of MAYV without the transmembrane region. The E2 gene region was cloned, including a 6× His tag at the N-terminal portion, into a pET-30a plasmid vector. The rE2-MAYV protein was expressed and purified from E. coli cells under native conditions by Biomatik Corporation (USA).
Indirect ELISA using the recombinant envelope protein 2 of MAYV.
The rE2-MAYV ELISA is an indirect assay performed in 96-well plates (Corning, USA) coated with rE2. The ELISA antigen rE2 of MAYV was tested in different concentrations, ranging from 0.5 µg/ml to 8 µg/ml, diluted in 0.05 M carbonate-bicarbonate buffer, pH 9.6, and incubated for 18 and 36 h in a wet chamber at 4°C (Sigma-Aldrich, USA). Plate wells were washed 3 to 5 times with 150 µl of PBS-T (phosphate-buffered saline with 0.05%, vol/vol, Tween 20). Plates were blocked with 150 µl of 10% (wt/vol) nonfat dry milk in PBS-T and incubated for 2 h to reduce unspecific background signals. All incubation steps were performed in a dark wet chamber at 37°C. MAYV mouse hyperimmune serum, diluted 1:100 to 1:800 in blocking solution, was added to plate wells as a positive control. Plates were incubated for 1 h and washed, and 50 µl of horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG Fab specific body (Sigma-Aldrich, USA), diluted 1:2,000 in blocking solution, was added to the wells. Plates were incubated for 1 h and washed, and 100 µl of 2,2'-azinobis (3-ethylbenzothiazoline-6-sulfonic acid)-diammonium salt (ABTS) peroxidase substrate (KPL, USA) was added to the wells. Plates were incubated for 15 min and read using a Multiscan MMC/340 microplate reader (Titertek, Germany) at an optical density (OD) of 405 nm.
Human samples.
A total of 68 human serum samples from patients suspected of arboviral infection were collected during 2017 in Mato Grosso State, Brazil, and tested by IgG and IgM rE2-MAYV ELISAs. The assays were performed as previously standardized, and E. coli cell extract was used as a negative control at the same dilution of the rE2-MAYV coating antigen in microplate wells. Human antibodies were detected using HRP-conjugated goat anti-human IgG Fab specific antibody (Sigma-Aldrich, USA) diluted 1:2,000 or HRP-conjugated goat anti-human IgM Fc5µ specific antibody (Merck Millipore, USA) at 1:25,000, both diluted in blocking solution. The cutoff value for positive serum was determined as that higher than the mean OD of negative controls added to three standard deviations (SDs) (24). The IgG-positive titers were determined for serum samples by testing its detection in dilutions ranging from 1:100 to 1:12,800 in duplicates.
Plaque reduction neutralization assay.
Human serum samples were tested by 50% plaque reduction neutralization assay (PRNT50) for MAYV and CHIKV. Briefly, 1 day prior to infection, 2 × 105 Vero cells were seeded in each well of a 12-well plate. Human sera were heat inactivated for 1 h at 56°C and serially diluted from 1/10 to 1/20,480 in Dulbecco’s modified Eagle’s medium (DMEM). Serum dilutions were mixed with 1 × 102 PFU of MAYV strain BeAr20290 or CHIKV strain S27-African and incubated for 1 h at 37°C. After incubation, 200 µl of serum-virus mixture was inoculated into the Vero cell monolayers and incubated for 1 h at 37°C under gentle rocking for viral adsorption. Subsequently, 1 ml of prewarmed 1% agar–DMEM containing 3% fetal bovine serum (FBS; Vitrocell, Brazil) was gently added to each well, and the plates were incubated at 37°C in 5% CO2 atmosphere for 2 days. Finally, cells were fixed with 2 ml of 10% formaldehyde solution for 2 h and stained with 1% crystal violet (Sigma-Aldrich, USA) for 30 min. Plaque reduction was calculated for each sample by comparing the number of plaques in wells inoculated with serum-virus mixtures with those in wells inoculated with 1 × 102 PFU of MAYV or CHIKV (positive control) assayed simultaneously.
Cross-reactivity of Alphavirus antibodies in the rE2-MAYV ELISA.
Polyclonal hyperimmune mouse sera specific to Aura virus (AURV), Eastern equine encephalitis virus (EEEV), Mucambo virus (MUCV), and Western equine encephalitis virus (WEEV), as well as to the rE2 of MAYV and rE2 of CHIKV, were produced. Briefly, 6-week-old female BALB/c mice were intraperitoneally inoculated with 1 × 106 PFU of alphaviruses or 30 µg of rE2-MAYV or rE2-CHIKV (GenBank accession number MG945127) with complete Freund’s adjuvant (Sigma-Aldrich, USA) (1:1, vol/vol) once a week for 4 weeks. One week after the last immunization, all mice were anesthetized and blood was collected by cardiac puncture. Specificity of the mouse sera against its respective alphavirus was confirmed by immunofluorescent assay (25), using infected Vero cells staining for nuclei, cytoplasm, and virus antigens. All mouse sera were tested at dilutions ranging from 1/100 to 1/800 in blocking buffer by IgG rE2-MAYV ELISA. The mouse sera produced against rE2 of CHIKV and MAYV were tested by rE2-MAYV ELISA.
Furthermore, in order to evaluate cross-reactivity for IgG detection by rE2-MAYV ELISA, 24 human serum samples having neutralizing antibodies to CHIKV were tested at 1/100 dilution by rE2-MAYV ELISA and by a previously standardized rE2-CHIKV ELISA (26).
Avidity assay.
All serum samples determined to be IgG or IgM positive by rE2-MAYV ELISA were subjected to an avidity assay (27). Briefly, ELISA procedures were performed as described above, except that after primary antibody incubation, PBS alone or 100 µl of a 6 M urea solution diluted in PBS was added to the wells. Plates were incubated for 10 min at 37°C in a wet chamber and washed four times with PBS-T, and subsequent steps were performed as previously described. Relative avidity (percent) index (RAI) was calculated for each sample by dividing the liquid OD in urea-treated wells by that in untreated wells (PBS). Samples with an RAI of >60% were considered to have high avidity, 40% to 60% was medium avidity, and <40% was low avidity.
Data availability.
The nucleotide sequence used in this study for expression of the recombinant envelope protein 2 of MAYV has been deposited in GenBank under accession number MH396439.
RESULTS
Standardization of rE2-MAYV ELISA.
The rE2-MAYV antigen was expressed and purified from E. coli and confirmed by SDS-PAGE and Western blot analysis, showing a molecular weight of ∼45 kDa (data not shown). The optimal concentration of rE2-MAYV antigen added to the plate wells was 4 µg/ml in a volume of 50 µl, and the coating period was 18 h at 4°C. Following these parameters, it was possible to detect anti-MAYV murine antibodies at 1:100 dilution. Results using higher concentrations of the antigen and longer coating periods did not increase sensitivity of the assay.
Detection of human antibodies against MAYV by rE2-MAYV ELISA.
Results of IgG and IgM detection in sera of 68 patients suspected of arboviral infection by rE2-MAYV ELISAs were compared to those of MAYV-specific PRNT50 (Table 1). Comparisons showed 100% sensitivity and 78.95% specificity for IgG detection (Table 2). The rE2-MAYV ELISA was able to detect all 11 MAYV-positive neutralizing samples, and false-negative results were not observed. IgG-positive samples in rE2-MAYV ELISA also were all negative for CHIKV-specific PRNT50 and showed ELISA detection titers of 100 to 3,200, but only MAYV-neutralizing samples showed titers higher than 800 (Table 1). The IgM rE2-MAYV ELISA was able to detect 29 positive samples. Furthermore, 69.5% (16/23) of the IgG-positive samples and 13.8% (4/29) of the IgM-positive samples presented high-avidity interactions to rE2-MAYV (Table 3).
TABLE 1.
Human serum sample antibody detection and titration by rE2-MAYV ELISA and neutralization titer by PRNT50
| Sample | Detection ofa: |
PRNT50 | |
|---|---|---|---|
| IgG/titer | IgM | ||
| 1 | − | − | <10 |
| 2 | − | + | <10 |
| 3 | − | + | <10 |
| 4 | − | − | <10 |
| 5 | − | + | <10 |
| 6 | − | + | <10 |
| 7 | − | + | <10 |
| 8 | − | − | <10 |
| 9 | − | + | <10 |
| 10 | − | + | <10 |
| 11 | − | − | <10 |
| 12 | − | − | <10 |
| 13 | − | + | <10 |
| 14 | − | + | <10 |
| 15 | − | + | <10 |
| 16 | − | + | <10 |
| 17 | − | + | <10 |
| 18 | − | + | <10 |
| 19 | − | + | <10 |
| 20 | − | + | <10 |
| 21 | − | + | <10 |
| 22 | − | − | <10 |
| 23 | +/800 | − | 1,280 |
| 24 | − | − | <10 |
| 25 | +/100 | − | <10 |
| 26 | +/400 | − | <10 |
| 27 | − | + | <10 |
| 28 | − | − | <10 |
| 29 | − | + | <10 |
| 30 | − | + | <10 |
| 31 | − | − | <10 |
| 32 | − | + | <10 |
| 33 | − | + | <10 |
| 34 | − | + | <10 |
| 35 | − | + | <10 |
| 36 | − | + | <10 |
| 37 | +/400 | − | <10 |
| 38 | − | + | <10 |
| 39 | − | − | <10 |
| 40 | − | + | <10 |
| 41 | − | − | <10 |
| 42 | +/400 | − | <10 |
| 43 | − | − | <10 |
| 44 | − | − | <10 |
| 45 | +/400 | − | 1,280 |
| 46 | +/1,600 | − | 2,560 |
| 47 | +/100 | − | <10 |
| 48 | +/100 | − | <10 |
| 49 | − | − | <10 |
| 50 | +/3,200 | − | 640 |
| 51 | +/3,200 | + | 640 |
| 52 | +/100 | − | <10 |
| 53 | +/200 | − | <10 |
| 54 | +/800 | − | 1,280 |
| 55 | +/3,200 | − | 320 |
| 56 | +3,200 | − | 1,280 |
| 57 | − | − | <10 |
| 58 | +/100 | − | <10 |
| 59 | +/100 | − | <10 |
| 60 | +/100 | + | <10 |
| 61 | +/400 | − | 640 |
| 62 | +/200 | + | <10 |
| 63 | +/800 | − | 5,120 |
| 64 | +/800 | − | 640 |
| 65 | − | − | <10 |
| 66 | − | − | <10 |
| 67 | − | − | <10 |
| 68 | − | − | <10 |
+, positive; −, negative.
TABLE 2.
Statistical indexes for IgG detection by rE2-MAYV ELISAa
| Assay | P | FP | TN | FN | Se (%) | Sp (%) |
|---|---|---|---|---|---|---|
| PRNT50 | Gold standard | |||||
| IgG detection | 11 | 12 | 45 | 0 | 100 | 78.95 |
Se, sensitivity; Sp, Specificity; P, positive; FP, false positive; N, negative; FN, false negative; GS, gold standard.
TABLE 3.
Relative avidity index for MAYV IgG and IgM samples
| Sample | Relative avidity index (%) for IgG | Avidity IgG classification | Relative avidity index (%) for IgM | Avidity IgM classification |
|---|---|---|---|---|
| 2 | Not detected | 19.16 | Low | |
| 3 | Not detected | 58.47 | Medium | |
| 5 | Not detected | 18.58 | Low | |
| 6 | Not detected | 15.19 | Low | |
| 7 | Not detected | 28.85 | Low | |
| 9 | Not detected | 3.06 | Low | |
| 10 | Not detected | 19.04 | Low | |
| 13 | Not detected | 35.17 | Low | |
| 14 | Not detected | 19.69 | Low | |
| 15 | Not detected | 14.44 | Low | |
| 16 | Not detected | 13.74 | Low | |
| 17 | Not detected | 83.50 | High | |
| 18 | Not detected | 74.92 | High | |
| 19 | Not detected | 12.13 | Low | |
| 20 | Not detected | 16.34 | Low | |
| 21 | Not detected | 0 | Low | |
| 23 | 92.62 | High | Not detected | |
| 25 | 71 | High | Not detected | |
| 26 | 34.82 | Low | Not detected | |
| 27 | Not detected | 45.40 | Medium | |
| 29 | Not detected | 4.29 | Low | |
| 30 | Not detected | 25.67 | Low | |
| 32 | Not detected | 7.10 | Low | |
| 33 | Not detected | 42.92 | Medium | |
| 34 | Not detected | 13.09 | Low | |
| 35 | Not detected | 5.70 | Low | |
| 36 | Not detected | 7.66 | Low | |
| 37 | 7.91 | Low | Not detected | |
| 38 | Not detected | 31.33 | Low | |
| 40 | Not detected | 30.95 | Low | |
| 42 | 49.52 | Medium | Not detected | |
| 45 | 100 | High | Not detected | |
| 46 | 100 | High | Not detected | |
| 47 | 100 | High | Not detected | |
| 48 | 100 | High | Not detected | |
| 50 | 100 | High | Not detected | |
| 51 | 100 | High | 100 | High |
| 52 | 54.63 | Medium | Not detected | |
| 53 | 22.08 | Low | Not detected | |
| 54 | 95.77 | High | Not detected | |
| 55 | 100 | High | Not detected | |
| 56 | 96.85 | High | Not detected | |
| 58 | 41.64 | Medium | Not detected | |
| 59 | 100 | High | Not detected | |
| 60 | 46.72 | Medium | 63.82 | Medium |
| 61 | 100 | High | Not detected | |
| 62 | 100 | High | 96.91 | High |
| 63 | 100 | High | Not detected | |
| 64 | 95.25 | High | Not detected |
Cross-reactivity detection in the rE2-MAYV ELISA.
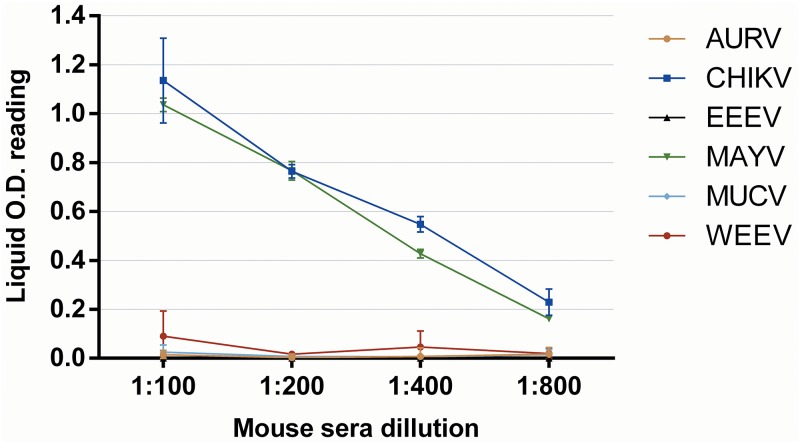
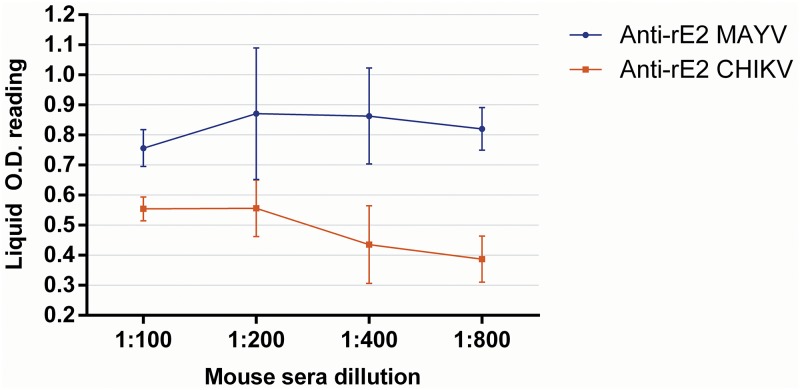
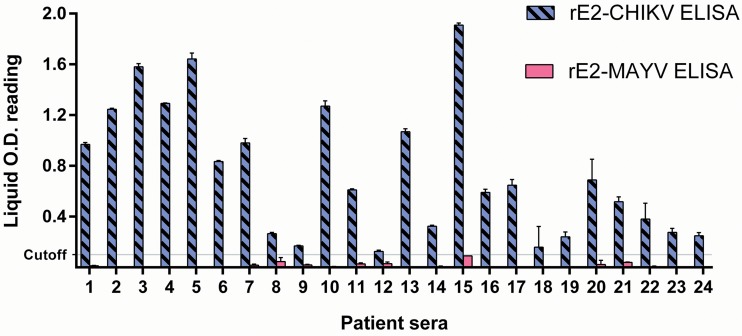
Cross-reactions were not observed in the IgG rE2-MAYV ELISA when testing AURV, EEEV, MUCV, and WEEV mouse hyperimmunized sera. However, the rE2-MAYV ELISA detected IgG murine antibodies to CHIKV, indicating cross-reactivity detection (Fig. 1). Additionally, rE2-CHIKV-specific mouse hyperimmune serum also reacted in the rE2-MAYV ELISA (Fig. 2). Furthermore, 24 CHIKV-neutralizing human serum samples, positive for IgG in a previously described rE2-CHIKV ELISA (26), were all negative for IgG detection in the rE2-MAYV ELISA (Fig. 3).
FIG 1.
FIG 1 Cross-reactivity analysis of IgG detection using different alphavirus polyclonal antibodies of AURV, CHIKV, EEEV, MUCV, and WEEV (including MAYV as a positive control) in the rE2-MAYV ELISA.
FIG 2.
Cross-reactivity of anti-rE2-MAYV and anti-rE2-CHIKV by IgG rE2-MAYV ELISA.
FIG 3.
Cross-reactivity of CHIKV-neutralizing IgG human serum samples by rE2-CHIKV and rE2-MAYV ELISAs.
DISCUSSION
MAYV diagnosis is preconized by virus isolation or virus genome detection assays as gold standards. However, these assays are limited to the acute viremic period, which usually does not last more than 5 days (6). After the viremic period, specific antibody detection is often used for diagnosis of virus infections. Thus, an in-house antibody detection assay using MAYV-infected cells (EIA-ICC) has been previously described, but this assay showed low sensitivity for IgM detection compared to that of immunoglobulin M antibody capture enzyme-linked immunosorbent assay (MAC-ELISA) (28). Currently, commercial assays for MAYV diagnosis are not available (29). Considering the lack of serological assays for MAYV and recognizing that the envelope protein 2 (E2) of alphaviruses is the main target for protective host immune response (30–33), and that recombinant E2 of CHIKV produced in Escherichia coli is an efficient antigen for serological diagnosis (26, 34), we expressed rE2 of MAYV in an E. coli system for use as the antigen in a diagnostic ELISA.
Antibody cross-reactivity among antigenically related alphaviruses is a major concern in serologic diagnostic assays (35). In previous studies, an rE2-CHIKV protein expressed and purified from E. coli did not show cross-reactivity detection with alphavirus murine hyperimmune sera (26). In the present study, using the rE2-MAYV and mouse hyperimmune antisera rather than human sera due to the difficulties in obtaining positive infected patient samples, we also did not observe cross-reactivity against AURV, EEEV, MUCV, and WEEV. However, the murine hyperimmune antiserum to CHIKV cross-reacted with rE2-MAYV in the ELISA.
MAYV and CHIKV cross-reactive antibodies are probably due to conserved and similar epitopes in both E2 proteins of these viruses, which are targets of the humoral immune system. This fact was corroborated when we tested specific murine antibodies produced against rE2 of CHIKV in the rE2-MAYV ELISA, which also demonstrated levels of cross-reactivity detection. Infection by other alphaviruses, which are phylogenetically more distant, does not induce such cross-reactive antibodies to rE2-MAYV. Interestingly, human sera with neutralizing IgG to CHIKV, which were also positive for IgG detection in the rE2-CHIKV ELISA, did not cross-react in the rE2-MAYV ELISA. Therefore, for serological diagnosis in human sera, the specificity of the rE2-MAYV antigen in the ELISA is an important feature to discard infections by other alphaviruses, even in the presence of cross-reaction with hyperimmune sera from animal origin. In this context, it is important to highlight that MAYV is a neglected arbovirus that circulates mainly in rural or forestal areas, but it has a high potential for urban adaptation. Therefore, precise diagnostic tools are essential to screen and differentiate MAYV infections from those produced by other arboviruses, such as CHIKV, which is also an ongoing public health concern in South America (36, 37), and they often circulate in the same geographical region and produce similar clinical symptoms (23). We show here a useful tool for human diagnosis of MAYV, for which there currently is no such efficient diagnostic assay, that might be helpful for serologic screening and during possible MAYV outbreaks.
A total of 68 serum samples from patients from a region where MAYV is endemic and clinically suspected of arboviral infection were tested at 1:100 dilution by IgG and IgM rE2-MAYV ELISA; 33.8% (23/68) of these samples were IgG positive, and 42.64% (29/68) were IgM positive. The IgG rE2-MAYV ELISA showed 100% sensitivity and 78.95% specificity compared to results obtained with PRNT50 to MAYV. IgG-positive serum samples showed titers of 100 to 3,200 in rE2-MAYV ELISA, and they were all negative for CHIKV-specific PRNT50, suggesting non-cross-reactive antibody detection between MAYV and CHIKV by the rE2-MAYV ELISA. Interestingly, only MAYV-positive neutralizing samples showed titers higher than 800, indicating that the rE2-MAYV capture antigen is sensitive in the ELISA. However, we recommend the 1:100 dilution for routine diagnosis, since specific nonneutralizing or subneutralizing antibodies may also be produced during viral infections but could not be confirmed in our study, since our gold standard detection assay is the PRNT50. These results were also corroborated by the avidity assay that showed an association of high-binding-affinity sera in the IgG rE2-MAYV ELISA and positive neutralizing antibodies to MAYV. Therefore, the IgG rE2-MAYV ELISA is a suitable tool for diagnosis of MAYV infection that in the acute phase could be performed in paired serum samples and also could be used in serologic surveys. Unfortunately, the IgM detection results obtained in the rE2-MAYV ELISA were not compared to those of other serological tests due to the absence of other suitable and trusted assays for MAYV. In addition, the avidity assay performed for IgM antibodies showed that the majority of positive samples have low-avidity interaction with the rE2-MAYV, which is an expected result for IgM antibodies due to the partial maturation of its affinity. Therefore, considering that rE2-MAYV demonstrated high specificity to IgG antibodies specific to MAYV, we believe that rE2-MAYV ELISA can be suitable for IgM antibody diagnosis.
We show here that rE2-MAYV ELISA represents a useful new assay for diagnosis of MAYV infection. This ELISA is fast, highly sensitive, and able to detect specific IgG human antibodies to MAYV without cross-reactivity to other alphaviruses in human samples. Future studies are needed to evaluate the potential IgM diagnosis by the rE2-MAYV ELISA, and its cross-reactivity detection to other alphavirus antibodies, besides those of CHIKV, should be assessed using human serum samples.
ACKNOWLEDGMENTS
The study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo, Brazil (grant no. 14/02438-6 and scholarship no. 16/01414-1, 12/24150-9, 17/13981-0, and 14/20851-8).
REFERENCES
- 1.Anderson CR, Downs WG, Wattley GH, Ahin NW, Reese AA. 1957. Mayaro virus: a new human disease agent. II. Isolation from blood of patients in Trinidad, B.W.I. Am J Trop Med Hyg 6:1012–1016. doi: 10.4269/ajtmh.1957.6.1012. [DOI] [PubMed] [Google Scholar]
- 2.King AMQ, Lefkowitz E, Adams MJ, Carstens EB. 2011. Family togaviridae In King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ (ed), Virus Taxonomy. Classification and nomenclature of viruses Ninth report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press, San Diego, CA. [Google Scholar]
- 3.Lavergne A, de Thoisy B, Lacoste V, Pascalis H, Pouliquen JF, Mercier V, Tolou H, Dussart P, Morvan J, Talarmin A, Kazanji M. 2006. Mayaro virus: complete nucleotide sequence and phylogenetic relationships with other alphaviruses. Virus Res 117:283–290. doi: 10.1016/j.virusres.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 4.de Thoisy B, Gardon J, Salas RA, Morvan J, Kazanji M. 2003. Mayaro virus in wild mammals, French Guiana. Emerg Infect Dis 9:1326–1329. doi: 10.3201/eid0910.030161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seymour C, Peralta PH, Montgomery GG. 1983. Serologic evidence of natural togavirus infections in Panamanian sloths and other vertebrates. Am J Trop Med Hyg 32:854–861. doi: 10.4269/ajtmh.1983.32.854. [DOI] [PubMed] [Google Scholar]
- 6.Pinheiro FP, Freitas RB, Travassos da Rosa JF, Gabbay YB, Mello WA, LeDuc JW. 1981. An outbreak of Mayaro virus disease in Belterra, Brazil. I. Clinical and virological findings. Am J Trop Med Hyg 30:674–681. doi: 10.4269/ajtmh.1981.30.674. [DOI] [PubMed] [Google Scholar]
- 7.Pinheiro FP, LeDuc JW. 1988. Mayaro virus disease In Monath TP. (ed), The arboviruses: epidemiology and ecology, vol 3 CRC Press, Boca Raton, FL. [Google Scholar]
- 8.Azevedo RS, Silva EV, Carvalho VL, Rodrigues SG, Nunes-Neto JP, Monteiro H, Peixoto VS, Chiang JO, Nunes MR, Vasconcelos PF. 2009. Mayaro fever virus, Brazilian Amazon. Emerg Infect Dis 15:1830–1832. doi: 10.3201/eid1511.090461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Izurieta RO, Macaluso M, Watts DM, Tesh RB, Guerra B, Cruz LM, Galwankar S, Vermund SH. 2011. Hunting in the rainforest and Mayaro virus infection: an emerging alphavirus in Ecuador. J Glob Infect Dis 3:317–323. doi: 10.4103/0974-777X.91049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navarrete-Espinosa J, Gómez-Dantés H. 2006. Arbovirus causing hemorrhagic fever at IMSS. Rev Med Inst Mex Seguro Soc 44:347–353. [PubMed] [Google Scholar]
- 11.Lednicky J, De Rochars VM, Elbadry M, Loeb J, Telisma T, Chavannes S, Anilis G, Cella E, Ciccozzi M, Okech B, Salemi M, Morris JG Jr. 2016. Mayaro virus in child with acute febrile illness, Haiti, 2015. Emerg Infect Dis 22:2000–2002. doi: 10.3201/eid2211.161015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Auguste AJ, Liria J, Forrester NL, Giambalvo D, Moncada M, Long KC, Moron D, de Manzione N, Tesh RB, Halsey ES, Kochel TJ, Hernandez R, Navarro JC, Weaver SC. 2015. Evolutionary and ecological characterization of Mayaro virus strains isolated during an outbreak, Venezuela, 2010. Emerg Infect Dis 21:1742–1750. doi: 10.3201/eid2110.141660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aitken TH, Downs WG, Anderson CR, Spence L, Casals J. 1960. Mayaro virus isolated from a Trinidadian mosquito, Mansonia venezuelensis. Science 131:986. doi: 10.1126/science.131.3405.986. [DOI] [PubMed] [Google Scholar]
- 14.Groot H, Morales A, Vidales H. 1961. Virus isolations from forest mosquitoes in San Vicente de Chucuri, Colombia. Am J Trop Med Hyg 10:397–402. doi: 10.4269/ajtmh.1961.10.397. [DOI] [PubMed] [Google Scholar]
- 15.Karbaat J, Jonkers AH, Spence L. 1964. Arbovirus infections in Dutch military personnel stationed in Surinam: a preliminary study. Trop Geogr Med 16:370–376. [PubMed] [Google Scholar]
- 16.Talarmin A, Chandler LJ, Kazanji M, de Thoisy B, Debon P, Lelarge J, Labeau B, Bourreau E, Vie JC, Shope RE, Sarthou JL. 1998. Mayaro virus fever in French Guiana: isolation, identification, and seroprevalence. Am J Trop Med Hyg 59:452–456. doi: 10.4269/ajtmh.1998.59.452. [DOI] [PubMed] [Google Scholar]
- 17.Terzian ACB, Auguste AJ, Vedovello D, Ferreira MU, da Silva-Nunes M, Sperança MA, Suzuki RB, Juncansen C, Araújo JP, Weaver SC, Nogueira ML. 2015. Isolation and characterization of Mayaro virus from a human in Acre, Brazil. Am J Trop Med Hyg 92:401–404. doi: 10.4269/ajtmh.14-0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forshey BM, Guevara C, Laguna-Torres VA, Cespedes M, Vargas J, Gianella A, Vallejo E, Madrid C, Aguayo N, Gotuzzo E, Suarez V, Morales AM, Beingolea L, Reyes N, Perez J, Negrete M, Rocha C, Morrison AC, Russell KL, Blair PJ, Olson JG, Kochel TJ, NMRCD Febrile Surveillance Working Group. 2010. Arboviral etiologies of acute febrile illnesses in western South America, 2000-2007. PLoS Negl Trop Dis 4:e787. doi: 10.1371/journal.pntd.0000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brunini S, Franca DDS, Silva JB, Silva LN, Silva FPA, Spadoni M, Rezza G. 2017. High frequency of Mayaro virus IgM among febrile patients, Central Brazil. Emerg Infect Dis 23:1025–1026. doi: 10.3201/eid2306.160929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mourão MPG, Bastos MDS, de Figueiredo RP, Gimaque JBL, Galusso EDS, Kramer VM, de Oliveira CMC, Naveca FG, Figueiredo LTM. 2012. Mayaro fever in the city of Manaus, Brazil, 2007-2008. Vector Borne Zoonotic Dis 12:42–46. doi: 10.1089/vbz.2011.0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zuchi N, Heinen LB, Santos MA, Pereira FC, Slhessarenko RD. 2014. Molecular detection of Mayaro virus during a dengue outbreak in the state of Mato Grosso, Central-West Brazil. Mem Inst Oswaldo Cruz 109:820–823. doi: 10.1590/0074-0276140108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vieira CJ, Silva DJ, Barreto ES, Siqueira CE, Colombo TE, Ozanic K, Schmidt DJ, Drumond BP, Mondini A, Nogueira ML, Bronzoni RV. 2015. Detection of Mayaro virus infections during a dengue outbreak in Mato Grosso, Brazil. Acta Trop 147:12–16. doi: 10.1016/j.actatropica.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 23.Figueiredo ML, Figueiredo LT. 2014. Emerging alphaviruses in the Americas: Chikungunya and Mayaro. Rev Soc Bras Med Trop 47:677–683. doi: 10.1590/0037-8682-0246-2014. [DOI] [PubMed] [Google Scholar]
- 24.Ramani S, Paul A, Saravanabavan A, Menon VK, Arumugam R, Sowmyanarayanan TV, Samuel P, Kang G. 2010. Rotavirus antigenemia in Indian children with rotavirus gastroenteritis and asymptomatic infections. Clin Infect Dis 51:1284–1289. doi: 10.1086/657069. [DOI] [PubMed] [Google Scholar]
- 25.Figueiredo LT. 1990. The use of Aedes albopictus C6/36 cells in the propagation and classification of arbovirus of the Togaviridae, Flaviviridae, Bunyaviridae and Rhabdoviridae families. Rev Soc Bras Med Trop 23:13–18. doi: 10.1590/S0037-86821990000100003. [DOI] [PubMed] [Google Scholar]
- 26.Fumagalli MJ, de Souza WM, Esposito DLA, Silva A, Romeiro MF, Martinez EZ, da Fonseca BAL, Figueiredo LTM. 2018. Enzyme-linked immunosorbent assay using recombinant envelope protein 2 antigen for diagnosis of Chikungunya virus. Virol J 15:112. doi: 10.1186/s12985-018-1028-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levett PN, Sonnenberg K, Sidaway F, Shead S, Niedrig M, Steinhagen K, Horsman GB, Drebot MA. 2005. Use of immunoglobulin G avidity assays for differentiation of primary from previous infections with West Nile virus. J Clin Microbiol 43:5873–5875. doi: 10.1128/JCM.43.12.5873-5875.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Figueiredo LT, Nogueira RM, Cavalcanti SM, Schatzmayr H, da Rosa AT. 1989. Study of two different enzyme immunoassays for the detection of Mayaro virus antibodies. Mem Inst Oswaldo Cruz 84:303–307. doi: 10.1590/S0074-02761989000300003. [DOI] [PubMed] [Google Scholar]
- 29.Mota MTO, Ribeiro MR, D V, Nogueira ML. 2015. Mayaro virus: a neglected arbovirus of the Americas. Future Virol doi: 10.2217/fvl.15.76. [DOI] [Google Scholar]
- 30.Porta J, Jose J, Roehrig JT, Blair CD, Kuhn RJ, Rossmann MG. 2014. Locking and blocking the viral landscape of an alphavirus with neutralizing antibodies. J Virol 88:9616–9623. doi: 10.1128/JVI.01286-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kam YW, Lee WW, Simarmata D, Harjanto S, Teng TS, Tolou H, Chow A, Lin RT, Leo YS, Renia L, Ng LF. 2012. Longitudinal analysis of the human antibody response to Chikungunya virus infection: implications for serodiagnosis and vaccine development. J Virol 86:13005–13015. doi: 10.1128/JVI.01780-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kam YW, Lum FM, Teo TH, Lee WW, Simarmata D, Harjanto S, Chua CL, Chan YF, Wee JK, Chow A, Lin RT, Leo YS, Le Grand R, Sam IC, Tong JC, Roques P, Wiesmuller KH, Renia L, Rotzschke O, Ng LF. 2012. Early neutralizing IgG response to Chikungunya virus in infected patients targets a dominant linear epitope on the E2 glycoprotein. EMBO Mol Med 4:330–343. doi: 10.1002/emmm.201200213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lum FM, Teo TH, Lee WW, Kam YW, Renia L, Ng LF. 2013. An essential role of antibodies in the control of Chikungunya virus infection. J Immunol 190:6295–6302. doi: 10.4049/jimmunol.1300304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho B, Jeon BY, Kim J, Noh J, Kim J, Park M, Park S. 2008. Expression and evaluation of Chikungunya virus E1 and E2 envelope proteins for serodiagnosis of Chikungunya virus infection. Yonsei Med J 49:828–835. doi: 10.3349/ymj.2008.49.5.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Powers AM, Brault AC, Shirako Y, Strauss EG, Kang W, Strauss JH, Weaver SC. 2001. Evolutionary relationships and systematics of the alphaviruses. J Virol 75:10118–10131. doi: 10.1128/JVI.75.21.10118-10131.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ministério da Saúde. 2018. Monitoramento dos casos de dengue, febre de chikungunya e febre pelo vírus Zika até a Semana Epidemiológica 51, 2017. Boletim Epidemiol 49:1–12. [Google Scholar]
- 37.Pan American Health Organization. 22 December 2017. Number of reported cases of Chikungunya fever in the Americas, by country or territory. Pan American Health Organization, Washington, DC: https://www.paho.org/hq/dmdocuments/2017/2017-dec-22-phe-CHIKV-cases-ew-51.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The nucleotide sequence used in this study for expression of the recombinant envelope protein 2 of MAYV has been deposited in GenBank under accession number MH396439.