Dientamoeba fragilis is a gastrointestinal trichomonad parasite whose pathogenicity is yet to be determined. The difficulty involved in microscopically diagnosing D. fragilis in feces led to the development of real-time PCR methodologies for the detection of D. fragilis in stool samples.
KEYWORDS: Dientamoeba, PCR validation, prevalence, real-time PCR, sensitivity thresholds
ABSTRACT
Dientamoeba fragilis is a gastrointestinal trichomonad parasite whose pathogenicity is yet to be determined. The difficulty involved in microscopically diagnosing D. fragilis in feces led to the development of real-time PCR methodologies for the detection of D. fragilis in stool samples. Prevalence studies in Europe show much higher levels of infection where a laboratory-developed real-time assay is the predominant assay for the detection of Dientamoeba fragilis than in regions that use the EasyScreen assay for detection of gastrointestinal pathogens. The aim of this study was to compare a commercially available Dientamoeba fragilis assay (Genetic Signatures EasyScreen assay) to a widely used laboratory-developed real-time PCR method. Two hundred fifty fecal samples were screened using the laboratory-developed real-time assay on four real-time PCR platforms producing a number of discrepant results. Limit-of-detection studies were undertaken to attempt to resolve sensitivity for each platform tested. The presence or absence of Dientamoeba fragilis DNA in discrepant samples was shown using PCR amplicon next-generation sequencing. Eukaryotic 18S diversity profiling was conducted on discrepant samples to identify the presence or absence of additional protozoan species in samples that may be responsible for cross-reactivity seen in these samples. The results revealed the potential for multiple false-positive results when using the laboratory-developed real-time assay across multiple real-time platforms using manufacturer default settings. This report provides recommendations to resolve these issues where possible and suggestions for future prevalence studies, and it emphasizes the EasyScreen assay as the molecular method of choice as well as the need for standardization of detection assays across all nations screening for D. fragilis.
INTRODUCTION
Dientamoeba fragilis is a trichomonad parasite that resides in the human bowel (1). It was first observed by Wenyon in 1909, although it was not until 1918 that it was first described in the literature by Jepps and Dobell, who characterized it as a harmless commensal (2). In the years that followed, much of what was known about D. fragilis remained highly speculative, and some aspects of its biology, including its mode of transmission, are still debated. The role of D. fragilis in human gut health is highly scrutinized, and its pathogenic potential is under investigation (3, 4). As a result, clinical management of patients harboring D. fragilis is confounded. Adding to confusion is the fact that manifestation of symptoms often varies between individuals and asymptomatic carriage has been reported.
Symptomatic patients experience a range of symptoms, including abdominal pain, diarrhea, fatigue, and flatulence (5). Dientamoeba fragilis can be easily passed between family members, with reinfection between parents and children causing some infections to continue for many years (6). Notably, these symptoms are not unique to dientamoebiasis and there lies the potential for D. fragilis infections to be misdiagnosed as other gastrointestinal diseases in regions where screening for D. fragilis is not routine. Traditionally, permanent staining of smears has been used to identify D. fragilis with trichrome stains being widely used in North America (7). The routine use of permanent staining brings a multitude of disadvantages. Dientamoeba fragilis is particularly difficult to identity in wet preparations, as the D. fragilis trophozoites appear as a nonspecific rounded mass whose nucleic structure cannot be visualized in saline or iodine preparations (8). Indeed, the difficulty of microscopically diagnosing D. fragilis led to the development of a number of real-time PCR methodologies for the detection of D. fragilis in feces. The benefits of real-time PCR are well publicized; consequently, real-time PCR has become the gold standard for gastrointestinal protozoan diagnostics. A number of real-time PCR assays have been described for the detection of D. fragilis, the most common of which is a laboratory-developed real-time PCR assay developed in 2007 (9). There are a number of commercially available assays for the detection of D. fragilis in stool samples, including the EasyScreen enteric parasite detection kit (Genetic Signatures), The Rida Gene Dientamoeba fragilis real-time PCR assay (R-Biopharm), the G-DiaFrag real-time PCR assay (Diagenode), and the LightMixModular Dientamoeba real-time PCR assay (Roche Diagnostics). The commercially available EasyScreen enteric parasite detection kit, developed by Genetic Signatures (Sydney, Australia), is an established assay used clinically within Australia, although international use is not yet widespread (10). The EasyScreen assay tests not only for D. fragilis but also for Giardia intestinalis, Cryptosporidium spp., Entamoeba histolytica, and Blastocystis hominis. These assays offer a highly sensitive alternative to traditional diagnostic methods; however, this high degree of sensitivity brings with it its own difficulties. Cross-reactivity of real-time PCR primers often becomes an issue, as was previously demonstrated by Chan et al. (11), who showed that the laboratory-developed real-time PCR assay was not useful for the study of animal specimens because the assay was shown to be nonspecific for D. fragilis, cross-reacting with Trichomonas foetus in animal stools (11).
Variation between Dientamoeba fragilis prevalence rates also complicates investigation. Cases of D. fragilis infection have been reported across multiple continents, with infections being more common in resource-rich countries than in resource-poor nations (12, 13), most likely due to budgetary limitations in resource-poor countries placing D. fragilis surveillance as a low priority and access to real-time PCR equipment being rare. Reported prevalence rates vary significantly between nations (14–24), from 1.8% in north central Venezuela when diagnosing via wet preparation (18) to 5% in the United States, where the trichrome stain is commonly used for identification (25), to as high as 42.7% in Denmark using real-time PCR (22).
Indeed, in certain demographics in Denmark, where the laboratory-developed assay is predominantly used, prevalence is much higher, with D. fragilis being detected in 68.3% of children ages 0 to 6 years old from a 1-year multi-day-care-center study (4). Incidentally, the high prevalence rates in these regions has led significant support to the conclusion of a commensal nature for D. fragilis (4). This is in stark contrast to prevalence rates found in Australia, where the prevailing diagnostic assay for D. fragilis is the Genetic Signatures EasyScreen real-time PCR, as only 12% of patients with gastroenteritis are found to be infected with D. fragilis (10). In additional to potential epidemiological differences between nations, differences in diagnostic assays may contribute to wide variation observed between prevalence rates, and therefore, direct comparison is difficult.
Real-time PCR is a powerful diagnostic tool, but the technology brings a set of criteria that must be satisfied when designing target specific primers and probes, especially when amplifying from a microbe-rich sample, such as stool. Specific attention to sensitivity thresholds must be given, as therein lies the potential for positive results with high cycle threshold (CT) values being beyond the theoretical sensitivity limit for a particular assay. In order to examine the accuracy of the two most prominent D. fragilis real-time PCR assays available, 250 fecal samples were collected and screened for the presence of D. fragilis on four platforms. Discrepant results were then characterized using MiSeq next-generation sequencing (NGS) to identify the full eukaryotic microbial diversity of each submission that returned positive results for D. fragilis. The presence of D. fragilis within each sample was confirmed or denied by targeted amplicon sequencing. The resulting data have highlighted multiple issues with the available assays and unveiled several contentious conflicts regarding the current literature on D. fragilis.
MATERIALS AND METHODS
Collection of stool specimens and DNA extraction.
Fecal samples submitted to SydPath at St Vincent’s Hospital Sydney for D. fragilis screening were included in this study. Screening for fecal pathogens at SydPath is done using the Genetic Signatures EasyScreen enteric parasite detection kit and the Genetic Signatures EasyScreen enteric prokaryote detection kit. Two groups of samples were collected. Group 1 consisted of 50 samples, all of which had tested positive for the presence of D. fragilis using the EasyScreen enteric protozoan detection kit. Group 2 consisted of 200 samples that had tested negative for the presence of D. fragilis, Blastocystis spp., Cryptosporidium spp., Entamoeba complex, and Giardia intestinalis, as well as bacterial pathogens, such as Salmonella spp., Shigella spp., Campylobacter spp., Yersinia enterocolitica, Listeria monocytogenes, enteroinvasive Escherichia coli, and Clostridium difficile, via multiplex real-time PCR (Genetic Signatures). Viral pathogens, including norovirus group I, norovirus group II, bocavirus, adenovirus 40/41, rotaviruses A and B, astrovirus (group 1 to 7), and sapovirus, were also excluded via quantitative PCR (qPCR) (26). DNA extraction for EasyScreen samples was performed using the GS1 automated DNA extraction machine as part of the EasyScreen sample pipeline, as per the manufacturer’s instructions.
DNA extraction for all stool samples screened using the laboratory-developed real-time assay was performed using a Qiagen DNA extraction robot. Briefly, 250 µl of G2 lysis buffer, 10 µl of proteinase K, and 10 mg of stool sample were added to an Eppendorf tube and vortexed for 30 s to mix thoroughly. Samples were then placed on a heating block at 95°C for 10 min. Samples were then spun at 13,000 relative centrifugal force (RCF) for 1 min Next, 200 µl of supernatant for each sample was removed and placed into an empty sample tube. This sample tube was then extracted using the EZ1 and the Qiagen EZ1 DNA tissue kit, following the manufacturer’s extraction protocol.
Real-time qPCR. (i) EasyScreen assay.
All fecal samples were originally screened using the Genetic Signatures EasyScreen enteric protozoan detection kit, with all real-time PCRs run according to the manufacturer’s specifications. Included in the protocol are internal positive controls and extraction controls to detect false negatives due to extraction errors or inhibition. The EasyScreen assay was run using the Bio-Rad CFX384 real-time PCR thermocycler platform as per the manufacturer’s recommendations.
(ii) Laboratory-developed real-time PCR assay.
Dientamoeba fragilis real-time PCR was performed on all samples using the laboratory-designed assay, amplifying a 98-bp fragment within the 5.8 rRNA gene sequence under the conditions defined by Verweij et al. (9). In short, amplification was done using the forward primer sequence Df-124F (5′-CAACGGATGTCTTGGCTCTTTA-3′) and reverse primer sequence Df-221R (5′-TGCATTCAAAGATCGAACTTATCAC-3′). A D. fragilis-specific MGB TaqMan probe (Df172revT) was also used, with the following sequence: 6-carboxyfluorescein (FAM)-5′-CAATTCTAGCCGCTTAT-3′-MGB (Thermo Fisher). Each reaction was conducted in a volume of 50 µl with PCR buffer (HotstarTaq master mix; Qiagen, Germany), 5 mM MgCl2, 3 pmol of each D. fragilis-specific primer, 5 pmol of D. fragilis-specific MGB probe, and 10 ml of the DNA sample. Amplification consisted of 15 min at 95°C followed by 50 cycles of 15 s at 95°C and 60 s at 60°C Each D. fragilis real-time PCR was performed on four different thermocyclers: the Cepheid SmartCycler II, the Roche LightCycler 480 (LC 480), the ABI 7500 (ABI), and the Bio-Rad CFX96.
(iii) LOD assessment.
To determine the limit of detection (LOD) for each platform, a D. fragilis culture sample with a known concentration of 100 trophozoites per µl was diluted in phosphate-buffered saline (PBS) for a series of 1:10 dilutions. These samples were then used as DNA templates in the laboratory-designed assay on each platform to assess assay sensitivity.
5.8S rDNA PCR amplicon sequencing.
Amplicon sequencing was performed on any sample that returned a positive qPCR result. This was performed by the service provider Australian Genome Research Facility (AGRF). PCR amplicon sequencing consisted of two stages, conventional PCR amplification followed by Illumina MiSeq next-generation amplicon sequencing. Each conventional PCR targeted a 98-bp fragment within the D. fragilis 5.8S ribosomal DNA (rDNA) region. The primers used were identical to those used in the laboratory-developed assay (9) as described above under “Laboratory-developed real-time PCR assay.” Amplification reactions were performed in a volume of 50 µl with PCR buffer (HotstarTaq master mix; Qiagen, Germany), 5 mM MgCl2, 3 pmol of each D. fragilis-specific primer, and 10 µl of the DNA sample. Amplification consisted of 15 min at 95°C followed by 50 cycles of 15 s at 95°C and 60 s at 60°C. The PCR product was analyzed by electrophoresis on 1.0% agarose gels for quality and concentration. Samples were then run through the Illumina MiSeq workflow as specified by the AGRF, with the Illumina bcl2fastq 2.20.0.422 pipeline used to generate sequence data.
Eukaryotic diversity profiling.
All samples that registered positive qPCR results were submitted to the AGRF for sequencing of the 18S ribosomal DNA to identify possible causes of cross-reactivity. The DNA samples were prepared according to AGRF instructions, at a concentration of greater than 10 ng/μl and with A260/A280 ratios in the range of 1.6 to 1.9. Upon receipt of these DNA samples, the AGRF performed a PCR targeting the 18S Euk1391F-EukBR target of the 18S ribosomal subunit DNA using the forward primer sequence 5′-GTACACACCGCCCGTC-3′ and the reverse primer sequence 5′-TGATCCTTCTGCAGGTTCACCTAC-3′. The amplicons from each sample were then sequenced in multiplex, on the Illumina MiSeq platform, utilizing Illumina’s Nextera XT v2 indices and paired-end sequencing chemistry. In addition to the paired-end .fastq sequence files generated, a report was generated including a description of the operational taxonomic units (OTUs), their relative abundances, and raw read counts for all eukaryotic taxa detected in each sample.
RESULTS
Real-time qPCR. (i) EasyScreen assay.
All 50 samples from group 1 returned positive results using the EasyScreen assay, with CT values ranging between 21.47 and 38.37 (Table 1). The EasyScreen assay returned negative results for all 200 group 2 samples (Table 2).
TABLE 1.
Group 1 real-time PCR results using the laboratory-developed real-time assay for the detection of Dientamoeba fragilisa
| Assay | Platform | No. positive | No. negative | % positive | CT range |
|---|---|---|---|---|---|
| EasyScreen (10) | Bio-Rad CFX96 | 50 | 0 | 100 | 21.47–38.37 |
| Laboratory-developed real-time PCR assay (9) | Cepheid SmartCycler II | 50 | 0 | 100 | 17.36–36.83 |
| Roche LightCycler 480 | 42 | 8 | 84 | 23.40–43.75 | |
| Bio-Rad CFX96 | 48 | 2 | 96 | 23.34–48.91 | |
| ABI 7500 | 49 | 1 | 98 | 17.94–38.07 |
Samples from this group had all previously tested positive for the presence of Dientamoeba fragilis using the EasyScreen assay.
TABLE 2.
Group 2 real-time PCR results using the laboratory-developed real-time assay for the detection of Dientamoeba fragilis prior to implementation of fluorescence thresholds or CT cutoff valuesa
| Assay | Platform | No. positive | No. negative | % positive | CT range |
|---|---|---|---|---|---|
| EasyScreen (10) | Bio-Rad CFX96 | 0 | 200 | 0 | 0–0 |
| Laboratory-developed real-time PCR assay (9) | Cepheid SmartCycler II | 15 | 185 | 8.1 | 32.00–45.16 |
| Roche LightCycler 480 | 4 | 196 | 2.0 | 39.59–45.00 | |
| Bio-Rad CFX96 | 14 | 186 | 7.5 | 37.53–47.61 | |
| ABI 7500 | 6 | 194 | 3.0 | 32.34–40.11 |
Samples from this group had all previously tested negative for the presence of Dientamoeba fragilis using the EasyScreen assay.
(ii) Laboratory-developed real-time PCR.
All 50 samples from group 1 returned positive results with 100% sensitivity using the laboratory-developed real-time assay on the SmartCycler (Cepheid), with CT values ranging between 17.36 and 36.83. The majority of CT values were below 35 (94%), with a mean CT value of 27.3 (Table 1). When tested on the Roche LC 480, 42 samples returned positive results, with 8 negatives. CT values were registered much later in general on the LC 480 than on the SmartCycler II (Cepheid), with ranges between 33.4 and 43.75 and a mean CT of 35.1. The ABI 7500 (ABI) platform returned positive results for all but one of the group 1 samples, with CT values ranging between 17.94 and 38.07 and a mean CT of 29.98. When run on the CFX96 (Bio-Rad) real-time PCR platform, 48 samples returned positive results, with 2 negatives. Cycle threshold ranges obtained from the CFX96 (Bio-Rad) were the latest of the three platforms tested with CT values registering from 23.34 up to a value of 47.61 with a mean of 39.3.
Two hundred samples from group 2 were screened using the laboratory-developed real-time assay on a variety of thermocycler platforms (Table 2). Fifteen of the 200 samples returned positive results for the presence of D. fragilis on the SmartCycler II (Cepheid). These positive results displayed CT values ranging from 32.00 to 45.16, although the majority of samples (73%) held CT values greater than 35. Only one sample held a CT value less than 37. Incidentally, the limit of detection for this platform was determined to be 1 trophozoite per ml, equating to a CT value of 36.9. By applying a cycle threshold cutoff value of 37, many false positives could be excluded (Table 3), raising the specificity of the assay from 94.5 to 99.5% and increasing the positive predictive value (PPV) of the laboratory-designed assay from 83.1% to 98.2% (Table 4). As shown in Fig. 1, fluorescence intensities for many group 2 samples were equal to or in excess of those from group 1, and therefore, no fluorescence threshold could be determined which would exclude only false-positive results.
TABLE 3.
Group 2 real-time PCR results using the laboratory-developed real-time assay for the detection of Dientamoeba fragilis following implementation of fluorescence thresholds or CT cutoff values
| Assay | Platform | No. positive | No. negative | % positive | CT range |
|---|---|---|---|---|---|
| EasyScreen (10) | Bio-Rad CFX96 | 0 | 200 | 0 | 0–0 |
| Laboratory-developed real-time PCR assay (9) | Cepheid SmartCycler II (CT cutoff, 37) | 5 | 195 | 2.5 | 32.00–45.16 |
| Roche LightCycler 480a | 4 | 196 | 2.0 | 39.59–45.00 | |
| Bio-Rad CFX96 (threshold, 12,000 RFU × 103) | 2 | 198 | 1.0 | 46.93–47.61 | |
| ABI 7500 (CT cutoff, 38) | 4 | 194 | 3.0 | 32.34–40.11 |
True-positive and false-positive results could not be distinguished via CT cutoff values or fluorescence thresholds on these platforms. (True positives were established via amplicon sequencing.)
TABLE 4.
Analytical sensitivity data of laboratory designed assay before and after recommendationsa
| Platform | Sensitivity (%) |
Specificity (%) |
PPV (%) |
NPV (%) |
||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |
| Cepheid SmartCycler II | 100.0 | 100.0 | 94.4 | 99.5 | 83.1 | 98.2 | 100.0 | 100.0 |
| Roche LightCycler 480b | 77.8 | 77.8 | 98.0 | 98.0 | 91.3 | 91.3 | 94.1 | 94.1 |
| Bio-Rad CFX96 | 88.9 | 88.9 | 92.9 | 99.0 | 77.4 | 96.0 | 96.8 | 97.0 |
| ABI 7500 | 92.6 | 98.0 | 97.4 | 98.0 | 90.9 | 92.5 | 97.9 | 99.5 |
PPV, positive predictive value; NPV, negative predictive value. True positives and negatives were defined by NGS amplicon sequencing results.
True-positive and false-positive results could not be distinguished via CT cutoff values or fluorescence thresholds on these platforms.
FIG 1.
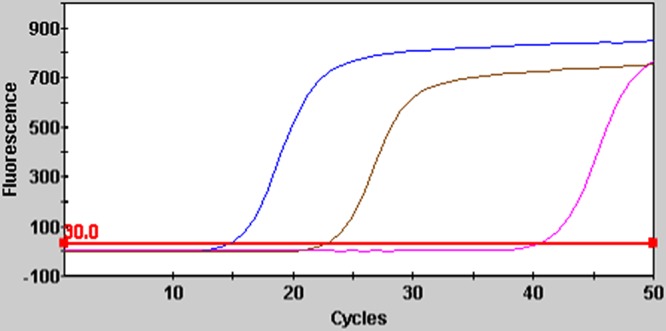
Fluorescence curves for laboratory developed real-time PCR using the Cepheid SmartCycler II. Blue, Dientamoeba fragilis positive control; gold, group 1 positive result (D. fragilis DNA presence confirmed via amplicon sequencing); pink, group 2 positive result (D. fragilis DNA not detected via amplicon sequencing).
Using the CFX96 platform (Bio-Rad), the laboratory-developed real-time PCR assay returned 14 positive results, all of which held CT values greater than 35. The limit of detection for this platform was determined to be 1 trophozoite per ml, equating to a CT of 35.39, although it was found that many group 1 samples had significantly higher CT values than this; therefore, no CT cutoff value could be implemented without excluding true-positive results. However, as shown in Fig. 2, group 2 positive samples exhibited lower fluorescence intensities than true positives. Therefore, by applying a fluorescence threshold of 12,200 (RFU × 103), all but 2 false-positive results were able to be excluded (Table 3), leaving only true-positive CT values. This threshold value could not be increased without excluding true positives. Application of the fluorescence threshold value increased sensitivity from 92.9 to 99.0% and increased the PPV of the CFX96 (Bio-Rad) platform from 77.4% to 96% (Table 4).
FIG 2.
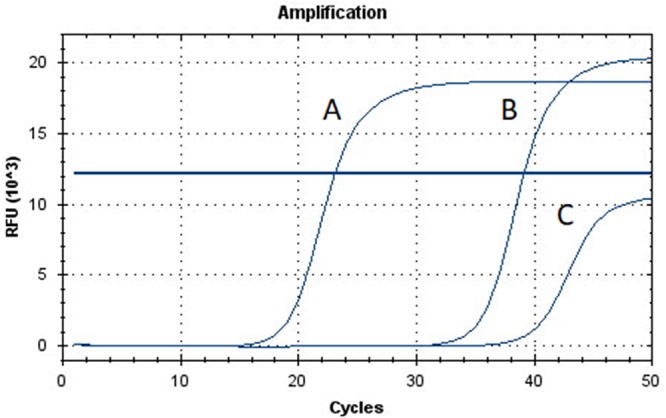
Fluorescence curves for laboratory-developed real-time PCR using the Bio-Rad CFX96. A, Dientamoeba fragilis positive control; B, group 1 positive result (D. fragilis DNA presence confirmed via amplicon sequencing); C, group 2 positive result (D. fragilis DNA not detected via amplicon sequencing). A fluorescence threshold of 12,000 RFU × 103 was used.
The ABI 7500 (ABI) platform returned 6 positive results (out of 200) from group 2 samples, with CT values ranging between 32.34 and 40.11. Sixty-seven percent of positive samples from group 2 were beyond a CT value of 35 on the ABI 7500. The limit of detection for this platform was determined to be 1 trophozoite per ml, equating to a CT of 34.2. Several samples shown to be positive for D. fragilis returned CT values higher than 34.2, although by applying a CT cutoff value of 38 two false positive results were able to be excluded, bringing the total amount of false-positive samples down to 4 on this platform (Table 3). This reduction increased the sensitivity of the assay from 97.4 to 98 and increased the PPV of the ABI7500 (ABI) platform from 90.9% to 92.5% (Table 4). Finally, the 200 samples were screened using the LightCycler 480 (Roche), returning only 4 positives, all with CT values greater than 35. The limit of detection for this platform was determined to be 1 trophozoite per ml, equating to a CT value of 35.16, but once again, several samples with D. fragilis positive next-generation sequencing (NGS) results returned significantly higher CT values than this; consequently, no CT cutoff value could be implemented without excluding true-positive results. Positive results from group 2 were not consistent across all platforms; individual results are shown in Table 5.
TABLE 5.
Individual laboratory-developed real-time PCR results for positive group 2 samples using multiple PCR platforms
| Sample | Cepheid SmartCycler II | Bio-Rad CFX96 | Roche LightCycler 480 | ABI 7500 | NGS |
|---|---|---|---|---|---|
| 3 | + | − | − | − | − |
| 4 | + | − | − | − | − |
| 7 | + | − | − | + | − |
| 8 | − | + | − | − | − |
| 11 | + | + | − | − | − |
| 13 | + | − | − | − | − |
| 16 | − | + | − | − | − |
| 23 | − | + | − | − | − |
| 28 | + | − | − | − | − |
| 31 | + | − | − | − | − |
| 40 | − | − | − | + | − |
| 49 | − | + | − | − | − |
| 51 | − | − | − | + | − |
| 59 | − | − | − | + | − |
| 52 | − | − | + | − | − |
| 61 | − | + | − | − | − |
| 64 | − | + | − | − | − |
| 77 | − | + | − | − | − |
| 94 | − | − | + | − | − |
| 102 | + | − | − | − | − |
| 106 | − | + | − | − | − |
| 109 | − | − | + | − | − |
| 111 | + | − | − | − | − |
| 114 | − | − | + | − | − |
| 122 | − | + | − | − | − |
| 124 | + | − | − | − | + |
| 130 | + | − | − | − | + |
| 140 | + | − | − | + | + |
| 141 | + | − | − | − | − |
| 154 | − | + | − | − | − |
| 163 | + | − | − | − | − |
| 170 | + | − | − | − | + |
| 178 | − | + | − | − | − |
| 185 | − | − | − | + | − |
| 186 | − | + | − | − | − |
| 195 | − | + | − | − | − |
5.8S rDNA PCR amplicon sequencing.
True and false positives were determined via PCR amplicon sequencing. In total, 82 samples were analyzed by PCR amplicon sequencing, 50 from group 1 and 32 from group 2. PCR amplification and sequencing were successful for all 50 samples sent from group 1, amplifying a 98-bp fragment within the D. fragilis 5.8S ribosomal DNA region. The number of reads generated varied between samples, ranging from 561 to 523,933. Of the 32 samples from group 2, only 4 resulted in successful amplification and sequencing of the 5.8S ribosomal DNA region, with the number of reads ranging between 8,322 and 87,192. BLAST analysis matched sequences from all samples that produced reads to D. fragilis 5.8S ribosomal DNA with 99% identity. These results led us to conclude that, in total, 54 of the 250 samples tested did in fact contain D. fragilis DNA (group 1, 50/50; group 2, 4/200). Only the SmartCycler II (Cepheid) returned positive results for all four group 2 samples where D. fragilis sequences were identified.
Eukaryotic diversity profiling.
In total, 18S diversity profiling on samples from group 1 yielded 42 individual eukaryotic observational taxonomical units (OTUs), although the majority of eukaryotic abundance was dominated by 12 OTUs, the remainder having a total relative abundance of less than 1%. Of the predominant 12 OTUs, only 3 were related to protozoa, consisting of an unknown trichomonad, followed by Blastocystis spp. and then D. fragilis. The 34 samples sequenced from group 2 yielded 38 eukaryotic OTUs but zero abundance of trichomonad-related OTUs.
DISCUSSION
Dientamoeba fragilis has been shown to be a prominent gastrointestinal protozoan worldwide, often surpassing Giardia in some demographics (17, 27). While its vast distribution cannot be denied, assertations on its pathogenesis are harder to establish. Multiple reports have associated D. fragilis carriage with abdominal pain, discomfort, and diarrhea (20, 27), followed by symptom resolution after elimination of D. fragilis trophozoites using therapy (28–30). Contradicting this partial fulfillment of Koch’s postulates are additional reports detailing asymptomatic carriage, sometimes even within immediate family members of symptomatic patients (1, 31, 32). Understandably, this makes establishing pathogenesis difficult, although this problem is not unique to D. fragilis: Giardia and E. histolytica exhibit similar etiologies, yet both are widely regarded as significant pathogens (33–35). Proponents for and against the pathogenesis of D. fragilis exist, with opinions largely centered around apparent prevalence rates.
Prevalence rates of 43% from routine screening (22) and 68.3% of children in Danish day care centers prompted those authors to ascertain that D. fragilis was indeed a commensal (4). Both of these publications cite the method described in reference 9 as their method for real-time PCR detection of D. fragilis but fail to mention CT ranges, sensitivity thresholds or which real-time PCR platform and analysis software were used to evaluate results. Considering the unusually high prevalence rates stemming from these studies and other research articles (4, 22, 36, 37) using the laboratory-developed real-time D. fragilis assay, it became prudent to validate the effectiveness of this assay using samples previously screened for the presence of D. fragilis using the EasyScreen assay on the CFX384 (Bio-Rad), one of several platforms for which the EasyScreen assay has been validated by the manufacturer (38). It is important to note that the EasyScreen assay is not yet widely used in Europe, and there are few prevalence studies and diagnostic laboratories that have utilized this assay in this region.
Fifty samples positive for D. fragilis (group 1) and 200 samples negative for D. fragilis (group 2) were rescreened using the laboratory-developed real-time assay on a variety of platforms. The results raise an important concern regarding the reproducibility and specificity of this assay if run without clearly defined conditions such as cycle threshold cutoff values, fluorescence thresholds, or standardized reagents. Samples from group 1 returned results analogous to those of the EasyScreen assay when tested on the SmartCycler II (Cepheid), although several samples did return negative results in the Roche and Bio-Rad systems. Amplicon sequencing across all samples revealed D. fragilis DNA in 54 samples across both groups tested. Considering that four samples from group 2 returned D. fragilis sequences, it was apparent that the Genetic Signatures EasyScreen assay initially missed these samples during routine screening. These four samples from group 2 were identified only using the laboratory-developed real-time assay on the SmartCycler II (Cepheid) platform, although CT values were relatively late, with values between 32 and 37, suggesting a very low parasite load in the original clinical sample, as CT values decrease with dilution of trophozoite concentration (11).
The multiple positive results from group 2 highlight the importance of sensitivity cutoff values and fluorescence thresholds being implemented where possible, and where not possible, these thermocyclers and associated analysis platforms must be avoided for particular assays. Conventional PCR and sequencing confirmed the presence of D. fragilis DNA within all group 1 samples, but only four group 2 samples, confirming the majority of group 2 PCR results to be false positives before CT cutoff values or fluorescence thresholds were implemented. The laboratory-developed assay specifies amplification to be run for 50 cycles but offers no value at which a positive result should be interpreted as beyond limits of detection (9). Sensitivity data indicated the laboratory-developed real-time assay to be reproducible to a limit of 1 trophozoites per ml of liquid stool, corresponding to a CT value of 36.9 when using the SmartCycler II (Cepheid). Based on this information, these findings suggest that CT values above 37 are implausibly detecting fractions of a single trophozoite when using this platform. Despite this, multiple samples from group 2 registered CT values much greater than 35, going as high as 47.61. A CT value of this level does not necessarily suggest a true detection of D. fragilis, and indeed this was proven for the majority of samples in group 2 via PCR amplicon sequencing. CT values for group 1 samples where D. fragilis 5.8S rDNA sequences were identified did not exceed a value of 37 on the SmartCycler, although true-positive results were often beyond the limit of detection on other platforms. In light of this, future use of the laboratory-developed real-time assay must be preempted by sensitivity and LOD testing before use. Of the five samples that remained after implementing a cutoff value of 37, only one returned a negative result after next-generation sequencing, indicating that implementation of this CT cutoff value excluded all but one of the false-positive results on the Cepheid thermocycler.
Limits of detection for each platform were exceeded by both positive and negative samples in many cases and highlight the difficulty in reproducing accurate results when using the laboratory-designed assay. Since no CT cutoff value could be found to distinguish true and false positives when using the Bio-Rad CFX96 platform, it is recommended that any future use of the D. fragilis laboratory-developed real-time assay on this platform implement an independently verified fluorescence threshold to minimize false-negative results. A fluorescence threshold of 12,000 RFU × 103 was implemented in this case, but it must be recognized that this is an unusually high threshold for fluorescence and could exclude true-positive results if not independently verified.
No CT cutoff value or fluorescence threshold could be found that reliably distinguished true and false positives on the Roche LC 480. By using a cutoff value of 38 when using the ABI 7500 (ABI), false positives were reduced to 4 samples, marginally increasing the effectiveness of this platform.
Eukaryotic 18S diversity profiling was performed to identify the presence of any potential species for cross-reactivity within each sample that returned a positive PCR result. Eukaryotic OTUs in group 2 were predominantly limited to a number of fungi and Chordata, likely remnants from the patient’s diet. The laboratory-developed real-time assay was shown to be cross-reactive with T. foetus (11) although no evidence of T. foetus sequences could be found, which is not surprising considering that zoonotic transmission to humans is opportunistic and uncommon (39). Ultimately, 18S diversity profiling ruled out the presence of any closely related trichomonad species that could account for cross-reactivity within the samples.
Without correct validation of detection assays, prevalence numbers can be significantly misrepresented in certain demographics, impeding accurate surveillance of potential pathogens. Epidemiological differences will always exist and must be accounted for, but epidemiology alone cannot be attributed to prevalence differences in the ranges of 30% to 40% for a protozoan with fecal-oral transmission in nations with well-developed sanitation. Incidentally, since the development of the laboratory-developed real-time assay in 2007, the highest D. fragilis prevalence rates have been attributed to nations where this assay is the primary diagnostic tool for the detection of D. fragilis (4, 36, 37, 40). Important details are often absent in these papers, with authors simply citing the original reference without specifying what real-time PCR platforms were used or what, if any, sensitivity cutoff values or fluorescence thresholds were used. The responsibility lies with both authors and publishers to ensure that sufficient details are included in research articles, so that results can be interpreted accurately and experiments can be replicated. Consequently, the use of diagnostic assays in an inappropriate way can lead researchers to make false assumptions regarding pathogenicity when making decisions on prevalence alone. In the absence of full genomic sequencing and data from animal models, declarations on the pathogenicity of D. fragilis must be resisted. Regardless of the pathogenicity of D. fragilis, a prevalence rate of 42.7% for an organism whose only known transmission is fecal-oral suggests an improbably poor standard of hygiene for a highly developed nation such as Denmark.
In conclusion, the Genetic Signatures EasyScreen assay for detection of gastrointestinal pathogens has been shown to be an accurate methodology for the measurement of D. fragilis prevalence, and we recommend that the EasyScreen assay be used in future studies. In cases where the Genetic Signatures EasyScreen assay is not available, researchers must take great care to validate the assay on their own platforms. Researchers must acknowledge that all laboratories are different and unless initial publications are thorough in their recommendations, any laboratory planning to run an assay must account for variation in results due to divergence from published reagents or techniques. Cycle thresholds can and will vary across different platforms and different master mix buffers, different polymerases, and different DNA extraction methods, in turn affecting cycle threshold cutoff values. Researchers using a laboratory-designed assay must first evaluate it accordingly and develop their own in-house thresholds for detection using a cohort of independently characterized positive and negative samples. If thresholds that clearly distinguish true from false positives cannot be ascertained, then that assay should not be used. In any case, if an assay is to be used, it is recommended that any deviations from previously published methods are reported when publishing prevalence data. Based on results from this study, and depending on the real-time PCR platform used, true prevalence rates for nations using the laboratory-developed real-time assay may be approximately 9% lower than reported unless specific cutoff values and thresholds are implemented. However, this is clearly a minimum suggestion, and the authors of those studies should review their findings with proper consideration of the data presented here.
REFERENCES
- 1.Stark D, Barratt J, Chan D, Ellis JT. 2016. Dientamoeba fragilis, the neglected trichomonad of the human bowel. Clin Microbiol Rev 29:553–580. doi: 10.1128/CMR.00076-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jepps MW, Dobell C. 1918. Dientamoeba fragilis n. g., n. sp., a new intestinal amoeba from man. Parasitology 10:352–367. doi: 10.1017/S0031182000003929. [DOI] [Google Scholar]
- 3.Barratt JL, Harkness J, Marriott D, Ellis JT, Stark D. 2011. A review of Dientamoeba fragilis carriage in humans: several reasons why this organism should be considered in the diagnosis of gastrointestinal illness. Gut Microbes 2:3–12. doi: 10.4161/gmic.2.1.14755. [DOI] [PubMed] [Google Scholar]
- 4.Jokelainen P, Hebbelstrup Jensen B, Andreassen BU, Petersen AM, Roser D, Krogfelt KA, Nielsen HV, Stensvold CR. 2017. Dientamoeba fragilis, a commensal in children in Danish day care centers. J Clin Microbiol 55:1707–1713. doi: 10.1128/JCM.00037-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz MD, Nelson ME. 2003. Dientamoeba fragilis infection presenting to the emergency department as acute appendicitis. J Emerg Med 25:17–21. doi: 10.1016/S0736-4679(03)00104-5. [DOI] [PubMed] [Google Scholar]
- 6.Stark D, Roberts T, Marriott D, Harkness J, Ellis JT. 2012. Detection and transmission of Dientamoeba fragilis from environmental and household samples. Am J Trop Med Hyg 86:233–236. doi: 10.4269/ajtmh.2012.11-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Windsor JJ, Johnson EH. 1999. Dientamoeba fragilis: the unflagellated human flagellate. Br J Biomed Sci 56:293–306. [PubMed] [Google Scholar]
- 8.Windsor JJ, Rafay AM. 1997. Laboratory detection of Dientamoeba fragilis. Br J Biomed Sci 54:223–224. [PubMed] [Google Scholar]
- 9.Verweij JJ, Mulder B, Poell B, van Middelkoop D, Brienen EA, van Lieshout L. 2007. Real-time PCR for the detection of Dientamoeba fragilis in fecal samples. Mol Cell Probes 21:400–404. doi: 10.1016/j.mcp.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Stark D, Roberts T, Ellis JT, Marriott D, Harkness J. 2014. Evaluation of the EasyScreen (TM) enteric parasite detection kit for the detection of Blastocystis spp., Cryptosporidium spp., Dientamoeba fragilis, Entamoeba complex, and Giardia intestinalis from clinical stool samples. Diagn Microbiol Infect Dis 78:149–152. doi: 10.1016/j.diagmicrobio.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Chan D, Barratt J, Roberts T, Phillips O, Šlapeta J, Ryan U, Marriott D, Harkness J, Ellis J, Stark D. 2016. Detection of Dientamoeba fragilis in animal faeces using species specific real time PCR assay. Vet Parasitol 227:42–47. doi: 10.1016/j.vetpar.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 12.Barratt JLN, Banik GR, Harkness J, Marriott D, Ellis JT, Stark D. 2010. Newly defined conditions for the in vitro cultivation and cryopreservation of Dientamoeba fragilis: new techniques set to fast track molecular studies on this organism. Parasitology 137:1867–1878. doi: 10.1017/S0031182010000764. [DOI] [PubMed] [Google Scholar]
- 13.Stark D, Beebe N, Marriott D, Ellis J, Harkness J. 2007. Dientamoeba fragilis as a cause of travelers’ diarrhea: report of seven cases. J Travel Med 14:72–73. doi: 10.1111/j.1708-8305.2006.00100.x. [DOI] [PubMed] [Google Scholar]
- 14.Calderaro A, Gorrini C, Montecchini S, Peruzzi S, Piccolo G, Rossi S, Gargiulo F, Manca N, Dettori G, Chezzi C. 2010. Evaluation of a real-time polymerase chain reaction assay for the detection of Dientamoeba fragilis. Diagn Microbiol Infect Dis 67:239–245. doi: 10.1016/j.diagmicrobio.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 15.Calderaro A, Montecchini S, Rossi S, Gorrini C, De Conto F, Medici MC, Chezzi C, Arcangeletti MC. 2014. Intestinal parasitoses in a tertiary-care hospital located in a non-endemic setting during 2006–2010. BMC Infect Dis 14:264. doi: 10.1186/1471-2334-14-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crotti D, D’Annibale ML. 2007. Intestinal infections caused by Dientamoeba fragilis and Giardia duodenalis in our experience. Recenti Prog Med 98:361–366. (In Italian.) [PubMed] [Google Scholar]
- 17.Girginkardesler N, Coskun S, Balcioglu IC, Ertan P, Ok UZ. 2003. Dientamoeba fragilis, a neglected cause of diarrhea, successfully treated with secnidazole. Clin Microbiol Infect 9:110–113. doi: 10.1046/j.1469-0691.2003.00504.x. [DOI] [PubMed] [Google Scholar]
- 18.Jimenez S, Cortez J, Diaz M, Duran C, Hidalgo G, Aguilera W, Nakal S, Albano C, Incani RN, Rodriguez-Morales A. 2010. Prevalence of Dientamoeba fragilis among asymptomatic individuals from North Central Venezuela. Int J Infect Dis 14:E295–E296. doi: 10.1016/j.ijid.2010.02.2142. [DOI] [Google Scholar]
- 19.Millet V, Spencer MJ, Chapin M, Stewart M, Yatabe JA, Brewer T, Garcia LS. 1983. Dientamoeba fragilis, a protozoan parasite in adult members of a semicommunal group. Dig Dis Sci 28:335–339. doi: 10.1007/BF01324950. [DOI] [PubMed] [Google Scholar]
- 20.Norberg A, Nord CE, Evengard B. 2003. Dientamoeba fragilis—a protozoal infection which may cause severe bowel distress. Clin Microbiol Infect 9:65–68. doi: 10.1046/j.1469-0691.2003.00459.x. [DOI] [PubMed] [Google Scholar]
- 21.Preiss U, Ockert G, Bromme S, Otto A. 1990. Dientamoeba-fragilis infection, a cause of gastrointestinal symptoms in childhood. Klin Padiatr 202:120–123. doi: 10.1055/s-2007-1025503. [DOI] [PubMed] [Google Scholar]
- 22.Roser D, Simonsen J, Nielsen HV, Stensvold CR, Molbak K. 2013. Dientamoeba fragilis in Denmark: epidemiological experience derived from four years of routine real-time PCR. Eur J Clin Microbiol Infect Dis 32:1303–1310. doi: 10.1007/s10096-013-1880-2. [DOI] [PubMed] [Google Scholar]
- 23.Vandenberg O, Peek R, Souayah H, Dediste A, Buset M, Scheen R, Retore P, Zissis G, van Gool T. 2006. Clinical and microbiological features of dientamoebiasis in patients suspected of suffering from a parasitic gastrointestinal illness: a comparison of Dientamoeba fragilis and Giardia lamblia infections. Int J Infect Dis 10:255–261. doi: 10.1016/j.ijid.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 24.Windsor JJ, Rafay AM, Shenoy AK, Johnson EH. 1998. Incidence of Dientamoeba fragilis in faecal samples submitted for routine microbiological analysis. Br J Biomed Sci 55:172–175. [PubMed] [Google Scholar]
- 25.Schuster H, Jackson RS. 2009. Prevalence of Dientamoeba fragilis among patients consulting complementary medicine practitioners in the British Isles. J Clin Pathol 62:182. doi: 10.1136/jcp.2008.059659. [DOI] [PubMed] [Google Scholar]
- 26.Siah SP, Merif J, Kaur K, Nair J, Huntington PG, Karagiannis T, Stark D, Rawlinson W, Olma T, Thomas L, Melki JR, Millar DS. 2014. Improved detection of gastrointestinal pathogens using generalised sample processing and amplification panels. Pathology 46:53–59. doi: 10.1097/PAT.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 27.Stark D, Barratt J, Roberts T, Marriott D, Harkness J, Ellis J. 2010. A review of the clinical presentation of dientamoebiasis. Am J Trop Med Hyg 82:614–619. doi: 10.4269/ajtmh.2010.09-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kean BH, Malloch CL. 1966. The neglected ameba: Dientamoeba fragilis. A report of 100 pure infections. Am J Dig Dis 11:735–746. doi: 10.1007/BF02239427. [DOI] [PubMed] [Google Scholar]
- 29.Stark DJ, Beebe N, Marriott D, Ellis JT, Harkness J. 2006. Dientamoebiasis: clinical importance and recent advances. Trends Parasitol 22:92–96. doi: 10.1016/j.pt.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Yang J, Scholten T. 1977. Dientamoeba fragilis: a review with notes on its epidemiology, pathogenicity, mode of transmission, and diagnosis. Am J Trop Med Hyg 26:16–22. doi: 10.4269/ajtmh.1977.26.16. [DOI] [PubMed] [Google Scholar]
- 31.Preiss U, Ockert G, Broemme S, Otto A. 1991. On the clinical importance of Dientamoeba fragilis infections in childhood. J Hyg Epidemiol Microbiol Immunol 35:27–34. [PubMed] [Google Scholar]
- 32.Lukeš J, Stensvold CR, Jirků-Pomajbíková K, Wegener Parfrey L. 2015. Are human intestinal eukaryotes beneficial or commensals? PLoS Pathog 11:e1005039. doi: 10.1371/journal.ppat.1005039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ish-Horowicz M, Korman SH, Shapiro M, Har-Even U, Tamir I, Strauss N, Deckelbaum RJ. 1989. Asymptomatic giardiasis in children. Pediatr Infect Dis J 8:773–779. doi: 10.1097/00006454-198911000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Coyle CM, Varughese J, Weiss LM, Tanowitz HB. 2012. Blastocystis: to treat or not to treat… . Clin Infect Dis 54:105–110. doi: 10.1093/cid/cir810. [DOI] [PubMed] [Google Scholar]
- 35.Marie C, Petri WA Jr. 2014. Regulation of virulence of Entamoeba histolytica. Annu Rev Microbiol 68:493–520. doi: 10.1146/annurev-micro-091313-103550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holtman GA, Kranenberg JJ, Blanker MH, Ott A, Lisman-van Leeuwen Y, Berger MY. 2017. Dientamoeba fragilis colonization is not associated with gastrointestinal symptoms in children at primary care level. Fam Pract 34:25–29. doi: 10.1093/fampra/cmw111. [DOI] [PubMed] [Google Scholar]
- 37.Enserink R, Scholts R, Bruijning-Verhagen P, Duizer E, Vennema H, De Boer R, Kortbeek T, Roelfsema J, Smit H, Kooistra-Smid M, Van Pelt W. 2014. High detection rates of enteropathogens in asymptomatic children attending day care. PLoS One 9:e89496. doi: 10.1371/journal.pone.0089496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Genetic Signatures. 2018. EasyScreen™ EP001 & EP001-HT enteric protozoan user guide ver 6.1. Genetic Signatures, Newtown, NSW, Australia. [Google Scholar]
- 39.Suzuki J, Kobayashi S, Osuka H, Kawahata D, Oishi T, Sekiguchi K, Hamada A, Iwata S. 2016. Characterization of a human isolate of Tritrichomonas foetus (cattle/swine genotype) infected by a zoonotic opportunistic infection. J Vet Med Sci 78:633–640. doi: 10.1292/jvms.15-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roser D, Nejsum P, Carlsgart AJ, Nielsen HV, Stensvold CR. 2013. DNA of Dientamoeba fragilis detected within surface-sterilized eggs of Enterobius vermicularis. Exp Parasitol 133:57–61. doi: 10.1016/j.exppara.2012.10.009. [DOI] [PubMed] [Google Scholar]


