Abstract
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disorder of the motor neuron, which selectively affects it both at central (first motor-neuron) and peripheral level (second motor-neuron). The disease shows up at a mean age of 56 years and the most affected are males. Although ALS may start as a bulbar or spinal disease, with the progression of the disease typically both become evident. Pharmacological approved treatments for ALS are still limited and include riluzole and edaravone which improve survival over time. Despite this, ALS leads to progressive muscle involvement and requires a complex multidisciplinary approach to manage increasing disability which goes beyond motor neurons. Sialorrhea is, amongst others, one of the most disabling symptoms in ALS. The complexity in managing saliva is due to a muscular spasticity and to a scarce palatino-lingual muscles control, rather than to an overproduction of saliva. These features could increase the risk of aspiration pneumonia and limit the use of noninvasive mechanical ventilation. We reviewed the treatment for sialorrhea in ALS patients that are available at this time, emphasizing pros and cons for each approach. Our purpose is to create a practical tool for the diagnosis, in order to facilitate the quantification and management of sialorrhea in everyday practice.
Keywords: amyotrophic lateral sclerosis, sialorrhea, botulinum toxin, radiotherapy salivary gland, anticholinergic drug
Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disorder of the motoneurone, which selectively affects the motor neurons, both at central (first moto-neuron) and peripheral level (second motor neuron). Its prevalence is around 4–6 people per 100,000 population .1 The disease begins at a mean age of 56 years and the most affected are males.2 ALS may begin as a bulbar or spinal disease but as with the progression of the disease, typically both become evident. The bulbar onset forms have a median survival of 20 months while a relatively longer survival is described for the spinal onset forms (29 months).1
Pharmacological approved treatments for ALS are still limited and include riluzole and edaravone which improve survival over time.2 Despite this, ALS leads to progressive muscle involvement and requires a complex multidisciplinary approach to manage increasing disability which goes beyond motor neurons.
Sialorrhea is amongst others, one of the most disabling symptoms in ALS.3 This is particularly relevant in patients with bulbar palsy experience in whom sialorrhea is associated with mucous secretions and saliva along with an impairment of ability to swallow secretions but not due to an increasing of saliva production: this pathological alteration is caused by tongue spasticity, orofacial and palatino-lingual muscle control failure, facial muscular weakness, as well as to an inability to maintain oral and buccal competence.3,4
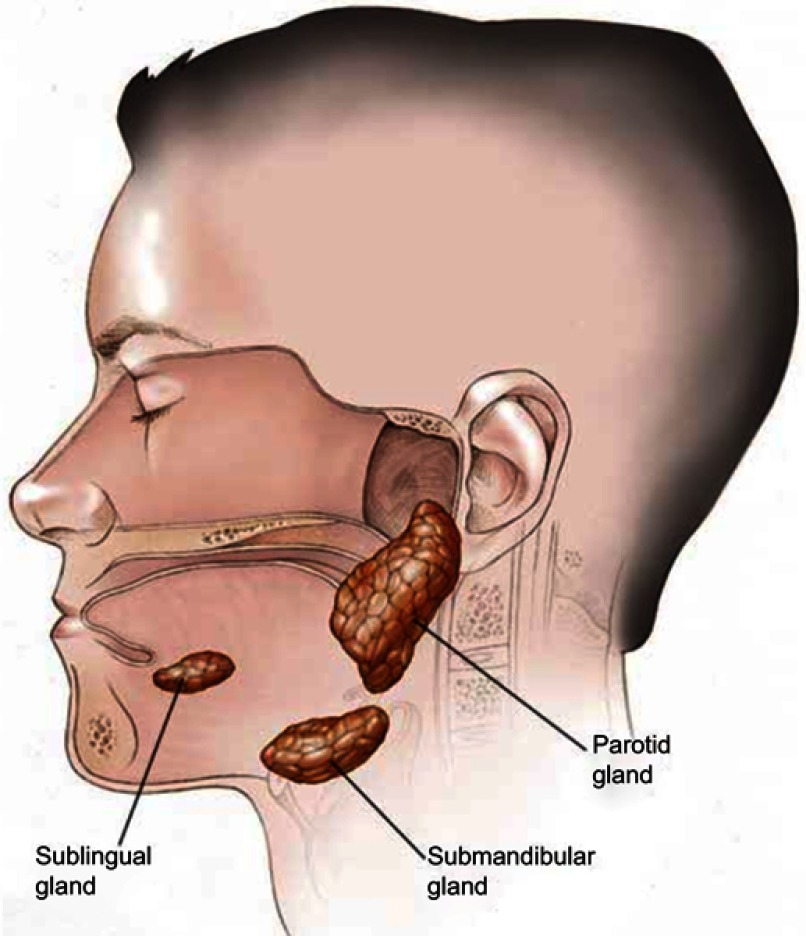
Major salivary glands producing saliva are three: parotid, submandibular and sublingual (Figure 1).
Figure 1.
Anatomy of salivary glands.
Moreover, there are several minor salivary glands diffused in the oral cavity. Saliva is mainly made up of water, electrolytes, proteins and enzymes. From the production to the secretive phase through the reabsorption of electrolytes, saliva is subjected to significant modifications. It changes from being isotonic in glandular acini to hypotonic in the glands ductal system. Salivar proteins are constantly produced and stored inside cellular granules; electrolytic secretion is mainly supported by parasimpatic stimulation, while protein secretion is activated by simpatic stimulation. However, there is an interaction between the two regulatory systems. Under stimulation, the main production of saliva comes from the parotid glands (66% of the total flow) and around 1.0–1.5 L of saliva are secreted everyday by a healthy person and this quantity consists in water, electrolytes, antimicrobials, enzymes and growth factors.5 The main functions of saliva are to facilitate mastication, deglutition and the beginning of digestion. Furthermore, saliva performs an important role in the protection of the oral mucosa and of the teeth.
The innervation of salivary glands is mainly constituted by parasympathetic nerves. The submandibular glands are the major responsibilities for the production of unstimulated saliva. This gland is a mixed but mostly serous gland, whereas the parotid gland, a purely serous gland, secretes saliva mainly during chewing. The sublingual gland is purely mucous.5
Fifty percent of ALS patients is affected by impairment to control saliva production during the course of the disease, and nearly a quarter of patients develop moderate-to-severe symptoms.6 Progressive sialorrhea and drooling can cause skin maceration, soaking of clothes and exacerbation of dysarthria. These patients could develop psychological stress and social embarrassment, decreasing quality of life. The risk of aspiration pneumonia is increased by the presence of secretions in the throat and bronchial tree and by the inability of the patient to perform an effective cough because of the respiratory muscle weakness.7 In ALS patients, pulmonary aspiration syndromes are often underdiagnosed, but they are associated with a high mortality rate.8 In a cohort of 40 consecutive patients with ALS, six aspiration pneumonia events were reported in five cases (13%).9 A study showed that the cause of death in 41% of cases was attributable to bronchopneumonia and in 11% aspiration pneumonia.10 In an analysis of 200 ALS Patients, the most frequent cause of death was a respiratory failure (61%) and the second was pneumonia (9,6%).11 In an Italian study based on discharge diagnosis, the incidence of aspiration pneumonia varied from 1 to 2,1%.12 Tolerance to noninvasive ventilation (NIV) is limited by excessive mucous and drooling and NIV is important for the management of ALS patients because an important increase of life expectancy and quality of life has been shown.13–21
There are substantial differences between the treatment of bronchial secretions and the serious secretion of salivary glands.
In 2001, the American Academy of Neurology recommended both pharmacologic interventions and non-pharmacologic approaches of sialorrhea in ALS patients.22 In 2011, a Cochrane review on the treatment for sialorrhea in people with motor neuron disease and ALS identified only one randomized controlled trial.23 The Authors’ conclusions stated that the use of botulinum toxin injections to salivary glands may be of benefit for the treatment of sialorrhea in MND. Yet emphasis was placed on the fact that more information and further research are needed. There are many reviews in the Cochrane Library which analyze the effectiveness of symptomatic treatment therapies for ALS patients.24 We reviewed treatments for sialorrhea in ALS that are available at this time for ALS emphasizing pros and cons for each approach with the purpose of creating a facilitating practical tool to the diagnosis, quantification and management of sialorrhea in everyday practice.
Measurement of sialorrhea in ALS
In order for clinicians and patients to appreciate the effectiveness of treatment, there needs to be a measurable tool that will be sensitive to changes. Currently, not many scales for the assessment of sialorrhea and particularly in ALS patients have been identified.
In the revised Amyotrophic Lateral Sclerosis Functional Rating Scale, a validated questionnaire to monitor disease progression in ALS, three items assessing the function of bulbar muscles, including one evaluating sialorrhea, have been inserted.25
The Oral Secretion Scale (OSS) has been specifically designed for the evaluation of hypersialorrhea in ALS.26,27 The OSS is not designed to evaluate the effectiveness of invasive treatments for hypersialorrhea.28
The Sialorrhea Scoring Scale: although it has been developed for Parkinson disease, it was used for ALS patients. It does not predict tolerance to NIV.28 These scores should be used before and after the therapy at least at a reasonable time to evaluate the effective therapeutic efficacy (at least two weeks for botulism)
Treatment options
Anticholinergic drugs
Few clinical studies were published about the effectiveness of these drugs on patients with ALS.29 There are five anticholinergic drugs usually used to manage sialorrhea problems in ALS patients: hyoscine hydrobromide/scopolamine (transdermal patch or oral preparation), oral amitriptyline, atropine (sublingual drops, transdermal patch, or tablets), oral propantheline and oral glycopyrronium bromide/glycopyrrolate.
Regarding dosage, this usually needs to be tailored for each patient, either because an initial dose may not provide sufficient symptomatic improvement or because there may be a need to reduce side effects. The selection of the best medication often depends upon the severity and frequency of the drooling and on the severity of dysphagia. In severe cases, the sublingual or transdermal form is clearly preferred. Of note, not all oral medications can be given through a PEG tube because of precipitation or inadequate absorption if ground. Data from the national ALS Patient CARE database indicate that over half of ALS patients responded to treatment with atropine, glycopyrrolate or amitriptyline.22 In a group of patients affected by chronic neurological diseases and drooling, discontinuation rate for scopolamine patches occurred in 13% of patients. The oral administration of these drugs is more often discontinued due to systemic side effects (eg, sedation and delirium), common in elderly patients rather than because these drugs are ineffective. Another side effect of these drugs is the possible thickening of mucous secretions in the throat and lungs, which is a severe complication, by far worse than drooling.30 An alternative to oral administration is the transdermal application. A recent study demonstrated that scopolamine patches are effective for the management of sialorrhea in 85% of the treated subjects.7 However, monotherapy was insufficient and about half of these subjects also required additional therapies 20% had to discontinue scopolamine patches, mostly because of skin reaction. Cutaneous reactions, pupillary abnormality and urinary retention are minor common adverse effects of scopolamine delivered by skin patches. The administration of subcutaneous glycopyrrolate (600 g over 12 hrs via a syringe driver) was reported anecdotally as beneficial in one patient with bulbar onset in whom traditional drugs had been ineffective with minimal side effects, also permitting the use of NIV at night.27
Anticholinergic drugs are not effective in more of 30% of the treated patients. In the long term, these medications are often not a sustainable therapy for sialorrhea in a great number of patients.31 Moreover, given the mean age of onset of ALS patients, there may be comorbidities like heart diseases, glaucoma, pyloric stenosis, prostatic hypertrophy and hepatic and/or renal insufficiency where the use of anticholinergic drugs is contraindicated.
At this time, anticholinergic drugs are still considered “first line” pharmacologic treatment for sialorrhea. Unfortunately, on this date, no randomized trial comparing the efficacy of these different agents in ALS patients was published. To our knowledge, only one double-blind comparative study has evaluated the sialorrhea treatment efficacy with transdermal scopolamine compared to placebo in ALS; this highlighted a decrease in the mean daily volume of saliva in patients treated with scopolamine, however without a significative difference in Visual Analogue Scale.32
Botulinum toxin
Comparing the results of the studies on Botulin toxin treatment is difficult, because there are several heterogeneity of toxin types, dosages, outcome measures and injection technique. One common objective measure in studies using botulinum toxin B was weighing cotton wool balls placed in the mouth before and after treatment. However, this measure is influenced by a number of factors (cotton wool ball position, duration of absorption assessment, methodology). Despite these limitations, the strongest evidence supports the use of botulinum toxin B injections for the management of drooling among ALS patients reduces, in significant and persistent manner, the saliva production. This is in agreement with the updated Practice Parameter of The American Academy of Neurology.22 The anatomical location of the injection can be identified by either anatomical markers, electromyography or ultrasound guidance. The latter is a more precise procedure.33–35 However, in some reported cases, patients were maintained on anticholinergic medications making, it difficult to distinguish between the effects of botulinum toxin injections from those of other medications. Side-effects were reported in a third of patients.33,36–38 The most frequent one was worsening of dysphagia and dysarthria.39,40
A number of issues related to botulinum B toxin are yet to be determined.41 There is no consensus on which glands should be injected and with how many units per dose (2.500 units?). There is limited information on how many sites within each gland should be considered and whether ultrasound guidance is really significantly more beneficial.
Radiotherapy
Head and neck radiation therapy (RT) typically tries to spare the parotid glands because it may reduce salivation and cause dry mouth symptoms in cancer patients.42 Because of this secondary effect, RT of the salivary gland could be an alternative and effective technique to reduce sialorrhea in ALS patients: in a retrospective study, there was a good response in 65% of subjects. Different techniques and doses have been reported in previous studies on radiotherapy and, for this reason, there is no consensus on the type of radiation (electron-based therapy vs photon-based therapy) and on the optimum total dose.43 It was obtained a positive effect on the administration of 20 Gy in 5 fractions on the whole of the submandibular gland and sparing the upper part of the parotid gland. Similar beneficial effects have been described by using computed tomography to target a single parotid gland and delivering a global dose of 15 Gy divided into 3 fractions.10 Single-dose radiotherapy (single fraction of 7.5 Gy) has also been described as beneficial in reducing excessive drooling in ALS [60]. On the other hand, there are other suggestions indicating that the delivery of a single dose of 8 Gy could be as effective and safe compared to higher fractionated doses and increasing the dose does not lead to additional improvement.,41,43Electron-based therapy appears to be better tolerated, more effective in the long term (persistent positive effects 4–6 months after treatment) compared with photon-based therapy.41,43 A recent review recommends dose-fractionation schemes of 12 Gy in 2 fractions or 20 Gy in 4 fractions with treatments twice a week.44 In addition, photon-based therapy can determine acute collateral effects (oral pain and mucositis during or immediately after irradiation) or delayed local reaction (edema or xerostomia up to one month after irradiation or oral pain three months after irradiation).45
Considering that life expectancy is reduced in ALS, the probability to have radiation-induced malignancy after RT is relatively low related to the course of the disease. Still, ALS patients with supposed extended survival need to be addressed at the time of radiation oncologist’s consultation, particularly in patients with a slower course of ALS.46,47
In conclusion, a great number of ALS patients with salivation problems respond well to therapeutic salivary gland irradiation and the improvement may last for several months. Neurologists should consider this treatment option for select ALS patients.
Surgical options
The surgical treatment for sialorrhea is the last-choice therapeutic option and it is suggested:
In case of persistent moderate sialorrhea not responding to conservative treatments.
In severe sialorrhea cases in whom previous treatment trials have failed or have had limited results with conservative treatment.
In cases where compliance to conservative treatments is limited due to cognitive impairment.
Neurectomy
The tympanic plexus nerve decreases the production of saliva. Together with the tympanic cord, they may be cut, bilaterally or unilaterally, alone or helped by other procedures as submandibular gland exeresis. The quantity of secretion from submaxillary and sublingual glands is reduced by the neurectomy of the tympanic cord. Complications, like auditory loss or decreased perception of taste in the first two sections of the tongue, can take place. Indeed, it is not suggested to patients who already have hearing problems before the therapy. Long-term results from isolated neurectomies are not definitive.48
Surgical procedures on the salivary duct and gland
Most known studies describing the surgical procedure to control excessive drooling were performed on children affected by cerebral palsy. The main procedure is made up by retropositioning the parotid ducts into the tonsillar fossa region along with the bilateral submandibular gland resection. In these patients, other effective variants of the main surgical procedure (Wilkie’s procedure) have been shown:
relocation of the submandibular gland duct into the tonsillar fossa;
ligation of the parotid ducts, along with the usual submandibular gland resection;
deviation of both submandibular and parotid ducts behind the anterior pillar of the soft palate;
bilateral submandibular duct relocation with or without sublingual gland excision;
a combination of homolateral parotid duct ligation and contralateral parotid duct repositioning;
ligation of both parotid and submandibular ducts (four-duct ligation).
In 2009, Reed et al, published a metanalysis that showed how bilateral submandibular duct rerouting, bilateral submandibular gland excision with bilateral parotid duct rerouting and bilateral submandibular gland excision with bilateral parotid duct ligation are similarly effective.49 Although being potentially less efficient, four-duct ligation allows a safe, quick and simple method that can help to control symptoms.50
In conclusion, although a surgical approach to sialorrhea is theoretically possible in other patients (cerebral palsy children), there is no evidence as yet in patients with ALS. Indeed, at this time this procedure is not standardized and therefore not recommended in ALS patients although there may be interested in the future for severe cases in whom a definitive approach is required.
Recommendations
The first sialorrhea therapeutic approach in ALS is made up by amitriptyline, oral or transdermal hyoscine, scopolamine, or sublingual atropine drops.51
Botulinum toxin injections into the parotid and/or submandibular gland are tolerated and effective when injected in patients with refractory sialorrhoea.51
In ALS patients with consequent sialorrhoea, injections of botulinum into the parotid gland reduce sialorrhoea and improves QOL up to four months, with an efficacy at four weeks from the injection.52
Avoiding direct injections of botulinum into salivary ducts is suggested because associated with significant adverse effects.52
In the case of pharmacological treatment failure, irradiation of salivary glands may be tried.51
Radiotherapy of salivary glands has shown its efficacy in reducing sialorrhoea at doses from 7 to 12.5 Gy up to six months.52
Surgical interventions are not recommended due to: low life expectancy of patients, and inability to tolerate surgical intervention. In selected cases, surgery could represent an alternative treatment because the advantages are no tachyphylaxis and no repeated therapeutic sessions.53
Consider the duration of the treatment: There is some evidence indicating that both botulinum and radiotherapy are well tolerated, effective treatments for persistent sialorrhea in patients with ALS and that the duration of action is up to three months with botulinum and six months with radiotherapy.52
Discuss with the patients the best options of treatment.6
There are different approaches into treatment timing: the EFNS guidelines start with amitriptyline, followed by oral or transdermal hyoscine, or sublingual atropine drops, botulinum toxin injections in utilized in refractory cases. Irradiation is considered as an option after pharmacological treatment failure.54 In for patients with cognitive impairment, the NICE guidelines introduce glycopyrrolate as a first-line of treatment because of fewer central nervous side effects.55
Conclusion
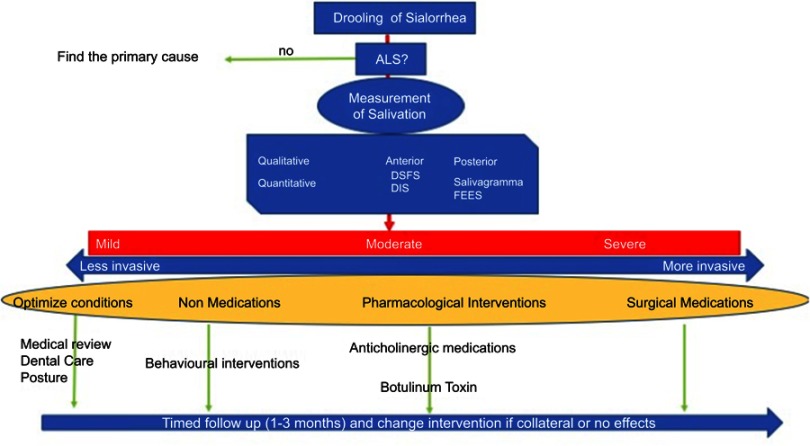
Evidence from previous studies is limited and there are still a number of open questions. How can we measure sialorrhea in a reliable and reproducible way? How can we measure the impact of this specific symptom on the patients’ perception of life quality? How long shall the treatment last?52 We should consider that there may be a need to include measures which specifically address the impact of drooling on quality of life, rather than hoping to determine efficacy measuring the impact on overall QOL. In any case, quantitative and qualitative disorders in salivary secretions are frequent in clinical practice and sialorrhea is burdensome for patients and caregivers. Currently, there are a number of drugs that may be, at least partially, beneficial, but side effects need to be considered. In this respect, it is important that treatment is tailored according to the severity and impact on the patient’s quality of life, balancing side effects. We believe that the stepwise structured approach described may prove to be a useful tool to guide clinical practice and better target treatment of sialorrhea in ALS (Figure 2).
Figure 2.
Diagnostic-therapeutic pathway for sialorrhea in ALS patients.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Warrell DA, Cox TM, Firth JD, Benz EJ, editors. Oxford Textbook of Medicine. Oxford: Oxford University Press; 2003. [Google Scholar]
- 2.Ackrivo J, Hansen-Flaschen J, Wileyto EP, Schwab RJ, Elman L, Kawut SM. Development of a prognostic model of respiratory insufficiency or death in amyotrophic lateral sclerosis. Eur Respir J. 2019. pii: 1802237 doi: 10.1183/13993003.02237-2018 [Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dash RP, Babu RJ, Srinivas NR. Two decades-long journey from riluzole to edaravone: revisiting the clinical pharmacokinetics of the only two amyotrophic lateral sclerosis therapeutics. Clin Pharmacokinet. 2018;57(11):1385–1398. doi: 10.1007/s40262-018-0655-4 [DOI] [PubMed] [Google Scholar]
- 4.Danel-Brunaud V, Touzet L, Chevalier L, et al. Ethical considerations and palliative care in patients with amyotrophic lateral sclerosis: a review. Rev Neurol (Paris). 2017;173(5):300–307. doi: 10.1016/j.neurol.2017.03.032 [DOI] [PubMed] [Google Scholar]
- 5.Kontis TC, ME Johns. Anatomy and physiology of the salivary glands In: Bailay BJ, editor. Head and Neck Surgery Otolaryngology, 4th ed Philadelphia: Lippincott Williams & Wilkins; 2006:531–539. [Google Scholar]
- 6.Tysnes O-B. Treatment of sialorrhea in amyotrophic lateral sclerosis. Acta Neurol Scand. 2008;117(Suppl. 188):77–81. doi: 10.1111/j.1600-0404.2008.01037.x [DOI] [PubMed] [Google Scholar]
- 7.Blackhall LJ. Amyotrophic lateral sclerosis and palliative care: where we are, and the road ahead. Muscle Nerve. 2012;45(3):311–318. doi: 10.1002/mus.22305 [DOI] [PubMed] [Google Scholar]
- 8.Robbins J. Swallowing in ALS and motor neuron disorders. Neurol Clin. 1987;5(2):213–229. [PubMed] [Google Scholar]
- 9.Sorenson EJ, Crum B, Stevens JC. Incidence of aspiration pneumonia in ALS in Olmsted County, MN. Amyotroph Lateral Scler. 2007;8(2):87–898. doi: 10.1080/17482960601147461 [DOI] [PubMed] [Google Scholar]
- 10.Kurian KM, Forbes RB, Colville S, Swingler RJ. Cause of death andclinical grading criteria in a cohort of amyotrophic lateral sclerosis cases undergoing autopsy from the Scottish Motor Neurone Disease Register. J Neurol Neurosurg Psychiatry. 2009;80(1):84–87. doi: 10.1136/jnnp.2008.149708 [DOI] [PubMed] [Google Scholar]
- 11.Wolf J, Safer A, Wöhrle JC, et al. [Causes of death in amyotrophic lateral sclerosis: results from the Rhineland-Palatinate ALS registry]. Nervenarzt. 2017;88(8):911–918. doi: 10.1007/s00115-017-0293-3 German. [DOI] [PubMed] [Google Scholar]
- 12.Pisa FE, Logroscino G, Giacomelli Battiston P, Barbone F. Hospitalizations due to respiratory failure in patients with amyotrophic lateral sclerosis and their impact on survival: a population-based cohort study. BMC Pulm Med. 2016;16(1):136 doi: 10.1186/s12890-016-0276-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Carvalho M, Matias T, Coelho F, Evangelista T, Pinto A, Sales Luís ML. Motor neuron disease presenting with respiratory failure. J Neurol Sci. 1996;139(Suppl):117–122. doi: 10.1016/0022-510X(96)00089-5 [DOI] [PubMed] [Google Scholar]
- 14.Farrero E, Prats E, Povedano M, Martinez-Matos JA, Manresa F, Escarrabill J. Survival in amyotrophic lateral sclerosis with home mechanical ventilation: the impact of systematic respiratory assessment and bulbar involvement. Chest. 2005;127(6):2132–2138. doi: 10.1378/chest.127.6.2132 [DOI] [PubMed] [Google Scholar]
- 15.Pinto AC, Evangelista T, Carvalho M, Alves MA, Sales Luís ML. Respiratory assistance with a non-invasive ventilator (Bipap) inMND/ALS patients: survival rates in a controlled trial. J Neurol Sci. 1995;129(Suppl):19–26. doi: 10.1016/0022-510X(95)00052-4 [DOI] [PubMed] [Google Scholar]
- 16.Kleopa KA, Sherman M, Neal B, Romano GJ, Heiman-Patterson T. Bipap improves survival and rate of pulmonary function decline in patients with ALS. J Neurol Sci. 1999;164(1):82–88. [DOI] [PubMed] [Google Scholar]
- 17.Lyall RA, Donaldson N, Fleming T, et al. A prospective study of quality of life in ALS patients treated with noninvasive ventilation. Neurology. 2001;57(1):153–156. [DOI] [PubMed] [Google Scholar]
- 18.Bourke SC, Bullock RE, Williams TL, Shaw PJ, Gibson GJ. Noninvasive ventilation in ALS: indications and effect on quality of life. Neurology. 2003;61(2):171–177. [DOI] [PubMed] [Google Scholar]
- 19.Sancho J, Servera E, Morelot-Panzini C, Salachas F, Similowski T, Gonzalez-Bermejo J. Non-invasive ventilation effectiveness and the effect of ventilatory mode on survival in ALS patients. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15(1–2):55–61. doi: 10.3109/21678421.2013.855790 [DOI] [PubMed] [Google Scholar]
- 20.Vandenberghe N, Vallet AE, Petitjean T, et al. Absence of airway secretion accumulation predicts tolerance to noninvasive ventilation in amyotrophic lateral sclerosis. Respir Care. 2013;58(9):1424–1432. doi: 10.4187/respcare.02103 [DOI] [PubMed] [Google Scholar]
- 21.Gordon PH, Salachas F, Bruneteau G, et al. Improving survival in a large French ALS center cohort. J Neurol. 2012;259(9):1788–1792. doi: 10.1007/s00415-011-6403-4 [DOI] [PubMed] [Google Scholar]
- 22.Bradley WG, Anderson F, Bromberg M, et al. Current management of ALS: comparison of the ALS CARE database and the AAN practice parameter. The American Academy of Neurology. Neurology. 2001;57(3):500–504. [DOI] [PubMed] [Google Scholar]
- 23.Young CA, Ellis C, Johnson J, Sathasivam S, Pih N Treatment for sialorrhea (excessive saliva) in people with motor neuron disease/amyotrophic lateral sclerosis. Cochrane Database of Syst Rev. 2011. doi: 10.1002/14651858.CD006981.pub2 [DOI] [PubMed] [Google Scholar]
- 24.Cedarbaum JM, Stambler N, Malta E, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci. 1999;169(1):13–21. [DOI] [PubMed] [Google Scholar]
- 25.Ng L, Khan F, Young CA, Galea M. Symptomatic treatments for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst Rev. 2017;1:CD011776. doi: 10.1002/14651858.CD011776.pu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brooks BR, Lewarski JL, McKim DA, Nakayama Y, Chatburn RL. Oral Secretion Score (OSS) predicts best care interventions and outcomes of patients with ALS/MND using noninvasive ventilation (NIV). Amyotroph Lateral Scler Frontotemporal Degener. 2013;14(Suppl 2):60. [Google Scholar]
- 27.Brooks BR, Lewarski JL, McKim DA, Chatburn RL. Oral Secretion Scale (OSS) score in amyotrophic lateral sclerosis patients is associated with tolerance of non-invasive positive pressure ventilation, need for hospice or transition to tracheal positive pressure ventilation (TPPV) and survival (abstract). Amyotroph Lateral Scler. 2010;11(Suppl 1):140.19551535 [Google Scholar]
- 28.Abdelnour-Mallet M, Tezenas Du Montcel S, Cazzolli PA, et al. Validation of robust tools to measure sialorrhea in amyotrophic lateral sclerosis: a study in a large French cohort. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14(4):302–307. doi: 10.3109/21678421.2012.735238 [DOI] [PubMed] [Google Scholar]
- 29.Jackson CE. Treating the symptoms of amyotrophic lateral sclerosis In: Mitsumoto H, editor. Amyotrophic Lateral Sclerosis: A Guide for Patients and Families. New York: Demos Medical Publishing; 2009:53–54. [Google Scholar]
- 30.McGeachan AH, Stephenson J, Shaw PJ, McDermott CA. Multicentre evaluation of secretion management in patients with motor neurone disease (MND) (abstract). Amyotroph Lateral Scler Frontotemporal Degener. 2013;14(2):59–60. [Google Scholar]
- 31.Andersen PM, Gro¨Nberg H, Franzen L, Funegård U. External radiation of the parotid glands significantly reduces drooling in patients with motor neurone disease with bulbar paresis. J Neurol Sci. 2001;191(1–2):111–114. [DOI] [PubMed] [Google Scholar]
- 32.Odachi K, Narita Y, Machino Y, et al. Efficacy of transdermal scopolamine for scialorrhea in patients with amyotrophic lateral sclerosis. Cogent Med. 2017;4:1365401 doi: 10.1080/2331205X.2017.1365401 [DOI] [Google Scholar]
- 33.Scott K, Kothari M, Venkatesh Y, Murphy T, Simmons Z. Parotid gland injections of botulinum toxin A are effective in treating sialorrhea in amyotrophic lateral sclerosis. J Clin Neuromuscul Dis. 2005;7:62–65. doi: 10.1097/01.cnd.0000188865.88167.62 [DOI] [PubMed] [Google Scholar]
- 34.Tan EK, Lo YL, Seah A, Auchus AP. Recurrent jaw dislocation after botulinum toxin treatment for sialorrhoea in amyotrophic lateral sclerosis. J Neurol Sci. 2001;190:95–97. doi: 10.1016/S0022-510X(01)00565-2 [DOI] [PubMed] [Google Scholar]
- 35.Contarino M, Pompili M, Tittoto P, et al. Botulinum toxin B ultrasound-guided injections for sialorrhea in amyotrophic lateral sclerosis and Parkinson’s disease. Parkinsonism Relat Disord. 2007;13:299–303. doi: 10.1016/j.parkreldis.2006.05.005 [DOI] [PubMed] [Google Scholar]
- 36.Weikamp J, Schinagl D, de Swart B, Schelhaas H, Zwarts M. A prospective, randomised controlled study comparing radiotherapywith botulinum toxin A as a treatment for drooling in ALS. Amyotroph Lateral Scler. 2008;9:152. [Google Scholar]
- 37.Jackson CE, Gronseth G, Rosenfeld J, et al. Randomized double-blind study of botulinum toxin type B for sialorrhea in ALS patients. Muscle Nerve. 2009;39:137–143. doi: 10.1002/mus.v39:2 [DOI] [PubMed] [Google Scholar]
- 38.Costa J, Rocha M, Ferreira J, Evangelista T, Coelho M, de Carvalho M. Botulinum toxin type-B improves sialorrhea and quality of life in bulbar onset amyotrophic lateral sclerosis. J Neurol. 2008;255:545–550. doi: 10.1007/s00415-008-0738-5 [DOI] [PubMed] [Google Scholar]
- 39.Meijer J, Van Kuijk A, Geurts A, Schelhaas H, Zwarts M. Acute deterioration of bulbar function after botulinum toxin treatment for sialorrhoea in amyotrophic lateral sclerosis. Am J Phys Med Rehabil. 2008;87:321–324. doi: 10.1097/PHM.0b013e318164a931 [DOI] [PubMed] [Google Scholar]
- 40.Winterholler M, Erbguth FJ, Wolf S, et al. Botulinum toxin for the treatment of sialorrhea in ALS: serious side effects of a transductal approach. J Neurol Neurosurg Psychiatry. 2001;70:417–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Squires N, Humberstone M, Wills A, Arthur A. The use of botulinum toxin injections to manage drooling in amyotrophic lateral sclerosis/motor neurone disease: a systematic review. Dysphagia. 2014;29:500–508. doi: 10.1007/s00455-014-9535-8 [DOI] [PubMed] [Google Scholar]
- 42.Guy N, Bourry N, Dallel R, et al. Comparison of radiotherapy types in the treatment ofsialorrhea in amyotrophic lateral sclerosis. J Palliat Med. 2011;14(4):391–395. doi: 10.1089/jpm.2010.0308 [DOI] [PubMed] [Google Scholar]
- 43.Bourry N, Guy N, Achard JL, Verrelle P, Clavelou P, Lapeyre M. Salivary glands radiotherapy to reduce sialorrhea in amyotrophic lateral sclerosis: dose and energy. Cancer Radiother. 2013;17(3):191–195. doi: 10.1016/j.canrad.2013.01.010 [DOI] [PubMed] [Google Scholar]
- 44.Nathan M, Hawkey BA, Nicholas G, Zaorsky MD, Thomas J, Galloway MD. The role of radiation therapy in the management of sialorrhea: a systematic review. Laryngoscope. 2016;126:80–85. doi: 10.1002/lary.25444 [DOI] [PubMed] [Google Scholar]
- 45.Harriman M, Morrison M, Hay J, Revonta M, Eisen A, Lentle B. Use of radiotherapy for control of sialorrhea in patients with amyotrophic lateral sclerosis. J Otolaryngol. 2001;30(4):242–245. [DOI] [PubMed] [Google Scholar]
- 46.Brenner DJ, Curtis RE, Hall EJ, Ron E. Second malignancies in prostate carcinoma patients after radiotherapy compared with surgery. Cancer Jan. 2000;88:398 doi: [DOI] [PubMed] [Google Scholar]
- 47.Dörr W, Herrmann T. Cancer induction by radiotherapy: dose dependence and spatial relationship to irradiated volume. J Radiol Prot Sep. 2002;22:A117–A121. doi: 10.1088/0952-4746/22/3A/321 [DOI] [PubMed] [Google Scholar]
- 48.Meningaud JP. Drooling of saliva: a review of the etiology and management options. Oral Surg Oral Med Oral Pathol Oral Endod. 2006;101:48–57. doi: 10.1016/j.tripleo.2005.08.018 [DOI] [PubMed] [Google Scholar]
- 49.Reed J, Mans CK, Brietzke SE. Surgical management of drooling: a meta-analysis. Arch Otolaryngol Head Neck Surg. 2009;135:924–931. [DOI] [PubMed] [Google Scholar]
- 50.Khan WU, Islam A, Fu A, et al. Four-duct ligation for the treatment of sialorrhea in children. JAMA Otolaryngol Head Neck Surg. 2016;142:278–283. doi: 10.1001/jamaoto.2015.3592 [DOI] [PubMed] [Google Scholar]
- 51.Wiley, John. Today’s research, tomorrow’s innovation. Wiley Online Library; 2018. Available from: onlinelibrary.wiley.com/.
- 52.Stone CA, O’Leary N. Systematic review of the effectiveness of botulinum toxin or radiotherapy for sialorrhea in patients with amyotrophic lateral sclerosis. J Pain Symp Manag. 2009. doi: 10.1016/j.jpainsymman.2008.02.006 [DOI] [PubMed] [Google Scholar]
- 53.Banfi P, Ticozzi N, Lax A, Guidugli GA, Nicolini A, Silani V. A review of options for treating sialorrhea in amyotrophic lateral sclerosis. Respir Care. 2015;60(3):446–454. doi: 10.4187/respcare.02856 [DOI] [PubMed] [Google Scholar]
- 54.EFNS Task Force on Diagnosis and Management of Amyotrophic Lateral Sclerosis, Andersen PM, Abrahams S, et al. EFNS guidelines on the clinical management of amyotrophic lateral sclerosis (MALS)–revised report of an EFNS task force. Eur J Neurol. 2012;19(3):360–375. doi: 10.1111/j.1468-1331.2011.03501.x Epub 2011 Sep 14. [DOI] [PubMed] [Google Scholar]
- 55.Oliver D, Radunovic A, Allen A, McDermott C. The development of the UK National Institute of Health and Care Excellence evidence-based clinical guidelines on motor neurone disease. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(5–6):313–323. doi: 10.1080/21678421.2017.1304558 Epub 2017 May 17. [DOI] [PubMed] [Google Scholar]