Insertion sequences play important roles in bacterial evolution and are frequently utilized in mutagenesis systems. However, the intrinsic insertion sequences of tetragenococci are not well characterized. Here, we identified three active insertion sequences of T. halophilus by transposition into the region around the arc operon. This report provides an example of insertion sequence-mediated generation and evolution of T. halophilus and primary information about their characteristics.
KEYWORDS: arginine deiminase system, ethyl carbamate, insertion sequence, Tetragenococcus halophilus
ABSTRACT
Tetragenococcus halophilus, a halophilic lactic acid bacterium, is often used as a starter culture in the manufacturing of soy sauce. T. halophilus possesses an arginine deiminase system, which is responsible for the accumulation of citrulline, the main precursor of the potential carcinogen ethyl carbamate. In this study, we generated five derivatives lacking arginine deiminase activity from T. halophilus NBRC 12172 by UV irradiation. Using these derivatives as a fermentation starter prevented arginine deimination in soy sauce. DNA sequence analysis of the derivatives revealed that novel IS4 family insertion sequences, designated ISTeha3, ISTeha4, and ISTeha5, were transposed into the region around the arginine deiminase (arc) operon in the mutants. These insertion sequences contain a single open reading frame encoding a putative transposase and 13- to 15-bp inverted repeats at both termini, which are adjacent to 7- to 9-bp duplications of the target sequence. Investigation of wild strains isolated from soy sauce mash incapable of arginine deimination also indicated that insertion sequences are involved in the disruption of the arginine deiminase system in T. halophilus.
IMPORTANCE Insertion sequences play important roles in bacterial evolution and are frequently utilized in mutagenesis systems. However, the intrinsic insertion sequences of tetragenococci are not well characterized. Here, we identified three active insertion sequences of T. halophilus by transposition into the region around the arc operon. This report provides an example of insertion sequence-mediated generation and evolution of T. halophilus and primary information about their characteristics.
INTRODUCTION
Insertion sequences (ISs) are small transposable elements of DNA, generally consisting of transposase and terminal inverted repeats (IRs), which function as the sites for recognition and cleavage by transposase (1). ISs are distributed in a wide range of bacteria, and many bacteria possess multiple ISs in their genome (2–4). It is well recognized that the transposition of ISs often causes gene inactivation or alteration of the expression levels of adjacent genes. Furthermore, two homologous ISs can cause recombination and generate chromosomal inversions or deletions, which lead to complex rearrangements (5). Thus, ISs contribute significantly to bacterial evolution. In addition, ISs have been utilized in transposon mutagenesis systems to disrupt genes randomly in many species, including lactic acid bacteria (6, 7). Nevertheless, no active IS has been reported in tetragenococci, although putative ISs have been found in their genome or plasmid sequences (8, 9).
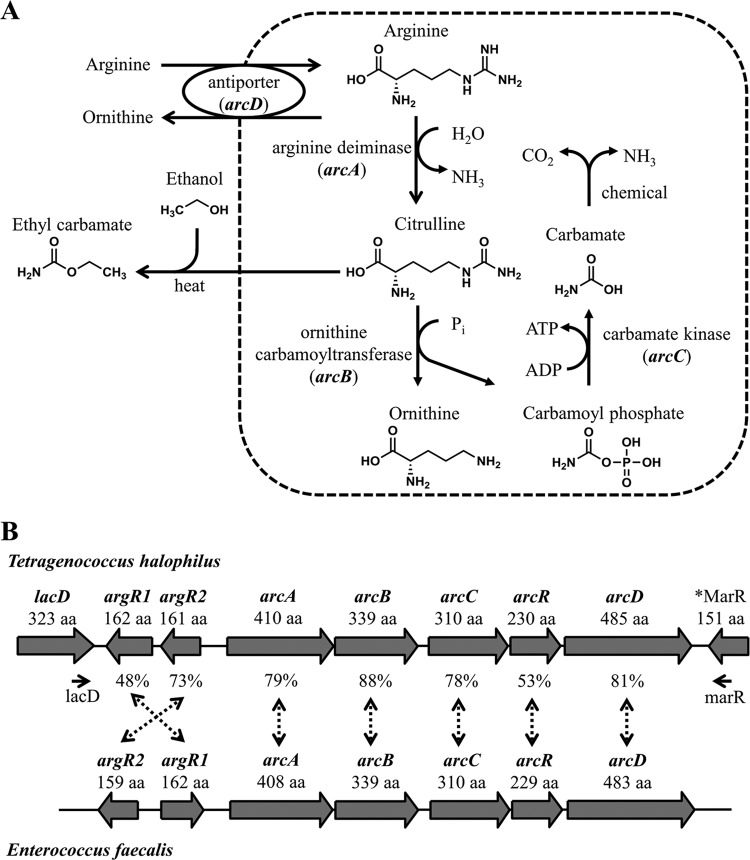
Tetragenococcus halophilus, a homofermentative, halophilic lactic acid bacterium, is isolated as the dominant microbe from various salted and fermented foods, including soy sauce and salted anchovies (10–12). T. halophilus plays an important role in the fermentation of soy sauce. Lactic acid produced in soy sauce mash causes a reduction in pH, which prepares the appropriate environment for halotolerant yeasts such as Zygosaccharomyces rouxii (13). On the other hand, some T. halophilus strains can raise the pH of soy sauce mash through arginine degradation via the arginine deiminase system (ADS) (14). The ADS converts arginine into ornithine, CO2, and NH3 releasing ATP (Fig. 1A). NH3 generation prevents pH reduction, which causes an increase of lactic acid, inhibiting the alcoholic fermentation. More importantly, citrulline, a metabolic intermediate of the ADS, is reported as the main precursor of ethyl carbamate (EC) in soy sauce (15). EC belongs to the 2A group of carcinogens and is formed through the reaction of citrulline and ethanol. Normally, the ADS converts arginine to ornithine inside the bacterial cells, and citrulline is not discharged outside the cell membrane. However, when the bacterial cells are destroyed while this pathway is in progress, citrulline leaks out of the cells and accumulates in soy sauce mash. Infection by bacteriophage is one of the factors that can induce cell lysis and citrulline accumulation (16). Therefore, selected strains of T. halophilus which are incapable of arginine deimination are preferred for the fermentation starter in soy sauce manufacturing.
FIG 1.
Arginine deimination pathway in T. halophilus and generation of EC (A) and schematic representation of the locus of the genes around the arc operon in the genome of T. halophilus NBRC 12172 and E. faecalis SD10 (B). (A) The broken line represents the cell membrane. Citrulline leakage out of the cell is caused by external factors, such as bacteriophage infection. (B) *MarR represents a putative MarR family transcriptional regulator gene. The arrows under lacD and MarR indicate the position on which the lacD and marR primers were designed. Percentages show the amino acid identities between T. halophilus and E. faecalis.
Most of the ADS genes studied to date are organized in a single operon known as the arc operon (17, 18). The three cytoplasmic enzymes participating in the ADS, arginine deiminase, ornithine transcarbamylase, and carbamate kinase, are encoded by the arcA, arcB, and arcC genes, respectively (Fig. 1A). In addition to these genes, other genes may be present in the arc operons of lactic acid bacteria, such as genes encoding arginine/ornithine antiporter (arcD) and Crp/Fnr-type regulator (arcR) (19, 20). Recent whole-genome analysis of T. halophilus revealed five genes of the arc operon, arcABCRD, sharing 53% to 88% of amino acids with those of Enterococcus faecalis (Fig. 1B). Two ArgR/AhrC-type regulators (argR1 and argR2), located upstream of the arc operon, were also reported to participate in the ADS by regulating the expression of arc operon genes (21).
In this study, we generated T. halophilus mutants lacking arginine deiminase activity by UV irradiation, and we identified novel ISs by transposition into the region around the arc operon. Information about the structure and transposition properties of the active ISs described here will contribute not only to the understanding of IS-mediated evolution in T. halophilus but also to the establishment of transposon mutagenesis systems in this species.
RESULTS
Isolation of NBRC 12172 derivatives lacking arginine deiminase activity.
Approximately 20,000 colonies of NBRC 12172 derivatives surviving UV exposure were picked up into arginine indicator broth, and five strains (M1, M2, M3, M4, and M5) changed the color of the broth to yellow (Table 1). The candidate strains were cultured in soy sauce medium containing 2% arginine for 7 days. The arginine content of the cultures was then analyzed, and it was confirmed that the derivatives did not decrease arginine content after incubation (see Fig. S1A in the supplemental material).
TABLE 1.
Strains used in this study
| Tetragenococcus halophilus strain | Description and genotype | Source |
|---|---|---|
| NBRC 12172 | ADS positive, genome sequenced | National Bio Resource Center |
| M1 | ADS negative, arcD::ISTeha3 | NBRC 12172 derivative generated in this study |
| M2 | ADS negative, arcA::ISTeha5 | NBRC 12172 derivative generated in this study |
| M3 | ADS negative, arcA_pr::ISTeha4 | NBRC 12172 derivative generated in this study |
| M4 | ADS negative, arcD::ISTeha3 | NBRC 12172 derivative generated in this study |
| M5 | ADS negative, arcD::ISTeha4 | NBRC 12172 derivative generated in this study |
| YA5 | ADS negative | Isolated from soy sauce mash, kept in our laboratory |
| A-30 | ADS negative | Isolated from soy sauce mash, kept in our laboratory |
Identification and structure of ISTeha3, ISTeha4, and ISTeha5.
We assumed that the derivatives lacking arginine deiminase activity would have base substitutions and/or base insertions/deletions around the arc operon. Thus, we amplified the DNA region covering the entire arc operon and two regulator genes (argR1 and argR2) by PCR using the lacD and marR primer sets, designed from lacD encoding tagatose 1,6-diphosphate aldolase and the putative MarR family transcriptional regulator gene (Table 2 and Fig. 1B). Surprisingly, all five derivatives showed elongated PCR products (over 8.0 kb) compared with that of the fragment amplified from the wild-type strain of NBRC 12172 (7.4 kb; Fig. S1B). These derivatives were expected to display active transposable elements inserted into this region.
TABLE 2.
Primers used in this study
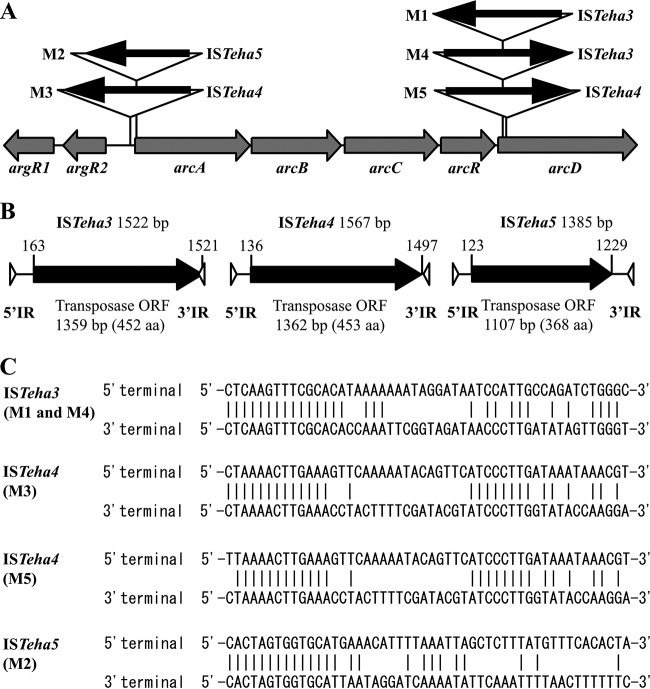
DNA sequence analysis revealed that the elongated PCR products carried the inserted DNA fragments, but their sequences and insertion sites were not identical among the derivatives (Fig. 2A). The 1,522-bp inserted elements of M1 and M4, designated ISTeha3, duplicated exactly the same 7-bp target sequence on arcD but integrated in the opposite direction of each other (Table 3). The inserts showed 100% identity to each other and to two putative ISs on the genome of NBRC 12172 itself (locus tags of transposase, TEH_16330 and TEH_20570). The inserts contain a single open reading frame (ORF) and 15-bp perfect IRs at both termini (Fig. 2B and C). The ORF encodes a putative transposase consisting of 452 amino acids. This amino acid sequence shows 43% identity to the putative transposase of ISDha5 in Desulfitobacterium hafniense DCB-2 (GenBank accession no. NC_011830; Fig. S2A). ISDha5 belongs to the IS4 family ISPepr1 subgroup, and the amino acid sequence of the putative transposase of ISTeha3 also shows 37% identity to that of ISPepr1 (NZ_AAJH01000015).
FIG 2.
Location and orientation of ISs transposed around the arc operon in each derivative (A) and genetic structures (B) and nucleotide sequences of the 5′ and 3′ termini (C) of ISTeha3, ISTeha4, and ISTeha5. (A) Black arrows indicate the direction of the putative transposase transcription. The derivative names and designated IS names are indicated on the left side and right side of the arrows, respectively. (B) The black arrows indicate ORFs that encode a putative transposase. The triangles indicate IRs at the 5′ and 3′ termini. The nucleotide numbers refer to the start and stop positions of the ORFs. (C) Vertical lines between the sequences denote homologous nucleotides.
TABLE 3.
DNA sequences of the insertion sites and their flanking regions of ISTeha3, ISTeha4, and ISTeha5
| IS transposed in each derivative | Flanking sequence at 5′ terminus | Insertion site (duplicated target sequence) | Flanking sequence at 3′ terminus |
|---|---|---|---|
| ISTeha3 | |||
| M1 | ATAAGAATAA | TAAATAA | AATACTAAAT |
| M4 | ATTTAGTATT | TTATTTA | TTATTCTTAT |
| ISTeha4 | |||
| M3 | CAACATAAGG | ATAATAA | ATTATCCAAT |
| M5 | TTAGGTATAC | TAACAATAA | TACTAAATGG |
| ISTeha5 | |||
| M2 | AACATTAATT | GGCTTACTC | ATAACTTAAG |
The 1,567-bp inserted elements of M3 duplicated a 7-bp target sequence between argR2 and arcA, while the same-sized inserted elements of M5 duplicated a 9-bp target sequence on arcD (Table 3). They show 96.2% identity to each other and 100% identity to the different putative ISs of NBRC 12172 (M3, TEH_05530 and TEH_21080; M5, TEH_08830). The inserts encode a putative transposase with a predicted 453 amino acid residues, sharing 98.2% identity with each other (Fig. 2B). According to ISfinder, ISs sharing more than 98% similarity in amino acid sequence or more than 95% similarity in DNA sequence are considered isoforms (22). Therefore, both elements transposed in M3 and M5 were designated ISTeha4. The insert of M3 contains 13-bp perfect IRs, whereas the IRs of M5 are imperfect (Fig. 2C). The amino acid sequence of the putative transposase in M3 shows 45% identity to that of ISLre1 in Lactobacillus reuteri 100-23 (NZ_AAPZ00000000; Fig. S2A). ISLre1 also belongs to the IS4 family ISPepr1 subgroup, and the amino acid sequence of the putative transposase of ISTeha4 shows 36% identity to ISPepr1. Therefore, we entered ISTeha3 and ISTeha4 as novel IS4 family ISPepr1 subgroup members in the ISfinder database.
The 1,385-bp element inserted in M2 was designated ISTeha5, and it duplicated the 9-bp target sequence on arcA (Table 3). The insert showed 100% identity to a putative IS of NBRC 12172 (TEH_24130) and contains a single ORF and 14-bp perfect IRs at both termini (Fig. 2B and C). The ORF encodes a putative transposase consisting of 368 amino acids showing 44% identity to the putative transposase of IS641 in Bacillus halodurans C-125 (NC_002570; Fig. S2B). IS641 belongs to the IS4 family IS4Sa subgroup, and the amino acid sequence of the putative transposase of ISTeha5 also shows 26% identity to that of IS4Sa (U38915). Hence, we entered ISTeha5 as a novel IS4 family IS4Sa subgroup member in the ISfinder database.
In the genome of NBRC 12172, at least one putative IS completely identical to the insert of each derivative is present. We amplified the DNA fragments covering these ISs from the genomic DNA of the wild-type strain and each derivative using primer sets designed at locations upstream and downstream of the fragments (Table S1). All derivatives showed the same size PCR products as the wild-type strain, suggesting that ISTeha3, ISTeha4, and ISTeha5 leave the parent sequences at the original sites and accumulate their copies in other sites (Fig. S3).
mRNA expression of arcA in derivatives.
M1, M2, M4, and M5 carried ISs transposed into arcA or arcD, the structural genes encoding arginine deiminase and arginine/ornithine antiporter, respectively. Therefore, it is understandable that these derivatives lose arginine deiminase activity. In M3, however, an IS was transposed into the region between arcA and argR2, and no alteration was found in any ORF of arcABCRD and two regulators (argR1 and argR2) by DNA sequence analysis (Fig. 2A). Therefore, we hypothesized that ISTeha4 of M3 disrupted the promoter region of the arc operon. To investigate this hypothesis, the expression of arcA was analyzed at the mRNA level by quantitative reverse transcription-PCR (RT-PCR) using a primer set designed from arcA. As expected, the mRNA expression level of arcA in M3 decreased to only 2% of that of the wild-type strain (Fig. 3). Upstream of the ISTeha4 transposition site in M3, five putative ArgR binding sequences, so-called Arg boxes [Escherichia coli consensus sequence, (T/A)NTGAAT(T/A)(T/A)(A/T)(A/T)ATTCAN(A/T)] (23), were found (Fig. S4A). In addition, a putative cre box [Bacillus subtilis consensus sequence, TG(T/A)NANCGNTN(T/A)CA] (24) was also found between Arg boxes, indicating that the ADS is regulated by CcpA, the protein that binds to the cre box and mediates catabolite repression in Gram-positive bacteria. Although the ADS regulation mechanism in T. halophilus was not clarified, it was estimated that the expression level of arcA in M3 was reduced because of the transposition of ISTeha4 into the region that is essential for the transcription of arc operon genes. The reduced arcA expression levels of the other derivatives compared to that of the wild-type strain may be explained by the mRNA destabilization triggered by IS transposition due to enhanced exonuclease activity (25).
FIG 3.
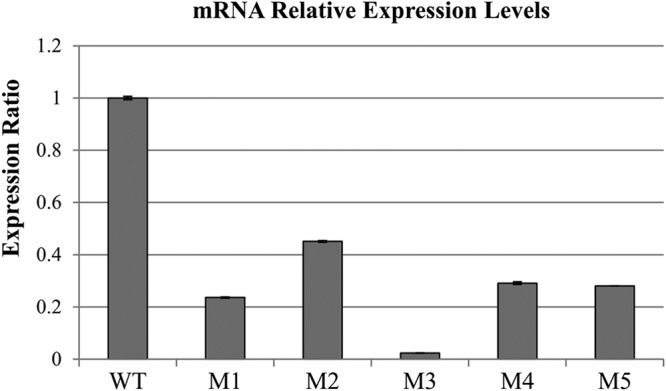
Relative expression levels of arcA in the strains determined by quantitative real-time PCR. The 16S rRNA gene was used as the reference. Data are expressed as means with error bars representing ±SD (n = 3). WT, wild-type strain.
Soy sauce fermentation with mutant strains.
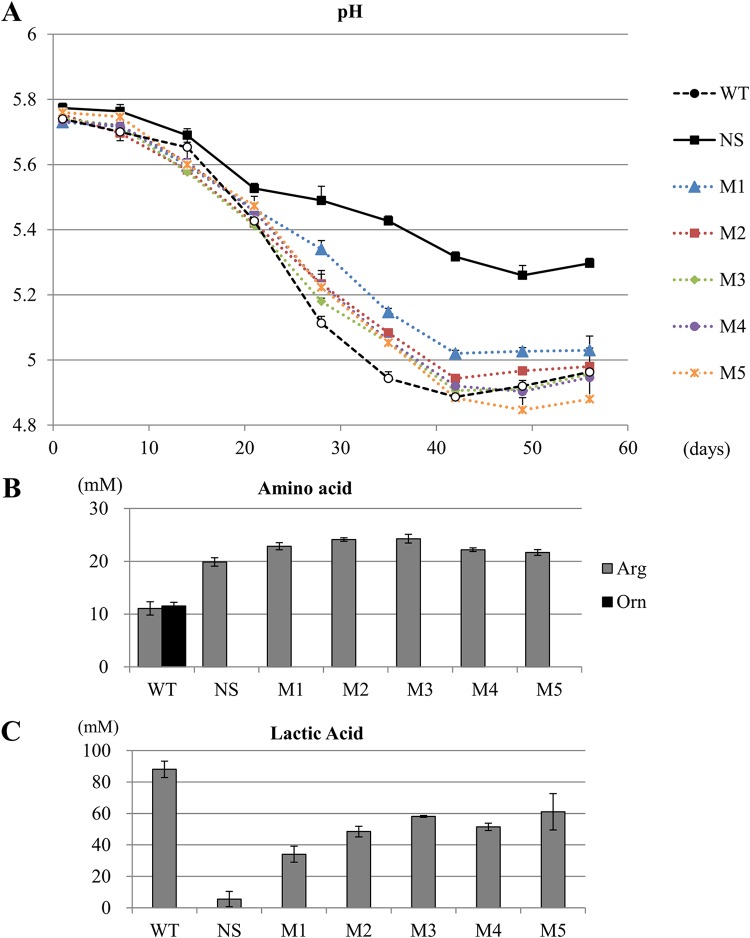
To evaluate the derivatives as soy sauce fermentation starters, we performed small-scale fermentation. The five derivatives and wild-type strain of NBRC 12172 were cultured in soy sauce medium and inoculated in soy sauce mash. All derivatives, as well as the wild-type strain, lowered the pH compared to that of the mash without starter cultures (Fig. 4A). After 8 weeks of fermentation, the amino acid content was analyzed. All five derivatives did not convert arginine to ornithine, whereas the wild-type strain produced 11.6 mM ornithine and reduced approximately the same amounts of arginine compared to the mash with the other strains and without starter cultures (Fig. 4B). Lactic acid content was lower in the mash inoculated with the derivatives than that of the mash inoculated with the wild-type strain (Fig. 4C).
FIG 4.
pH transition of soy sauce mash treated with the strains (A) and arginine and ornithine content (B) and lactic acid content (C) in the soy sauce after 8 weeks of fermentation. (A) Data are expressed as the means with error bars representing +1 SD (n = 3). WT, wild-type strain. NS, without starter cultures. (B) Gray bar and black bar indicate arginine and ornithine, respectively. Data are expressed as the means with error bars representing ±SD. Ornithine contents were below the detection limit, except for those of the samples treated with the WT. (C) Data are expressed as the means with error bars representing ±SD.
Wild strains lacking arginine deiminase activity.
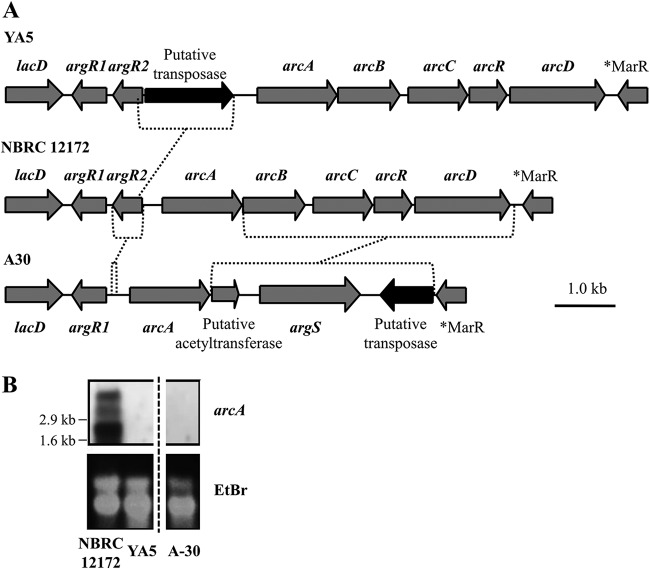
The DNA region around the arc operon of two wild strains lacking arginine deiminase activity isolated from soy sauce mash was amplified as described above. The amplified DNA fragments from these two strains, YA5 and A-30, had different lengths from that of NBRC 12172; the fragment was elongated in YA5 and shortened in A-30 (data not shown). DNA sequences of amplified fragments were analyzed (Fig. 5A). Compared to NBRC 12172, the arc operon genes of YA5 were completely preserved, with the exception of alterations of six amino acids on ArcA and two amino acids on ArcB. However, a 1,522-bp element, sharing 1,521 identical nucleotide bases with ISTeha3 transposed in M1 and M4, was inserted into argR2. On the other hand, in A-30 argR2 was not present in the region between argR1 and arcA. The amino acid homology of ArcA between A-30 and NBRC 12172 was 80%, and arcBCRD was replaced by three ORFs encoding a putative acetyltransferase, arginyl-tRNA synthetase (argS), and a putative transposase.
FIG 5.
Schematic representation of the locus of the genes between lacD and putative MarR family transcriptional regulator gene (A) and Northern blot analysis for arcA (B). (A) Broken lines represent the insertion or the replacement. *MarR represents a putative MarR family transcriptional regulator gene. (B) The result of Northern blot analysis is shown in the upper panel, designated arcA, and the total RNA stained with ethidium bromide is shown in the lower panel, designated EtBr. The RNA size on the membrane was estimated by the 16S rRNA (1.6 kb) and 23S rRNA (2.9 kb) patterns.
ArgR2 was identified as an essential activator of arc operon genes in Streptococcus pneumoniae (26). Therefore, we assessed the arcA expression by Northern blot analysis, so that it could be detected even if it did not share a highly homologous nucleotide sequence with NBRC 12172, and found no arcA expression in YA5 and A-30 (Fig. 5B). NBRC 12172 produced three mRNAs of different lengths, which probably correspond to the transcripts of the entire or partial arcABCRD and are also reported in E. faecalis (21). A highly stable potential transcription termination hairpin (estimated minimum free energy, −250 kJ/mol) follows arcD, and weaker stem-loops are predicted after arcB and arcR (−113 and −26 kJ/mol, respectively), which probably act as partial terminators. Although the precise transcription start site and stop site are not clarified, the length of the three mRNA products from NBRC 12172 is not contradictory to the expected length of the fragments generated (Fig. S4B).
DISCUSSION
EC is a topic of significant concern, especially in alcoholic beverages like wine and sake, in which urea mainly reacts with ethanol to make EC (27). The generation of non-urea-producing yeasts could minimize the accumulation of EC (28). However, the generation of yeasts or bacteria able to prevent EC accumulation in soy sauce has not been previously attempted. The main precursor of EC in soy sauce is citrulline, and here we provide an example of IS-mediated generation of non-citrulline-producing strains in T. halophilus, one of the main microorganisms used in the production of soy sauce. DNA sequencing of the wild strains also suggested that the transposition of ISs into argR2, the putative activator of arc operon genes, disrupted the ADS, which highlighted the contribution of ISs to the natural evolution in this species.
T. halophilus shows physiological diversity not only in arginine deimination but also in other processes, such as amino acid decarboxylation (14), carbohydrate fermentation (29), organic acid metabolism (30), and aggregation (31). These traits severely affect the quality of fermented food products. For instance, histidine decarboxylation and tyrosine decarboxylation are responsible for histamine and tyramine accumulation, respectively (32, 33). Furthermore, bacterial bodies of T. halophilus can cause turbidity in soy sauce, but aggregated strains can be trapped during the squeezing process and do not flow out into soy sauce (34). The difficulty to acquire strains possessing favorable traits in all these aspects underscores the need to develop techniques for the modification of T. halophilus. Higuchi et al. prepared phage-insensitive strains, and theirs is one of the few reports about modifying T. halophilus, but they did not describe the mutation mechanism or the genes in which the mutation occurred (35).
In this report, we identified three active IS4 family ISs, ISTeha3, ISTeha4, and ISTeha5. Transposases of the IS4 family belong to the DDE transposase group, which displays three acidic residues: aspartic acid (D), aspartic acid (D), and glutamic acid (E). ISTeha3, ISTeha4, and ISTeha5 conserved these residues (see Fig. S2 in the supplemental material). Furthermore, the main hallmark of this family's transposases is the Y-(2)-R-(3)-E-(6)-(K) motif (36). The three novel ISs displayed the complete YREK motif, suggesting that they are IS4 family members.
The IS4 family can be divided into seven subgroups based mainly on the amino acid sequence of transposases (36). ISTeha3 and ISTeha4 belong to the ISPepr1 subgroup, and ISTeha5 belongs to the IS4Sa subgroup. IR sequences of ISTeha3 (5′-CTCAAGTTTCGCACA-3′) and ISTeha4 (5′-(C/T)TAAAACTTGAAA-3′) match strongly with the consensus sequence of ISPepr1 subgroup IRs (5′-CTCAA+TTTCGCAG-3′). IS size (1,522 to 1,567 bp) and direct repeat (DR) length (7 to 9 bp) also show mostly typical features of the ISPepr1 subgroup (1,500 to 1,600 bp and 7 to 8 bp, respectively). ISTeha4 duplicated 7- or 9-bp DRs in this study, and putative ISTeha4 elements conserved in the genome of NBRC 12172 show 7- or 8-bp DRs (Table S1). ISTeha3 in the genome also shows 6- or 7-bp DRs. The length of the DRs is generally fixed, but certain ISs are reported to generate atypical lengths of DRs (37).
The IR sequence of ISTeha5 (5′-CACTAGTGGTGCAT-3′) matches strongly with the consensus sequence of the IS4Sa subgroup IRs (5′-CACTAGTGTCCGAT-3′). IS and DR length (1,385 bp and 9 bp) also supports this subgroup classification (1,150 to 1,750 bp and 8 to 10 bp in the IS4Sa subgroup). The DR length is fixed to 9 bp, including those conserved in the genome of NBRC 12172 (Table S1).
Among the 18 ISs deposited as the ISPepr1 subgroup in the ISfinder database, only IS2621 of Deinococcus radiodurans (GenBank accession no. AB001611) was demonstrated to possess transposition activity (38). Among the 41 ISs belonging to the IS4Sa subgroup, IS4Bsu1 of B. subtilis (AB031551) and IS5377 of Bacillus stearothermophilus (X67862) were demonstrated to be active (39, 40). Members of the IS4 family preferentially use a conservative (cut-and-paste) mechanism of transposition (1), but some examples, such as IS4Bsu1 of B. subtilis, showed IS4 family members using the replicative (copy-and-paste) mechanism (39). PCR amplification of the genomic region covering the ISs identified in this study supported the replicative mechanism of ISTeha3, ISTeha4, and ISTeha5 (Fig. S4).
The fact that all derivatives showed transposed ISs around the arc operon suggests that the inactivation of the ADS in T. halophilus is caused mainly by the transposition of transposable elements rather than by the spontaneous mutations, such as base substitutions or deletions, associated with UV irradiation. However, although UV irradiation has indeed been reported to induce the transposition of ISs (41), there is no direct evidence that UV irradiation actually caused the transposition in this case. It might have occurred spontaneously during the culturing before the irradiation. Further research is necessary to determine the induction factors of IS transposition.
NBRC 12172 possesses 71 copies of putative transposases in 19 different categories, except for the truncated fragments. ISTeha1 and ISTeha2 (AP012046) have been deposited in ISfinder, belonging to the ISLre2 family and the IS6 family, respectively. However, the transposition activity of ISs in this species has not been reported previously. In tetragenococci, some reports demonstrated plasmid DNA transfer by conjugation (42, 43), but the transposon mutagenesis systems are still undeveloped. The transposon mutagenesis system is advantageous for the comprehensive characterization of gene functions that contribute to objective phenotypes. Hence, for the development of such a system in tetragenococci, the identification and characterization of active ISs are important. Other active ISs of T. halophilus, in addition to the three elements described here, are expected to be identified in the future.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
Bacterial strains used in this study are shown in Table 1. YA5 and A-30 were selected from the strain collection of Yamasa Corporation, which were isolated from soy sauce mash of the factory. They were confirmed not to decrease arginine content in soy sauce medium containing 2% arginine after 7 days of cultivation (data not shown). T. halophilus was cultured in MRS-10, soy sauce medium, arginine indicator broth, or LA13 medium, as specified in individual experiments. MRS-10 is Lactobacilli MRS broth (Difco, Detroit, MI) supplemented with 10% NaCl. Soy sauce medium is the medium for halophilic lactic acid bacteria, using soy sauce (Yamasa, Choshi, Japan) and NaCl so that the final concentration becomes 0.4% total nitrogen and 10% NaCl. The pH was adjusted to 8.0 before sterilization by autoclave. When necessary, soy sauce medium was supplemented with 2% arginine. Arginine indicator broth contains 0.5% beef extract, 0.5% Hipolypepton (Nihon Pharmaceutical, Tokyo, Japan), 0.5% yeast extract, 0.1% thioglycolic acid, 10% NaCl, 0.1% glucose, 0.004% bromocresol purple, and 1% arginine. The pH was adjusted to 7.5 before sterilization by autoclave. LA13 medium contains 1% Hipolypepton (Nihon Pharmaceutical), 0.4% yeast extract, 1% KH2PO4, 12% NaCl, 0.5% glucose, 5% soy sauce (Yamasa), and 1.5% agar (14). Liquid media were incubated at 30°C without stirring, and agar plates were incubated at the same temperature with an AnaeroPack (Mitsubishi Gas Chemical, Tokyo, Japan) in a hermetically sealed box.
UV irradiation and isolation of mutants lacking arginine deiminase activity.
UV irradiation was used to induce random mutations that could affect the genes encoding the ADS. One ml of MRS-10 cultures of fully grown T. halophilus NBRC 12172 (Table 1) was resuspended in 9 ml of fresh MRS-10 and grown for 16 h. The cultures were then centrifuged (6,000 × g) for 10 min at 4°C. The pellets were washed once and resuspended in 10 ml of 10% NaCl. The 5-ml suspensions in sterile dishes (diameter, 55 mm) were UV irradiated using a germicidal lamp (GL-15; Panasonic, Kadoma, Japan) at a distance of 260 mm for 10 to 30 s with constant stirring to yield a 0.1 to 0.01% survival rate after exposure. Before and after irradiation, part of the suspension was properly diluted, spread on the LA13 medium plates, and incubated for 5 days. The survival rate was determined by counting the number of colonies on the plates. Mutants lacking arginine deiminase activity were isolated in arginine indicator broth containing bromocresol purple. pH reduction by lactic acid production changes the color of this medium to yellow, but when arginine is deiminated and the pH is increased, the color remains purple. UV-irradiated derivatives were inoculated into 80 μl of this medium in 384-well microplates, which were sealed with aluminum films (AlumaSeal II; Excel Scientific, CA, USA). After 5 days of incubation, strains which changed the color of arginine indicator broth to yellow were isolated as candidate strains for mutants lacking arginine deiminase.
DNA manipulations and sequence analysis.
The oligonucleotide primers used in this study are listed in Table 2. PCR was performed using DNA polymerase KOD FX Neo (Toyobo, Osaka, Japan) under the optimal conditions recommended by the supplier. PCR products were separated on 0.9% agarose gel and purified using the Wizard SV gel and PCR clean-up system (Promega, Madison, WI). The DNA sequences of the fragments were determined by a commercial DNA sequencing service (Fasmac, Atsugi, Japan).
mRNA expression analysis by quantitative RT-PCR.
Cells grown in 500 μl of soy sauce medium supplemented with 2% arginine were pelleted by centrifugation (6,000 × g) and suspended in 1 ml of Isogen RNA extraction kit medium (Nippon Gene, Tokyo, Japan). Cells were lysed with approximately 0.5 mg of glass beads (0.1 mm in diameter) by a bead cell disrupter at room temperature and 3,000 rpm for 3 min, and RNA extraction was carried out according to the manufacturer's instructions. The yield and purity of RNA were determined by spectrophotometric measurements of the UV absorbance at 260 and 280 nm. Total RNA was reverse transcribed with a PrimeScript RT reagent kit with genomic DNA eraser (TaKaRa, Kusatsu, Japan), using random primers, by following the manufacturer’s instructions. The primer set arcAf and arcAr was used for the arcA gene expression analysis. The 16S rRNA gene was used as the housekeeping control gene with the primer set 16Sf and 16Sr (Table 2). Quantitative real-time PCR was performed using SYBR premix Ex Taq II (TaKaRa) using 1 μg of the cDNA as the template. The results were analyzed using the thermal cycler Dice real-time system TP900 (TaKaRa). The cycle threshold value for each PCR was calculated by the second derivative maximum method, and the relative expression of arcA was calculated by the calibration curve. The specificity of reactions was determined by melting curve analysis and the electrophoresis of the PCR products. The PCR was performed in triplicate.
Northern blotting.
Total RNA was extracted as described above. The probe was amplified by PCR using the primer set probe-arcAf and probe-arcAr (Table 2) and the genomic DNA of NBRC 12172 as a template and labeled with digoxigenin (DIG)-dUTP using PCR DIG labeling mix (Roche, Basel, Switzerland). RNA was incubated in formaldehyde morpholinepropanesulfonic acid buffer for 15 min at 65°C for denaturation, subjected to electrophoresis (0.4 μg per lane) on a formaldehyde-containing 0.9% agarose gel, stained with ethidium bromide, and transferred to a Hybond-N+ nylon membrane (GE Healthcare, Chicago, IL). Hybridization was performed with the DIG Easy Hyb system (Roche) at 50°C for 16 h with a DIG-labeled probe. For detection, the membranes were treated with anti-DIG-AP Fab fragments and CDP-Star (Roche), exposed to BioMax light film (Carestream Health, Rochester, USA), and developed by a Kodak GBX developer/fixer (Kodak China, Wuxi, China). The product size on the membrane was estimated by the 16S rRNA (1.6 kb) and 23S rRNA (2.9 kb) patterns stained with ethidium bromide.
Soy sauce manufacturing.
Soy sauce mash was prepared as described previously (11). Briefly, steamed soy beans and roasted wheat were mixed with Aspergillus oryzae and incubated for 40 h to make koji, which was then mixed with brine to make soy sauce mash (final NaCl concentration, 17%). Starter strains were cultured in soy sauce medium for 5 days, and 1 ml of the cultures was added to 1 liter of soy sauce mash. The mash was fermented at 15°C for 2 weeks and then at 28°C for 6 weeks. The fermentation experiment was performed in triplicate.
Chemical analysis.
Bacterial cultures or soy sauce mash were filtered through filter paper (no. 2; Toyo Roshi, Tokyo, Japan), and the pH of the filtrate was determined using an F52 pH meter (Horiba, Kyoto, Japan). Amino acid composition was analyzed by high-performance liquid chromatography (HPLC) with ninhydrin detection. The HPLC system used was the Hitachi ELITE LaChrom (Hitachi High-Technologies, Tokyo, Japan) equipped with a pump (L-2130), an autosampler (L-2200), a column oven (L-2300), a UV detector (L-2420), and a cation-exchange column (no. 2619PH). Lactic acid content was determined using a Shodex OA organic acid analysis system (Shodex, Tokyo, Japan) by following the instructions of the manufacturer.
Bioinformatics.
The DNA and amino acid sequences were analyzed using BLAST and ISfinder (44). The thermodynamic stability of RNA secondary structures was estimated using free-energy calculations from the RNA Websuite (45).
Data availability.
Sequence data were deposited in the DDBJ/EMBL/GenBank databases under accession numbers LC430896 to LC430900 (M1 to M5) and LC458667 to LC458668 (YA5 and A-30).
Supplementary Material
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.00208-19.
REFERENCES
- 1.Mahillon J, Chandler M. 1998. Insertion sequences. Microbiol Mol Biol Rev 62:725–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baliga NS, Bonneau R, Facciotti MT, Pan M, Glusman G, Deutsch EW, Shannon P, Chiu Y, Weng RS, Gan RR, Hung P, Date SV, Marcotte E, Hood L, Ng WV. 2004. Genome sequence of Haloarcula marismortui: a halophilic archaeon from the Dead Sea. Genome Res 14:2221–2234. doi: 10.1101/gr.2700304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blattner FR, Plunkett G, Bloch CA, Perna NT, Burland V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF, Gregor J, Davis NW, Kirkpatrick HA, Goeden MA, Rose DJ, Mau B, Shao Y. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 4.Tanizawa Y, Tohno M, Kaminuma E, Nakamura Y, Arita M. 2015. Complete genome sequence and analysis of Lactobacillus hokkaidonensis LOOC260 T, a psychrotrophic lactic acid bacterium isolated from silage. BMC Genomics 16:240. doi: 10.1186/s12864-015-1435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee H, Doak TG, Popodi E, Foster PL, Tang H. 2016. Insertion sequence-caused large-scale rearrangements in the genome of Escherichia coli. Nucleic Acids Res 44:7109–7119. doi: 10.1093/nar/gkw647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gury J, Barthelmebs L, Cavin JF. 2004. Random transposon mutagenesis of Lactobacillus plantarum by using the pGh9: ISS1 vector to clone genes involved in the regulation of phenolic acid metabolism. Arch Microbiol 182:337–345. doi: 10.1007/s00203-004-0705-1. [DOI] [PubMed] [Google Scholar]
- 7.Maguin E, Prevost H, Ehrlich SD, Gruss A. 1996. Efficient insertional mutagenesis in lactococci and other gram-positive bacteria. J Bacteriol 178:931–935. doi: 10.1128/jb.178.3.931-935.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishimura I, Shiwa Y, Sato A, Oguma T, Yoshikawa H, Koyama Y. 2018. Comparative genomics of Tetragenococcus halophilus. J Gen Appl Microbiol 63:369–372. doi: 10.2323/jgam.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Satomi M, Furushita M, Oikawa H, Yano Y. 2011. Diversity of plasmids encoding histidine decarboxylase gene in Tetragenococcus spp. isolated from Japanese fish sauce. Int J Food Microbiol 148:60–65. doi: 10.1016/j.ijfoodmicro.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 10.Tanasupawat S, Thongsanit J, Okada S, Komagata K. 2002. Lactic acid bacteria isolated from soy sauce mash in Thailand. J Gen Appl Microbiol 48:201–209. doi: 10.2323/jgam.48.201. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka Y, Watanabe J, Mogi Y. 2012. Monitoring of the microbial communities involved in the soy sauce manufacturing process by PCR-denaturing gradient gel electrophoresis. Food Microbiol 31:100–106. doi: 10.1016/j.fm.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Villar M, de Ruiz Holgado AP, Sanchez JJ, Trucco RE, Oliver G. 1985. Isolation and characterization of Pediococcus halophilus from salted anchovies (Engraulis anchoita). Appl Environ Microbiol 49:664–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamada T, Ishiyama T, Motai H. 1989. Continuous fermentation of soy sauce by immobilized cells of Zygosaccharomyces rouxii in an airlift reactor. Appl Microbiol Biotechnol 31:346–350. [Google Scholar]
- 14.Wakinaka T, Iwata S, Takeishi Y, Watanabe J, Mogi Y, Tsukioka Y, Shibata Y. 2019. Isolation of halophilic lactic acid bacteria possessing aspartate decarboxylase and application to fish sauce fermentation starter. Int J Food Microbiol 292:137–143. doi: 10.1016/j.ijfoodmicro.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Matsudo T, Aoki T, Abe K, Fukuta N, Higuchi T, Sasaki M, Uchida K. 1993. Determination of ethyl carbamate in soy sauce and its possible precursor. J Agric Food Chem 41:352–356. doi: 10.1021/jf00027a003. [DOI] [Google Scholar]
- 16.Nakadai T. 2015. Isolation of soy sauce lactic acid bacteria from soy sauce mash and their breeding. J Jpn Soy Sauce Res Inst 41:358–376. [Google Scholar]
- 17.Baur H, Luethi E, Stalon V, Mercenier A, Haas D. 1989. Sequence analysis and expression of the arginine-deiminase and carbamate-kinase genes of Pseudomonas aeruginosa. Eur J Biochem 179:53–60. doi: 10.1111/j.1432-1033.1989.tb14520.x. [DOI] [PubMed] [Google Scholar]
- 18.D'Hooghe I, Vander Wauven C, Michiels J, Tricot C, de Wilde P, Vanderleyden J, Stalon V. 1997. The arginine deiminase pathway in Rhizobium etli: DNA sequence analysis and functional study of the arcABC genes. J Bacteriol 179:7403–7409. doi: 10.1128/jb.179.23.7403-7409.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Divol B, Tonon T, Morichon S, Gindreau E, Lonvaud-Funel A. 2003. Molecular characterization of Oenococcus oeni genes encoding proteins involved in arginine transport. J Appl Microbiol 94:738–746. doi: 10.1046/j.1365-2672.2003.01907.x. [DOI] [PubMed] [Google Scholar]
- 20.Zúñiga M, del Carmen Miralles M, Pérez-Martínez G. 2002. The product of arcR, the sixth gene of the arc operon of Lactobacillus sakei, is essential for expression of the arginine deiminase pathway. Appl Environ Microbiol 68:6051–6058. doi: 10.1128/AEM.68.12.6051-6058.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barcelona-Andrés B, Marina A, Rubio V. 2002. Gene structure, organization, expression, and potential regulatory mechanisms of arginine catabolism in Enterococcus faecalis. J Bacteriol 184:6289–6300. doi: 10.1128/JB.184.22.6289-6300.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siguier P, Varani A, Perochon J, Chandler M. 2012. Exploring bacterial insertion sequences with ISfinder: objectives, uses, and future developments, p 91–103. In Bigot Y. (ed), Mobile genetic elements. Methods in Molecular Biology (Methods and Protocols), vol 859 Humana Press, Hoboken, NJ. doi: 10.1007/978-1-61779-603-6_5. [DOI] [PubMed] [Google Scholar]
- 23.Maas WK. 1994. The arginine repressor of Escherichia coli. Microbiol Rev 58:631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weickert MJ, Chambliss GH. 1990. Site-directed mutagenesis of a catabolite repression operator sequence in Bacillus subtilis. Proc Natl Acad Sci U S A 87:6238–6242. doi: 10.1073/pnas.87.16.6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skorski P, Proux F, Cheraiti C, Dreyfus M, Denmat SH-L. 2007. The deleterious effect of an insertion sequence removing the last twenty percent of the essential Escherichia coli rpsA gene is due to mRNA destabilization, not protein truncation. J Bacteriol 189:6205–6212. doi: 10.1128/JB.00445-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schulz C, Gierok P, Petruschka L, Lalk M, Mäder U, Hammerschmidt S. 2014. Regulation of the arginine deiminase system by ArgR2 interferes with arginine metabolism and fitness of Streptococcus pneumoniae. mBio 5:e01858-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao X, Du G, Zou H, Fu J, Zhou J, Chen J. 2013. Progress in preventing the accumulation of ethyl carbamate in alcoholic beverages. Trends Food Sci Technol 32:97–107. doi: 10.1016/j.tifs.2013.05.009. [DOI] [Google Scholar]
- 28.Yoshiuchi K, Watanabe M, Nishimura A. 2000. Breeding of a non-urea producing sake yeast with killer character using a kar1-1 mutant as a killer donor. J Ind Microbiol Biotechnol 24:203–209. doi: 10.1038/sj.jim.2900797. [DOI] [Google Scholar]
- 29.Uchida K. 1982. Multiplicity in soy pediococci carbohydrate fermentation and its application for analysis of their flora. J Gen Appl Microbiol 28:215–223. doi: 10.2323/jgam.28.215. [DOI] [Google Scholar]
- 30.Kanbe C, Uchida K. 1982. Diversity in the metabolism of organic acids by Pediococcus halophilus. Agric Biol Chem 46:2357–2359. doi: 10.1080/00021369.1982.10865436. [DOI] [Google Scholar]
- 31.Ueki T, Izawa T, Ohba K, Noda Y. 2000. Isolation and characterization of halotolerant lactic acid bacteria with flocculent. J Jpn Soy Sauce Res Inst 26:197–203. [Google Scholar]
- 32.Satomi M, Furushita M, Oikawa H, Yoshikawa-Takahashi M, Yano Y. 2008. Analysis of a 30 kbp plasmid encoding histidine carboxylase gene in Tetragenococcus halophilus isolated from fish sauce. Int J Food Microbiol 126:202–209. doi: 10.1016/j.ijfoodmicro.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 33.Satomi M, Shozen K-I, Furutani A, Fukui Y, Kimura M, Yasuike M, Funatsu Y, Yano Y. 2014. Analysis of plasmids encoding the tyrosine decarboxylase gene in Tetragenococcus halophilus isolated from fish sauce. Fish Sci 804:849–858. doi: 10.1007/s12562-014-0756-4. [DOI] [Google Scholar]
- 34.Ueki T, Izawa T, Ohba K, Noda Y. 2002. Mechanisms of floc formation by the halotolerant lactic acid bacteria with flocculent and its use in soy sauce brewing. J Jpn Soy Sauce Res Inst 28:105–110. [Google Scholar]
- 35.Higuchi T, Uchida K, Abe K. 1999. Preparation of phage-insensitive strains of Tetragenococcus halophila and its application for soy sauce fermentation. Biosci Biotechnol Biochem 63:415–417. doi: 10.1271/bbb.63.415. [DOI] [PubMed] [Google Scholar]
- 36.De Palmenaer D, Siguier P, Mahillon J. 2008. IS 4 family goes genomic. BMC Evol Biol 8:18. doi: 10.1186/1471-2148-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hickman AB, Dyda F. 2015. Mechanisms of DNA transposition. Microbiol Spectr 3:MDNA3. doi: 10.1128/microbiolspec.MDNA3-0034-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Narumi I, Cherdchu K, Kitayama S, Watanabe H. 1997. The Deinococcus radiodurans uvrA gene: identification of mutation sites in two mitomycin-sensitive strains and the first discovery of insertion sequence element from deinobacteria. Gene 198:115–126. doi: 10.1016/S0378-1119(97)00301-6. [DOI] [PubMed] [Google Scholar]
- 39.Nagai T, Tran LSP, Inatsu Y, Itoh Y. 2000. A new IS4 family insertion sequence, IS4Bsu1, responsible for genetic instability of poly-γ-glutamic acid production in Bacillus subtilis. J Bacteriol 182:2387–2392. doi: 10.1128/JB.182.9.2387-2392.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu K, He ZQ, Mao YM, Sheng RQ, Sheng ZJ. 1993. On two transposable elements from Bacillus stearothermophilus. Plasmid 29:1–9. doi: 10.1006/plas.1993.1001. [DOI] [PubMed] [Google Scholar]
- 41.Eichenbaum Z, Livneh Z. 1998. UV light induces IS10 transposition in Escherichia coli. Genetics 149:1173–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toda A, Kayahara H, Yasuhira H, Sekiguchi J. 1989. Conjugal transfer of pIPSOl from Enterococcus faecalis to Pediococcus halophilus. Agric Biol Chem 53:3317–3318. doi: 10.1271/bbb1961.53.3317. [DOI] [Google Scholar]
- 43.Benachour A, Flahaut S, Frère J, Novel G. 1996. Plasmid transfer by electroporation and conjugation in Tetragenococcus and Pediococcus genera and evidence of plasmid-linked metabolic traits. Curr Microbiol 32:188–194. doi: 10.1007/s002849900034. [DOI] [Google Scholar]
- 44.Siguier P, Pérochon J, Lestrade L, Mahillon J, Chandler M. 2006. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res 34:D32–D36. doi: 10.1093/nar/gkj014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gruber AR, Lorenz R, Bernhart SH, Neuböck R, Hofacker IL. 2008. The Vienna RNA Websuite. Nucleic Acids Res 36:W70–W74. doi: 10.1093/nar/gkn188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequence data were deposited in the DDBJ/EMBL/GenBank databases under accession numbers LC430896 to LC430900 (M1 to M5) and LC458667 to LC458668 (YA5 and A-30).