The gut microbiome has an important role in both health and disease of the host. The mammalian gut microbiome is often dominated by bacteria from the Bacteroidales, an order that includes Bacteroides and Prevotella. In this study, we have identified an acylated amino acid, called glycine lipid, produced by Bacteroides thetaiotaomicron, a beneficial bacterium originally isolated from the human gut. In addition to identifying the genes required for the production of glycine lipids, we show that glycine lipids have an important role during the adaptation of B. thetaiotaomicron to a number of environmental stresses, including exposure to either bile or air. We also show that glycine lipids are important for the normal colonization of the murine gut by B. thetaiotaomicron. This work identifies glycine lipids as an important fitness determinant in B. thetaiotaomicron and therefore increases our understanding of the molecular mechanisms underpinning colonization of the mammalian gut by beneficial bacteria.
KEYWORDS: glycine lipid, stress
ABSTRACT
Acylated amino acids function as important components of the cellular membrane in some bacteria. Biosynthesis is initiated by the N-acylation of the amino acid, and this is followed by subsequent O-acylation of the acylated molecule, resulting in the production of the mature diacylated amino acid lipid. In this study, we use both genetics and liquid chromatography-mass spectrometry (LC-MS) to characterize the biosynthesis and function of a diacylated glycine lipid (GL) species produced in Bacteroides thetaiotaomicron. We, and others, have previously reported the identification of a gene, named glsB in this study, that encodes an N-acyltransferase activity responsible for the production of a monoacylated glycine called N-acyl-3-hydroxy-palmitoyl glycine (or commendamide). In all of the Bacteroidales genomes sequenced so far, the glsB gene is located immediately downstream from a gene, named glsA, that is also predicted to encode a protein with acyltransferase activity. We use LC-MS to show that the coexpression of glsB and glsA results in the production of GL in Escherichia coli. We constructed a deletion mutant of the glsB gene in B. thetaiotaomicron, and we confirm that glsB is required for the production of GL in B. thetaiotaomicron. Moreover, we show that glsB is important for the ability of B. thetaiotaomicron to adapt to stress and colonize the mammalian gut. Therefore, this report describes the genetic requirements for the biosynthesis of GL, a diacylated amino acid species that contributes to fitness in the human gut bacterium B. thetaiotaomicron.
IMPORTANCE The gut microbiome has an important role in both health and disease of the host. The mammalian gut microbiome is often dominated by bacteria from the Bacteroidales, an order that includes Bacteroides and Prevotella. In this study, we have identified an acylated amino acid, called glycine lipid, produced by Bacteroides thetaiotaomicron, a beneficial bacterium originally isolated from the human gut. In addition to identifying the genes required for the production of glycine lipids, we show that glycine lipids have an important role during the adaptation of B. thetaiotaomicron to a number of environmental stresses, including exposure to either bile or air. We also show that glycine lipids are important for the normal colonization of the murine gut by B. thetaiotaomicron. This work identifies glycine lipids as an important fitness determinant in B. thetaiotaomicron and therefore increases our understanding of the molecular mechanisms underpinning colonization of the mammalian gut by beneficial bacteria.
INTRODUCTION
Members of the phylum Bacteroidetes, including genera containing important human gut commensal bacteria such as Bacteroides, Parabacteroides, and Prevotella, dominate the healthy human gut microbiota (1). The gut-associated Bacteroidetes are required to digest complex dietary glycans into short-chain fatty acids (SCFA) (such as acetate and propionate) that are accessible to the host (2–4). A longitudinal study in infants revealed the presence of Bacteroides in the infant gut within 1 week of birth, and some species of Bacteroides have been shown to utilize the polysaccharides present in human breast milk (5, 6). Therefore, it has been suggested that Bacteroides may have an important role during the early development of the infant gut (6).
Acylated amino acids can be found in the membranes of many bacteria (7, 8). The best-characterized, and most widespread, acylated amino acid is ornithine lipid (OL). OL contains a 3′-hydroxy fatty acid group attached by an amide linkage to the α-amino group of ornithine, with a second fatty acid group ester linked to the 3′-hydroxy group of the first fatty acid (9). The genetics of OL production were first described for Sinorhizobium meliloti, where it was shown that an N-acyltransferase encoded by olsB catalyzed the attachment of a hydroxylated fatty acid group to ornithine, resulting in monoacylated ornithine or lyso-OL (10). The second fatty acid was subsequently attached to the hydroxyl group of lyso-OL through the activity of an O-acyltransferase encoded by olsA, resulting in the production of OL (11). An enzyme called OlsF, containing functionally independent domains with N-acyltransferase and O-acyltransferase activities, has recently been shown to produce OL in Serratia proteamaculans (12). Activities that further modify the OL by hydroxylation or methylation have also been identified (13–15). OL have been shown to be important for growth during acid and temperature stress in Rhizobium tropici, and depletion of OL results in an increase in the speed of crown gall tumor formation in plants infected with Agrobacterium (13, 16). Therefore, OL are important during the interactions between bacteria and their environment, including hosts.
We, and others, have previously identified a gene from Bacteroides, initially named choA, that encodes an N-acyltransferase required for the production of a monoacylated glycine species, called N-acyl-3-hydroxy-palmitoyl glycine or commendamide (17, 18). We have previously reported that choA encodes a protein with homology to the N-acyltransferase domain of OlsF, suggesting a potential role for choA in the production of a diacylated glycine lipid (GL) species (17). In this study, we use liquid-chromatography-mass spectrometry (LC-MS) to show that choA is required for the production of a diacylated GL in Bacteroides thetaiotaomicron, and in line with previous nomenclature, we have renamed choA as glsB. Using heterologous expression in Escherichia coli, we show that GL production also requires glsA, a gene predicted to encode an O-acyltransferase that is located immediately upstream from glsB in the B. thetaiotaomicron genome. Finally, we show that glsB is important for the ability of B. thetaiotaomicron to adapt to stress and colonize the mammalian gut.
(This article was submitted to an online preprint archive [19].)
RESULTS
ChoA and ChoB are required to produce diacylated GL in E. coli.
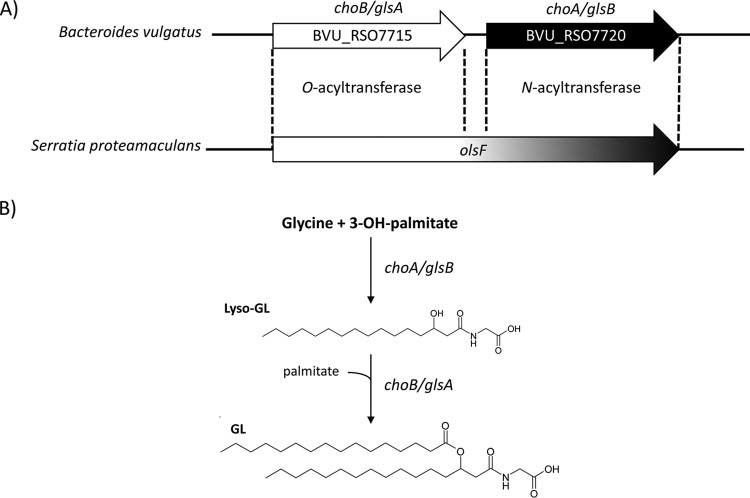
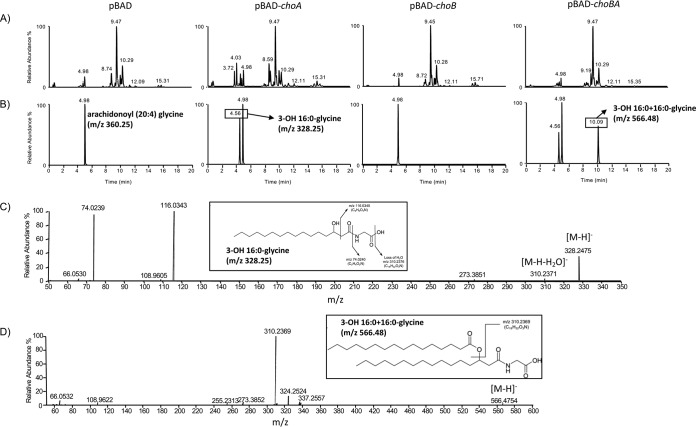
We have previously shown that choA, when expressed in E. coli, results in the production of commendamide, an N-acylated (3-OH C16:0) derivative of glycine with hemolytic activity and the ability to solubilize cholesterol micelles (17). In all members of the Bacteroidales, the choA gene is located immediately downstream from another gene (nominally called choB) predicted to encode an O-acyltransferase (17). Together, choA and choB are homologous to the bifunctional amino acid acyltransferase OlsF that is responsible for the production of OL in S. proteamaculans (Fig. 1). Therefore, we wanted to determine whether choA and choB might work together to produce diacylated glycine lipids (GL). To do this, we amplified BVU_RSO7720 (choA), BVU_RS07715 (choB), and choB-choA from Bacteroides vulgatus and cloned the genes into pBAD24 for arabinose-controlled expression in E. coli (resulting in the formation of pBAD-choA, pBAD-choB, and pBAD-choBA, respectively). The cells were cultured in LB broth, and gene expression was induced by the addition of 0.2% (wt/vol) l-arabinose to the cultures (as described in Materials and Methods). Cells were harvested, and methanol extracts of the cell pellets were subjected to high-resolution LC-MS analysis. In cells overexpressing choA, we detected lipid species that corresponded to a glycine with an N-acyl substitution of various carbon chain lengths and degrees of saturation, primarily 14:0, 16:0 (i.e., commendamide), 16:1, and 18:1 (Fig. 2 and Table 1). Notably, overexpression of choA also resulted in the production of very low levels of diacylated glycine, i.e., 0.7% of the total acylated glycine pool (Table 1). When cells carrying pBAD-choBA were analyzed, we detected a range of both monoacylated and diacylated glycine species (Fig. 2 and Table 1). The identity of these lipids was confirmed by tandem MS (MS/MS) fragmentation, although some diacylated glycine species appeared to have mixed fatty acid compositions; e.g., in the peak with a mass-to-charge ratio (m/z) value of 510.4, there was a mixture of diacylated glycines substituted with 3-OH-16:0 + 12:0 and 3-OH-14:0 + 14:0 (3-OH-16:0 + 12:0 indicates a glycine that is substituted with 2 acyl chains, C16:0 and C12:0) (Table 1). The total level of monoacylated glycine production in cells overexpressing choBA was approximately 4.5-fold lower than the level observed in cells overexpressing choA alone. Moreover, diacylated glycine production in choBA-expressing cells accounted for 92.7% of the total acylated glycine pool (Table 1). Importantly, we could not detect monoacylated or diacylated glycine in cells overexpressing choB alone (Fig. 2). Therefore, we propose that, in a mechanism analogous to OL biosynthesis, ChoA N-acylates glycine, resulting in the formation of lyso-GL, which is subsequently O-acylated by ChoB to produce diacylated GL. In accordance with the nomenclature used for the genes involved in OL biosynthesis, we propose that choA and choB be renamed glsB (encoding glycine N-acyltransferase) and glsA (encoding lyso-GL O-acyltransferase), respectively (Fig. 1B).
FIG 1.
(A) The protein encoded by the choB gene in Bacteroides vulgatus has predicted homology (30.2% identity over 273 amino acids) with the N terminus of OlsF (Spro_2569), carrying the O-acyltransferase activity required for the biosynthesis of ornithine lipids (OL). Similarly, the choA gene is predicted to encode a protein with homology (32.6% identity over 264 amino acids) to the C terminus of OlsF, carrying the N-acyltransferase activity involved in OL biosynthesis. (B) Predicted pathway for the production of glycine lipid (GL) in Bacteroides based on homology with OL production in Serratia proteamaculans. In this schematic, the N- and O-acylations involve 3-OH-palmitate and palmitate, respectively.
FIG 2.
Identification of glycine lipids in E. coli overexpressing choA and choB. E. coli containing pBAD24, pBAD-choA, pBAD-choB, or pBAD-choBA was cultured in the presence of 0.2% l-arabinose until mid-exponential phase, and extracted lipids were analyzed by LC-MS, as described in Materials and Methods. All samples were spiked with 500 pmol arachidonyl (20:4) glycine as an internal standard (Rf = 4.98 min; m/z 360.25). (A) Base peak intensity chromatogram showing the range of lipids present in the E. coli membrane. All lipid profiles appear to be qualitatively similar, with the exception of that for pBAD-choA, which shows increased peaks eluting with an Rf of approximately 4 min, where monoacylated glycine molecules would be expected to be eluted. (B) Extracted ion chromatograms of peaks eluting with Rf values corresponding to mono- or diacylated glycine species. (For clarity, only 3-OH-16:0 [Rf = 4.56 min; m/z 328.25] and 3-OH-16:0 + 16:0 [Rf = 10.09 min; m/z 566.48] are shown. The full list of identified molecules can be found in Table 1.) (C) MS/MS fragmentation of the molecule eluting with an Rf of 4.56 confirming its identification as 3-OH-16:0-glycine. The same peak in both pBAD-choA and pBAD-choBA gave identical fragmentation profiles. (D) MS/MS fragmentation confirming the O-acylation of 3-OH-16:0 resulting in a structure of the m/z 566.48 compound that is consistent with a diacylated glycine (in this case, 3-OH-16:0 + 16:0). All diacylated glycine molecules detected are listed in Table 1.
TABLE 1.
Glycine lipids in E. coli expressing glsB and glsAc
| Predicted size of acyl group (m/z) | Mean concn (pmol/109 cells) ± SD |
|
|---|---|---|
| pBAD-choA/glsB | pBAD-choBA/glsAB | |
| 3-OH-12:0 | 35.3 ± 9.0 | ND |
| 3-OH-14:0 (300.2)b | 4,070.3 ± 220.9 | 221.3 ± 145.2 |
| 3-OH-14:1 (298.2)b | 118.0 ± 13.3 | ND |
| 3-OH-15:0 (314.2)b | 52.4 ± 14.4 | 9.2a |
| 3-OH-16:0 (328.2)b | 3,954.6 ± 215.3 | 1,584.9 ± 843.4 |
| 3-OH-16:1 (326.2)b | 6,831.9 ± 264.0 | 382.3 ± 267.1 |
| 3-OH-18:0 (356.1)b | 7.8 ± 1.1 | 37.4 ± 33.7 |
| 3-OH-18:1 (354.2)b | 2,539.2 ± 227.5 | 1,697.4 ± 994.2 |
| Total monoacylated glycine | 17,609.5 ± 965.5 | 3,923.3 ± 2,283.6 |
| 3-OH-14:0 + 12:0 (482.4)b | ND | 436.6 ± 228.5 |
| 3-OH-16:0 + 12:0/3-OH-14:0 + 14:0 (510.4)b | ND | 5,864.1 ± 2,999.9 |
| 3-OH-16:1 + 12:0 (508.4)b | ND | 652.0 ± 356.9 |
| 3-OH-29:0 | ND | 240.5 ± 122.1 |
| 3-OH-16:0 + 14:0 (538.4)b | 16.3 ± 5.7 | 17,407.0 ± 6,125.0 |
| 3-OH-16:1 + 14:0/3-OH-16:0 + 14:1 (536.4)b | ND | 5,154.6 ± 2,257.9 |
| 3-OH-30:2 | ND | 452.0 ± 238.0 |
| 3-OH-16:0 + 15:0 (552.4)b | ND | 458.8 ± 228.3 |
| 3-OH-31:1 | ND | 152.3 ± 47.2a |
| 3-OH-16:0 + 16:0 (566.4)b | 44.5 ± 10.8 | 3,901.3 ± 141.9 |
| 3-OH-16:0 + 16:1/3-OH-18:1 + 14:0 (564.4)b | 39.4 ± 14.6 | 10,673.8 ± 3,157.3 |
| 3-OH-16:1 + 16:1 (562.4)b | ND | 1,322.0 ± 429.6 |
| 3-OH-33:1 | ND | 131.4 ± 74.3 |
| 3-OH-16:0 + 18:1/3-OH-18:1 + 16:0 (592.5)b | 27.7 ± 15.2 | 1,540.3 ± 23.7 |
| 3-OH-18:1 + 16:1 (590.4)b | ND | 1,275.5 ± 218.8 |
| 3-OH-36:2 | ND | 179.5 ± 43.9 |
| Total diacylated glycine | 127.9 ± 46.3 | 49,841.7 ± 16,693.3 |
| Total acylated pool | 17,737.4 | 53,765.0 |
Not detected in all biological replicates.
Acylated glycine confirmed by MS/MS fragmentation. The acyl group designation is indicative, based on the predicted number of carbons in the lipid species.
No acylated glycine molecules could be detected in cells carrying the pBAD vector alone. ND, not detected.
GlsB is required for the production of commendamide in B. thetaiotaomicron.
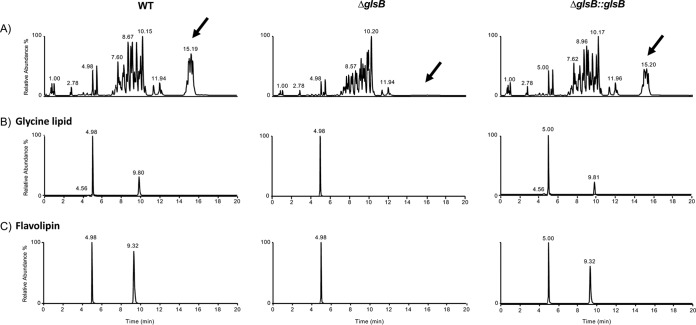
We wanted to confirm that glsA and glsB were involved in the production of GL in Bacteroides. To do this, we decided to take advantage of the genetic tools available from Bacteroides thetaiotaomicron VPI-5482 in order to make a deletion mutant of the glsB (BT_3459) homologue in this bacterium. We also constructed a strain whereby a native copy of glsB was inserted into the genome of the ΔglsB mutant strain (ΔglsB::glsB). Unfortunately, despite several attempts, we were unable to construct a knockout mutation of glsA (BT_3458), or a double knockout of glsA glsB, in B. thetaiotaomicron. Nonetheless, we were able to identify lipid species in both the wild type (WT) and the complemented ΔglsB::glsB strain that were consistent with commendamide and other N-acylated derivatives of glycine (Fig. 3 and Table 2). Further mass spectrometric analysis indicated the presence of diacylated GL in both the WT and the complemented ΔglsB::glsB strain but not in the ΔglsB mutant (Fig. 3B and Table 2). These species showed differences in their retention times compared to the GL found in E. coli and gave rise to multiple, partially resolved chromatographic peaks. Bacteroides strains are known to produce branched-chain fatty acids, although from our MS/MS analysis, it was not possible to definitively assign whether the glycines were acylated with straight- or branched-chain acyl groups (e.g., 16:0 or methyl-15:0) or if they were iso or anteiso branched (20). Importantly, we could not detect any acylated glycine in the ΔglsB mutant (Table 2). Therefore, glsB is required for the production of all acylated glycine species in B. thetaiotaomicron. Interestingly, a comparison of the total lipid chromatograms revealed both qualitative and quantitative differences between the ΔglsB mutant and both the WT and ΔglsB::glsB strains (Fig. 3A). Although a comprehensive analysis of these differences is not the objective of this study, we determined that an ion with the same mass as lipid 654 is produced by B. thetaiotaomicron but absent from the ΔglsB mutant (Fig. 3C). Lipid 654 is an acylated serine-glycine dipeptide that has been detected in many members of the Bacteroidetes (21). Lipid 654, also called flavolipin, was first described in members of the Flavobacterium and Cytophaga genera (22–24). Some recent studies with Porphyromonas gingivalis have implicated lipid 654 in osteoblast differentiation and atherosclerosis in humans and have also identified lipid 654 as a potential microbiome-associated biomarker for multiple sclerosis (21, 25–27). In addition, a series of molecules with retention times of approximately 14 to 16 min and m/z ratios ranging from 1,200 to 1,300 were also completely absent from the ΔglsB mutant. The identity of these species is under investigation. Therefore, our data confirm that glsB is required for the production of GL and lipid 654 in B. thetaiotaomicron, and a mutation in glsB results in significant qualitative and quantitative changes in the lipid profile of the membranes of this bacterium.
FIG 3.
Identification of glycine lipids in B. thetaiotaomicron. Cells (as indicated) were cultured in BHIS broth until mid-exponential phase, and extracted lipids were analyzed by LC-MS, as described in Materials and Methods. All samples were spiked with 500 pmol arachidonyl (20:4) glycine as an internal standard (Rf = 4.98 min; m/z 360.25). (A) Base peak intensity chromatogram showing the range of lipids present in the membrane of B. thetaiotaomicron. Clear qualitative (indicated by arrows) and quantitative differences are observed between the profiles of the WT, ΔglsB, and ΔglsB::glsB strains. (B) Extracted ion chromatograms of peaks eluting with Rf values corresponding to mono- or diacylated glycine species. For clarity, the internal standard (arachidonoyl [20:4] glycine [Rf = 4.98 to 5.00 min; m/z 360.25]) and 2 ions corresponding to 3-OH-16:0 (Rf = 4.56 min; m/z 328.25) and C32:0 (Rf = 9.8 min; m/z 566.4) are shown. The full list of identified molecules can be found in Table 2. (C) Extracted ion chromatograms of peaks eluting with Rf values corresponding to flavolipin (lipid 654). The internal standard and the ion corresponding to C32:0 flavolipin (Rf = 9.32 min; m/z 653.51) are shown.
TABLE 2.
Glycine lipids in B. thetaiotaomicron
| Predicted size of acyl group (m/z) | Mean concn (pmol/109 cells) ± SDc |
||
|---|---|---|---|
| WT | ΔglsB | ΔglsB::glsB | |
| 3-OH-15:0/3-OH-methyl 14:0 (314.2)b | 67.9 ± 23.8a | ND | 64.6 ± 16.9a |
| 3-OH-16:0/3-OH-methyl 15:0 (328.2)b | 252.1 ± 36.3a | ND | 137.2 ± 98.1 |
| 3-OH-17:0/3-OH-methyl 16:0 (342.2)b | 429.9 ± 71.6a | ND | 223.0 ± 157.0 |
| Total monoacylated glycine | 749.9 ± 131.7 | 0 | 424.8 ± 272 |
| 3-OH-methyl 15:0 + 13:0/3-OH-methyl 14:0 + 14:0 (524.4)b | 223.2 ± 25.5 | ND | 169.4 ± 30.9 |
| 3-OH-methyl 15:0 + 14:0/3-OH-methyl 14:0 + 15:0 (538.4)b | 792.1 ± 81.2 | ND | 635.4 ± 92.0 |
| 3-OH-methyl 15:0 + 15:0/3-OH-methyl 16:0 + 14:0 (552.4)b | 2,000.3 ± 139.3 | ND | 1,372.0 ± 181.3 |
| 3-OH-methyl 16:0 + 15:0/3-OH-methyl 15:0 + 16:0 (566.4)b | 2,602.6 ± 118.9 | ND | 1,768.0 ± 259.6 |
| Total diacylated glycine | 5,618.2 ± 364.9 | 0 | 3,944.8 ± 563.8 |
| Total acylated pool | 6,368.1 | 0 | 4,369.6 |
Detected in only 2 (out of 3) biological replicates.
Acylated glycine confirmed by MS/MS fragmentation. The acyl group designation is indicative, based on the predicted number of carbons in the lipid species.
ND, not detected.
Expression of glsB and glsA is constitutive in B. thetaiotaomicron.
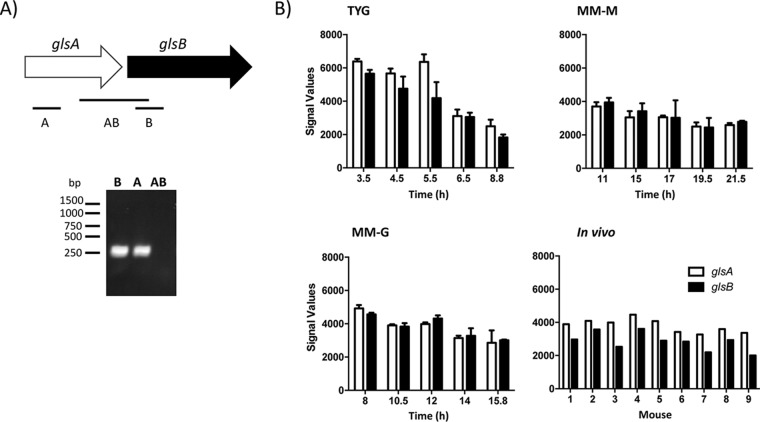
We wanted to examine the expression levels of glsA and glsB in B. thetaiotaomicron during growth. Cells were cultured to mid-exponential phase in either rich (brain heart infusion-supplemented [BHIS]) or defined (defined minimal medium [DMM]) growth medium, and total RNA was isolated. Reverse transcription-PCR (RT-PCR) analysis suggested that glsA and glsB are on different transcripts in B. thetaiotaomicron (Fig. 4A). To determine the expression profile of glsA and glsB, we examined the data from previously reported microarray experiments undertaken using B. thetaiotaomicron cultured under different in vitro and in vivo conditions (28, 29). In TYG broth, a rich growth medium composed of tryptone, yeast extract, and glucose, the levels of expression of both glsA and glsB are highest during early exponential phase, and the expression of both genes decreases over time (Fig. 4B). A similar trend is observed during growth in minimal medium, but the decrease in glsA and glsB expression over time is not as strong (Fig. 4B). Therefore, the expression of glsA and glsB may be linked to the growth rate. Moreover, glsA and glsB are also expressed during colonization of the cecum of mice by B. thetaiotaomicron (Fig. 4B). Therefore, glsA and glsB appear to be constitutively expressed in B. thetaiotaomicron during growth in vitro and in vivo.
FIG 4.
Analysis of the expression of glsA and glsB in B. thetaiotaomicron. (A) RT-PCR transcript analysis of expression from the glsAB locus. B. thetaiotaomicron cells were grown to mid-exponential phase in BHIS broth, and RNA was extracted and back-transcribed into cDNA (as described in Materials and Methods). Transcript analysis was undertaken using primer combinations that amplify a region specific to glsA (A) or glsB (B) or the intergenic region (AB). (B) Expression of glsA and glsB under different in vitro and in vivo growth conditions. Normalized microarray data were extracted from data sets available in the GEO database (accession number GSE2231). Sample preparation and analysis were described previously (28, 29). For the in vitro samples, B. thetaiotaomicron was cultured in chemostats using different media (TYG broth, minimal medium with maltose [MM-M], and minimal medium with glucose [MM-G]). At the indicated times, cells were harvested, RNA was extracted, and expression profiling was undertaken using custom B. thetaiotaomicron GeneChips. The data presented are the means of results from 2 biological replicates, and the error bars represent the standard deviations. For the in vivo experiments, individual germfree NMRI mice (n = 9) were monoassociated with B. thetaiotaomicron and fed a standard chow diet for 10 days. RNA was extracted from the cecal contents of each mouse and used for expression profiling using the custom B. thetaiotaomicron GeneChips.
The glsB gene is required for normal growth in vitro.
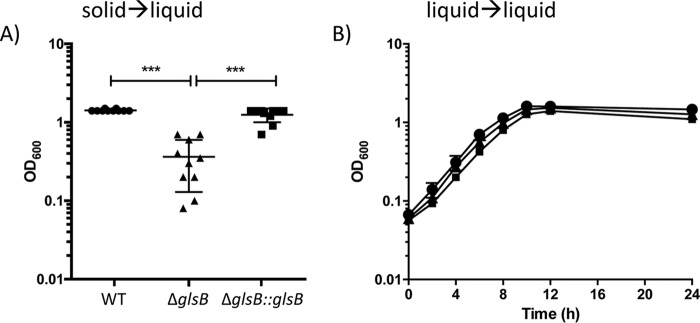
During preliminary experiments, we observed that when colonies were inoculated from agar plates into BHIS broth and incubated overnight, there was significantly reduced growth of the ΔglsB mutant compared to WT cultures. This suggested that glsB might be important for the normal growth of B. thetaiotaomicron. In order to quantify this observation, we set up 10 cultures, from fresh BHIS agar plates inoculated with the WT, the ΔglsB mutant, or the ΔglsB::glsB strain, and the cultures were incubated at 37°C for 18 h, at which point the final optical density at 600 nm (OD600) was taken as a measurement of growth. WT and ΔglsB::glsB cultures grown under these conditions reached mean OD600 values of 1.42 ± 0.042 and 1.25 ± 0.25, respectively (Fig. 5). However, the OD600 values of cultures inoculated with the ΔglsB mutant were significantly lower (0.36 ± 0.23; P < 0.001), confirming that the ΔglsB mutant has a strong growth defect under these conditions. Interestingly, when ΔglsB mutant cells from the broth cultures grown overnight were inoculated into fresh broth cultures, there was no observed defect in growth rates between the WT and the ΔglsB mutant (Fig. 5B). Therefore, our data suggest that glsB may be required to facilitate the adaptation of B. thetaiotaomicron to the transition from growth on a solid surface to growth in liquid broth.
FIG 5.
The ΔglsB mutant is unable to normally transition from solid to liquid growth media. (A) B. thetaiotaomicron (WT, ΔglsB, and ΔglsB::glsB strains) was grown on BHIS agar, and individual colonies (n = 10) were inoculated into fresh BHIS broth (solid→liquid). The cultures were incubated at 37°C for 24 h, and the OD600 was used to measure growth. Each point represents data for an individual culture, and the means (± standard deviations) are presented (***, P < 0.0001, as determined using one-way ANOVA with Tukey’s posttest for multiple comparisons). (B) Cells cultured as described above for panel A were used to inoculate fresh BHIS broth, cells were grown at 37°C, and the OD600 was measured at the indicated times. Each strain was grown in triplicate, each point is the mean from the replicates, and errors bars represent the standard deviations.
The glsB gene is required for adaptation to stress in vitro.
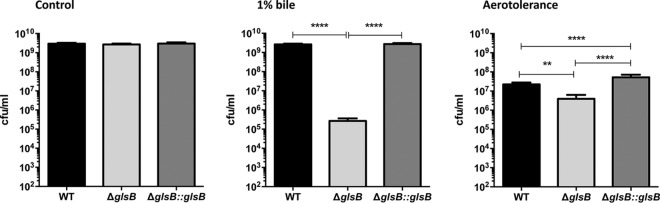
The transition from growth in solid medium to growth in liquid medium may represent a stress to the bacterium. Therefore, we decided to assess the sensitivity of the ΔglsB mutant to different stresses that would normally be encountered by B. thetaiotaomicron, i.e., bile stress and the presence of oxygen in air. B. thetaiotaomicron was cultured in BHIS broth to mid-exponential phase before the cells were transferred to fresh medium supplemented with 1% (wt/vol) porcine bile, and the cells were incubated, anaerobically, for a further 14 h. Under these conditions, WT and ΔglsB::glsB cultures reached final cell densities of 2.6 × 109 CFU ml−1 and 2.7 × 109 CFU ml−1, respectively (Fig. 6A). These cell densities are only marginally lower than the cell densities achieved when cells are grown under the same conditions but in the absence of bile (2.98 × 109 CFU ml−1 and 2.9 × 109 CFU ml−1, respectively), and this reflects the high level of bile tolerance associated with Bacteroides (30). In contrast, the ΔglsB mutant achieved a cell density of only 2.7 × 105 CFU ml−1 when cultured in the presence of 1% (wt/vol) porcine bile (in contrast to 2.7 × 109 CFU ml−1 when cultured in the absence of bile). Therefore, the ΔglsB mutant is approximately 104-fold more sensitive to porcine bile than the WT (Fig. 6A). Similarly, the ΔglsB mutant exhibited a 10-fold increased sensitivity to exposure to air for 14 h compared to both the WT and ΔglsB::glsB strains (Fig. 6B). Therefore, the glsB gene is important in B. thetaiotaomicron to allow adaptation to a variety of stresses, including exposure to bile and air.
FIG 6.
The glsB gene is required for adaptation to stress. B. thetaiotaomicron (WT, ΔglsB, and ΔglsB::glsB strains) was cultured to mid-exponential phase (OD600 = 0.2) before (i) inoculation into BHIS broth followed by anaerobic incubation for 14 h (control), (ii) inoculation into BHIS broth supplemented with 1% (wt/vol) porcine bile followed by anaerobic incubation for 14 h (1% bile), or (iii) inoculation into BHIS broth followed by aerobic incubation (with vigorous shaking) for 14 h (aerotolerance). Viable cell counts were determined using serial dilutions that were plated onto BHIS agar followed by anaerobic incubation for 24 to 48h. The experiment was repeated 3 times, and the error bars represent the standard deviations. Significance was determined using one-way ANOVA with Tukey’s posttest for multiple comparisons (**, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
The glsB gene is required for normal colonization of the murine gut.
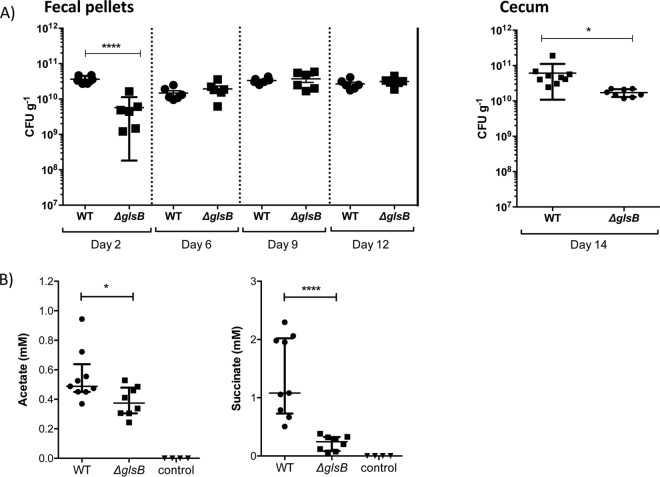
We wanted to determine the role, if any, of glsB during colonization of the mammalian gut. Therefore, germfree (GF) C57BL/6 mice were subjected to a single oral gavage of 108 CFU of either WT B. thetaiotaomicron or ΔglsB mutant bacteria. Fecal pellets were collected on days 2, 6, 9, and 12 postgavage, and bacteria were enumerated by viable plate counting on BHIS agar. On day 2, the level of WT B. thetaiotaomicron bacteria in fecal pellets was 3.6 × 1010 CFU g−1 feces, compared to a significantly lower level of 5.7 × 109 CFU g−1 feces for the ΔglsB mutant (Fig. 7A). However, by day 4, the ΔglsB mutant was found to be present in fecal pellets at the same level as the WT. Analysis of the cecal contents of mice collected on day 14 indicated that there is a small, but significant, decrease in the level of the ΔglsB mutant in the cecum compared to WT B. thetaiotaomicron (Fig. 7B). Therefore, the ΔglsB mutant is affected in its ability to colonize the murine gut, particularly during the early stages of colonization.
FIG 7.
B. thetaiotaomicron requires the glsB gene for normal colonization of the gut of mice. Germfree C57BL/6 mice were gavaged with a single dose of 108 CFU of the B. thetaiotaomicron WT (n = 9) or ΔglsB mutant (n = 8) strain, as indicated. Control mice (n = 4) were not colonized. (A) At the indicated times postgavage, fecal pellets were collected, and bacteria were enumerated by plating fecal homogenates onto BHIS agar. Bacteria in the cecum of colonized mice were also enumerated on day 14 postgavage. Control mice did not contain any bacteria at this point. Error bars represent the standard deviations of colonization levels in at least 5 mice (*, P < 0.05; ****, P < 0.001 [by an unpaired t test]). (B) The contents of the cecum from each colonized and control mouse were examined for the presence of acetate and succinate by HPLC analysis. The error bars represent the 25th to 75th percentile values from the median, and significance was determined using the Mann-Whitney test (*, P < 0.05; ****, P < 0.0001).
An important role for B. thetaiotaomicron in the gut is the conversion of dietary glycans into SCFA such as acetate and other organic acids, e.g., succinate (4, 31). Therefore, we decided to use acetate and succinate production as markers of B. thetaiotaomicron metabolism in the host. Using high-performance liquid chromatography (HPLC), we measured the levels of acetate and succinate in the cecal contents collected from germfree mice infected with either the WT or the ΔglsB mutant. As expected, we could not detect any acetate or succinate in the cecal contents of uninfected mice, confirming that these metabolites are exclusively derived from microbial activity in the gut (Fig. 7C). There was a small, but significant (P = 0.036 by a Mann-Whitney test), decrease in the level of acetate present in the cecal contents of mice colonized with the ΔglsB mutant compared to the WT (0.37 mM [0.24 to 0.53 mM] versus 0.49 mM [0.37 to 0.94 mM], respectively). The level of succinate was also significantly (P < 0.0001 by a Mann-Whitney test) reduced in the cecal contents of mice infected with the ΔglsB mutant compared to WT bacteria (0.25 mM [0.06 to 0.38 mM] versus 1.1 mM [0.51 to 2.3 mM], respectively). Therefore, the metabolism of the ΔglsB mutant is different from that of WT B. thetaiotaomicron during growth in the murine gut.
DISCUSSION
The acylated amino acids GL and flavolipin have previously been identified in the membranes of several different members of the phylum Bacteroidetes (8, 21, 32). In this study, we have identified the genes required for the production of these acylated amino acids. Using genetics and high-resolution LC-MS, we show that glsB (BT_3459) encodes a glycine N-acyltransferase that is required for the production of both GL and flavolipin in B. thetaiotaomicron. We also present evidence that glsA (BT_3458), a gene found immediately upstream from glsB on the B. thetaiotaomicron genome, encodes an O-acyltransferase that is required for the efficient production of GL. Interestingly, the overproduction of glsA and glsB in E. coli results in the synthesis of only GL, suggesting the presence of another activity in B. thetaiotaomicron that may convert GL to flavolipin.
Flavolipin (i.e., lipid 654) has been reported to signal to eukaryotic cells through an interaction with Toll-like receptor 2 (TLR2) (27, 33). Diacylated lipid 654 has been shown to be converted into a more potent monoacylated derivative, lipid 430 (or lyso-lipid 654), through the action of phospholipase A2 activity in the host (21). N-Acyl amino acids are important endogenous signaling molecules produced in the human host; e.g., N-arachidonoyl glycine has been shown to block pain perception in mice (34–36). Genes encoding N-acyl synthases are enriched in the human gut microbiome, and both lipid 654 and lipid 430 have been found in tissues that are distal from the gut, indicating that these molecules can be distributed around the human host (21, 36). Therefore, there is accumulating evidence supporting an important role for the gut microbiota in the production of acylated amino acids, such as flavolipin, that can act as signaling molecules in the host.
During periods of phosphate starvation, some bacteria access cellular phosphate reserves by using acylated amino acids such as OL to replace the phospholipids normally found in bacterial membranes (37–39). On the other hand, OL production in Rhizobium tropici and Burkholderia cepacia has been shown to be important for the normal tolerance of the bacterial cell to acid and temperature stress (13, 40, 41). Our data suggest a role for GL/flavolipin during the response of B. thetaiotaomicron to different stresses, and a glsB deletion mutant was compromised in its ability to adapt to various stresses, including the transition from liquid to solid media, exposure to bile, and exposure to air. Interestingly, we could not construct a deletion in glsA, suggesting that this gene might be essential in B. thetaiotaomicron. However, IN-Seq analysis of B. thetaiotaomicron did not identify either glsA or glsB as an essential gene, although in the same study, both genes were identified as being important for normal growth in vitro (42). Therefore, it is clear that glsA and glsB have an important role in B. thetaiotaomicron. Interestingly, orthologues of glsA and glsB have been successfully deleted in Bacteroides fragilis, suggesting that the importance of glsA and glsB during growth might also be species dependent (43).
In this study, we show that the ΔglsB mutant in B. thetaiotaomicron is affected in its ability to colonize the gut of a GF mouse. A mutant with a deletion of both the glsA and glsB orthologues in B. fragilis (named hlyB and hlyA, respectively) was also attenuated for virulence in a mouse abscess model, supporting a role for these genes during in vivo growth (44). We have shown that, in contrast to the WT, the ΔglsB mutant produces decreased levels of both acetate and succinate while in the cecum, indicating that there are differences in metabolism between the WT and the ΔglsB mutant. Acetate and succinate are important end products of carbohydrate metabolism in B. thetaiotaomicron. Acetate is produced from acetyl-CoA via the acetate kinase (AckA)-phosphate acetyltransferase (Pta) pathway, resulting in the generation of ATP. The reduction in acetate production in the ΔglsB mutant is small and may not by physiologically important. Succinate production is via phosphoenolpyruvate, oxaloacetate, malate, and fumarate and is linked to the production of reducing equivalents [through the regeneration of NAD(P)+] and the formation of a proton motive force. Fumarate reductase (Frd) catalyzes the production of succinate from fumarate, and this protein complex is localized to the inner membrane of Bacteroides. Therefore, one possible explanation for the reduced level of succinate observed in the glsB mutant of B. thetaiotaomicron is that the activity of Frd is compromised by the altered membrane composition of this mutant. Nonetheless, the significantly reduced levels of succinate produced by the ΔglsB mutant would be expected to compromise metabolic flux and, therefore, reduce fitness in the gut. In support of this, both glsA (BT_3458) and glsB (BT_3459) have been identified as important determinants for the colonization of the GF mouse gut during an IN-Seq screen with B. thetaiotaomicron (42). Therefore, we have shown that glsB, and presumably the production of GL and/or flavolipin, is an important fitness factor in Bacteroides, required for adaptation to stress and normal colonization of the mammalian gut, particularly in the presence of a competing microbiota.
MATERIALS AND METHODS
Strains, plasmids, primers, and growth conditions.
Bacteroides thetaiotaomicron VPI 5482 was cultured anaerobically at 37°C in brain heart infusion medium (Sigma) supplemented with hemin (5 μg ml−1), 0.1% (wt/vol) cysteine, and 0.2% (wt/vol) sodium bicarbonate. Escherichia coli EPI300 (Epicentre) was routinely cultured in LB broth at 37°C (Merck). For agar plates, 1.5% (wt/vol) agar was added to the liquid media. Where appropriate, the following antibiotics were added to the media: ampicillin (Amp) at 100 μg/ml, chloramphenicol (Cm) at 12.5 μg/ml, gentamicin (Gm) at 50 μg/ml or 200 μg/ml, and erythromycin (Ery) at 25 μg/ml. Plasmids and primers used in this study are shown in Tables 3 and 4.
TABLE 3.
Strains and plasmids used in this study
| Strain or plasmid | Characteristics | Source or reference |
|---|---|---|
| Strains | ||
| Escherichia coli EPI300 | F− mcrA Δ(mrr-hsdRMS-mcrBC) Φ80dlacZΔM15 ΔlacX74 recA1 endA1 araD139 Δ(ara leu)7697 galU galK λ− rpsL (Strr) nupG trfA dhfr | Epicentre |
| B. thetaiotaomicron | VPI 5482 with tdk deletion; Gmr FUdRr | Eric Martens, University of Michigan |
| B. thetaiotaomicron ΔglsB | Derivative of Δtdk strain with glsB deletion; Gmr FUdRr | This study |
| B. thetaiotaomicron ΔglsB::glsB | ΔglsB strain with complementing glsB + 500 bp of upstream sequence; Gmr Ermr | This study |
| Plasmids | ||
| pBAD24 | Arabinose-inducible expression vector; Ampr | 47 |
| pBAD24-choA | Arabinose-inducible expression vector; Ampr | This study |
| pBAD24-choB | Arabinose-inducible expression vector; Ampr | This study |
| pBAD24-choBA | Arabinose-inducible expression vector; Ampr | This study |
| pEXCHANGE-Δtdk | Carrying cloned tdk; Ampr Ermr | 45 |
| pEXCHANGE-Δtdk ΔglsB | Carrying cloned tdk; Ampr Ermr | This study |
| pNbu2-bla-ermGb | Inserts into NBU2 att1 and/or att2 site; Ermr Ampr | 45 |
| pNbu2-bla-ermGb-pglsB | Chromosomal insertion vector; inserts into NBU2 att1 and/or att2 site; Ermr Ampr | This study |
TABLE 4.
Primers used in this study
| Primer | Sequence (5′–3′) | Use in study |
|---|---|---|
| RTfwB300thet | ATACTTCGGACGAAAGCTCC | RT-PCR for glsA fragment from B. thetaiotaomicron |
| RTrvB300thet | CTTTACTTTCCCGTCATAATGG | RT-PCR for glsA fragment from B. thetaiotaomicron |
| RTfwA300thet | AAGAAATATTAGGTGGTTACCG | RT-PCR for glsB fragment from B. thetaiotaomicron |
| RTrvA300thet | GATAGCTGGGATACATAGTC | RT-PCR for glsB fragment from B. thetaiotaomicron |
| FwBA630thet | TTCCCAAGATGGTGGAAGCC | RT-PCR for glsAB fragment from B. thetaiotaomicron |
| RvBA630thet | TATTCGTCGATATCCATCGAC | RT-PCR for glsAB fragment from B. thetaiotaomicron |
| FwchoAFLANK1 | CTGCTGGGATCCTCATCAGGACGAGATTAATG | glsB deletion from B. thetaiotaomicron |
| RvchoAFLANK1 | GCACTCGATCTTTACCGGAAAATTACATATTCTTGTTATAGTGTTCTATC | glsB deletion from B. thetaiotaomicron |
| FwchoAFLANK2 | GATAGAACACTATAACAAGAATATGTAATTTTCCGGTAAAGATCGAGTGC | glsB deletion from B. thetaiotaomicron |
| RvchoAFLANK2 | CTGCTGTCTAGATACCCCTTTTCATCGAGCC | glsB deletion from B. thetaiotaomicron |
| FwchoAKOcheck | GGTTTCTTATCTGAAGAAAATAG | Checking glsB deletion in B. thetaiotaomicron |
| RvchoAKOcheck | TCAACGCTTGCCTCCATCG | Checking glsB deletion in B. thetaiotaomicron |
Construction of gene knockouts in B. thetaiotaomicron.
Gene deletions were carried out using B. thetaiotaomicron Δtdk, as previously described (45). Briefly the DNA regions flanking the gene to be deleted were amplified and fused by PCR, cloned into the pEXCHANGE-tdk vector, and transformed into E. coli S17-1 λpir. The donor (E. coli) and recipient (B. thetaiotaomicron) strains were mixed, plated onto BHIS agar containing 10% (vol/vol) horse blood (BHIS blood agar), and incubated aerobically at 37°C for 24 h. The biomass was resuspended in 5 ml TYG broth, before plating onto BHIS blood agar supplemented with Gm and Ery. The plates were incubated anaerobically for 48 h at 37°C, before 5 to 10 colonies were restreaked onto BHIS blood agar (Gm Ery). After 48 h at 37°C, single colonies were picked into TYG broth and grown for 20 h without antibiotics before plating onto BHIS blood agar supplemented with 200 μg ml−1 5-fluoro-2′-deoxyuridine (FUdR) for vector counterselection. The plates were incubated anaerobically for 72 h and restreaked onto BHIS blood agar plates containing FUdR. Colony PCR, using primers that were designed outside the flanking regions, was used to identify potential knockout mutants, before confirmation by sequencing. For complementation experiments, the glsB (choA) gene, plus 500 bp upstream from the proposed translation start site, was amplified and cloned into the pNbu2-bla-ermGb insertion vector. The cloned DNA fragment was inserted into the B. thetaiotaomicron Δtdk ΔglsB genome in either of the two Nbu2-targeted tRNASer loci, via conjugation from E. coli S17-1 λpir. The resulting complemented strain, B. thetaiotaomicron Δtdk ΔglsB::glsB, was selected by plating onto BHIS blood agar supplemented with Gm and Ery, and the presence of the glsB gene was confirmed by PCR.
Colonization of germfree C57BL/6NTac mice.
All experiments involving animals were performed at the Biological Services Unit at University College Cork and were approved by the University College Cork Animal Experimentation Ethics Committee. For colonization experiments, 6-week-old germfree female C57BL/6NTac mice were gavaged with 20 μl of 5 × 109 CFU ml−1 of the appropriate bacterial strain (n = 9 for WT B. thetaiotaomicron, n = 8 for B. thetaiotaomicron ΔglsB, and n = 4 for the uninoculated control). The mice were housed in groups of 2 to 3 in individually ventilated cages (IVC), and bacterial enumerations were carried out by serial dilution and plating of homogenized fecal pellets collected from each IVC on days 2, 6, 9, and 12 postgavage. All mice were euthanized on day 14, the ceca were harvested, and cecal contents were collected for further analysis, including bacterial enumeration.
Analysis of short-chain fatty acids.
The level of short-chain fatty acids (SCFA) in the cecal contents was determined by HPLC using a protocol described previously (46). Cecal contents were weighed and resuspended in sterile MilliQ water (1:10 [wt/vol]) containing several 3- to 4-mm sterile glass beads (Sigma). The samples were vortexed for 1 min, and homogenates were centrifuged at 10,000 × g for 10 min. The supernatants were filter sterilized using a 0.22-μm filter and analyzed using HPLC with a refractive index detector (Agilent 1200 HPLC system). A Rezex 8μ, 8% H, 300- by 7.8-mm organic acid column (Phenomenex, USA) was used with 0.01 N H2SO4 as the elution fluid, at a flow rate of 0.6 ml min−1. The temperature of the column was maintained at 65°C, and 20 μl of each sample was injected for analysis. End product peaks were identified by comparison of their retention times with those of pure compounds, and concentrations were determined from standards of known concentrations.
Identification and quantification of glycine lipids.
Cultures of E. coli, with the appropriate plasmids, or B. thetaiotaomicron grown overnight were inoculated into fresh medium (LB broth with 0.2% [wt/vol] l-arabinose for E. coli or BHIS broth for B. thetaiotaomicron) at an OD600 of 0.05 and allowed to grow at 37°C until the OD600 reached 0.5 to 0.6. At this point, 1-ml samples were centrifuged (5 min at 12,000 × g), the pellets were resuspended in HPLC-grade methanol (Sigma), and 500 pmol N-arachidonyl glycine (NAGly) (20:4) (Cayman Chemicals, Ann Arbor, MI, USA) was added as an internal standard (ISTD). Ethyl acetate was added, and the mixture was left at 4°C for 30 min before being centrifuged at 2,000 × g for 5 min to remove denatured proteins. The supernatant was collected, evaporated to dryness under nitrogen gas, and reconstituted in methanol containing 5 mM ammonium formate (Sigma). LC-MS analyses were performed using a Thermo Exactive Orbitrap mass spectrometer (Thermo Scientific, Hemel Hempsted, UK) equipped with a heated electrospray ionization (HESI) probe and coupled to a Thermo Accela 1250 ultra-high-pressure liquid chromatography (UHPLC) system. Samples were injected onto a Thermo Hypersil gold C18 column (2.1 mm by 100 mm; 1.9 μm) maintained at 50°C. Mobile phase A consisted of water containing 10 mM ammonium formate and 0.1% (vol/vol) formic acid. Mobile phase B consisted of a 90:10 mixture of isopropanol-acetonitrile containing 10 mM ammonium formate and 0.1% (vol/vol) formic acid. The initial conditions for analysis were 65% mobile phase A–35% mobile phase B, and the percentage of mobile phase B was increased from 35% to 65% over 4 min, followed by 65% to 100% over 15 min, with a hold for 2 min before reequilibration to the starting conditions over 6 min. The flow rate was 400 μl/min. Samples were analyzed in negative-ion mode over the mass-to-charge ratio (m/z) range of 250 to 2,000 at a resolution of 100,000. The signals corresponding to the accurate m/z values for [M − H] ions of glycine lipid molecular species were extracted from raw LC-MS data sets with the mass error set to 5 ppm. Quantification was achieved by relating the peak area of the glycine lipid species to the peak area of the NAGly (20:4) ISTD. Tandem mass spectrometry (MS/MS) was employed to confirm the identity of glycine lipid species. Samples were infused at a rate of 5 μl/min into a Thermo LTQ-Orbitrap XL mass spectrometer and subjected to higher-energy collision dissociation (HCD) in the Orbitrap analyzer. Additional MS3 analyses were performed through collision-induced dissociation (CID) in the ion trap. Collision energies ranged from 40 to 65%, and helium was used as the collision gas.
Reverse transcription-PCR.
Bacteroides cultures grown overnight were subcultured into fresh BHIS broth to an OD600 of 0.05 and incubated anaerobically at 37°C until the cultures reached mid-exponential phase (OD600 = 0.3 to 0.5). At this stage, a 5-ml aliquot was removed and centrifuged, and the cell pellet was resuspended in RNAprotect (Qiagen). RNA extractions were carried out using a High Pure RNA isolation kit (Roche), according to the manufacturer’s instructions. For the qualitative determination of gene expression, RNA was reverse transcribed into cDNA using a QuantiTect reverse transcription kit (Qiagen), according to the manufacturer’s instructions. This cDNA was subsequently used as a template for PCR using 0.5 μl DNA template, 2.5 μl CoralLoad PCR buffer (Qiagen), 100 pmol of the appropriate primers (Table 4), 0.2 mM deoxynucleoside triphosphate (dNTP) mix (Promega), 0.125 μl Taq DNA polymerase (Qiagen), and sterile MilliQ distilled water (dH2O), in a final volume of 25 μl. The following PCR conditions were used: 95°C for 5 min (initial denaturation), followed by 35 cycles of 95°C for 30 s, the primer-specific annealing temperature for 30 s, and 72°C for the template-specific length of time (1 min per 1 kbp DNA). This was followed by a final extension step at 72°C for 10 min.
Stress assays.
Cultures of the appropriate B. thetaiotaomicron strains grown overnight were adjusted to an OD600 of 0.05 in fresh BHIS broth and incubated for 7 h at 37°C anaerobically. At this point, the OD600 was adjusted to 0.2, and each culture was split into three equal aliquots, whereby one aliquot was cultured anaerobically, another aliquot was exposed to air, and the final aliquot was incubated in BHIS broth with 1% (wt/vol) porcine bile (Sigma). Following incubation for 14 h under the appropriate stress conditions, viable cells were enumerated by serial dilutions and plating onto BHIS agar.
Statistical analysis.
All statistical analysis was performed using GraphPad Prism 6.0e for Mac software. All experiments were carried out using biological triplicate samples, unless stated otherwise. Student’s t test or a Mann-Whitney test was used to compare two different groups of data, as indicated. One-way analysis of variance (ANOVA), with Tukey’s posttest, was used to compare three or more groups of data, and differences were considered to be significant if the P value was <0.05.
ACKNOWLEDGMENTS
This work was funded by an investigator award from Science Foundation Ireland (SFI) to D.J.C. (12/IP/1493) and by funding received through APC Microbiome Ireland, a research institute supported by the SFI (SFI/12/RC/2273). S.R.T., M.K.D., and P.D.W. gratefully acknowledge the financial support of the European Regional Development Fund, Scottish Funding Council, and Highlands and Islands Enterprise.
D.J.C. conceived the study, and A.L. carried out all of the experiments except for the lipidomics. P.D.W., S.R.T., and M.K.D. carried out all lipidomic experiments and analyses. A.L. and D.J.C. analyzed the data, and D.J.C. wrote the manuscript with the help of A.L. and P.D.W. All authors reviewed the manuscript.
REFERENCES
- 1.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto J-M, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Doré J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J, et al. 2010. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grondin JM, Tamura K, Déjean G, Abbott DW, Brumer H. 2017. Polysaccharide utilization loci: fueling microbial communities. J Bacteriol 199:e00860-16. doi: 10.1128/JB.00860-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porter NT, Martens EC. 2017. The critical roles of polysaccharides in gut microbial ecology and physiology. Annu Rev Microbiol 71:349–369. doi: 10.1146/annurev-micro-102215-095316. [DOI] [PubMed] [Google Scholar]
- 4.Wexler AG, Goodman AL. 2017. An insider’s perspective: Bacteroides as a window into the microbiome. Nat Microbiol 2:17026. doi: 10.1038/nmicrobiol.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marcobal A, Barboza M, Sonnenburg ED, Pudlo N, Martens EC, Desai P, Lebrilla CB, Weimer BC, Mills DA, German JB, Sonnenburg JL. 2011. Bacteroides in the infant gut consume milk oligosaccharides via mucus-utilization pathways. Cell Host Microbe 10:507–514. doi: 10.1016/j.chom.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill CJ, Lynch DB, Murphy K, Ulaszewska M, Jeffery IB, O’Shea CA, Watkins C, Dempsey E, Mattivi F, Tuohy K, Ross RP, Ryan CA, O’Toole PW, Stanton C. 2017. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET cohort. Microbiome 5:4. doi: 10.1186/s40168-016-0213-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geiger O, González-Silva N, López-Lara IM, Sohlenkamp C. 2010. Amino acid-containing membrane lipids in bacteria. Prog Lipid Res 49:46–60. doi: 10.1016/j.plipres.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Sohlenkamp C, Geiger O. 2016. Bacterial membrane lipids: diversity in structures and pathways. FEMS Microbiol Rev 40:133–159. doi: 10.1093/femsre/fuv008. [DOI] [PubMed] [Google Scholar]
- 9.Vences-Guzmán MÁ, Geiger O, Sohlenkamp C. 2012. Ornithine lipids and their structural modifications: from A to E and beyond. FEMS Microbiol Lett 335:1–10. doi: 10.1111/j.1574-6968.2012.02623.x. [DOI] [PubMed] [Google Scholar]
- 10.Gao J-L, Weissenmayer B, Taylor AM, Thomas-Oates J, López-Lara IM, Geiger O. 2004. Identification of a gene required for the formation of lyso-ornithine lipid, an intermediate in the biosynthesis of ornithine-containing lipids. Mol Microbiol 53:1757–1770. doi: 10.1111/j.1365-2958.2004.04240.x. [DOI] [PubMed] [Google Scholar]
- 11.Weissenmayer B, Gao J-L, López-Lara IM, Geiger O. 2002. Identification of a gene required for the biosynthesis of ornithine-derived lipids. Mol Microbiol 45:721–733. doi: 10.1046/j.1365-2958.2002.03043.x. [DOI] [PubMed] [Google Scholar]
- 12.Vences Guzmán MÁ, Guan Z, Escobedo Hinojosa WI, Bermúdez Barrientos JR, Geiger O, Sohlenkamp C. 2015. Discovery of a bifunctional acyltransferase responsible for ornithine lipid synthesis in Serratia proteamaculans. Environ Microbiol 17:1487–1496. doi: 10.1111/1462-2920.12562. [DOI] [PubMed] [Google Scholar]
- 13.Vences-Guzmán MÁ, Guan Z, Ormeño-Orrillo E, González-Silva N, López-Lara IM, Martínez-Romero E, Geiger O, Sohlenkamp C. 2011. Hydroxylated ornithine lipids increase stress tolerance in Rhizobium tropici CIAT899. Mol Microbiol 79:1496–1514. doi: 10.1111/j.1365-2958.2011.07535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hölzl G, Sohlenkamp C, Vences Guzmán MÁ, Gisch N. 2018. Headgroup hydroxylation by OlsE occurs at the C4 position of ornithine lipid and is widespread in proteobacteria and bacteroidetes. Chem Phys Lipids 213:32–38. doi: 10.1016/j.chemphyslip.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Escobedo Hinojosa WI, Vences Guzmán MÁ, Schubotz F, Sandoval-Calderón M, Summons RE, López-Lara IM, Geiger O, Sohlenkamp C. 2015. OlsG (Sinac_1600) is an ornithine lipid N-methyltransferase from the planctomycete Singulisphaera acidiphila. J Biol Chem 290:15102–15111. doi: 10.1074/jbc.M115.639575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vences Guzmán MÁ, Guan Z, Bermúdez Barrientos JR, Geiger O, Sohlenkamp C. 2013. Agrobacteria lacking ornithine lipids induce more rapid tumour formation. Environ Microbiol 15:895–906. doi: 10.1111/j.1462-2920.2012.02867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lynch A, Crowley E, Casey E, Cano R, Shanahan R, McGlacken G, Marchesi JR, Clarke DJ. 2017. The Bacteroidales produce an N-acylated derivative of glycine with both cholesterol-solubilising and hemolytic activity. Sci Rep 7:13270. doi: 10.1038/s41598-017-13774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen LJ, Kang H-S, Chu J, Huang Y-H, Gordon EA, Reddy BVB, Ternei MA, Craig JW, Brady SF. 2015. Functional metagenomic discovery of bacterial effectors in the human microbiome and isolation of commendamide, a GPCR G2A/132 agonist. Proc Natl Acad Sci U S A 112:E4825–E4834. doi: 10.1073/pnas.1508737112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lynch A, Tammireddy SR, Doherty MK, Whitfield PD, Clarke DJ. 2018. Characterization of the role of glycine lipids in Bacteroides thetaiotaomicron. bioRxiv. doi: 10.1101/371807. [DOI] [PMC free article] [PubMed]
- 20.Mayberry WR. 1980. Hydroxy fatty acids in Bacteroides species: d-(−)-3-hydroxy-15-methylhexadecanoate and its homologs. J Bacteriol 143:582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nemati R, Dietz C, Anstadt EJ, Cervantes J, Liu Y, Dewhirst FE, Clark RB, Finegold S, Gallagher JJ, Smith MB, Yao X, Nichols FC. 2017. Deposition and hydrolysis of serine dipeptide lipids of Bacteroidetes bacteria in human arteries: relationship to atherosclerosis. J Lipid Res 58:1999–2007. doi: 10.1194/jlr.M077792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawazoe R, Okuyama H, Reichardt W, Sasaki S. 1991. Phospholipids and a novel glycine-containing lipoamino acid in Cytophaga johnsonae Stanier strain C21. J Bacteriol 173:5470–5475. doi: 10.1128/jb.173.17.5470-5475.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawazoe R, Monde K, Reichardt W, Okuyama H. 1992. Lipoamino acids and sulfonoplipids [sic] in Cytophaga johnsonae Stanier strain C21 and six related species of Cytophaga. Arch Microbiol 158:171–175. doi: 10.1007/BF00290812. [DOI] [Google Scholar]
- 24.Kawai Y, Yano I, Kaneda K. 1988. Various kinds of lipoamino acids including a novel serine-containing lipid in an opportunistic pathogen Flavobacterium. Their structures and biological activities on erythrocytes. Eur J Biochem 171:73–80. doi: 10.1111/j.1432-1033.1988.tb13760.x. [DOI] [PubMed] [Google Scholar]
- 25.Farrokhi V, Nemati R, Nichols FC, Yao X, Anstadt E, Fujiwara M, Grady J, Wakefield D, Castro W, Donaldson J, Clark RB. 2013. Bacterial lipodipeptide, lipid 654, is a microbiome-associated biomarker for multiple sclerosis. Clin Transl Immunol 2:e8. doi: 10.1038/cti.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark RB, Cervantes JL, Maciejewski MW, Farrokhi V, Nemati R, Yao X, Anstadt E, Fujiwara M, Wright KT, Riddle C, La Vake CJ, Salazar JC, Finegold S, Nichols FC. 2013. Serine lipids of Porphyromonas gingivalis are human and mouse Toll-like receptor 2 ligands. Infect Immun 81:3479–3489. doi: 10.1128/IAI.00803-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y-H, Nemati R, Anstadt E, Liu Y, Son Y, Zhu Q, Yao X, Clark RB, Rowe DW, Nichols FC. 2015. Serine dipeptide lipids of Porphyromonas gingivalis inhibit osteoblast differentiation: relationship to Toll-like receptor 2. Bone 81:654–661. doi: 10.1016/j.bone.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sonnenburg JL, Xu J, Leip DD, Chen C-H, Westover BP, Weatherford J, Buhler JD, Gordon JI. 2005. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science 307:1955–1959. doi: 10.1126/science.1109051. [DOI] [PubMed] [Google Scholar]
- 29.Sonnenburg ED, Sonnenburg JL, Manchester JK, Hansen EE, Chiang HC, Gordon JI. 2006. A hybrid two-component system protein of a prominent human gut symbiont couples glycan sensing in vivo to carbohydrate metabolism. Proc Natl Acad Sci U S A 103:8834–8839. doi: 10.1073/pnas.0603249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wexler HM. 2007. Bacteroides: the good, the bad, and the nitty-gritty. Clin Microbiol Rev 20:593–621. doi: 10.1128/CMR.00008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Louis P, Flint HJ. 2017. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol 19:29–41. doi: 10.1111/1462-2920.13589. [DOI] [PubMed] [Google Scholar]
- 32.Moore EK, Hopmans EC, Rijpstra WIC, Villanueva L, Sinninghe Damsté JS. 2016. Elucidation and identification of amino acid containing membrane lipids using liquid chromatography/high-resolution mass spectrometry. Rapid Commun Mass Spectrom 30:739–750. doi: 10.1002/rcm.7503. [DOI] [PubMed] [Google Scholar]
- 33.Olsen I, Nichols FC. 2018. Are sphingolipids and serine dipeptide lipids underestimated virulence factors of Porphyromonas gingivalis? Infect Immun 86:e00035-18. doi: 10.1128/IAI.00035-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Connor M, Vaughan CW, Vandenberg RJ. 2010. N-Acyl amino acids and N-acyl neurotransmitter conjugates: neuromodulators and probes for new drug targets. Br J Pharmacol 160:1857–1871. doi: 10.1111/j.1476-5381.2010.00862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bradshaw HB, Rimmerman N, Hu SSJ, Burstein S, Walker JM. 2009. Novel endogenous N‐acyl glycines: identification and characterization. Vitam Horm 81:191–205. doi: 10.1016/S0083-6729(09)81008-X. [DOI] [PubMed] [Google Scholar]
- 36.Cohen LJ, Esterhazy D, Kim S-H, Lemetre C, Aguilar RR, Gordon EA, Pickard AJ, Cross JR, Emiliano AB, Han SM, Chu J, Vila-Farres X, Kaplitt J, Rogoz A, Calle PY, Hunter C, Bitok JK, Brady SF. 2017. Commensal bacteria make GPCR ligands that mimic human signalling molecules. Nature 549:48–53. doi: 10.1038/nature23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barbosa LC, Goulart CL, Avellar MM, Bisch PM, von Kruger WMA. 2018. Accumulation of ornithine lipids in Vibrio cholerae under phosphate deprivation is dependent on VC0489 (OlsF) and PhoBR system. Microbiology 164:395–399. doi: 10.1099/mic.0.000607. [DOI] [PubMed] [Google Scholar]
- 38.Diercks H, Semeniuk A, Gisch N, Moll H, Duda KA, Hölzl G. 2015. Accumulation of novel glycolipids and ornithine lipids in Mesorhizobium loti under phosphate deprivation. J Bacteriol 197:497–509. doi: 10.1128/JB.02004-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewenza S, Falsafi R, Bains M, Rohs P, Stupak J, Sprott GD, Hancock REW. 2011. The olsA gene mediates the synthesis of an ornithine lipid in Pseudomonas aeruginosa during growth under phosphate-limiting conditions, but is not involved in antimicrobial peptide susceptibility. FEMS Microbiol Lett 320:95–102. doi: 10.1111/j.1574-6968.2011.02295.x. [DOI] [PubMed] [Google Scholar]
- 40.Taylor CJ, Anderson AJ, Wilkinson SG. 1998. Phenotypic variation of lipid composition in Burkholderia cepacia: a response to increased growth temperature is a greater content of 2-hydroxy acids in phosphatidylethanolamine and ornithine amide lipid. Microbiology 144:1737–1745. doi: 10.1099/00221287-144-7-1737. [DOI] [PubMed] [Google Scholar]
- 41.González-Silva N, López-Lara IM, Reyes-Lamothe R, Taylor AM, Sumpton D, Thomas-Oates J, Geiger O. 2011. The dioxygenase-encoding olsD gene from Burkholderia cenocepacia causes the hydroxylation of the amide-linked fatty acyl moiety of ornithine-containing membrane lipids. Biochemistry 50:6396–6408. doi: 10.1021/bi200706v. [DOI] [PubMed] [Google Scholar]
- 42.Goodman AL, McNulty NP, Zhao Y, Leip D, Mitra RD, Lozupone CA, Knight R, Gordon JI. 2009. Identifying genetic determinants needed to establish a human gut symbiont in its habitat. Cell Host Microbe 6:279–289. doi: 10.1016/j.chom.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robertson KP, Smith CJ, Gough AM, Rocha ER. 2006. Characterization of Bacteroides fragilis hemolysins and regulation and synergistic interactions of HlyA and HlyB. Infect Immun 74:2304–2316. doi: 10.1128/IAI.74.4.2304-2316.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lobo LA, Jenkins AL, Smith CJ, Rocha ER. 2013. Expression of Bacteroides fragilis hemolysins in vivo and role of HlyBA in an intra-abdominal infection model. Microbiologyopen 2:326–337. doi: 10.1002/mbo3.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koropatkin NM, Martens EC, Gordon JI, Smith TJ. 2008. Starch catabolism by a prominent human gut symbiont is directed by the recognition of amylose helices. Structure 16:1105–1115. doi: 10.1016/j.str.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huda-Faujan N, Abdulamir AS, Fatimah AB, Anas OM, Shuhaimi M, Yazid AM, Loong YY. 2010. The impact of the level of the intestinal short chain fatty acids in inflammatory bowel disease patients versus healthy subjects. Open Biochem J 4:53–58. doi: 10.2174/1874091X01004010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guzman LM, Belin D, Carson MJ, Beckwith J. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose pBAD promoter. J Bacteriol 177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]