Abstract
Complete surgical resection is the potentially curative treatment for pancreatic cancer, but only fewer than 20% of those individuals will be found to be eligible for surgery. Here we report a 49-year-old man with locally advanced pancreatic cancer successfully treated with high-dose radiotherapy using helical tomotherapy (66 Gy/33 fractions, 2 Gy per day over 6.5 weeks). To our knowledge, this is the first reported case of locally advanced pancreatic cancer curatively treated with helical tomotherapy alone.
Keywords: Pancreatic neoplasms; Radiation; Radiotherapy, image-guided
Introduction
High-dose image-guided intensity-modulated radiation therapy (IMRT) to the pancreatic carcinoma was performed using helical tomotherapy that provides both highly conformed therapeutic doses as well as image guidance. The prognosis for pancreatic cancer remains poor and has an overall survival rate of less than 6% [1]. Surgery is usually the only way pancreatic cancer can be completely cured. However, the majority of patients with pancreatic cancer initially present with locally advanced and metastatic disease, and a fewer than 20% have resectable tumors [2]. Helical tomotherapy is able to treat complex malignant cancer with high-precision radiotherapy, and it can reduce the damage to normal tissues and improve treatment effects. Concurrent chemoradiotherapy delivered via helical tomotherapy has been reported in patients with inoperable pancreatic cancer [3], but monotherapy with helical tomotherapy and successful treatment outcome of locally advanced pancreatic cancer has not been reported.
Here, we report a case of locally advanced pancreatic cancer successfully treated with high-dose helical tomotherapy. As far as we know, this is the first case report of locally advanced pancreatic cancer definitively treated with helical tomotherapy alone.
Case report
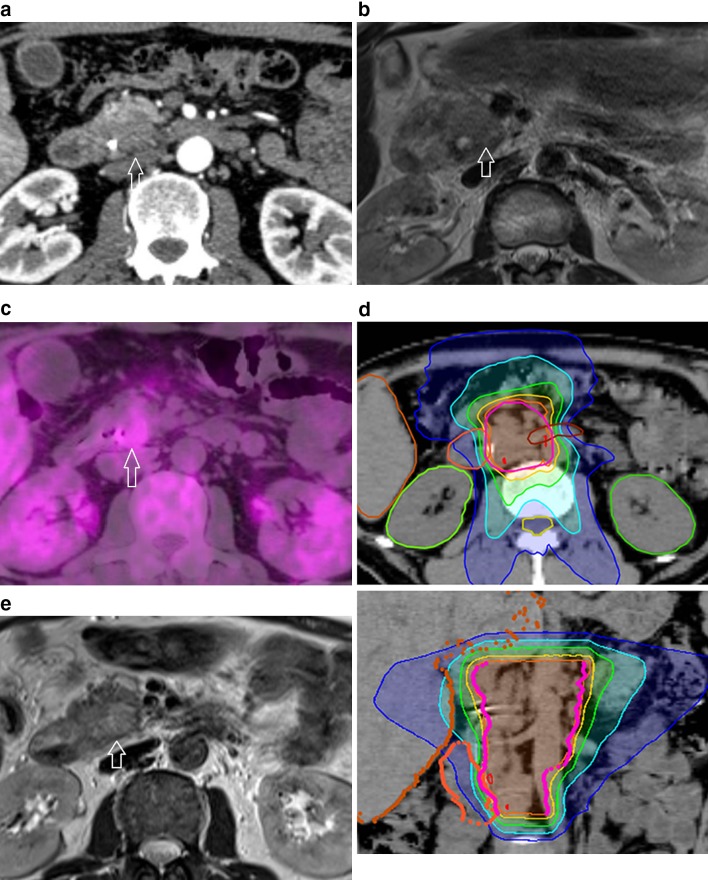
A 49-year-old male with jaundice, weight loss, and upper abdominal pain was hospitalized after ultrasonically suspected infiltration in the pancreatic head. The patient was found to have dilated intrahepatic ducts of at least 6 mm and serum total bilirubin level was 27.4 mg/dl (normal 0.2–1.2 mg/dl). A biliary drainage tube was placed in the common bile duct after endoscopic retrograde cholangiopancreatography (ERCP). ERCP showed narrowing in the common bile duct that might be due to pancreatic cancer. Endoscopic biopsy of the pancreas revealed pancreatic ductal adenocarcinoma. The serum tumor marker carbohydrate antigen 19–9 (CA19–9) level was elevated to 105.6 U/mL (normal limit: 37 U/ml). Contrast-enhanced CT showed a hypovascular mass in the pancreatic head extending beyond the pancreas and involvement of the superior mesenteric vein but without involvement of the celiac axis or the superior mesenteric artery (Fig. 1a), with slightly enlarged precaval lymph node. The tumor (23 mm in diameter) presented with intermediate-to-high signal intensity on T2-weighted MR image compared to the surrounding normal pancreas (Fig. 1b), and it appeared as a hypermetabolic lesion on 18F-FDG PET/CT (Fig. 1c). Based on the imaging findings, the patient was diagnosed with locally advanced borderline resectable pancreatic cancer [4] and curative surgical resection was initially planned. However, after he was given detailed explanations and full details of treatment options, he decided not to undergo surgery or chemotherapy but to receive radiation therapy.
Fig. 1.
a Axial contrast-enhanced CT in the arterial phase shows a hypo-enhanced ill-demarcated mass (arrow) in the pancreatic head with peripancreatic fat infiltration. b On T2-weighted turbo spin-echo MR image, the pancreatic tumor (arrow) appears hyperintense compared to the surrounding healthy pancreas. c Axial 18F-FDG PET/CT image shows a hypermetabolic focus in the pancreatic head (arrow). d Axial (upper) and coronal (lower) slice showing planning target volume (PTV) and isodose lines. PTV, pink line. Isodose lines—107% of prescription dose, red; 100%, orange; 90%, yellow; 70%, green; 50%, light blue; 30%, blue. e At 6 months after therapy, T2-weighted turbo spin-echo MR image shows that the pancreatic cancer disappeared (arrow)
High-dose helical tomotherapy was delivered at 2 Gy per fraction every day, with a total dose of 66 Gy (Fig. 1d). To inhibit intestinal peristalsis and prevent stomach expansion, we instructed a 3-h dietary restriction before radiotherapy. Clinical target volume (CTV) included the primary mass, superior mesenteric artery (SMA) origin with 5–7 mm margin, SMA and superior mesenteric vein (SMV) and neural plexus adjacent to the pancreatic head, enlarged lymph nodes and celiac axis. Internal target volume (ITV) of primary mass was also created using 4D-CT, and then, the ITV plus 3 mm margin was added to the CTV. Initial planning target volume (PTV) was created by asymmetrically expanding the CTV by 4–8 mm in three directions. The patient received radiotherapy to the primary site and the regional lymph nodes until a cumulative dose of 46 Gy. After 46 Gy, the PTV included the primary site and the enlarged regional lymph nodes. The goal target coverage for the PTV was volume of target receiving ≥ 95% of prescription dose. Organs at risk (OAR) dosimetric constraints included the small bowel (maximum dose: < 67 Gy; volume of organ receiving ≥ 45 Gy [V45]: < 150 cm3; V30: < 300 cm3), duodenum (maximum dose: < 67 Gy); liver (mean dose: < 25 Gy; V30: < 60%; volume of organ receiving ≤ 30 Gy [CV30]: >700 cm3), kidney (V18: < 10%), and spinal cord (maximum dose: < 46 Gy). During radiotherapy, treatment-related adverse events were not noted. Follow-up MR imaging 6 months after treatment showed that the pancreatic cancer completely disappeared (Fig. 1e), and his serum CA19–9 level decreased to 18.7 U/mL. He has been disease-free with no adverse events in subsequent follow-up since that time and has achieved an overall survival of 50 months with a good performance status (Karnofsky Performance Status 100%). MR imaging follow-up revealed no evidence of recurrent tumor and his serum CA 19–9 level was 26.6 U/mL.
Discussion
Several retrospective studies have suggested the feasibility and effectiveness of dose-escalated radiotherapy for pancreatic cancer [5–7]. It has been considered that a higher radiation dose can result in better local control of pancreatic cancer. In order to deliver higher radiation dose while minimizing damage to surrounding normal tissue, IMRT or stereotactic body radiotherapy (SBRT) has been applied [5–7]. In addition, image-guided radiation therapy (IGRT) also tends to be most useful in tumors where the surrounding normal tissue tolerance such as intestine is lower than the dose needed for tumor control. The intrinsic features of helical tomotherapy for CT imaging for IMRT image guidance is capable of delivering high dose of radiation to the pancreas without increasing the risk of normal tissue toxicity. It has been reported that helical tomotherapy shows significantly better sparing of the stomach and small bowel compared to step-and-shoot IMRT, and the decreased dose to OAR with helical tomotherapy is likely to improve the therapeutic ratio [8]. As far as we know, this is the first report of locally advanced pancreatic cancer successfully treated with helical tomotherapy alone [9].
One limitation of this report is that it is impossible to image tumors and surrounding normal tissues while the patient receives radiotherapy. However, MR-guided radiation therapy which is capable of continuous imaging during radiotherapy might be a solution. Another limitation is the optimal dose/fractionation for high-dose radiotherapy or combination therapy of chemotherapeutic agents has yet to be established. Since local tumor control is associated with longer survival [10, 11], local tumor control by high-dose radiotherapy using helical tomotherapy may be a promising treatment approach.
In conclusion, the findings of single case report cannot be generalized to others without further scientific verification; however, high-dose helical tomotherapy may provide effective local control of pancreatic cancer with minimal adverse events.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013 CA. Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg. 1993;165:68–72. doi: 10.1016/S0002-9610(05)80406-4. [DOI] [PubMed] [Google Scholar]
- 3.Ji JS, Han CW, Jang JW, et al. Helical tomotherapy with concurrent capecitabine for the treatment of inoperable pancreatic cancer. Radiat Oncol. 2010;5:60. doi: 10.1186/1748-717X-5-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Callery MP, Chang KJ, Fishman EK, et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1727–1733. doi: 10.1245/s10434-009-0408-6. [DOI] [PubMed] [Google Scholar]
- 5.Zschaeck S, Blumke B, Wust P, et al. Dose-escalated radiotherapy for unresectable or locally recurrent pancreatic cancer: dose volume analysis, toxicity and outcome of 28 consecutive patients. PLoS One. 2017;12:e0186341. doi: 10.1371/journal.pone.0186341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crane CH. Hypofractionated ablative radiotherapy for locally advanced pancreatic cancer. J Radiat Res. 2016;57(Suppl 1):i53–i57. doi: 10.1093/jrr/rrw016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Geus SWL, Eskander MF, Kasumova GG, et al. Stereotactic body radiotherapy for unresected pancreatic cancer: a nationwide review. Cancer. 2017;123:4158–4167. doi: 10.1002/cncr.30856. [DOI] [PubMed] [Google Scholar]
- 8.Taylor R, Opfermann K, Jones BD, et al. Comparison of radiation treatment delivery for pancreatic cancer: Linac intensity-modulated radiotherapy versus helical tomotherapy. J Med Imaging Radiat Oncol. 2012;56:332–337. doi: 10.1111/j.1754-9485.2012.02373.x. [DOI] [PubMed] [Google Scholar]
- 9.Chang JS, Wang ML, Koom WS, et al. High-dose helical tomotherapy with concurrent full-dose chemotherapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2012;83:1448–1454. doi: 10.1016/j.ijrobp.2011.10.050. [DOI] [PubMed] [Google Scholar]
- 10.Neoptolemos JP, Stocken DD, Dunn JA, et al. European Study Group for Pancreatic Cancer. Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Ann Surg. 2001;234:758–768. doi: 10.1097/00000658-200112000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neoptolemos JP, Palmer DH, Ghaneh P, et al. European Study Group for Pancreatic Cancer. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011–1024. doi: 10.1016/S0140-6736(16)32409-6. [DOI] [PubMed] [Google Scholar]