Abstract
Regulated necrosis (necroptosis) plays a pivotal role in the extent of cardiomyocyte loss and the development of post-ischemic adverse remodelling and cardiac dysfunction following myocardial I/R injury. Although HIIT has been reported to give rise to cardioprotection against MI, but the detailed knowledge of its molecular targets for treatment of MI is still not available. The LAD of Male Wistar rats was occluded to induce MI for 30 min and reperfusion for eight weeks. We investigated the effect of long-term HIIT for eight weeks on lipid peroxidation, SOD activity and GSH content using ELISA assay. Cardiac function, fibrosis, and infarct size were assessed by echocardiography, Masson’s trichrome and Evans Blue/TTC dual staining respectively. The expressions of gene markers of myocardial hypertrophy, fibrosis and key mediators of necroptosis were measured using RT-PCR and western blotting assay respectively. The results indicated that HIIT reduced lipid peroxidation, infarct size and improved endogenous antioxidant system and heart function. Significant decreases in mRNA levels of procollagen α1(I), α1(III), and fibronectin1were observed following HIIT. Moreover, that HIIT significantly decreased the expression of key mediators of necroptosis induced by MI (P < 0.05). There were no significant differences in β-MHC mRNA level in different groups. The findings of study suggest that HIIT might exert cardioprotective effects against post-ischemic adverse remodeling through targeting necroptosis process. Likewise, cardioprotective effects of HIIT in coping with myocardial I/R injury may be associated with RIP1-RIP3-MLKL axis. These findings establish a critical foundation for higher efficiency of exercise-based cardiac rehabilitation post–MI and future research.
Keywords: Necroptosis, High-intensity interval training, Myocardial infarction, Oxidative stress, Post-ischemic adverse remodelling
Introduction
MI is a growing health problem and a leading cause of disability, morbidity and mortality around the world which is closely associated with life-style changes, increasing urbanization, and socioeconomic conditions (Levine et al. 2016; Amani et al. 2017b; Rakhshan et al. 2018). Although early reperfusion therapy using coronary intervention technologies and pharmacological agents has been demonstrated to increase survivors by decreasing acute phase mortality of MI, a high rate of mortality is observed in post-MI patients owing to post-ischemic adverse remodelling and subsequent the prevalence of congestive heart failure among them (Mao et al. 2017). Although reperfusion might contribute to cell survival in the site of interest (Pazoki-Toroudi et al. 2003; Ghadernezhad et al. 2016), it may exacerbate I/R injury via initiation of pathophysiological cascades including massive release of intracellular Ca2+ ions, neutrophil recruitment, excessive generation of ROS and acute inflammatory response (Neri et al. 2015; Ajami et al. 2016; Pazoki-Toroudi et al. 2016; Ajami et al. 2013). Numerous cellular signaling pathways are involved in post-ischemic adverse remodelling during myocardial I/R injury (Song et al. 2014). Necroptosis is a mode of cell death that occurs based on a caspase independent programmed necrosis and is involved in myocardial I/R injury (Koshinuma et al. 2014; Li et al. 2014). The ventricular remodelling can occur as a result of necroptosis and irreversible cardiomyocyte loss which, in turn, leads to cardiac dysfunction after MI (Dorn 2008). It is found that RIP1 and/or RIP3-containing necroptosome are master mediators of necroptosis and their assembly is required for the initiation of this specific subform of programmed necrosis (Linkermann et al. 2012). RIP1 is a death-domain containing kinase that is involved in several cellular signaling pathway including NF-κB activation, apoptosis and necroptosis. Serine/threonine kinase activity of RIP1 plays a critical role in initiation of necroptosis in target cells (Christofferson and Yuan 2010). Necroptosis has attracted much attention as an utmost important molecular target because it is leading cause of aging related myocardial vulnerability. It is found that the autophagy substrate Sequestosome1 (p62/SQSTMl) elevates during myocardial ischemia, and then degrades in reperfusion. In the case of aged hearts subjected to myocardial ischemia, degradation of p62/SQSTMl is much lower than young hearts which, in turn, results in conjugation of p62 to RIP1, formation of RIP1-RIP3 complex (necrosome) and subsequent cell membrane damage in a RIP1-RIP3- MLKL axis dependent mechanism (Li et al. 2017). Indeed, phosphorylation and trimerization of MLKL by RIP3 lead to its translocation to plasma membrane and disruption of plasma membrane integrity in a TRPM7- dependent mechanism which, in turn, results in a TNF-induced necroptosis (Cai et al. 2014). Exercise therapy has been shown great promise to improve outcomes after MI. Exercise can ameliorate infarct size and collagen content of left ventricular by targeting matrix metalloproteinase 9 and pro-inflammatory cytokines (Puhl et al. 2015). Moreover, some studies have shown that exercise training results in cardioprotection in coping with AMI by modulating mitochondrial biogenesis and energy metabolism via targeting PGC-1α signaling pathway (Tao et al. 2015). HIIT has been described as alternating short bursts of high aerobic exercise with passive or active mild recovery periods. HIIT has several advantages such as using fat as a source of energy, reinforcement of enzyme activity and reduction of cardiometabolic risk factors (Guiraud et al. 2012; Batacan et al. 2016). A recent clinical trial study indicated that both HIIT and MICT contribute to improvement of quality of life and functional capacity in post-MI patients (Ulbrich et al. 2016). Emerging evidences have shown that cardioprotective effects of HIIT against pulmonary hypertension are more than CExT. HIIT can remarkably increase cardiac index and reduce fibrosis while CExT is unable to provide these advantages (Brown et al. 2016). The detailed mechanisms about cardioprotective effects of HIIT against MI are still not available. In present study, we examined the effects of HIIT on necroptosis process in a rat model of MI. we hypothesized that HIIT attenuates long-term adverse ischemic remodelling by targeting necroptosis process.
Using western blotting assay, we evaluated the expression of the master mediators of necroptosis in different groups. Likewise, we investigated effect of HIIT on infarct size, cardiac function and the expression of gene markers of myocardial fibrosis and hypertrophy. Here, we indicated that long-term HIIT for eight weeks contributed to the improvement of cardiac function and cardiomyocyte survival in rats subjected to MI through targeting oxidative stress and the master mediators of necroptosis.
Materials and methods
Chemical
Rip3 polyclonal antibody (14–6048-82) was purchased from EBioscience Company (San Diego, CA, USA). RIPK1/RIP1 antibody (Nbp1–77,077) was obtained from Novus Biologicals Company (Littleton, CO). MLKL antibody (MBS858387) was purchased from MyBioSource Company (SanDiego, USA). Anti-rabbit IgG-Peroxidase (A0545), Immobilon®-FL PVDF membrane pore size 0.45 μm, protease inhibitor cocktail, thiopentalsodium, Evans Blue and TTC were purchased from Sigma (St. Louis, MO, USA). Trizol was obtained from Invitrogen company (Carlsbad, CA). cDNA Synthesis Kit was purchased from EURx Company (Gdańsk, Poland). SYBR® Premix Ex Taq ™ II (TliRNaseH Plus,RR820Q) was supplied by Takara company (Japan).
Animals and ethical statement
Six-week old male Wistar rats weighing 200–250 g were obtained from animal laboratory of Iran University of Medical Sciences. The animals were kept in controlled room temperature, exposed to 12 h: 12 h light: dark schedule, 60 ± 5% humidity, and fed with a standard food and free access to sterile water. The present study was approved by Animal Ethical Committee of Iran University of Medical Sciences.
Experimental design
The male Wistar rats were randomly divided into four cohorts containing 10 rats each cohort. The sham-operated animal cohort (sham) received all procedures except ligation of the LAD. In the case of ischemia cohort (MI-CTL), LAD was tied and recirculation was performed after 30 min to induce ischemia/reperfusion injury for 8 weeks. In the Baseline cohort, LAD was tied and recirculation was allowed for 1 week. Fractional shortening at ≤35%, was inclusion criteria for MI groups in the study. The treatment cohort (MI-HIIT) received ischemia/reperfusion + eight week HIIT. In the case of control cohort (HIIT), healthy rats received only HIIT for 8 weeks.
Exercise training program
After echocardiographic evaluation at 1 week after surgery, MI groups have a four recovery period that performed on the motorized treadmill (Iranian model, Tehran, Iran) at a slow pace (5 m/min) for 5 min/day, 3 days per week in the third and the fourth weeks of recovery period. After the fourth week of surgery and at the end of recovery period, MI-operated animals were randomly divided into the MI-Sedentary (MI-CTL) and the MI-Trained (MI-HIIT) cohorts. Moreover, a group of healthy rats received exercise training program (HIIT cohort). Three steps were devoted to each session consisted of 40 min exercise:
warming up: running for 5 min at 50–60% of VO2max
Main training: running for 30 min composed of 5 intervals, alternating between 4 min of high-intensity running at 85%–90% VO2max and 2 min active recovery at 50%–60% VO2max.
-
Cool-down: running for 5 min at 50–60% VO2max.
Calculation of the intensity of the training program for each week was based on the relation of the running speed and VO2max as described in a previous study (Kraljevic et al. 2013). Likewise, the running speed was increased gently over weeks of training by 0.02 m/s per week. Treadmill slope was 25° during training and testing.
Surgical procedures
A rat model of MI was established by ligating LAD. To make MI, rats were intraperitoneally anesthetized with sodium thiopental (50 mg/kg) and were placed in the supine sleeping position. To maintain their body temperature at 37 °C, we monitored body temperature by thermal pad and heating lamp means. To provide ventilation with room air via a rodent ventilator (tidal volume 2–3 mL, respiratory rate 65–70 per min), tube was inserted into trachea of animal. Then, at the fourth intercostal space; a left thoracotomy was carried out to expose heart and incise pericardium. A 6–0 silk suture slip knot was placed below the main branch of the LAD to ligate it1–2 mm distal from tip of the left atrial appendix. Then, to induce ischemia, a small vinyl tube was passed into both ends of the silk suture; the snare was pulled and was tightened using mosquito hemostat. A successful LAD occlusion was approved by regional cyanosis of the myocardial surface and ST segment elevation after ligation of LAD.
Restoration of blood flow to cardiac tissue was allowed after 30 min of ischemia. At the end of surgical procedures, the chest was closed; positive end expiratory pressure was increased to inflate the lungs. Then, the ventilator was removed and the animals were allowed to recover.
All procedures except ligation of LAD were performed in sham-operated animals.
Assessment of cardiac function
Transthoracic two dimensional (2D) guided M-mode echocardiography at a sweep speed of 100 mm/s using a 10 MHz echocardiogram (linear transducer, GEVing med Ultrasound) was recorded in animals 1 and 8 weeks after reperfusion. Investigated groups were anesthetized with sodium thiopental (50 mg/kg, ip). To assay some of the parameters such as LVIDd, LVIDs, we used parasternal 2D short-axis view at the level of papillary muscles. Calculation of FS was based on the following formula:[LVIDd – LVIDs)/LVIDd] × 100(Hochhauser et al. 2007). EF was calculated as: (LVIDd2 -LVIDs2)/LVIDd2 formula. Echo Pac software (GE Healthcare) was applied to analyze data of 3–5 consecutive heart cycles. Echocardiography was performed by an observer blinded to investigated groups.
Anesthesia and tissue collection
In the last period at the end of HIIT, the rats were sacrificed under deep anesthesia and hearts were quickly excised and stored at −80 °C for western blotting assay.
Infarct size assessment
The myocardial infarct volume and area at risk (AAR) were determined by Evans Blue/TTC dual staining 8 weeks after reperfusion.
In the last period at the end of HIIT, LAD was ligated again and 1 ml of 2% Evans Blue dye was injected through the femoral vein. The animals were sacrificed under deep anesthesia and the heart was rapidly removed. Then the aorta was removed and hearts were kept at −20 °C for 24 h and subsequently cut into 2 mm cross-sectional slices. To visualize infarct regions, the sections were incubated at 37 °C for 15–20 min in 1% TTC. To improve the contrast between infarct and non-infarct regions, the sections were fixed in 10% formalin for overnight.
Area at risk was presented as a percentage of left ventricle (AAR/LV) and infarct size was expressed as a percentage of area at risk (IS/AAR). A digital camera was used to photograph slices. Stained deep blue regions by Evans indicate the non-ischemic myocardium.
The software Image J (National Institutes of Health, Bethesda, MD, USA) was used to determine infarct size.
Western blotting
A complete protease and phosphatase inhibitor was used to homogenize small pieces of the rat heart tissues in cell lysis buffer. Protein concentration was measured using nanodrop. Samples were denaturized at 95 °C for 5 min, and then 30 μg of total protein was separated by 8 to 10% SDS-PAGE gel and transferred onto polyvinylidene fluoride membrane. To block nonspecific sites, membranes were immersed in 5% non-fat milk in TBS containing 0.1% Tween 20 for 1 h. Then, membranes were incubated with primary antibodies (dilution 1:1000) for 2 h and after washing in PBS, they were exposed to a secondary antibody conjugated by peroxidase for 1 h and then were visualized by ECL reagent.
RT –PCR analysis
Total RNA was extracted from tissue samples using Trizol according to the manufacturer’s instructions. A Dart cDNA kit was used to reversely transcribe RNA to cDNA according to the manufacturer’s protocol. Real-Time PCR were run for 30–40 cycles using SYBR® Premix Ex Taq ™ II (TliRNaseH Plus,RR820Q) on a Rotor-Gene Q 5plex System. GAPDH was employed as internal control. The 2-∆∆Ct method was used to normalize the expression levels of each target gene to internal control expression. Real-time PCR primer pairs are listed in Table 1.
Table 1.
Primer Sequences used for RT-PCR
| Gene | Primer sequence | Sequence detected |
|---|---|---|
| Procollagen I-α1 | forward 5’-CACCTACAGCACGCTTG-3’ | 191 bp |
| reverse 5’-GGATGGAGGGAGTTTACAC -3’ | ||
| Procollagen III-α1 | forward 5’-CCTCCCAGAACATTACATAC-3’ | 258 bp |
| reverse 5’-CAATGTCATAGGGTGCGAT-3’ | ||
| Fibronectin 1 | forward 5’-GTGAAGAACGAGGAGGATGTG-3’ | 267 bp |
| reverse 5’-GTGATGGCGGATGATGTAGC-3’ | ||
| β-MHC | forward 5′- ATCAAGGGAAAGCAGGAAGC-3’ | 196 bp |
| reverse 5′- CCTTGTCTACAGGTGCATCA-3’ | ||
| GAPDH | forward 5’-AGTTCAACGGCACAGTCAAG-3’ | 118 bp |
| reverse 5′- TACTCAGCACCAGCATCACC-3’ |
Histological assessment
To evaluate interstitial fibrosis, we performed Masson’s trichrome staining. In brief, the ventricles first were fixed in formalin. Then, they were sliced and embedded in paraffin. The tissues were serially cut into 5 μm thick sections from base to apex. Data were quantified using the Image J software (NIH, Bethesda, MD, USA) through its color deconvolution plugin.
Assessment of lipid peroxidation
The TBARS method was used to measure MDA level (Esterbauer and Cheeseman 1990). Heart tissues were (100 mg) were homogenized in 5 mL of 1.15% KCl. Then, 2 ml of cold 10% trichloroacetic acid and 2 ml of cold 10% thiobarbituric acid were added to tissue homogenates. The resulting solution was heated at 100 °C for 1 h. After removing the precipitate, MDA content of reaction mixture was determined according to its extinction coefficient of 155 mM−1 cm−1.
Assessment of SOD activity
Heart tissues were (100 mg) homogenized in 100 mM potassium phosphate buffer solution (PBS, pH = 7.0) at 1600 rpm for 2 min. After centrifugation at 7000 rpm for 30 min at +4 °C, the supernatant was separated to estimate soluble contents of SOD by measuring inhibition of the photochemical reduction of NBT described by Beauchamp and Fridovich (1971).
Assessment of GSH content
Heart tissues were (100 mg) were homogenized in 5% metaphosphoric acid solution at 1600 rpm for 2 min. After centrifugation at 3500 rpm for 10 min at +4 °C, 30 ul supernatant was incubated with 30 ul 5, 50 -dithiobis-(2-nitrobenzoic acid), β-NADPH and GSH reductase. The changes in optical density at 412 nm was measured to estimate GSH content (Boyne and Ellman 1972).
Statistical analysis
Results are expressed as the mean ± the standard error of the mean (SEM). Data analysis was performed using GraphPad Prism-5 statistic software (LaJolla, CA, USA). Differences in three or more groups were tested by one-way ANOVA. Post-hoc tests were carried out using tukey correction when a remarkable difference was detected with ANOVA. A value of P < 0.05 was accepted to be statistically significant.
Results
Effects of HIIT on body weight, heart weight, heart weight /body weight ratio and LV/body weight ratio
There are no statistically significant differences in the early body weight among experimental cohorts. Significant differences were found in body weight, heart weight, Heart weight /body weight ratio and LV/body weight ratio following long-term HIIT among experimental cohorts (Table 2).
Table 2.
Post long-term HIIT animal body and heart weights and heart to body and LV/body weight ratios in experimental cohorts
| Criteria | Sham | HIIT | MI-CTL | MI-HIIT |
|---|---|---|---|---|
| Body weight (g) | 214.4 ± 4.5 | 228.6 ± 5.9 | 229.6 ± 7.8 | 232.2 ± 7.6 |
| Final body weight (g) | 270.8 ± 13.4 | 287 ± 7.3 | 287.6 ± 9.4 | 314.8 ± 6.2** |
| Heart weight (g) | 950 ± 20.9 | 1108 ± 55.2* | 992 ± 44.8 | 1292 ± 40.3** |
| Heart /body weight ratio (mg/g) | 3.5 ± 0.1 | 3.8 ± 0.1 | 3.4 ± 0.1 | 3.9 ± 0.3$ |
| LV/body weight ratio (mg/g) | 2.4 ± 0.1 | 2.8 ± 0.1& | 2.4 ± 0.1 | 3.1 ± 0.3*** |
*P < 0.05 compared with sham cohort; **P < 0.01 compared with other cohorts; $P < 0.05 compared with MI-CTL cohort; ***P < 0.001 compared with sham and MI-CTL cohorts; &P < 0.05 compared with sham and MI-CTL cohorts
Effects of HIIT on infarct volume
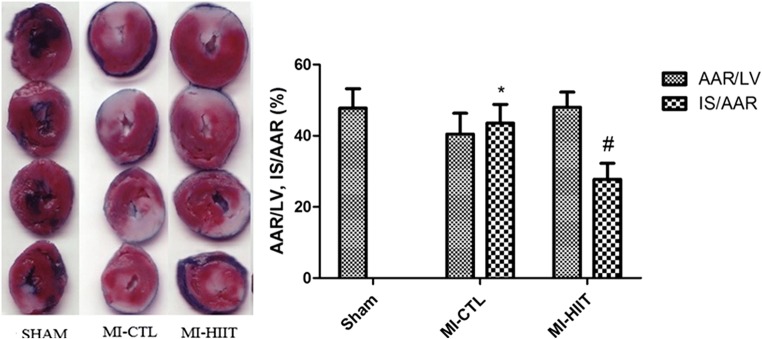
There is no remarkable difference in the size of area at risk (AAR) among sham (47.80 ± 5.44), MI-CTL (49.40 ± 5.85) and MI-HIIT (48.00 ± 4.30) cohorts. No formed infarction was observed in sham and HIIT cohorts. As shown in the representative figure of TTC-stained cardiac tissue sections (Fig. 1), the amount of infarct size caused by MI was 43.60 ± 5.22. Myocardial infarct volume following HIIT was significantly smaller relative to MI-CTL cohorts (27.80 ± 4.47).
Fig. 1.
HIIT for 8 weeks reduced infarct size caused by MI. There are no statistical differences among different cohorts for AAR. The myocardial infarct volume was significantly formed after MI induction (* P < 0.001 compared with sham cohort). TTC staining indicated that Long-term HIIT for 8 weeks could significantly reduce infarct volume compared with MI-CTL cohort (# P < 0.05)
Effects of HIIT on oxidative stress after MI
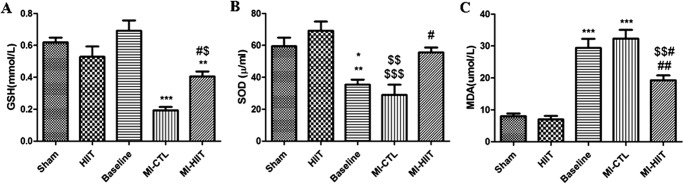
In order to identify whether HIIT can affect oxidative stress after MI, we measured SOD activity and levels of GSH and MDA using ELISA assay. We found that GSH tissue content was significantly decreased in MI-CTL cohorts. The alteration in GSH tissue content was caused following HIIT after MI (Fig. 2a). Likewise, SOD activity was markedly attenuated in MI rats 1 and 8 weeks after MI. HIIT remarkably restored SOD activity after 8 weeks (Fig. 2b). A significant elevated level of MDA was found in rats 1 and 8 weeks after MI. Long- term HIIT after MI markedly decreased MDA levels (Fig. 2c).
Fig. 2.
HIIT after MI exerts cardioprotective effects via attenuation of oxidative stress. a GSH content was significantly decreased in MI-CTL compared with sham, HIIT and Baseline (***P < 0.001). HIIT significantly restored GSH content (#P < 0.05 compared with MI-CTL; $P < 0.05 compared with sham; ** P < 0.01 compared with Baseline). b Significant decreased activity of SOD was found in Baseline and MI-CTL (* P < 0.05 and $$ P < 0.01 compared with sham; ** P < 0.01 and $$$ P < 0.001compared with HIIT). HIIT treatment significantly restored SOD activity compared with MI-CTL (#P < 0.05). c The levels of MDA in heart were significantly elevated in Baseline and MI-CTL compared with sham and HIIT (*** P < 0.001). Significant reduced MDA level was observed after treatment with HIIT (# P < 0.05 compared Baseline; ## P < 0.01 compared with MI-CTL; $$ P < 0.01 compared with sham and HIIT
Effects of HIIT on cardiac function after MI
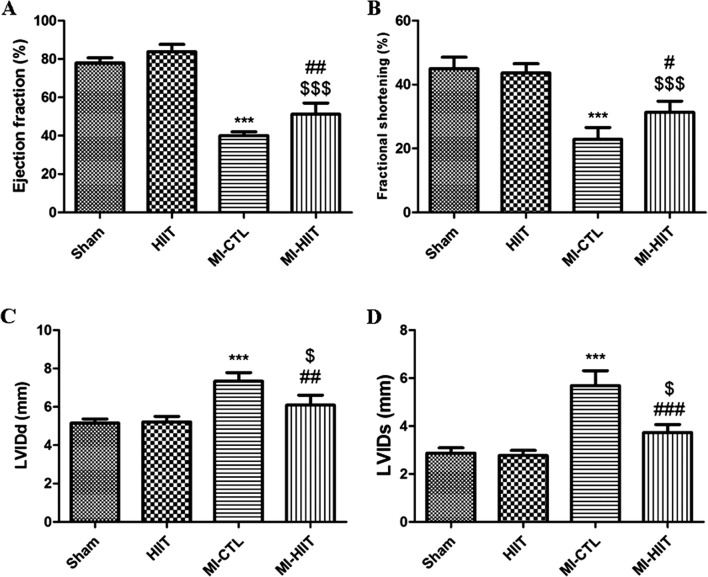
Evaluation of Left ventricular function was performed by echocardiography after 30 min ischemia and 8-week reperfusion at the end of HIIT. A significant decreased EF and FS were found in MI-CTL compared with sham. As depicted in Fig. 3a and b, HIIT after MI preserved left ventricular function and prevented remodelling by restoring EF and FS.
Fig. 3.
HIIT for 8 weeks contributed to the restoration of heart function. a, b Remarkable decreased EF and FS were created by LAD ligation (*** P < 0.001 compared with sham and HIIT cohorts). A significant increased percentage of EF and FS was found in MI-HIIT cohort (##P < 0.01 and # P < 0.05 compared with MI-CTL; $$$ P < 0.001 compared with sham and HIIT cohorts). c, d HIIT for 8 weeks markedly inhibited the increase of LVIDd and LVIDs after MI (*** P < 0.001 compared with sham and HIIT cohorts; ## P < 0.01 and ### P < 0.001 compared with MI-CTL; $ P < 0.05 compared with sham and HIIT cohorts)
As shown in Fig. 3c and d, LVIDd and LVIDs were dramatically increased in MI-CTL relative to sham and HIIT. Statistically significant differences were found between MI-CTL and MI-HIIT for LVIDd parameter. Likewise, in the case of MI-HIIT cohort, HIIT markedly blunted increasing of LVIDs relative to MI-CTL.
The effects of HIIT on post-ischemic adverse remodelling and myocardial hypertrophy
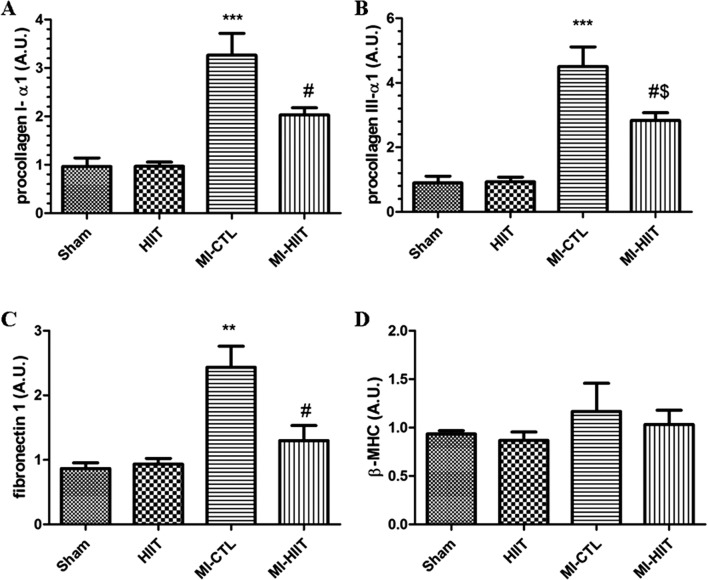
To evaluate effects of HIIT on post-ischemic adverse remodelling, we measured mRNA levels of gene markers of fibrosis such as procollagen α1 (I), procollagen α1 (III), and fibronectin1. As shown in Fig. 4a, b and c, our results showed that the mRNA levels of procollagen α1 (I), procollagen α1 (III), and fibronectin1 were markedly increased in MI-CTL compared with sham and HIIT cohorts. Long- term HIIT significantly decreased mRNA levels of these markers. In order to evaluate whether HIIT for 8 weeks might affect pathological hypertrophy, we measured mRNA levels of β-MHC, a gene marker of myocardial hypertrophy. As shown in Fig. 4d, there were no significant changes in LV β-MHC mRNA level between different groups.
Fig. 4.
HIIT for 8 weeks attenuated post-ischemic adverse remodelling. Effects of HIIT for 8 weeks on mRNA levels of a procollagen α1(I), b procollagen α1(III), and c fibronectin1 (*** P < 0.001 and ** P < 0.01 compared with sham and HIIT cohorts; # P < 0.05 compared with MI-CTL; $ P < 0.05 compared with sham and HIIT cohorts). d Alternatively, mRNA levels of β-MHC as gene marker of myocardial hypertrophy did not change in different groups
The effect of HIIT on interstitial fibrosis
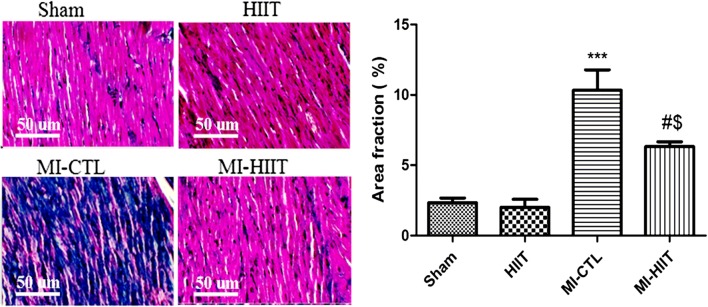
In order to prove protective effects of the eight-week HIIT training post-ischemic adverse remodelling, we performed Masson’s trichrome staining. As depicted in Fig. 5, histological analysis showed that the interstitial collagen deposition in the myocardium was significantly increased in MI-CTL compared with sham and HIIT cohorts. Long- term HIIT markedly reduced the interstitial collagen deposition and scar formation in the myocardium.
Fig. 5.
Histological assessment of cardiac fibrosis using Masson’s trichrome staining. The interstitial collagen deposition markedly increased in MI-CTL compared with sham and HIIT cohorts (*** P < 0.001). The eight-week HIIT training blunted the interstitial collagen deposition (# P < 0.05 compared with MI-CTL; $ P < 0.05 compared with sham and HIIT cohorts)
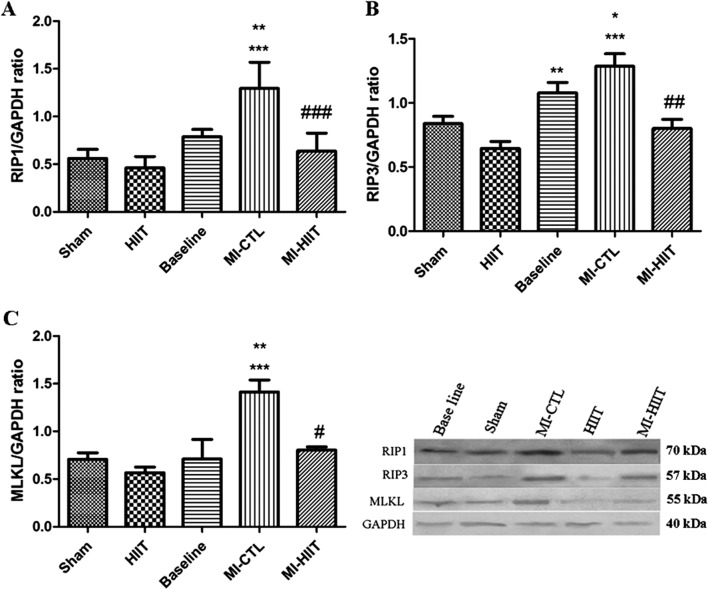
The effect of HIIT on key mediators of necroptosis
Effects of the eight-week HIIT training on protein levels of RIP1in cardiac tissue were evaluated using western blotting assay. As shown in Fig. 6a, our results showed that the expression level of RIP1 protein was significantly increased in MI-CTL cohort compared to sham and HIIT. A significant decreased level of RIP1 protein was found in MI-HIIT cohort, suggesting cardioprotective effects of HIIT against myocardial ischemia by targeting necroptosis. To obtain greater insight into effects of HIIT on necroptosis, we then examined the expression changes of RIP3 protein. As depicted in Fig. 6b, remarkable increased level of RIP3 protein was found in MI-CTL relative to sham and HIIT. Compared with MI-CTL, HIIT reversed the increased amount of RIP3 protein eight weeks after reperfusion, again confirming that HIIT exerts protection against MI by targeting necroptosis. To confirm the above findings on necroptosis targeted by HIIT after MI, we next investigated whether eight-week HIIT after reperfusion affected the expression levels of MLKL protein, downstream of RIP1 and RIP3. Figure 6c illustrates the findings of western blotting with MLKL antibody eight weeks after reperfusion. These findings indicated that induction of MI in rats significantly increased expression level of MLKL protein. A significant decreased level of MLKL was observed in MI-HIIT cohort.
Fig. 6.
Western blotting assay demonstrated that HIIT for 8 weeks accounted for cardioprotection in coping with MI by targeting necroptosis. a Protein level of RIP1 was significantly increased in MI-CTL relative to sham, HIIT (*** P < 0.001) and baseline cohorts (**P < 0.01). HIIT for 8 weeks markedly decreased Protein level of RIP1, indicating inhibition of necroptosis by HIIT after MI (### P < 0.001). b The expression of RIP3 protein was remarkably enhanced in Baseline and MI-CTL cohorts (** P < 0.01, *** P < 0.001 compared with HIIT, *P < 0.05 compared with HIIT). HIIT for 8 weeks significantly blunted the expression of RIP3 protein relative to MI-CTL (## P < 0.01). c Remarkable expression of MLKL protein was found in post-MI rats (*** P < 0.001 relative to sham and HIIT cohorts; ** P < 0.01 compared with baseline). HIIT for 8 weeks markedly decreased protein level of MLKL in post-MI rats, again confirming suppression of necroptosis following HIIT treatment (# P < 0.05 compared with MI-CTL cohort)
Discussion
In this study, for the first time we demonstrated eight-week HIIT could markedly reduce infarct size and prevent cardiac remodelling by targeting necroptosis in a rat model of MI. Indeed, for the first time, present study showed necroptosis is a long-term process that might be accounted for long-term adverse outcomes after MI.
Our results show that necroptosis plays an important role in cardiomyocyte loss and subsequent cardiac malfunction. Up-regulation of key mediators of necroptosis was found eight weeks after reperfusion in MI-CTL group. There was a significant correlation between HIIT following MI and down-regulation of key mediators of necroptosis. Indeed, HIIT exerted significant changes in molecular targets and cellular pathway to prevent abnormal changes in mass, size, cardiac geometry and function of the heart after injury. In recent years, researchers have introduced many new options for targeted therapy of MI and other diseases (Mahmoudi et al. 2017; Amani et al. 2017a; Jazayeri et al. 2016a; Jazayeri et al. 2016b; Pazoki-Toroudi et al. 2010).
Although some previous studies have shown that exercise after MI exerts detrimental effects on cardiac rehabilitation and heart function, there are many emerging evidences that demonstrate exercise can accelerate wound healing and restoration of heart function following MI (Garza et al. 2015; Giallauria et al. 2013). In a recent study, it is found that HIIT for 8 weeks contribute to the improvement of mechanical energy efficiency, energy expenditure and function in cardiac muscle through down-regulation of UCPs and up-regulation of Enos (Fallahi et al. 2016). Moreover, the same group reported that HIIT following MI ameliorated infarct and serum level of myocardial injury markers such as CK and plasma LDH (Rahimi et al. 2015). Our findings confirmed these previous data that HIIT for 8 weeks decreases the extent of infarct size. In a recent report by Barbosa-da-Silva et al. long-term HIIT for 8 weeks alleviated cardiac structural and functional remodelling caused by high-fat or high-fructose diets by mitigating LV hypertrophy in a left ventricular renin-angiotensin-system dependent mechanism (de Oliveira Sá et al. 2017). One goal of this study was to determine the effects of long-term HIIT on cardiac remodelling induced by MI. To this end, we investigated myocardial interstitial fibrosis as hallmark of cardiac remodelling. In this study, we demonstrated that mRNA levels of gene markers of fibrosis markedly decreased after long-term HIIT for 8 weeks. These findings confirm the protective effects of long-term HIIT for 8 weeks on cardiac remodelling induced by MI. In order to prove protective effects of long-term HIIT on cardiac remodelling induced by MI, we investigated myocardial interstitial fibrosis using Masson’s trichrome staining. Our result showed that long-term HIIT reduced the interstitial collagen deposition and scar formation in the myocardium. In consistent with our finding, a recent study showed that HIIT improved right ventricular dysfunction in a rat model of pulmonary hypertension through fibrosis reduction (Brown et al. 2016). On the other hand, transthoracic echocardiography data showed that HIIT significantly blunted increasing of LVIDs following MI and restored EF and FS which, in turn, resulted in the improvement of heart function. In keeping with our findings, Hu & et al. reported that both CMT and HIIT improve heart function after MI through expression changes in circulating microRNAs (Ding et al. 2016). Moreover, recent studies have shown that HIIT improves heart function in some diseases such as non-alcoholic fatty liver (Hallsworth et al. 2015). Pathological hypertrophy is independent risk factor for morbidity and mortality and is usually linked to the upregulation of some fetal genes such β-MHC and cardiac dysfunction (Reiser et al. 2001). In this study, our results showed that long-term HIIT markedly increased heart/body weight ratio and LV/body weight ratio compared to that in MI-CTL. To evaluate if heart/body weight ratio and LV/body weight ratio are associated with the physiological or pathological cardiac hypertrophy, we determined mRNA levels of β-MHC. Our results did not show any significant differences of β-MHC mRNA level between different groups. In keeping with our finding, a recent study showed that long-term exercise for 12 weeks after MI did not confer any change in β-MHC mRNA level (Wolff et al. 2017).The authors mentioned that it was unsurprising because the response of this marker depends on exact time -point chosen for analysis and infarct volume (Mair et al. 1994). On the other hand, a previous study showed that enhanced cardiac function is a hallmark of physiological hypertrophy that occurs after long-term exercise (McMullen and Jennings 2007). This study confirms our finding because cardiac function was improved after HIIT for 8 weeks. Then, we went on to clarify possible mechanisms by which long-term HIIT may exert protective effects against MI. Many cellular signaling pathways are involved in cell fate after MI; among them, necroptosis plays a crucial role in the modulation of post-ischemic adverse remodelling and cardiac dysfunction after MI (Luedde et al. 2014). Similar to apoptotic cell death, distinct molecules mediate necroptosis and similar to necrosis cell death initiation of necroptosis results in disruption of membrane integrity, and inflammation (Vandenabeele et al. 2010).
The detailed knowledge of involved mechanisms in cardio-protectiveness of HIIT following MI is still not available. In this study, we also sought to elucidate whether the possible mechanisms of HIIT in cardioprotection against cardiac functional remodelling after MI might be linked to necroptosis targeting. It has been recognized that necroptosis process is based on the activation of RIP1-RIP3-MLKL axis which, in turn, leads to the rupture of cell membrane, the release of cell contents and cell death (Kaczmarek et al. 2013).
RIP1is a master mediator of cardiomyocyte loss and its interaction with RIP3 results in necrosome formation, phosphorylation of MLKL, and necroptosis. Moreover, activation of RIP1 can create a pathophysiological chain including the increase of STAT3 phosphorylation, interaction of phosphorylated STAT3 with GRIM-19 (a subunit of mitochondrial complex I), STAT3 translocation to the mitochondria, generation of ROS and finally TNF-induced necroptosis (Shulga and Pastorino 2012). On the other hand, RIP1-dependent necrosis contributes to development of long-term adverse cardiac remodelling after MI. It has been found that Necrostatin-1, a specific inhibitor of necroptosis, can hamper interaction between RIP1 and RIP3 to reduce this programmed cell death, the inflammatory response, ROS generation and subsequent adverse cardiac remodelling (Zhang et al. 2018; Oerlemans et al. 2012). In this study, in agreement with previous studies the expression level of RIP1 was elevated following MI and reversed by HIIT. These results suggest that HIIT can guard against long-term adverse cardiac remodelling after MI by targeting RIP1-dependent necroptosis. RIP3 plays a critical role in the modulation of short-term adverse cardiac remodelling and heart malfunction. RIP3-dependent myocardial remodelling is associated with its co-localization with mitochondria and subsequent mitochondrial ROS generation. It is not known if translation of these short-term effects of RIP3 into long-term adverse ischemic remodelling mediates through its abundant and constant expression or by ROS generation. Overexpression of RIP3 leads to its interaction with RIP1, the formation of RIP1/RIP3 complex, inflammation and subsequent necroptosis cell death of cardiomyocytes (Luedde et al. 2014).
A recent study indicated that RIP3 can induce cardiac necrosis in the absence of RIP1 and MLKL proteins. It is recognized that RIP3 can mediate oxidative stress–induced myocardial necroptosis via activation of CaMKII (Zhang et al. 2016a). Therefore, we ask if HIIT might affect RIP3 and related mechanisms. The expression of RIP3 was remarkably increased following MI and reversed by HIIT. These findings were consistent with previous studies that RIP3 converted NF-induced cell death from apoptosis to necrosis (Zhang et al. 2009; Luedde et al. 2014; Newton et al. 2016). Previous reports have shown that MLKL activation leads to its translocation to the plasma membrane, membrane rupture and cell death (Dondelinger et al. 2016). A previous study indicated that Necrostatin-1 exerted cardioprotection paraquat-induced cardiac contractile dysfunction by targeting RIP1-RIP3-MLKL axis. It is seem MLKL contribute to development of adverse cardiac remodelling through the rupture of cell membrane (Zhang et al. 2018). In this study, MLKL was recognized as a molecular target of long-term HIIT. Our results indicated that down-regulation of MLKL by HIIT contributes to the inhibition of cardiac remodelling and dysfunction.
On the other hand, previous studies have shown that oxidative stress has been linked to TNFα-induced necroptosis. In fact, RIP1 activation can indirectly result in induction of oxidative stress and ROS accumulation in NF-κB activation-deficient cells (Shindo et al. 2013). However, depending on the cell type or the type of stimulus, ROS might be involved in necroptosis (Chtourou et al. 2015). The detailed knowledge about how ROS induces necroptosis is unknown (Han et al. 2018). A recent study has been shown that oxidative stress–induced myocardial necroptosis is associated activation of downstreams of RIP3 such as CaMKII (Zhang et al. 2016b). On the other hand, RIP kinase activity is required for function of glycogen phosphorylase and glutamate dehydrogenase 1 that utilizes glutamate or glutamine as substrates for ATP production in oxidative phosphorylation. A consequence of this process is ROS production which, in turn, results in a slippage of myofibrils and LV dilation (Declercq et al. 2009; Luedde et al. 2014). Therefore, we planned a strategy to examine whether HIIT exert cardioprotective effect after MI by targeting oxidative stress–induced myocardial necroptosis. Our findings demonstrated that HIIT caused noticeable changes in oxidative stress factors. HIIT contributed to reinforcement of endogenous antioxidant system and reduced lipid peroxidation.
Conclusion
Collectively, for the first time our results showed that HIIT gave rise to cardioprotection against adverse cardiac remodelling after MI by targeting oxidative stress–induced myocardial necroptosis. Discovery of these molecular targets of HIIT in MI can establish a substantial foundation for future drug design and research.
Acknowledgements
Present work was funded by a research grant from Physiology Research Center in Iran University of Medical Science.
Abbreviations
- MI
Myocardial infarction
- I/R
Ischemia/reperfusion
- ROS
Reactive oxygen species
- RIP1
Receptor-interacting protein kinase1
- NF-κB
Nuclear factor-κB
- MLKL
The mixed lineage kinase domain-like protein
- TRPM7
Transient receptor potential melastatin related 7
- HIIT
High-intensity interval training
- MICT
Moderate intensity continuous training
- CExT
Customary continuous exercise training
- LVIDd
Left ventricular diameter in diastole
- LVIDs
Left ventricular diameter in systole
- FS
Fractional shortening
- EF
Ejection fraction
- TBARS
The thiobarbituric acid reactive substance
- TTC
2,3,5- triphenyl-2H-tetrazolium chloride
- MDA
Malondialdehyde
- LAD
Left anterior descending coronary artery
- KCl
Potassium chloride
- NBT
Nitroblue tetrazolium
- GSH
Reduced glutathione
- UCPs
Uncoupling proteins
- eNOS
Endothelial nitric oxide synthase
- CaMKII
Ca2 + _calmodulin–dependent protein kinase
- LDH
Lactate dehydrogenase
- CK
Creatine kinase
- CMT
Continuous Moderately Training
- SOD
Superoxide dismutase
- GAPDH
Glyceraldehyde-3-phosphate Dehydrogenase
- β-MHC
Beta-myosin heavy chain
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Alireza Ghardashi Afousi, Email: Ghardashi.a@ut.ac.ir.
Abbasali Gaeini, Email: Aagaeini@ut.ac.ir.
Kamran Rakhshan, Email: Rakhshan.k@iums.ac.ir.
Nasim Naderi, Email: dr.naderi@irhfa.com.
Amir Darbandi Azar, Email: Amir.doc60@gmail.com.
Nahid Aboutaleb, Phone: 989123856305, Email: dr_nabo40@yahoo.com.
References
- Ajami M, Davoodi SH, Habibey R, Namazi N, Soleimani M, Pazoki-Toroudi H. Effect of DHA+ EPA on oxidative stress and apoptosis induced by ischemia-reperfusion in rat kidneys. Fundam Clin Pharmacol. 2013;27:593–602. doi: 10.1111/j.1472-8206.2012.01066.x. [DOI] [PubMed] [Google Scholar]
- Ajami M, Pazoki-Toroudi H, Amani H, Nabavi SF, Braidy N, Vacca RA, Atanasov AG, Mocan A, Nabavi SM. Therapeutic role of sirtuins in neurodegenerative disease and their modulation by polyphenols. Neurosci Biobehav Rev. 2016;73:39–47. doi: 10.1016/j.neubiorev.2016.11.022. [DOI] [PubMed] [Google Scholar]
- Amani H, Ajami M, Maleki SN, Pazoki-Toroudi H, Daglia M, Sokeng AJT, Di Lorenzo A, Nabavi SF, Devi KP, Nabavi SM. Targeting signal transducers and activators of transcription (STAT) in human cancer by dietary polyphenolic antioxidants. Biochimie. 2017;142:63–79. doi: 10.1016/j.biochi.2017.08.007. [DOI] [PubMed] [Google Scholar]
- Amani H, Habibey R, Hajmiresmail SJ, Latifi S, Pazoki-Toroudi H, Akhavan O. Antioxidant nanomaterials in advanced diagnoses and treatments of ischemia reperfusion injuries. J Mater Chem B. 2017;5:9452–9476. doi: 10.1039/C7TB01689A. [DOI] [PubMed] [Google Scholar]
- Batacan RB, Duncan MJ, Dalbo VJ, Tucker PS, Fenning AS. Br J Sports Med: bjsports-2015-095841. 2016. Effects of high-intensity interval training on cardiometabolic health: a systematic review and meta-analysis of intervention studies; pp. 494–503. [DOI] [PubMed] [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Boyne AF, Ellman GL. A methodology for analysis of tissue sulfhydryl components. Anal Biochem. 1972;46:639–653. doi: 10.1016/0003-2697(72)90335-1. [DOI] [PubMed] [Google Scholar]
- Brown MB, Neves E, Long G, Graber J, Gladish B, Wiseman A, Owens M, Fisher AJ, Presson RG, Petrache I. High-intensity interval training, but not continuous training, reverses right ventricular hypertrophy and dysfunction in a rat model of pulmonary hypertension. Am J Phys Regul Integr Comp Phys. 2016;312:R197–R210. doi: 10.1152/ajpregu.00358.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Jitkaew S, Zhao J, Chiang H-C, Choksi S, Liu J, Ward Y, Wu L-g, Liu Z-G. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol. 2014;16:55–65. doi: 10.1038/ncb2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christofferson DE, Yuan J. Necroptosis as an alternative form of programmed cell death. Curr Opin Cell Biol. 2010;22:263–268. doi: 10.1016/j.ceb.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chtourou Y, Slima AB, Makni M, Gdoura R, Fetoui H. Naringenin protects cardiac hypercholesterolemia-induced oxidative stress and subsequent necroptosis in rats. Pharmacol Rep. 2015;67:1090–1097. doi: 10.1016/j.pharep.2015.04.002. [DOI] [PubMed] [Google Scholar]
- de Oliveira Sá G, dos Santos Neves V, de Oliveira Fraga SR, Souza-Mello V, Barbosa-da-Silva S. High-intensity interval training has beneficial effects on cardiac remodeling through local renin-angiotensin system modulation in mice fed high-fat or high-fructose diets. Life Sci. 2017;189:8–17. doi: 10.1016/j.lfs.2017.09.012. [DOI] [PubMed] [Google Scholar]
- Declercq W, Vanden Berghe T, Vandenabeele P. RIP kinases at the crossroads of cell death and survival. Cell. 2009;138:229–232. doi: 10.1016/j.cell.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Ding R, Xia K, Hu D (2016) Study on the effect of different exercise intensity on cardiac function of rats with acute myocardial infarction and differential expression of circulating MiRNAs. In: Am Heart Assoc. Circulation 134(Suppl 1):A20726
- Dondelinger Y, Hulpiau P, Saeys Y, Bertrand MJM, Vandenabeele P. An evolutionary perspective on the necroptotic pathway. Trends Cell Biol. 2016;26:721–732. doi: 10.1016/j.tcb.2016.06.004. [DOI] [PubMed] [Google Scholar]
- Dorn GW. Apoptotic and non-apoptotic programmed cardiomyocyte death in ventricular remodelling. Cardiovasc Res. 2008;81:465–473. doi: 10.1093/cvr/cvn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterbauer H, Cheeseman KH (1990) '[42] determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol 186:407–421. 10.1016/0076-6879(90)86134-H [DOI] [PubMed]
- Fallahi AA, Shekarfroush S, Rahimi M, Jalali A, Khoshbaten A (2016) Alteration in cardiac uncoupling proteins and eNOS gene expression following high-intensity interval training in favor of increasing mechanical efficiency. Iran J Basic Med Sci 19(3):258–264 [PMC free article] [PubMed]
- Garza MA, Wason EA, Zhang JQ. Cardiac remodeling and physical training post myocardial infarction. World J Cardiol. 2015;7:52–64. doi: 10.4330/wjc.v7.i2.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghadernezhad N, Khalaj L, Pazoki-Toroudi H, Mirmasoumi M, Ashabi G. Metformin pretreatment enhanced learning and memory in cerebral forebrain ischaemia: the role of the AMPK/BDNF/P70SK signalling pathway. Pharm Biol. 2016;54:2211–2219. doi: 10.3109/13880209.2016.1150306. [DOI] [PubMed] [Google Scholar]
- Giallauria F, Acampa W, Ricci F, Vitelli A, Torella G, Lucci R, Del Prete G, Zampella E, Assante R, Rengo G. Exercise training early after acute myocardial infarction reduces stress-induced hypoperfusion and improves left ventricular function. Eur J Nucl Med Mol Imaging. 2013;40:315–324. doi: 10.1007/s00259-012-2302-x. [DOI] [PubMed] [Google Scholar]
- Guiraud T, Nigam A, Gremeaux V, Meyer P, Juneau M, Bosquet L. High-intensity interval training in cardiac rehabilitation. Sports Med. 2012;42:587–605. doi: 10.2165/11631910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Hallsworth K, Thoma C, Hollingsworth KG, Cassidy S, Anstee QM, Day CP, Trenell MI. Modified high-intensity interval training reduces liver fat and improves cardiac function in non-alcoholic fatty liver disease: a randomized controlled trial. Clin Sci. 2015;129:1097–1105. doi: 10.1042/CS20150308. [DOI] [PubMed] [Google Scholar]
- Han CH, Guan ZB, Zhang PX, Fang HL, Li L, Zhang HM, Zhou FJ, Mao YF, Liu WW. Oxidative stress induced necroptosis activation is involved in the pathogenesis of hyperoxic acute lung injury. Biochem Biophys Res Commun. 2018;495:2178–2183. doi: 10.1016/j.bbrc.2017.12.100. [DOI] [PubMed] [Google Scholar]
- Hochhauser E, Cheporko Y, Yasovich N, Pinchas L, Offen D, Barhum Y, Pannet H, Tobar A, Vidne BA, Birk E. Bax deficiency reduces infarct size and improves long-term function after myocardial infarction. Cell Biochem Biophys. 2007;47:11–19. doi: 10.1385/CBB:47:1:11. [DOI] [PubMed] [Google Scholar]
- Jazayeri MH, Amani H, Pourfatollah AA, Avan A, Ferns GA, Pazoki-Toroudi H (2016a) Enhanced detection sensitivity of prostate-specific antigen via PSA-conjugated gold nanoparticles based on localized surface plasmon resonance: GNP-coated anti-PSA/LSPR as a novel approach for the identification of prostate anomalies. Cancer Gene Ther 23(10):365–369. 10.1038/cgt.2016.42 [DOI] [PubMed]
- Jazayeri MH, Amani H, Pourfatollah AA, Pazoki-Toroudi H, Sedighimoghaddam B (2016b) Various methods of gold nanoparticles (GNPs) conjugation to antibodies. Sensing and bio-sensing research 9:17–22. 10.1016/j.sbsr.2016.04.002
- Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity. 2013;38:209–223. doi: 10.1016/j.immuni.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Koshinuma S, Miyamae M, Kaneda K, Kotani J, Figueredo VM. Combination of necroptosis and apoptosis inhibition enhances cardioprotection against myocardial ischemia–reperfusion injury. J Anesth. 2014;28:235–241. doi: 10.1007/s00540-013-1716-3. [DOI] [PubMed] [Google Scholar]
- Kraljevic J, Marinovic J, Pravdic D, Zubin P, Dujic Z, Wisloff U, Ljubkovic M. Aerobic interval training attenuates remodelling and mitochondrial dysfunction in the post-infarction failing rat heart. Cardiovasc Res. 2013;99:55–64. doi: 10.1093/cvr/cvt080. [DOI] [PubMed] [Google Scholar]
- Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, Granger CB, Lange RA, Mack MJ, Mauri L. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Thorac Cardiovasc Surg. 2016;152:1243–1275. doi: 10.1016/j.jtcvs.2016.07.044. [DOI] [PubMed] [Google Scholar]
- Li L, Chen Y, Doan J, Murray J, Molkentin JD, Liu Q (2014) A TAK1 signaling pathway critically regulates myocardial survival and remodeling. Circulation 130(24):2162–2172. 10.1161/CIRCULATIONAHA.114.011195 [DOI] [PMC free article] [PubMed]
- Li C, Xue H, Yang Z, Shi Z, Zhang B, Yu L, Ma H. GW28-e0894 impaired autophagosome clearance triggers myocardial necroptosis in ischemia/reperfusion injury. J Am Coll Cardiol. 2017;70:C33. doi: 10.1016/j.jacc.2017.07.114. [DOI] [Google Scholar]
- Linkermann A, Bräsen JH, Himmerkus N, Liu S, Huber TB, Kunzendorf U, Krautwald S. Rip1 (receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int. 2012;81:751–761. doi: 10.1038/ki.2011.450. [DOI] [PubMed] [Google Scholar]
- Luedde M, Lutz M, Carter N, Sosna J, Jacoby C, Vucur M, Gautheron J, Roderburg C, Borg N, Reisinger F. RIP3, a kinase promoting necroptotic cell death, mediates adverse remodelling after myocardial infarction. Cardiovasc Res. 2014;103:206–216. doi: 10.1093/cvr/cvu146. [DOI] [PubMed] [Google Scholar]
- Mahmoudi M, Yu M, Serpooshan V, Wu JC, Langer R, Lee RT, Karp JM, Farokhzad OC. Multiscale technologies for treatment of ischemic cardiomyopathy. Nat Nanotechnol. 2017;12:845. doi: 10.1038/nnano.2017.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair J, Wagner I, Jakob G, Lechleitner P, Dienstl F, Puschendorf B, Michel G. Different time courses of cardiac contractile proteins after acute myocardial infarction. Clin Chim Acta. 1994;231:47–60. doi: 10.1016/0009-8981(94)90253-4. [DOI] [PubMed] [Google Scholar]
- Mao Y, Koga J-i, Tokutome M, Matoba T, Ikeda G, Nakano K, Egashira K. Nanoparticle-mediated delivery of pitavastatin to monocytes/macrophages inhibits left ventricular remodeling after acute myocardial infarction by inhibiting monocyte-mediated inflammation. Int Heart J. 2017;58:615–623. doi: 10.1536/ihj.16-457. [DOI] [PubMed] [Google Scholar]
- McMullen JR, Jennings GL. Differences between pathological and physiological cardiac hypertrophy: novel therapeutic strategies to treat heart failure. Clin Exp Pharmacol Physiol. 2007;34:255–262. doi: 10.1111/j.1440-1681.2007.04585.x. [DOI] [PubMed] [Google Scholar]
- Neri M, Fineschi V, Di Paolo M, Pomara C, Riezzo I, Turillazzi E, Cerretani D. Cardiac oxidative stress and inflammatory cytokines response after myocardial infarction. Curr Vasc Pharmacol. 2015;13:26–36. doi: 10.2174/15701611113119990003. [DOI] [PubMed] [Google Scholar]
- Newton K, Dugger DL, Maltzman A, Greve JM, Hedehus M, Martin-McNulty B, Carano RAD, Cao TC, van Bruggen N, Bernstein L. RIPK3 deficiency or catalytically inactive RIPK1 provides greater benefit than MLKL deficiency in mouse models of inflammation and tissue injury. Cell Death Differ. 2016;23:1565–1576. doi: 10.1038/cdd.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oerlemans MIFJ, Liu J, Arslan F, den Ouden K, van Middelaar BJ, Doevendans PA, Sluijter JPG. Inhibition of RIP1-dependent necrosis prevents adverse cardiac remodeling after myocardial ischemia–reperfusion in vivo. Basic Res Cardiol. 2012;107(270):270. doi: 10.1007/s00395-012-0270-8. [DOI] [PubMed] [Google Scholar]
- Pazoki-Toroudi HR, Hesami A, Vahidi S, Sahebjam F, Seifi B, Djahanguiri B. The preventive effect of captopril or enalapril on reperfusion injury of the kidney of rats is independent of angiotensin II AT1 receptors. Fundam Clin Pharmacol. 2003;17:595–598. doi: 10.1046/j.1472-8206.2003.00188.x. [DOI] [PubMed] [Google Scholar]
- Pazoki-Toroudi HR, Ajami M, Habibey R. Pre-medication and renal pre-conditioning: a role for alprazolam, atropine, morphine and promethazine. Fundam Clin Pharmacol. 2010;24:189–198. doi: 10.1111/j.1472-8206.2009.00743.x. [DOI] [PubMed] [Google Scholar]
- Pazoki-Toroudi H, Amani H, Ajami M, Nabavi SF, Braidy N, Kasi PD, Nabavi SM. Targeting mTOR signaling by polyphenols: a new therapeutic target for ageing. Ageing Res Rev. 2016;31:55–66. doi: 10.1016/j.arr.2016.07.004. [DOI] [PubMed] [Google Scholar]
- Puhl S-L, Müller A, Wagner M, Devaux Y, Böhm M, Wagner DR, Maack C. Exercise attenuates inflammation and limits scar thinning after myocardial infarction in mice. Am J Phys Heart Circ Phys. 2015;309:H345–HH59. doi: 10.1152/ajpheart.00683.2014. [DOI] [PubMed] [Google Scholar]
- Rahimi M, Shekarforoush S, Asgari AR, Khoshbaten A, Rajabi H, Bazgir B, Mohammadi MT, Sobhani V, Shakibaee A. The effect of high intensity interval training on cardioprotection against ischemia-reperfusion injury in wistar rats. EXCLI J. 2015;14:237. doi: 10.17179/excli2014-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakhshan K, Azizi Y, Naderi N, Afousi AG, Aboutaleb N (2018) ELABELA (ELA) peptide exerts Cardioprotection against myocardial infarction by targeting oxidative stress and the improvement of heart function. Int J Pept Res Ther 1–9. 10.1007/s10989-018-9707-8
- Reiser PJ, Portman MA, Ning X-H, Moravec CS. Human cardiac myosin heavy chain isoforms in fetal and failing adult atria and ventricles. Am J Phys Heart Circ Phys. 2001;280:H1814–H1H20. doi: 10.1152/ajpheart.2001.280.4.H1814. [DOI] [PubMed] [Google Scholar]
- Shindo R, Kakehashi H, Okumura K, Kumagai Y, Nakano H. Critical contribution of oxidative stress to TNFα-induced necroptosis downstream of RIPK1 activation. Biochem Biophys Res Commun. 2013;436:212–216. doi: 10.1016/j.bbrc.2013.05.075. [DOI] [PubMed] [Google Scholar]
- Shulga N, Pastorino JG. GRIM-19-mediated translocation of STAT3 to mitochondria is necessary for TNF-induced necroptosis. J Cell Sci. 2012;125:2995–3003. doi: 10.1242/jcs.103093. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Song L, Yang H, Wang H-X, Tian C, Liu Y, Zeng X-J, Gao E, Kang Y-M, Du J, Li H-H. Inhibition of 12/15 lipoxygenase by baicalein reduces myocardial ischemia/reperfusion injury via modulation of multiple signaling pathways. Apoptosis. 2014;19:567–580. doi: 10.1007/s10495-013-0946-z. [DOI] [PubMed] [Google Scholar]
- Tao L, Bei Y, Lin S, Zhang H, Zhou Y, Jiang J, Chen P, Shen S, Xiao J, Li X. Exercise training protects against acute myocardial infarction via improving myocardial energy metabolism and mitochondrial biogenesis. Cell Physiol Biochem. 2015;37:162–175. doi: 10.1159/000430342. [DOI] [PubMed] [Google Scholar]
- Ulbrich AZ, Angarten VG, Netto AS, Sties SW, Bündchen DC, de Mara LS, Cornelissen VA, de Carvalho T (2016) Comparative effects of high intensity interval training versus moderate intensity continuous training on quality of life in patients with heart failure: study protocol for a randomized controlled trial. Clinical Trials and Regulatory Science in Cardiology 13:21–28. 10.1161/CIRCULATIONAHA.114.011195
- Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- Wolff AM, Rasmussen TP, Wichern CR, Peterson MR, Stayton MM, Thomas DP. Effects of pericardiectomy on training-and myocardial infarction-induced left ventricular hypertrophy, chamber dimensions and gene expression. Int J Sports Med. 2017;38:27–34. doi: 10.1055/s-0042-115567. [DOI] [PubMed] [Google Scholar]
- Zhang D-W, Shao J, Lin J, Zhang N, Lu B-J, Lin S-C, Dong M-Q, Han J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- Zhang T, Zhang Y, Cui M, Jin L, Wang Y, Lv F, Liu Y, Zheng W, Shang H, Zhang J. CaMKII is a RIP3 substrate mediating ischemia-and oxidative stress-induced myocardial necroptosis. Nat Med. 2016;22:175–182. doi: 10.1038/nm.4017. [DOI] [PubMed] [Google Scholar]
- Zhang T, Zhang Y, Cui M, Jin L, Wang Y, Lv F, Liu Y, Zheng W, Shang H, Zhang J. CaMKII is a RIP3 substrate mediating ischemia-and oxidative stress–induced myocardial necroptosis. Nat Med. 2016;22:175–182. doi: 10.1038/nm.4017. [DOI] [PubMed] [Google Scholar]
- Zhang L, Feng Q, Wang T (2018) Necrostatin-1 protects against Paraquat-induced cardiac contractile dysfunction via RIP1-RIP3-MLKL-dependent necroptosis pathway. Cardiovasc Toxicol 18(4):346–355. 10.1007/s12012-017-9441-z [DOI] [PubMed]