Abstract
We report a case in which combination therapy with albumin-bound paclitaxel (nab-paclitaxel) and gemcitabine converted unresectable pancreatic cancer with peritoneal metastases into resectable disease. The patient was a 71-year-old woman with anorexia. Enhanced abdominal computed tomography (CT) showed an atrophic pancreatic body and tail, dilated main pancreatic duct, peritoneal dissemination, portal vein stricture, bile duct stricture and wall thickening, and blockage of the right ureter. She was diagnosed with pancreatic cancer with peritoneal metastases. Curative resection was initially impossible. Combination therapy consisting of nab-paclitaxel and gemcitabine was initiated. The regimen consisted of 28-day cycles of albumin-bound paclitaxel (nab-paclitaxel) (125 mg/m2 intravenously over 30 min on days 1, 8, 15) and gemcitabine (1000 mg/m2 intravenously over 30 min on days 1, 8, 15). After 8 cycles of chemotherapy, enhanced CT showed no evidence of the tumor in the pancreatic body and tail or peritoneal metastases. Positron emission tomography with CT (PET-CT) showed no abnormal fluorodeoxyglucose uptake. After pre-operative chemotherapy for 8 months, the patient was underwent distal pancreatectomy with resection of soft tissue that corresponded to the right ureteral tumor seen on enhanced CT. This case showed that chemotherapy consisting of nab-paclitaxel and gemcitabine can be well-tolerated and can convert unresectable pancreatic cancer with peritoneal metastases into resectable disease.
Keywords: Case report, Peritoneal metastases, Pancreatic cancer, Conversion surgery
Introduction
Pancreatic cancer is one of the most common malignancies worldwide. As a result of the advanced stage at the time of diagnosis, rapid tumor growth, and high potential for distant metastases, the overall 5-year survival rate is only 1 to 4% [1]. However, a recent report suggested that adjuvant surgery for initially unresectable pancreatic cancer with a favorable response to chemotherapy or chemoradiation often contributes to a better prognosis than non-surgical treatment alone [2]. We present a case of peritoneal metastases from pancreatic cancer with clinical complete response to nab-paclitaxel and gemcitabine chemotherapy. This chemotherapy has the potential to convert unresectable pancreatic cancer with peritoneal metastases into resectable disease.
Case report
A 71-year-old woman visited her primary physician for anorexia in November 2015. Enhanced abdominal computed tomography (CT) revealed a tumor in the body and tail of the pancreas, stomach, and right ureter, as well as right hydronephrosis. She was referred to a nearby medical center, where she was diagnosed with unresectable pancreatic body and tail cancer with peritoneal metastases, right ureteral stricture, and peritoneal metastases infiltrating the stomach by PET-CT (Fig. 1). Upper endoscopy showed submucosal tumors with the red color sign at the gastric angle, greater curvature, and anterior wall. Pathological examination revealed adenocarcinoma and invasion from peritoneal metastases arising from pancreatic adenocarcinoma. Curative resection was impossible. The patient sought a second opinion at our hospital in January 2016.
Fig. 1.
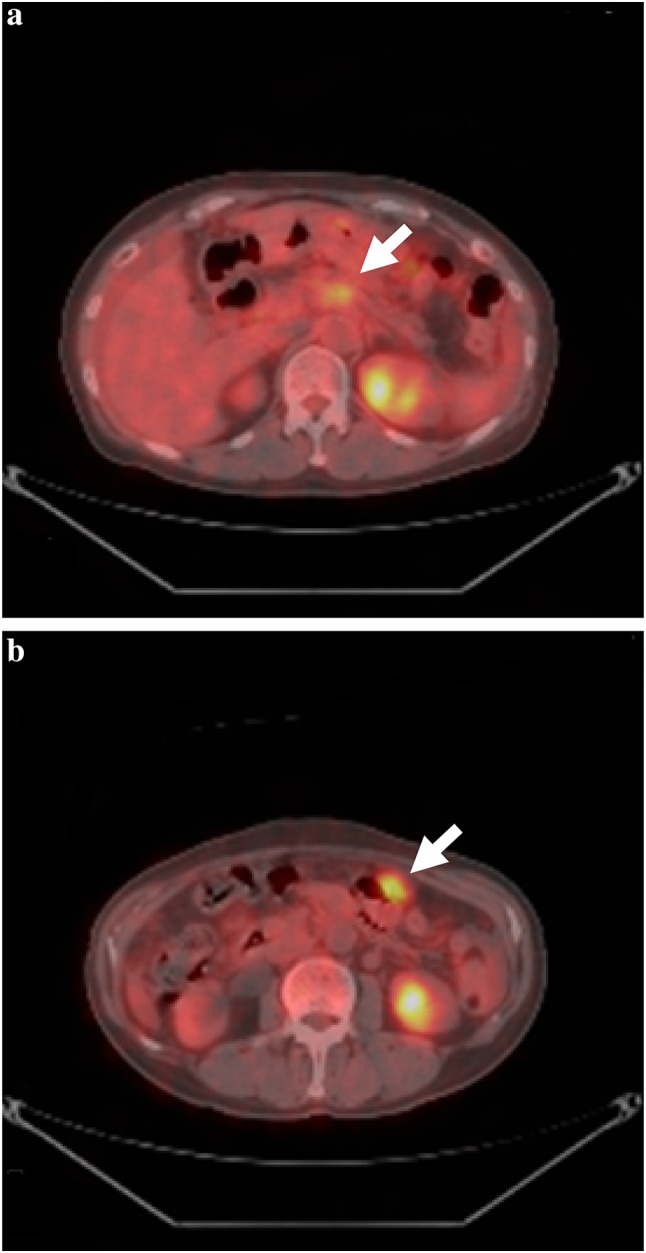
Whole-body PET-CT before preoperative chemotherapy. a Abnormal fluorodeoxyglucose uptake in the body of the pancreas (arrow). b Abnormal fluorodeoxyglucose uptake in the nodal desease in the abdomen (arrow)
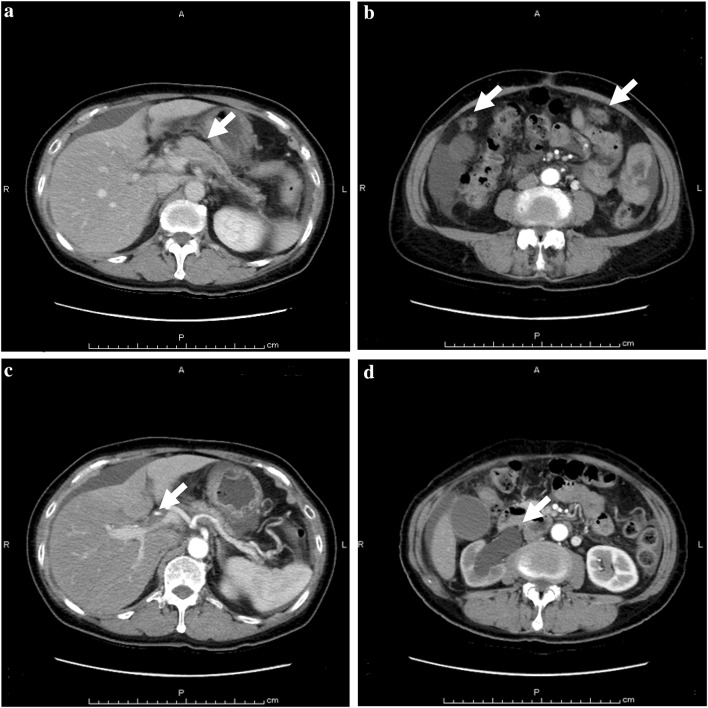
When she visited our hospital for the first time, carcinoembryonic antigen (CEA) was 3.1 ng/dl, serum carbohydrate antigen 19-9 (CA19-9) was 424 µ/ml, pancreas cancer-associated antigen (DUPAN-2) was 243 µ/ml, and hemoglobin was 4.4 g/dl. She was admitted emergently for evaluation and treatment of anemia. Her performance status score was 3 at the time. Abdominal CT on admission showed a suspicious tumor in the body of the pancreas with a dilated main pancreatic duct and atrophy of the pancreatic parenchyma distal to the tumor, peritoneal dissemination, portal vein stricture, stricture and wall thickening of the bile duct, blockage of the right ureter (Fig. 2), and accumulation of ascitic fluid in the pouch of Douglas. We decided that her general condition needed improvement before any cancer treatment. After blood transfusion, her performance status score improved from 3 to 1, and we initiated chemotherapy consisting of nab-paclitaxel (125 mg/m2 intravenously over 30 min on days 1, 8, 15) and gemcitabine (1000 mg/m2 intravenously over 30 min on days 1, 8, 15). This schedule was repeated at 28-day intervals. After 2 courses, CEA, CA19-9, DUPAN-2 decreased to within normal limits. Starting with the third course, we modified the schedule. Nab-paclitaxel and gemcitabine were given on days 1 and 8 in a 21-day cycle because this schedule was more convenient for the patient. After 8 courses of chemotherapy, enhanced CT showed no evidence of tumor in the body and tail of the pancreas or peritoneal metastases. Hydronephrosis was partially resolved (Fig. 3). Whole-body PET-CT also showed no abnormal uptake of fluorodeoxyglucose throughout the body (Data not shown). The patient agreed with our recommendation of surgical resection to confirm the effect of chemotherapy and examine the residual lesions. She received chemotherapy for 8 months, consisting of a total of 15.0 g of gemcitabine and 1.62 g of nab-paclitaxel. No detectable ascites, peritoneal metastasis and liver metastasis and being negative for the peritoneal lavage cytology were confirmed by the laparoscopic staging laparotomy. There were no neoplastic lesions evident in the gastric wall and the body and tail of the pancreas. She underwent distal pancreatectomy with regional lymph node dissection and resection of soft tissue that corresponded to the right ureteral tumor on enhanced CT before chemotherapy (Fig. 4). Intraoperative pathological examinations with tissue samples near the stomach, portal vein, and soft tissues around right urinary duct were negative for cancer cells. Operative time and intraoperative blood loss were 310 min and 80 ml, respectively.
Fig. 2.
Enhanced abdominal computed tomography before preoperative chemotherapy. a The atrophic body and tail and the interruption of the main pancreatic duct (arrow). b Peritoneal dissemination in the abdomen (arrow). c Portal vein stricture (arrow). d Blockage and dilation of the right ureter (arrow)
Fig. 3.
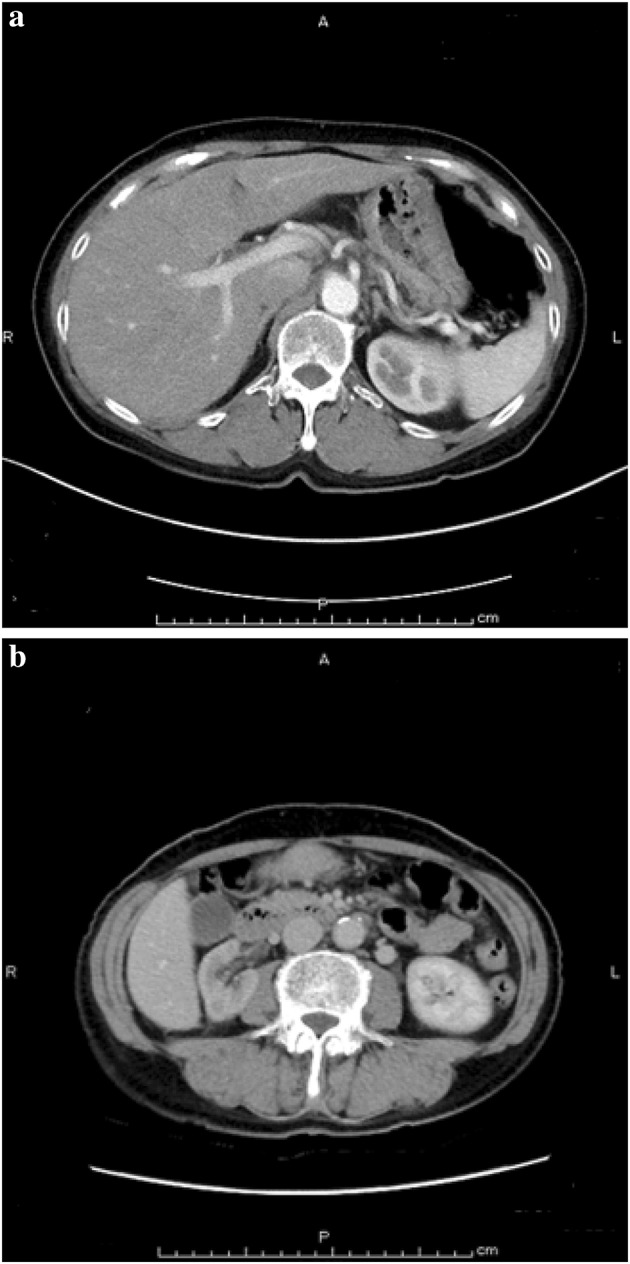
Enhanced abdominal computed tomography after chemotherapy. a Portal vein stricture and acites were disappereed (almost same lebel of Fig. 2c). b Hydronephrosis was resolved (almost same level of Fig. 2d)
Fig. 4.
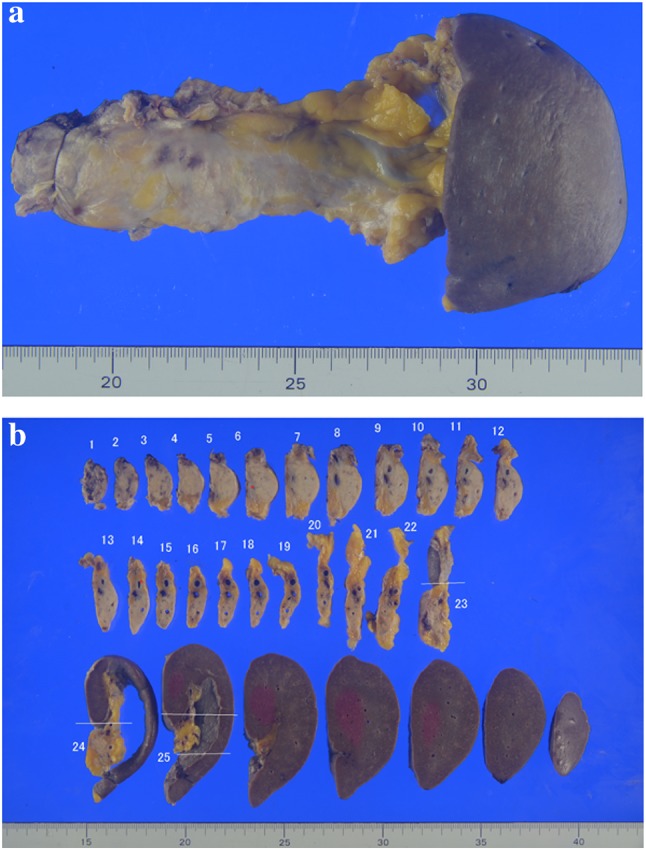
Macroscopic appearance of the formalin-fixed surgical specimen. a Reseted pancreastic body and tail, and spleen. b Serially sectioned speciemns of the resected pancreass and spleen
Histopathological evaluation showed only fibrous tissue in the pancreatic body and residual tumor cells located 1 mm deep from the adventitia to the media of the splenic vein. Furthermore, a lesion diagnosed as carcinoma in situ was detected in the distal pancreatic duct, which was considered a residual lesion of invasive carcinoma in the pancreatic duct or high-grade pancreatic intraepithelial neoplasia (Fig. 5). Of 32 resected lymph nodes, only one metastatic lymph node around the common hepatic artery was detected. The patient made a satisfactory recovery without complications. She was discharged on postoperative day 20. The patient received post-operative adjuvant chemotherapy consisting of the same regimen for 6 months becase the regimen was effective. However, PET-CT of 2 months ater finishing the postoperative adjuvant chemotherapy showed recurrence arround the accending colon as a peritoneal metastasis. She started to recieve FOLFIRINOX regimen [3] and is still alive after 22 months from diagnosis.
Fig. 5.
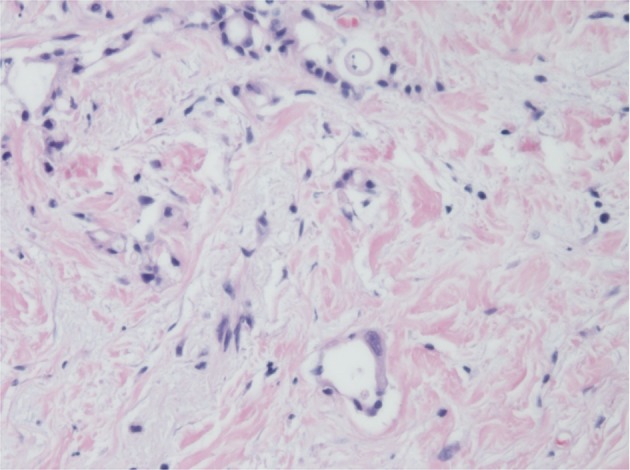
Microscopic appearance of the pancreatic tumor with hematoxylin-eosin staining. A lesion corresponding to carcinoma in situ was detected in the distal pancreatic duct, which was diagnosed as residual carcinoma invading the pancreatic duct or high-grade pancreatic intraepithelial neoplasia
Discussion
Pancreatic cancer is a lethal disease that contributes to the increasing number of cancer deaths worldwide. Although surgical resection is the only curative option for pancreatic cancer, the majority of patients are considered to have unresectable disease [1]. The overall 5-year survival rate is less than 5%; only 20% of patients can be treated with surgery [1]. Since 1997, gemcitabine has been the standard first-line treatment for patients with unresectable locally advanced or metastatic pancreatic cancer [4], but the rate of complete or partial response with gemcitabine ranges from 7 to 9.4% [3, 5]. Few patients have undergone conversion surgery after chemotherapy for unresectable pancreatic cancer [6–13]. However, 2 recent trials of systemic chemotherapy in metastatic pancreatic cancer have shown promise. The PRODIGE trial showed that the FOLFIRINOX regimen had an absolute survival benefit of 4.3 months compared with gemcitabine [3]. The addition of nab-paclitaxel to gemcitabine in the MPACT trial also demonstrated a survival benefit of 1.8 months compared with gemcitabine alone [5]. In subgroup analyses of these 2 trials, the objective response rate was higher in the FOLFIRINOX and nab-paclitaxel-gemcitabine groups than in the gemcitabine alone group.
Some reports have documented unresectable pancreatic cancer being converted into resectable disease with chemotherapy and prolonged overall survival after surgery [6–13]. We found 12 patients who underwent conversion surgery after neoadjuvant chemotherapy for pancreatic cancer initially diagnosed as unresectable (Table 1). The median overall survival after conversion surgery among these 12 patients was 14 months, which is better than the median survival observed in the PRODIGE and MPACT trials [3, 5]. Patients who undergo chemotherapy and conversion surgery have a survival benefit. Among the 12 patients, 8 had unresectable disease due to metastasis in the liver, 2 had extensive lymph node metastasis around the pancreas, 1 had metastasis in both liver and peritoneum, and 1 had peritoneal metastasis. Eleven patients received FOLFIRINOX or gemcitabine-based chemotherapy. One patient with peritoneal metastasis received intravenous and intraperitoneal paclitaxel with S-1 [12]. Although the addition of nab-paclitaxel to gemcitabine is now widely accepted as one of the most effective chemotherapy regimens for unresectable pancreatic cancer [14], there have been no reports of patients successfully treated with surgery after chemotherapy that included nab-paclitaxel. The case with peritoneal metastasis showed that paclitaxel-based chemotherapy, including nab-paclitaxel, can be effective for pancreatic cancer with peritoneal metastases.
Table 1.
Patients who underwent conversion surgery after chemotherapy for initially unresectable pancreatic cancer
| Reason for initial unresectability | Preoperative therapy | Durations of preoperative treatments (months) | Operative procedure | Combined resection | Outcome of surgery (months) | Reported |
|---|---|---|---|---|---|---|
| Liver | GEM + S-1 | 6 | PD | – | 37 | Kato [6] |
| Liver | GEM + S-1 | 5 | PD | PV | 11 | |
| Liver | GEM + S-1 | 9 | DP | Liver | 9 | |
| PALN | GEM + 5-FU | 7 | DP | Adrenal | 10 | |
| PALN | GEM | 16 | DP | – | 14 | Matsuda [7] |
| Liver | FOLFOX | 9 | PD | – | 24 | Buc [8] |
| Liver | FOLFIRINOX→GEM | 15 | PD | – | 24 | |
| Liver | FOLFIRINOX | ?(9 cycles) | DP | Liver | 24 | Neofytou [9] |
| Liver | FOLFIRINOX | 3 | DP | Liver | 14 | |
| Liver/P | FOLFIRINOX | 7 | DP | Liver | 9 | Schneitler [10] |
| Liver | FOLFIRINOX | 6 | DP | Liver | 39 | Sodergre [11] |
| P | PTX + S-1 | 5 | DP | Transverse mesocolon + stomach |
13 | Kitayama [12] |
| Liver (n = 13) | GEM or S-1 | > 6 | PD (n = 7) DP (n = 6) |
PV (n = 3) Liver (n = 5) Adrenal (n = 1) CA (n = 1) |
NS | Satoi [13] |
| PALN (n = 3) | GEM or S-1 | > 6 | DP (n = 3) | PV (n = 1) | NS | |
| P | GEM or S-1 | > 6 | DP | – | NS |
n number of the patients, P peritoneal metastases, PALN para-aortic lymph node, PD pancreaticoduodenectomy, DP distal pancreatomy, DPCAR DP with celiac axis resection, PV portal vein, CA celiac artery, GEM gemcitabine, NS not shown, FOLFIRINOX combination regimen consisting of oxaliplatin, irinotecan, fluorouracil, and leucovorin, PTX paclitaxel
Conversion surgery, which is defined as therapy achieving transformation of unresectable disease to resectable disease, has been established in colon cancer management [15]. Furthermore, there are some reports that conversion surgery for other types of cancer might also prolong overall survival. Gastrectomy in patients with gastric cancer and peritoneal metastasis has been reported to increase response to chemotherapy and thereby improve patient prognosis [16–19]. In Japan, conversion surgery for initially unresectable pancreatic cancer with a long-term favorable response to non-surgical anticancer treatment is considered to be an effective treatment [13]. Seventeen patients who initially had distant organ metastases underwent conversion surgery. The median survival and overall survival rates at 2 years among the 17 patients with conversion surgery were 39 months and 77%, respectivly, compared with 19 months and 33% among 45 patients that initially had unresectable pancreatic cancer with a long-term favorable response to non-surgical anticancer treatments but no surgical resection. These results suggested that conversion surgery might confer a significant benefit among some patients successfully treated with chemotherapy as compared to patients treated with chemotherapy alone. Furthermore, duration of the pre-operative chemotherapy should be an important factor for longer survival of the patients underwent conversion surgery [13]. Conversion surgery should be attempted according to not only the therapeutic effect but also duration of the pre-operative therapy. However, it still remains unclear whether better median survival and overall survival rates were the result of conversion surgery or just a good response to chemotherapy.
We present a case of metastatic pancreatic cancer in which the patient underwent R0 resection following 10 cycles of gemcitabine + nab-paclitaxel therapy and had long-term survival. To the best of our knowledge, this is the first case published in the literature of conversion surgery for initially unresectable pancreatic cancer using gemcitabine + nab-paclitaxel. From this case alone, it is not clear whether conversion surgery prolongs overall survival, but we think this case shows that it is important to consider surgery as part of the treatment strategy for patients with initially unresectable pancreatic cancer who respond favorably to chemotherapy. Further investigation is required to determine whether pancreatic tumors should be resected after a satisfactory response to chemotherapy when there are distant organ metastases.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Conlon KC, Klimstra DS, Klimstra DS, et al. Long-term survival after curative resection for pancreatic ductal adenocarcinoma: clinicopathological analysis of 5-year survivors. Ann Surg. 1996;223:273–279. doi: 10.1097/00000658-199603000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wright GP, Poruk KE, Zenati MS, et al. Primary tumor resection following favorable response to systemic chemotherapy in stage IV pancreatic adenocarcinoma with synchronous metastases: a bi-institutional analysis. J Gastrointest Surg. 2016;20:1830–1835. doi: 10.1007/s11605-016-3256-2. [DOI] [PubMed] [Google Scholar]
- 3.Conroy V, Desseigne T, Ychou F, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 4.Burris HA III, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 5.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nabpaclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kato K, Kondo S, Hirano S, et al. Adjuvant surgical therapy for patients with initially-unresectable pancreatic cancer with long-term favorable responses to chemotherapy. J Hepatobiliary Pancreat Sci. 2011;18:712–716. doi: 10.1007/s00534-011-0391-8. [DOI] [PubMed] [Google Scholar]
- 7.Matsuda T, Taniguchi F, Minato H, et al. Successful resection of advanced pancreatic tail cancer after neoadjuvant gemcitabine chemotherapy: report of a case. Surg Today. 2006;36(8):754–757. doi: 10.1007/s00595-006-3227-4. [DOI] [PubMed] [Google Scholar]
- 8.Buc E, Orry D, Antomarchi O, et al. Resection of pancreatic ductal adenocarcinoma with synchronous distant metastasis: is it worthwhile? World J Surg Oncol. 2014;12:347. doi: 10.1186/1477-7819-12-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neofytou K, Giakoustidis A, Smyth EC, et al. A case of metastatic pancreatic adenocarcinoma with prolonged survival after combination of neoadjuvant FOLFIRINOX therapy and synchronous distal pancreatectomy and hepatectomy. J Surg Oncol. 2015;111:768–770. doi: 10.1002/jso.23867. [DOI] [PubMed] [Google Scholar]
- 10.Schneitler S, Kropil P, Riemer J, et al. Metastasized pancreatic carcinoma with neoadjuvant FOLFIRINOX therapy and R0 resection. World J Gasteroenterol. 2015;21:6384–6390. doi: 10.3748/wjg.v21.i20.6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sodergren M, Brammer K, Cunningham D, et al. Long-term Disease-free Survival Following Combination Multi-visceral and Metastatic Resection with Neoadjuvant FOLFIRINOX for Pancreatic Adenocarcinoma: A Case Report. Cureus. 2015;7(12):e429. doi: 10.7759/cureus.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitayama K, Tsuji Y, Kondo T, et al. Conversion therapy for pancreatic cancer with peritoneal metastases using intravenous and intraperitoneal paclitaxel with S-1. Mol Clin Oncol. 2016;5:779–782. doi: 10.3892/mco.2016.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Satoi S, Yamaue H, Kato K, et al. Role of adjuvant surgery for patients with initially unresectable pancreatic cancer with a long-term favorable response to non-surgical anti-cancer treatments: results of a project study for pancreatic surgery by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2013;20:590–600. doi: 10.1007/s00534-013-0616-0. [DOI] [PubMed] [Google Scholar]
- 14.NCCN Clinical Practice Guidelines in Oncology Pancreatic Cancer v.2 (2017). https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. Accessed Apr 2017
- 15.Power DG, Kemeny NE. Chemotherapy for the conversion of unresectable colorectal cancer liver metastases to resection. Crit Rev Oncol Hematol. 2011;79:251 264. doi: 10.1016/j.critrevonc.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Kim SW. The Result of Conversion Surgery in Gastric Cancer Patients with Peritoneal Seeding. J Gastric Cancer. 2014;14(4):266–270. doi: 10.5230/jgc.2014.14.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugarbaker PH, Yonemura Y. Clinical pathway for the management of resectable gastric cancer with peritoneal seeding: best palliation with a ray of hope for cure. the International Society for Cellular. 2000;58:96–107. doi: 10.1159/000012086. [DOI] [PubMed] [Google Scholar]
- 18.Kuramoto M, Shimada S, Ikeshima S, et al. Extensive intraoperative peritoneal lavage as a standard prophylactic strategy for peritoneal recurrence in patients with gastric carcinoma. Ann Surg. 2009;250:242–246. doi: 10.1097/SLA.0b013e3181b0c80e. [DOI] [PubMed] [Google Scholar]
- 19.Stern JL, Denman S, Elias EG, et al. Evaluation of palliative resection in advanced carcinoma of the stomach. Surgery. 1975;77:291–298. [PubMed] [Google Scholar]