Abstract
Low-grade appendiceal mucinous neoplasm (LAMN) is rare disease, and the absence of characteristic clinical symptoms makes preoperative diagnosis difficult. The strategy of treatment for LAMN has not been established. Surgical approach and lymph node (LN) dissection are still controversial. We herein present a case of LAMN with difficulties in making the preoperative diagnosis, which exhibited invagination and was treated by laparoscopy-assisted ileocecal resection with LN dissection. When cystic mass is detected in the bowel, LAMN or mucinous adenocarcinoma should be considered as a different diagnosis. And the laparoscopy-assisted ileocecal resection is a feasible operation for LAMN with careful procedure.
Keywords: Appendix, Appendiceal mucocele, Low-grade appendiceal mucinous neoplasm
Introduction
Low-grade appendiceal mucinous neoplasm (LAMN) is included in appendiceal mucinous neoplasm by definition of WHO classification. Pathologically, appendiceal mucinous neoplasm is designated if over 50% of the region consists of extracellular mucin, and classified as LAMN or as mucinous adenocarcinoma. LAMN is rare disease constituting about 1% of all colorectal malignancies [1], 8% of all appendiceal tumors and 0.3–0.7% of all appendectomy specimens [2]. Preoperative diagnosis of LAMN is difficult due to rarity of the disease and the absence of characteristic clinical symptoms [3]. And then, laboratory tests are not specific in patients with LAMN.
Surgical resection is the first choice for LAMN, and a chemotherapy has not yet been established [5]. Recently, the laparoscopic surgery is increasing; however, surgical approach and lymph node (LN) dissection are still controversial because of the difficulty of preoperative malignancy diagnosis and the risk of intraoperative injury of the mucinous tumor [6].
We present a case of LAMN with difficulties in making the preoperative diagnosis that exhibited invagination, and was treated by laparoscopy-assisted ileocecal resection.
Case report
The patient was a 40-year-old female, who had a gradually increasing pain in right lower quadrant with nausea and vomiting. Her body temperature was 37.3 °C and her abdomen was soft, but palpable mass was present in the hypogastric region with tenderness. In the laboratory tests, carbohydrate antigen 125 and carcinoembryonic antigen (CEA) levels were elevated to 54 U/ml and 7.7 ng/ml, respectively. Computed tomography (CT) showed a concentric circles containing cystic structure with calcification at the left side of the lesion (Fig. 1a, b). We diagnosed the invagination of intestine and an emergency laparoscopic examination was performed; nevertheless intestinal tract showed no abnormal finding.
Fig. 1.
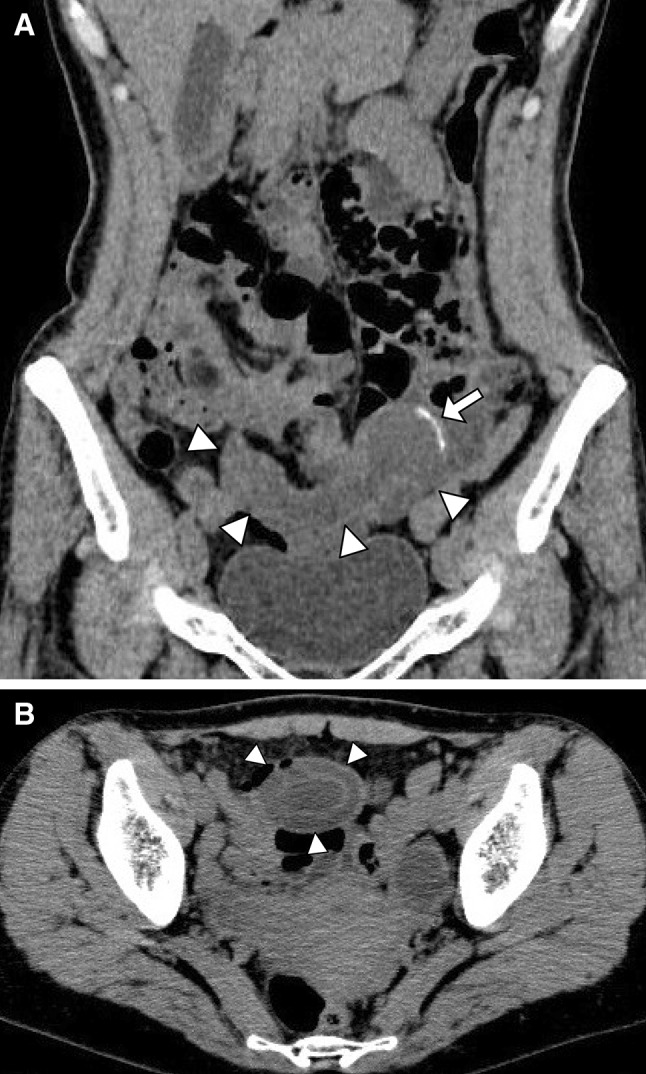
Abdominal CT scan (a and b) showed smooth cystic bulge with calcification at the left side of the lesion (arrow). It showed a concentric circles structure (b)
After laparoscopic examination, the abdominal pain persisted. Total colonoscopy revealed a 5-cm submucosal-tumor-like lesion in transverse colon, and it was moved easily to cecum (Fig. 2a). CT colonography showed smooth hemispheric defect of the cecum (Fig. 2b). The mucinous adenocarcinoma of the appendix or LAMN was suspected, and laparoscopy-assisted ileocecal resection was performed. On entering the peritoneal cavity, swollen cecum and appendix with smooth serosal surface were found. Laparoscopy-assisted ileocecal resection with D2 LN dissection was performed. Ileum was excised at 5-cm oral side from terminal ileum, and ascending colon was excised at 8-cm anal side from tumor by electrocautery. Reconstruction of ileum and ascending colon was performed by end-to-end anastomosis using a two-layer anastomosis technique.
Fig. 2.
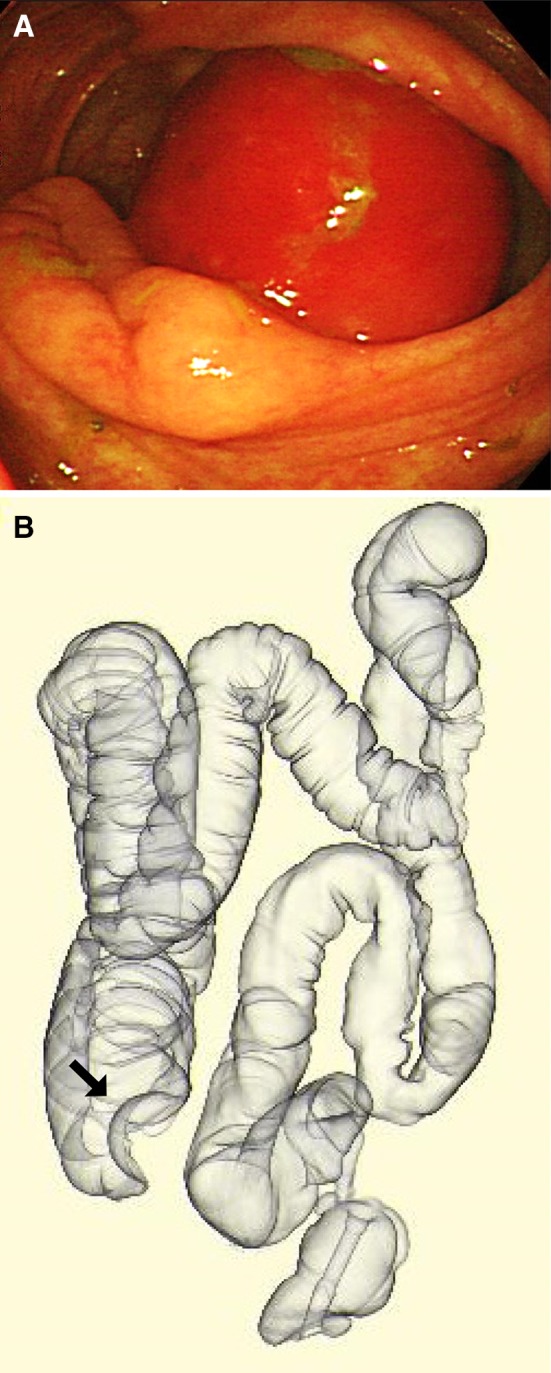
Colonoscopy revealed a submucosal tumor in the transverse colon, and it was moved easily to cecum (a). CT colonography showed smooth hemispheric defect of the cecum (b)
The appendiceal tumor measured 10 cm in length and 4 cm in diameter, and it continued into the cecum. The tumor was filled with jelly-like mucus (Fig. 3a, b). Histopathological examination revealed the papillary growing glandular epithelium with tall columnar mucinous cells. It showed elongated swollen nuclei at the base and low-grade dysplasia (Fig. 3c). Final pathologic diagnosis of the mass was LAMN.
Fig. 3.
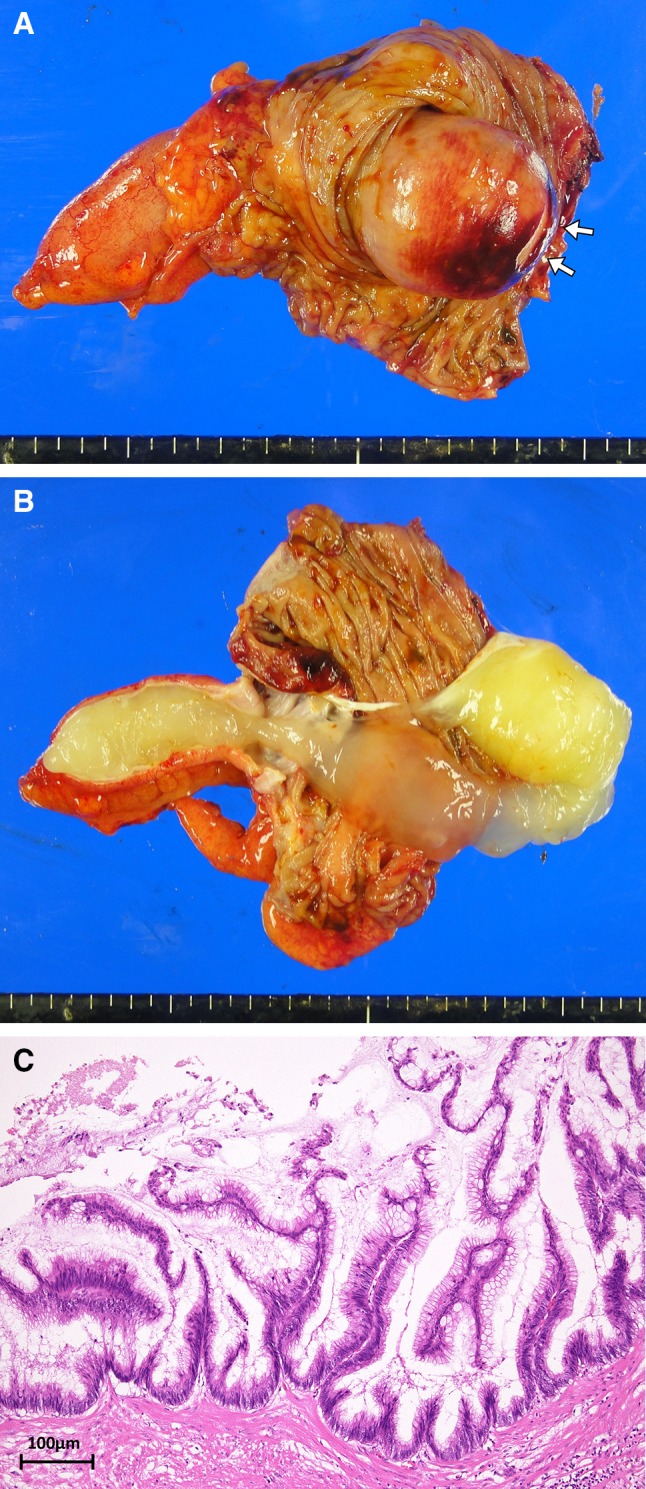
Macroscopic view of the resected specimen showed the sub-mucosal tumor originated from appendix and the appendiceal orifice (arrows). The tumor was filled with jelly-like mucus (a and b). Histopathologic image demonstrating the papillary growing glandular epithelium covered by a single layer of columnar mucinous cells, and elongate swelling nuclei at the base (c)
The postoperative course was uneventful; patient was discharged 8 days after surgery and remained well with no recurrence for 16 months.
Discussion
WHO classification of tumors 2010 divided appendiceal mucinous neoplasm into mucinous adenocarcinoma and LAMN, previously called “mucinous cystadenoma.” Pathologically, LAMN exhibits mostly epithelial villous adenomatous changes without malignancy and the prognosis is good. And mucinous adenocarcinoma demonstrates glandular stromal invasion, desmoplastic reaction, and/or presence of epithelial cells in the peritoneal implants and associated with a very poor survival rate and a high rate of lymph nodes or liver metastases [5]. While the nomenclature is controversial because LAMN can lead to diffuse peritoneal dissemination of the cells within the peritoneal cavity, known as pseudomyxoma peritonei, even distant metastases [7]. Like our case, LAMN is incidentally discovered at surgery or during imaging for unrelated symptoms, in patients with chronic abdominal pain, palpable mass in the right lower abdomen, or in patients with an intestinal tract invagination [4, 8–11]. Laboratory tests are not specific but some case reports showed that high serum CEA levels could be detected in patients with LAMN [12].
The diagnosis of LAMN is established basically by abdominal CT scan; its appearances include a well-encapsulated, round, and thin-walled cystic mass. Calcification is found in 50% of cases [13] and mucoceles less than 2 cm are rarely malignant but larger mucoceles (6 cm or more) are usually associated with adenocarcinoma and a higher perforation rate (20%) [5]. The enhancing nodules in the mucocele wall are suggestive of mucinous adenocarcinoma [5] but preoperative diagnosis of malignancy was difficult [3]. Colonoscopy may show a pathognomonic image called “volcano sign” [14]; the appendiceal orifice may be seen in the center of a mound-like elevation of the cecal wall [15]. In our case, the cecum was loosely fixed to retroperitoneum, and appendix was in an abnormal position by invagination, so we could not suppose appendiceal mucocele. However, LAMN or mucinous adenocarcinoma should be considered as a different diagnosis by primary CT findings. And we should have inspected the cecum and appendix more carefully at laparoscopic examination.
Surgical resection is considered as the only curative treatment for LAMN and appendectomy is performed when the appendiceal root is intact [16]. Even in the case of appendiceal mucinous adenocarcinoma, González et al. reported that overall survival rate of appendectomy was similar to that of right hemicolectomy and suggested hence appendectomy or cecectomy is preferable [17]. The incidence of LN metastasis has been reported in 1.7% cases of LAMN [18]; however, that of mutinous adenocarcinoma ranging from 25 to 50% [19] and preoperative definitive diagnosis may be difficult [20]. Therefore, ileocecal resection or right hemicolectomy with LN dissection is often performed [4]. In our case, laparoscopy-assisted ileocecal resection with D2 LN dissection was performed since perioperative diagnosis of progression was difficult.
Recently, the laparoscopic resection for LAMN has been increasing, and this approach is minimally invasive as demonstrated by minimal postoperative pain and quick recovery. The rupture of LAMN leads to pseudomyxoma peritonei that worsens the outcome of the disease [22] and previous report showed cases of pseudomyxoma peritonei due to perioperative perforation in both open and laparoscopic surgery [23]. Some authors recommended the open surgery to avoid the rupture of the mucocele [6, 24]. But there is no comparative study between open and laparoscopic surgery. To prevent the injury of the mucocele, surgeon should not grasp a tumor directly and ensure the margin especially in the invagination case [11], and sometimes it needs mobilizing the entire right hemi-colon [25]. In the accredited facilities, laparoscopic surgery including mobilization of the colon and LN dissection is standard procedure, And in this case laparoscopy-assisted ileocecal resection was safely performed without grasping the appendix, and D2 LN dissection was successfully completed.
In conclusion, we reported a case of LAMN with difficulties in making the preoperative diagnosis that exhibit invagination. And the laparoscopy-assisted ileocecal resection and D2 LN dissection were performed safely for LAMN.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Farah-Klibi F, Kourda-Boujemaa J, Bouaskar I, et al. Cystadenocarcinoma of the appendix: an incidental perioperatory finding in a patient with adenocarcinoma of the ascending and sigmoid colon: case report and review of literature. Pathologica. 2009;101(6):255. [PubMed] [Google Scholar]
- 2.Kalogiannidis I, Mavrona A, Grammenou S, et al. Endometrial adenocarcinoma and mucocele of the appendix: an unusual coexistence. Case Rep Obstet Gynecol. 2013 doi: 10.1155/2013/892378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murata Y, Goshima H, Kato H, et al. Two cases of mucocele of the appendix with acute abdomen. Mie-igaku. 2006;49(3):63–66. [Google Scholar]
- 4.Nakatani K, Tokuhara K, Sakaguchi T, et al. Low-grade mucinous neoplasia in a cecal diverticulum: a case report. Int J Surg Case Rep. 2015;15:66–69. doi: 10.1016/j.ijscr.2015.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spyropoulos C, Rentis A, Alexaki E, et al. Appendiceal mucocele and pseudomyxoma peritonei; the clinical boundaries of a subtle disease. Am J Case Rep. 2014;15:355. doi: 10.12659/AJCR.890837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moreno SG, Shmookler B, Sugarbaker P. Appendiceal mucocele. Surg Endosc. 1998;12(9):1177–1179. doi: 10.1007/s004649900811. [DOI] [PubMed] [Google Scholar]
- 7.Nakao A, Sato S, Nakashima A, et al. Appendiceal mucocele of mucinous cystadenocarcinoma with a cutaneous fistula. J Int Med Res. 2002;30(4):452–456. doi: 10.1177/147323000203000416. [DOI] [PubMed] [Google Scholar]
- 8.Shibata Y, Fukaya M. A case of intussusception caused by mucinous cystadenoma of the appendix. Jpn J Gastroenterol Surg. 2001;34(3):272–276. doi: 10.5833/jjgs.34.272. [DOI] [Google Scholar]
- 9.Krebs TL, Daly BD, Wong-You-Cheong J, et al. General case of the day. Mucinous cystadenocarcinoma of the appendix. Radiographics. 1998;18(4):1049–1050. doi: 10.1148/radiographics.18.4.9672990. [DOI] [PubMed] [Google Scholar]
- 10.Endo I, Misumi T. A case of intussuception caused by mucinouscystadenocarcinoma of the appendix. J Jpn Surg Assoc. 2008;69(12):3200–3203. doi: 10.3919/jjsa.69.3200. [DOI] [Google Scholar]
- 11.Yukawa N, Rino Y, Sugano N, et al. A case of appendiceal mucinous cystadenoma resection in laparoscopy assisted partial cecectomy. Jpn Coll Surg. 2011;36(4):658–664. doi: 10.4030/jjcs.36.658. [DOI] [Google Scholar]
- 12.Akasaka Y, Hanamura N, Kida H, et al. A case of mucocele of theappendix associated with an increase in serum CEA level. J Jpn Surg Assoc. 1997;58(2):419–424. [Google Scholar]
- 13.Pickhardt PJ, Levy AD, Rohrmann CA, Jr, et al. Primary neoplasms of the appendix: radiologic spectrum of disease with pathologic correlation 1. Radiographics. 2003;23(3):645–662. doi: 10.1148/rg.233025134. [DOI] [PubMed] [Google Scholar]
- 14.Rojnoveanu G, Ghidirim G, Mishin I, et al. Preoperatively diagnosed mucocele of the appendix. Chirurgia (Bucharest, Romania: 1990) 2013;109(3):416–20. [PubMed] [Google Scholar]
- 15.Minni F, Petrella M, Morganti A, et al. Giant mucocele of the appendix. Dis Colon Rectum. 2001;44(7):1034–6. doi: 10.1007/BF02235492. [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa H, Takamura H, Kuwata H, et al. Case report of mucinous cystadenocarcinoma of the appendix afterappendectomy. Jpn J Gastroenterol Surg. 1986;19(1):67–70. doi: 10.5833/jjgs.19.67. [DOI] [Google Scholar]
- 17.González-Moreno S, Sugarbaker P. Right hemicolectomy does not confer a survival advantage in patients with mucinous carcinoma of the appendix and peritoneal seeding. Br J Surg. 2004;91(3):304–311. doi: 10.1002/bjs.4393. [DOI] [PubMed] [Google Scholar]
- 18.Chua TC, Moran BJ, Sugarbaker PH, et al. Early-and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30(20):2449–2456. doi: 10.1200/JCO.2011.39.7166. [DOI] [PubMed] [Google Scholar]
- 19.McGory ML, Maggard MA, Kang H, et al. Malignancies of the appendix: beyond case series reports. Dis Colon Rectum. 2005;48(12):2264–71. doi: 10.1007/s10350-005-0196-4. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto A, Shibutani K, Inoue M, et al. A Case of mucinous cystadenoma in appendix performed laparoscopic partial excision of cecum. Jpn Soc Gastroenterol Surg. 2001;34(11):1650–1654. doi: 10.5833/jjgs.34.1650. [DOI] [Google Scholar]
- 21.Nakatsuji N, Yagura K, Ochi Y. A case of mucinous cystadenocarcinoma of the appendix. J Nara Med Assoc. 2012;63(3–4):71–77. [Google Scholar]
- 22.Pitiakoudis M, Tsaroucha A, Mimidis K, et al. Mucocele of the appendix: a report of five cases. Tech Coloproctol. 2004;8(2):109–112. doi: 10.1007/s10151-004-0067-3. [DOI] [PubMed] [Google Scholar]
- 23.Honore C, Caruso F, Dartigues P, et al. Strategies for preventing pseudomyxoma peritonei after resection of a mucinous neoplasm of the appendix. Anticancer Res. 2015;35(9):4943–4947. [PubMed] [Google Scholar]
- 24.Demetrashvili Z, Chkhaidze M, Khutsishvili K, et al. Mucocele of the appendix: case report and review of literature. Int Surg. 2012;97(3):266–269. doi: 10.9738/CC139.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsui H, Igarashi N, Okamura A, et al. Laparoscopy-assisted resection of an appendiceal mucinous cystadenoma. Tokai J Exp Clin Med. 2007;32(4):140–143. [PubMed] [Google Scholar]


