Abstract
Gallbladder cancer (GBC) is a rare malignancy, associated with poor disease prognosis with a 5-year survival of only 20%. This has been attributed to late presentation of the disease, lack of early diagnostic markers and limited efficacy of therapeutic interventions. Elucidation of molecular events in GBC can contribute to better management of the disease by aiding in the identification of therapeutic targets. To identify aberrantly activated signaling events in GBC, tandem mass tag-based quantitative phosphoproteomic analysis of five GBC cell lines was carried out. Proline-rich Akt substrate 40 kDa (PRAS40) was one of the proteins found to be hyperphosphorylated in all the invasive GBC cell lines. Tissue microarray-based immunohistochemical labeling of phospho-PRAS40 (T246) revealed moderate to strong staining in 77% of the primary gallbladder adenocarcinoma cases. Regulation of PRAS40 activity by inhibiting its upstream kinase PIM1 resulted in a significant decrease in cell proliferation, colony forming and invasive ability of GBC cells. Our results support the role of PRAS40 phosphorylation in GBC cell survival and aggressiveness. This study also elucidates phospho-PRAS40 as a clinical marker in GBC and the role of PIM1 as a therapeutic target in GBC.
Electronic supplementary material
The online version of this article (10.1007/s12079-018-00503-5) contains supplementary material, which is available to authorized users.
Keywords: Cell survival, Gastrointestinal cancer, mTOR signaling, Phosphoproteomics, SGI-1776, Targeted therapy
Introduction
Gallbladder cancer (GBC) represents the most prevalent form of biliary tract tumors. Although uncommon, this malignancy is aggressive and rapidly metastatic (Misra et al. 2006). Early detection is rare and incidental, with surgical resection being the only potential curative approach (Lazcano-Ponce et al. 2001). Five year survival rate is 32% for lesions confined to gallbladder mucosa and 1 year survival rate is 10% for advanced stages (Lazcano-Ponce et al. 2001). GBC patients present with poor prognosis since the disease is difficult to diagnose and treat. Majority of the GBC cases are diagnosed at advanced stages, where resection cannot be an option of treatment. In addition, the response to traditional methods of chemotherapy along with radiotherapy is limited (Bizama et al. 2015). New therapeutic options with improved outcome as well as reduced cytotoxicity and off-target effects need to be investigated. Recent clinical trials examining the effect of angiogenesis inhibitors such as bevacizumab and vandetanib on progression-free survival in GBC patients have not improved disease outcome (Jordan et al. 2016). Besides, majority of the ongoing trials are mainly focused on biliary tract cancers and not on gallbladder cancer alone (Jordan et al. 2016). Signaling pathways involving estrogen receptor, hedgehog, MIF and mTOR have been put forward as potential therapeutic targets (Zhang et al. 2013; Matsushita et al. 2014; Subbannayya et al. 2015a), albeit warranting clinical validation. Hence, it is important to further evaluate the underlying molecular events involved in GBC tumorigenesis.
Cellular processes including growth, proliferation, differentiation, migration and apoptosis, are known to be mediated through signaling events. These complex signaling networks are regulated by post-translational modifications including protein phosphorylation. Kinases are important regulators of these signaling events. Earlier studies have shown that protein kinases are frequently involved in tumorigenesis and can act as cancer drug targets. Currently, there are 28 FDA approved small molecule kinase inhibitors (Wu et al. 2015). Therapies targeting aberrantly activated protein kinases have been routinely used to treat lung (Kris et al. 2003; Lynch et al. 2004), gastric (Subbannayya et al. 2015b), head and neck (Radhakrishnan et al. 2016), breast (Rabindran et al. 2004) and pancreatic cancers (Harsha et al. 2008), as well as chronic myelogenous leukemia (Druker et al. 2001). Given the role of kinases in cell signaling and their potential to serve as therapeutic targets, phosphoproteome profiling is a promising approach to identify activated kinases associated with gallbladder tumorigenesis (Harsha and Pandey 2010). To this end, we have employed high-resolution mass spectrometry coupled with TMT-based labeling approach on five GBC cell lines. Using this panel of 4 invasive and 1 non-invasive GBC cell lines, a total of 2623 phosphosites from 1343 proteins were identified. Of these, 55 phosphosites were hyperphosphorylated and 35 phosphosites were hypophosphorylated in both replicates and in all 4 invasive GBC cell lines. Interestingly, our bioinformatics analysis of the hyperphosphorylated proteins identified mTOR and Class I PI3K signaling events mediated by AKT to be among the top biological pathways dysregulated in the invasive GBC cell lines, whereas proteins involved in mTOR signaling (such as proline-rich AKT substrate of 40 kDa (PRAS40), FOXO3A and RPS6) were hyperphosphorylated in all four invasive cells analysed. PRAS40 is a negative regulator of mTOR signaling and has been previously reported to be hyperphosphorylated in multiple solid malignancies (Madhunapantula et al. 2007; Yuan et al. 2015; Lu et al. 2014). Therefore, we thought to study and validate the role of PRAS40-mediated signaling in GBC tumorigenesis.
PRAS40, also known as AKT1 substrate 1 (AKT1S1), is a known substrate of AKT and a 14–3-3 binding protein (Kovacina et al. 2003). PRAS40 acts as a negative regulator of mTOR signaling by binding to mTOR and thus preventing interaction with its substrates. Phosphorylation of PRAS40 by AKT at T246 and by mTORC1 at S183 disrupts the binding between PRAS40 and mTORC1 and relieves its inhibitory effect (Chong 2016). PIM1, a serine threonine kinase, has also been reported to phosphorylate PRAS40 at T246 independent of AKT (Zhang et al. 2009). PRAS40 has been found to be hyperphosphorylated in malignant melanoma (Madhunapantula et al. 2007), HER2-positive breast cancer (Yuan et al. 2015) and gastric cancer (Lu et al. 2014). PIM-phosphorylated PRAS40, along with phospho-FOXO3A and 14–3-3, has been implicated to regulate radiation-induced radioresistance in non-small cell lung cancer (Kim et al. 2011). Taken together, these studies indicate a role of phospho-PRAS40 (T246) in cancer progression and resistance to therapy. However, the role of PRAS40 in regulating GBC tumorigenesis has not been defined. In this study, we have assessed the role of PRAS40 in the molecular events involved in GBC and evaluated its biomarker potential.
Materials and methods
Cell culture
GBC cell lines - TGBC24TKB, TGBC2TKB, G-415 (RIKEN Bio Resource Center, Ibaraki, Japan), OCUG-1 (Health Science Research Resources Bank, Osaka, Japan), SNU-308 (Korean Cell Line Bank, Seoul, Korea) and GB-d1 were used in the study. GB-d1 was authenticated by short tandem repeat analysis. Properties and culture conditions of these GBC cell lines are provided in Supplementary Table 1. All cell lines were maintained in a humidified incubator with 5% CO2 at 37 °C. Cells were periodically monitored for mycoplasma using the MycoDetect kit (Greiner Bio-One) and authenticated using genetic fingerprinting (Identifiler, Applied Biosystems) before use.
Protein extraction and TMT labeling
The GBC cell lines were grown to a confluence of ~80%. The cells were then rinsed thrice with phosphate buffered saline (PBS), devoid of Ca2+ and Mg2+ to remove traces of serum and growth factors. This was followed by an 8 h starvation in serum-free medium containing 1% penicillin-streptomycin. The cells were rinsed with PBS and lysed using SDS-containing lysis buffer (2% SDS, 5 mM sodium fluoride, 1 mM β-glycerophosphate, 1 mM sodium orthovanadate in 50 mM triethyl ammonium bicarbonate (TEABC)). The cell lysates were sonicated, heated at 95 °C for 5 min and centrifuged at 12,000 rpm for 10 min. Protein concentration was determined using bicinchoninic acid assays (BCA) method (Smith et al. 1985). Filter aided sample preparation (FASP) was employed to remove SDS as described previously (Kim et al. 2014). Briefly, equal amounts of protein from each cell line were reduced using dithiothreitol (DTT) at 60 °C for 20 min. SDS micelles and protein detergent complexes were disrupted using 8 M urea. Ultrafiltration using 30 kDa cut-off filters (Merck Millipore) was assisted in filtering out the detergent and excess reagents. The peptides were alkylated with iodoacetamide (IAA) for 10 min at room temperature and the excess reagents were filtered out by centrifugation. A second buffer exchange further reduced the SDS concentration using 50 mM TEABC. Proteins digestion was carried out using TPCK-treated trypsin (Worthington) at a 1:20 enzyme to protein ratio for 12 h at 37 °C. The samples were then dried and reconstituted in 50 mM TEABC buffer. Peptides from each sample were differentially labeled using TMT labeling reagents as per manufacturer’s instructions (catalog # 90110, Thermo Fisher Scientific) as follows: TGBC24TKB with 126, SNU-308 with 127C, OCUG-1 with 128C, GB-d1 with 129C and G-415 with 130C.
Basic reversed-phase liquid chromatography (bRPLC) phosphopeptide enrichment using TiO2
The TMT-labeled peptides were subjected to bRPLC fractionation, as described previously (Nagarajha Selvan et al. 2014). The 96 fractions obtained were concatenated into 12 fractions. From these pooled fractions, 100 μg equivalent peptides were taken for total proteome analysis and the remaining were lyophilized and subjected to TiO2-based enrichment (Larsen et al. 2005). The phosphopeptide-enriched TiO2 beads were washed two times with 80% ACN in 3% TFA, eluted using 4% ammonia solution and neutralized with 3% TFA. Eluted peptides were vacuum dried, desalted using C18 Stage Tips and stored at -20 °C till further analysis.
LC-MS/MS analysis
The total proteome and enriched phosphopeptide fractions were analyzed on LTQ-Orbitrap Velos mass spectrometer (Thermo Scientific) interfaced with Proxeon Easy nLC II system (Thermo Scientific) as described previously (Subbannayya et al. 2015a). The following parameters were used for the LC-MS/MS analysis: MS and MS/MS scans were acquired at a mass resolution of 60,000 and 15,000 at 400 m/z, respectively. Full MS scans were acquired in m/z range of 350–1800. Twenty most abundant precursor ions with charge state ≥2 were selected for fragmentation using higher energy collision dissociation as the activation method with 42% normalized collision energy. Isolation width was set to 1.9 m/z. The phosphopeptide fractions were analyzed twice on the mass spectrometer to obtain two technical replicates.
Data analysis
The mass spectrometry-derived raw data obtained were searched using Sequest and Mascot (version 2.2.0, Matrix Science) search algorithms against human protein database NCBI RefSeq (Release 65 containing 36,211 protein entries and known contaminants) using Proteome Discoverer (version 1.4) software suite (Thermo Fisher Scientific). The search parameters used for the data search are as follows: trypsin as the proteolytic enzyme with a maximum two missed cleavages, oxidation at methionine and phosphorylation at serine, threonine and tyrosine as dynamic modifications, alkylation (carbamidomethyl) at cysteine, TMT 6-plex (+229.163) modification at N-terminus of peptide and lysine as static modifications. Precursor and fragment mass tolerance were set at 10 ppm and 0.05 Da, respectively. PhosphoRS (version 3.1) was used to calculate the confident mapping of the phosphosite within the identified peptide (PhosphoRS score ≥ 75) (Taus et al. 2011). The data was also searched against a decoy database to calculate the false discovery rate (FDR). Peptide spectral matches (PSMs) at 1% FDR were used for identification of proteins. TMT quantitation was done using reporter ion intensities. The ratios, invasive neoplastic/non-invasive neoplastic, were obtained as follows – 127C (SNU-308)/126 (TGBC24TKB), 128C (OCUG-1)/126 (TGBC24TKB), 129C (GB-d1)/126 (TGBC24TKB) and 130C (G-415)/126 (TGBC24TKB). Scatter plot was created using the log2 of ratio of both the replicates for each cell line. Pearson’s correlation coefficient (r) was then calculated to evaluate the linear relationship between the two technical replicates for each cell line.
Bioinformatics analysis
The top biological pathways and processes associated with the hyperphosphorylated and overexpressed proteins in this study were identified through the use of Functional Enrichment Analysis tool (FunRich) (http://funrich.org). Proteins identified in this study were classified based on their primary localization, alternate localization, molecule class, molecular function, and biological process using the Gene Ontology (GO) compliant Human Protein Reference Database (HPRD; http://www.hprd.org) (Muthusamy et al. 2013; Goel et al. 2012).
Expression data processing
RNA-Seq data was retrieved from TCGA database. Data preprocessing and normalization steps were performed in R version 3.1.0 using DEseq package from Bioconductor. To adjust for the possible batch and processing effect, we have employed XPN algorithm (R package, CONOR), as previously described (Shabalin et al. 2008). The resulting matrix contained mRNA expression information for over 20 K genes across all analyzed samples. Normalized gene expression data were loaded into iPANDA (Ozerov et al. 2016; Makarev et al. 2017). The software enables calculation of the Pathway Activation Score (PAS) for each of the pathways analyzed, a value which serves as a quantitative measure of differential pathway activation. A collection of intracellular signaling pathways strongly implicated with various solid malignancies was obtained from the NCI Pathway Interaction Database, and used for the computational algorithm as described previously (Ozerov et al. 2016, Makarev et al. 2017).
Accessibility of proteomic data
The mass spectrometry-based raw data obtained in this study has been submitted to ProteomeXchange Consortium via the PRIDE public data repository (Vizcaino et al. 2016) and can be accessed using the data identifier – PXD007946.
Immunohistochemistry
The paraffin blocks of gallbladder adenocarcinoma and cholecystitis cases were obtained from Cancer Hospital and Research Institute, Gwalior, India. The approval from Institutional Human Ethics Committee and informed consent of the patients were obtained. Tissue microarrays (TMAs) were constructed at Lab Surgpath, Mumbai. IHC was carried out on these TMAs as described previously (Subbannayya et al. 2015a). The sections were incubated with anti-PIM1 antibody at 1:100 dilution (Cat#HPA003941, Sigma-Aldrich) and anti-phosphoPRAS40 (Cat#2997, Cell Signaling Technology) antibody at 1:100 dilution overnight at 4 °C in a humidified chamber. The immunohistochemical staining of the tissue sections was assessed by an experienced pathologist. The intensity of staining was scored on a grading scale ranging from 0 to 3+ (0 - negative staining, 1+ − weak staining, 2+ − moderate staining and 3+ − strong staining). The statistical significance of PIM1 and phospho-PRAS40 (T246) expression in gallbladder adenocarcinoma and cholecystitis was determined using two-tailed Chi-square test. Representative images were photographed at 20x magnification.
Western blotting
Whole cell extract of GBC cell lines were prepared using modified RIPA lysis buffer containing protease inhibitors (Roche) and phosphatase inhibitors (Thermo Scientific). Cells were either treated with vehicle control or with PIM1 antagonist, SGI-1776 (Cat#S2198, Selleckchem) (5 μM) or phosphatidylinositol 3 kinase (PI3K) inhibitor, LY294002 (Cat#9901, Cell Signaling Technology) (10 μM) and western blot analysis was carried out using 30 μg protein lysates as described previously (Chang et al. 2011). The following antibodies were obtained from Cell Signaling Technology: anti-PIM1 (Cat#2907), anti-phospho PRAS40 (T246, Cat#2997), anti-PRAS40 (Cat#2691), anti-phospho AKT (S473, Cat#4058), anti-AKT (Cat#9272), anti-FOXO3A (Cat#2497), anti-14-3-3 (pan) (Cat#8312), anti-phospho RPS6 (S240, Cat#2215) and anti-RPS6 (Cat#2217). Anti-phospho FOXO3A (S253, ab154786) antibody was obtained from Abcam. β-actin (Cat#A5316, Sigma-Aldrich) was used as the loading control. ImageJ software was used to obtain densitometry of the resultant bands (Schneider et al. 2012).
Cell viability assays
GBC cells (8 × 103 cells/well) were seeded in a 96-well plate. Cells were either treated with vehicle control or with PIM1 antagonist, SGI-1776 (2.5 μM) for 96 h in complete medium at 37 °C in 5% CO2 incubator. Cell viability was determined every 24 h using MTT (3-(4, 5-dimethylthiazol-2yl)-2, 5-diphenyl tetrazolium bromide) assays as previously described (Chatterjee et al. 2006). All experiments were performed in triplicate. Paired t-test was executed to evaluate the difference between the control and treated groups. P value <0.05 was considered to be significant and p value <0.001 was considered to be highly significant.
Colony formation assays
GBC cell lines (3 × 103 cells/well) were seeded in a 6-well plate. After 24 h, the cells were treated with PIM1 inhibitor, SGI-1776 (2.5 μM). The growth of cell colonies was monitored for up to 14 days. Colonies were fixed and stained with 4% methylene blue (Sigma) in 50% methanol. Counting of colonies formed was carried out in ten fields and representative images were photographed at 2.5x magnification. All experiments were performed in triplicate. Paired t-test was executed to evaluate the difference between the control and treated groups. P value <0.05 was considered to be significant and p value <0.001 was considered to be highly significant.
Cell invasion assays
Cell invasion assays were performed in a transwell system using cell culture inserts as previously described (Subbannayya et al. 2015a). 2x104 GBC cells were used for the assay. Number of cells invaded was counted using a light microscope and representative images were photographed at 4x magnification. All experiments were carried out in triplicate. Paired t-test was executed to evaluate the difference between the control and treated groups. P value <0.05 was considered to be significant and p value <0.001 was considered to be highly significant.
Results
Identification of dysregulated phosphoproteins in GBC cells
We studied the altered signaling events in GBC using a mass spectrometry-based phosphoproteomic approach. Five cell lines (TGBC24TKB, SNU-308, OCUG-1, GB-d1 and G-415) were selected to study GBC cell proteome based on their invasive abilities. TGBC24TKB is a non-invasive cell line (Subbannayya et al. 2015a), whereas SNU-308, OCUG-1, GB-d1 and G-415 have varied invasive abilities ranging from moderate to highly invasive (Fig. 1a) (Subbannayya et al. 2015a). We carried out TMT-based quantitative phosphoproteomic analysis to identify dysregulated phosphoproteins in the invasive GBC cell lines as compared to the non-invasive TGBC24TKBcells (Fig. 1b). On applying false discovery rate (FDR) cut-off of 1% and PhosphoRS probability cut-off of 75%, we identified a total of 2623 unique phosphosites corresponding to 2766 unique phosphopeptides from 1343 proteins (Supplementary Table 2). Among the phosphosites identified, 2290 (87%) were serine phosphorylated sites, 320 (12%) were threonine phosphorylated sites and 13 (0.5%) were tyrosine phosphorylated sites (Fig. 1c). Majority of the peptides were found to be phosphorylated at one site (Fig. 1c). Using a 1.5-fold cut-off, we identified 55 phosphosites to be hyperphosphorylated and 35 phosphosites to be hypophosphorylated in both the replicates and in all 4 invasive GBC cell lines as compared to the non-invasive TGBC24TKB cells. These correspond to 49 hyperphosphorylated and 31 hypophosphorylated proteins, respectively. A partial list of hyperphosphorylated proteins in invasive GBC cell lines is provided in Table 1. A scatter plot (Fig. 1d) depicts the correlation between the two technical replicates of the phosphoproteome data. High correlation between the technical replicates was found in all the GBC cell lines used in the phosphoproteomic experiment (r = 0.85 or higher).
Fig. 1.
Experimental design and proteomic results.a Invasive property of GBC cell lines – TGBC24TKB – non-invasive; SNU-308 – less invasive; OCUG-1 – moderately invasive; GB-d1 – highly invasive; G-415 – highly invasive. b Workflow for TMT-based quantitative phosphoproteomic analysis of GBC cell lines. c Pie chart depicting the distribution of phosphoserine, phosphothreonine and phosphotyrosine containing peptides (left panel); pie chart depicting the distribution of singly and multiple phosphorylated peptides (right panel). d Scatter plot showing correlation between the two MS technical replicates. Pearson’s correlation coefficient (r) was calculated to evaluate the linear relationship between the two technical replicates for each cell line. (r = 0.85 or higher)
Table 1.
Partial list of hyperphosphorylated proteins identified in GBC
| Phospho peptide sequence | Gene symbol | Protein | Phosphosite (Protein) | SNU-308/TGBC24TKB | OCUG-1/TGBC24TKB | GB-d1/TGBC24TKB | G-415/TGBC24TKB | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R1 | R2 | R1 | R2 | R1 | R2 | R1 | R2 | ||||
| LNtSDFQK | AKT1S1 | Proline-rich AKT1 substrate 1 (PRAS40) | T246 | 8.9 | 9.2 | 3.4 | 3.4 | 8.3 | 8.6 | 3.8 | 4.4 |
| AVsMDNSNK | FOXO3 | Forkhead box protein O3 | S253 | – | 4.9 | – | 2.1 | – | 3.4 | – | 1.4 |
| LSSLRAsTSK | RPS6 | 40S ribosomal protein S6 | S240 | 5.8 | 6.7 | 2.3 | 2.7 | 4.5 | 4.8 | 5.9 | 6.3 |
| VADPDHDHTGFLtEpYVATR | MAPK1 | Mitogen-activated protein kinase 1 | T185; Y187 | 1.6 | – | 2.9 | – | 3.4 | – | 3.4 | – |
| ALPNNTSSsPQPK | TP53 | Cellular tumor antigen p53 | S156 | 7.4 | 6.6 | 7.2 | 6.6 | 3.1 | 3.2 | 25.6 | 24.6 |
| HQLFRGFsFVATGLM | RPS6KA1 | Ribosomal protein S6 kinase alpha-1 | S380 | 4.2 | 4.9 | 2.8 | 2.9 | 8.9 | 12.1 | 2.1 | 2.5 |
| RVSIRLPsTSGSEGV | EPHA2 | Ephrin type-A receptor 2 | S897 | 1.7 | 1.9 | 1.8 | 1.8 | 6.7 | 7.8 | 2.7 | 2.7 |
R1 = Technical replicate 1; R2 = Technical replicate 2
The total proteome analysis led to the identification of a total of 2836 proteins, of which, 49 proteins were found to be overexpressed (≥1.5-fold) and 73 were downregulated (≤1.5-fold) in the invasive GBC cell lines when compared to the non-invasive control, TGBC24TKB. The complete list of identified proteins and their corresponding peptides is provided in Supplementary Table 3 and Supplementary Table 4, respectively.
Bioinformatics analysis of the hyperphosphorylated and overexpressed proteins (1.5-fold) identified in the phosphoproteome and total proteome data, respectively, was carried out using FunRich (Pathan et al. 2015). The top biological pathways identified are depicted in Fig. 2a, which includes mTOR signaling and Class I PI3K signaling events mediated by AKT. Cell growth and/or maintenance was among the top biological processes identified (Fig. 2b). The phosphoproteomic data revealed hyperphosphorylation of proteins involved in the mTOR signaling, including PRAS40, FOXO3A and RPS6 (Table 1). PRAS40 was hyperphosphorylated at T246 in both the replicates in all invasive GBC cell lines used in this study. The MS/MS spectrum for PRAS40 has been depicted in Fig. 2c. The total proteome data revealed that the PRAS40 expression remained unchanged in all the invasive GBC cell lines as compared to the non-invasive control, suggesting that phosphorylation is the dominant mechanism of PRAS40 regulation.
Fig. 2.
Bioinformatics analysis of proteomic data and expression pattern of phospho-PRAS40 (T246) and PIM1 in primary tissue. a Graphical representation of the top 10 biological pathways of the hyperphosphorylated and overexpressed proteins identified in this study using FunRich. The columns represent the percentage of genes identified from each pathway. The line graph represents the –log10 of the p value of all the genes in each pathway calculated based on hypergeometric test. b Graphical representation of the top 5 biological processes of the hyperphosphorylated and overexpressed proteins identified in this study using FunRich. The columns represent the percentage of genes identified from each pathway. The line graph represents the –log10 of the p value of all the genes in each pathway calculated based on hypergeometric test. c Representative MS/MS spectra of hyperphosphorylated protein PRAS40 in invasive GBC cell lines SNU-308, OCUG-1, GB-d1 and G-415 and the non-invasive GBC cell line, TGBC24TKB. Inset peaks represent tandem mass tag (TMT) label intensities representing fold change of the p-PRAS40 in all the GBC cell lines analyzed d Validation of phospho-PRAS40 (T246) and PIM1 by IHC in gallbladder adenocarcinoma and cholecystitis. Representative sections from cholecystitis – (i) probed with anti-PIM1 antibody (weak staining); (ii) probed with anti-phospho-PRAS40 (T246) antibody (weak staining). Representative sections from gallbladder adenocarcinoma – (iii) probed with anti-PIM1 antibody (strong staining); (iv) probed with anti-phospho-PRAS40 (T246) antibody (moderate staining). e Box plot showing the differences in mRNA expression levels of PIM1 and mTOR in cholangiocarcinoma and normal specimens from TCGA dataset. (f) iPANDA software suite was used to estimate the level of the indicated signaling pathways in TCGA cholangiocarcinoma dataset. TCGA transcriptomic data from tissue-specific non-cancerous samples was used as a reference after proper normalization. The heat map of indicates differentially activated pathways in all samples analyses. Downregulation is indicated in blue, upregulation is shaded in red
Immunohistochemical validation of PIM1 and phospho-PRAS40 in neoplastic gallbladder tissue
Since PRAS40 has been previously reported to be hyperphosphorylated in multiple solid malignancies (Madhunapantula et al. 2007; Yuan et al. 2015; Lu et al. 2014), we next assessed the expression of phospho-PRAS40 (T246) and its upstream kinase, PIM1 (Zhang et al. 2009) in primary gallbladder adenocarcinoma and cholecystitis tissue using tissue microarray-based immunohistochemical analysis (Fig. 2d). PIM1 staining showed cytoplasmic localization. Forty three percent (13 out of 30) of the gallbladder adenocarcinoma tissues demonstrated moderate to strong staining (2+ to 3+) and 33% (4 out of 12) of the cholecystitis cases showed negative to weak staining (0 to 1+) for PIM1 (Fig. 2d and Table 2). Although Chi-square test indicated that the difference in PIM1 overexpression between gallbladder adenocarcinoma and cholecystitis cases was not significant, none of the cholecystitis cases showed strong staining (3+), suggesting a trend towards higher PIM1expression in tumors. To further assess this suggestion, we have checked the level of PIM1 mRNA expression in cholangiocarcinoma (n = 36) and normal (n = 9) samples available in The Cancer Genome Atlas (TCGA) database. Since the number of gallbladder cancers in TCGA is very limited due to the very rare and uncommon nature of this disease, we have used the cholangiocarcinoma cohort, a group of malignancies of the biliary epithelium that share common etiology, to conduct statistically appropriate analyses. Expression of PIM1 mRNA was significantly higher in tumors compared to the non-cancerous controls (Fig. 2e), supporting the trend seen in our immunohistochemical analysis.
Table 2.
Summary of immunohistochemical validation of phospho-PRAS40 (T246) and PIM1 in GBC
| Staining intensity | Gallbladder adenocarcinoma | Cholecystitis |
|---|---|---|
| PIM1 | ||
| 0–1+ (Negative to weak) | 17 | 4 |
| 2+ − 3+ (Moderate to Strong) | 13 | 8 |
| p value of significance [Chi-square test (two-tailed)] | 0.17 (Statistically not significant) | |
| Subcellular localization of staining | Predominantly cytoplasm | |
| Phospho-PRAS40 (T246) | ||
| 0–1+ (Negative to weak) | 9 | 7 |
| 2+ − 3+ (Moderate to Strong) | 30 | 2 |
| p value of significance [Chi-square test (two-tailed)] | 0.0017 (Statistically significant) | |
| Subcellular localization of staining | Predominantly nucleus | |
In contrast to PIM1, phospho-PRAS40 (T246) showed predominantly nuclear staining. About 77% (30 out of 39) of the gallbladder adenocarcinoma showed moderate to strong staining (2+ to 3+) and 78% (7 out of 9) of the cholecystitis showed negative to weak staining (0 to 1+), whereas none of the cholecystitis showed strong staining (3+) (Fig. 2d and Table 2). A Chi-square test indicated that there was a significant overexpression of phospho-PRAS40 in gallbladder adenocarcinomas compared to the cholecystitis cases (p value = 0.0017), indicating that gallbladder cancer tissue displays strong phospho-PRAS40 expression.
As PRAS40 phosphorylation by PIM1 and AKT is associated with mTOR activation (Chong 2016) and mTOR mRNA expression is significantly upregulated in TCGA cholangiocarcinoma cohort (Fig. 2e), we next used iPANDA, a bioinformatics software suite for qualitative analysis of intracellular signaling pathway activation using transcriptomic data (Ozerov et al. 2016; Makarev et al. 2017), to assess the level of mTOR and AKT pathways activation in TCGA cholangiocarcinoma dataset. The transcriptomic data from tissue-specific non-cancerous samples (n = 9) was used as a reference. mTOR and AKT signaling axes were derived from the Pathway Interaction Database (PID) curated by NCI/Nature. Interestingly, 77% and 97% of cholangiocarcinoma patients were predicted to have upregulated mTOR and AKT signaling pathways, respectively (Fig. 2f). While the correlative analysis of PRAS40 phosphorylation with mTOR and AKT pathway activation status was not possible since the proteomic data is not yet available in TCGA, our results pose PRAS40 phosphorylation as an important event in gallbladder tumorigenesis. Interestingly, although PRAS40 mRNA levels did not differ significantly between the cholangiocarcinoma samples and normal controls (data not shown), further indicating that PRAS40 phosphorylation is most likely required for efficient downstream signaling, the overall patient survival and disease-free status was substantially better in patients with low mRNA level of PRAS40 (Supplementary Fig. 1).
Validation of expression of phospho-PRAS40 and its upstream kinases in GBC cells
PRAS40 is phosphorylated at T246 by two upstream kinases, AKT (Kovacina et al. 2003) and PIM1 (Zhang et al. 2009). We validated the expression of phospho-PRAS40 (T246) and two of its known upstream kinases – PIM1 and AKT1, in non-invasive GBC cell line, TGBC24TKB, and invasive GBC cell lines - SNU-308, OCUG-1, GB-d1, G-415 and TGBC2TKB using western blot analysis. Western blot analysis of phospho-PRAS40 (T246) expression correlated with the mass spectrometry results (Fig. 3a and b), with all invasive cell lines demonstrating higher phospho-PRAS40 levels compared to the non-invasive control. Since PIM1 was not identified in the proteomic data, we evaluated its expression using western blot in GBC cell lines. Both AKT1 (phospho and total) and PIM1 proteins revealed a heterogeneous expression pattern across cell lines analyzed (Fig. 3a and b). However, there was no direct association of phospho-PRAS40 expression with either AKT1 or PIM1levels (Supplementary Fig. 2a and 2b). In addition, the data revealed no direct association of phospho-PRAS40 (T246) expression with the colony forming and invasive ability of the GBC cell lines (Supplementary Fig. 2c and d). Owing to the diverse nature of GBC cell lines, this result does not rule out the role of phospho-PRAS40 (T246) in the GBC carcinogenesis. Hence, we next studied whether inhibition of PRAS40 phosphorylation affects cell survival, colony forming and invasive ability of GBC cell lines.
Fig. 3.
Inhibition of PRAS40 phosphorylation reduces cell proliferation of GBC cells. a Western blot analysis depicting the expression pattern of phospho-PRAS40 (T246), PRAS40, PIM1, phospho-AKT1 (S473) and AKT1 in GBC cell lines. β-actin was used as loading control. b Graphical representation of the densitometry of p-PRAS40 (T246), PIM1 and p-AKT (S473). p-PRAS40 and p-AKT normalized to their total expression levels, PIM1 normalized to β-actin (*p < 0.05; **p < 0.01); AU-Relative abundance. c Cellular proliferation of GBC cells on inhibition with PIM1 inhibitor SGI-1776 (2.5 μM) using DMSO as a control (*p < 0.05; **p < 0.01; ***p < 0.001)
Inhibition of PIM1 reduces cellular proliferation, colony formation and invasive ability of GBC cells via inhibition of PRAS40 phosphorylation
Having observed PRAS40 hyperphosphorylation at T246 in GBC cell lines using mass spectrometry as well as western blot, we evaluated the effect of inhibition of PIM1 and/or AKT onPRAS40 phosphorylation pattern in GBC cell lines – TGBC24TKB, TGBC2TKB, OCUG-1, GB-d1 and G-415. Western blot analysis revealed a significant decrease in phospho-PRAS40 (T246) expression upon treatment with either SGI-1776 (PIM1 inhibitor) or LY294002 (AKT inhibitor) or both (Supplementary Fig. 3). Since previous studies have reported that inhibition of AKT leads to a decreased viability in GBC cells (Leal et al. 2013), we did not evaluate the effect of AKT inhibition on phenotypic characters of our GBC cell panel. Upon treatment with PIM inhibitor SGI-1776, a significant decrease in cell proliferation (p value <0.05) (Fig. 3c), colony forming (p value <0.001) (Fig. 4a and b) and invasive ability (p value <0.001) (Fig. 4c and d) was observed in all GBC cell lines analyzed. These results suggest that inhibition of PRAS40 phosphorylation at T246 might remarkably decrease the tumorigenic ability of GBC cells regardless of their invasive potential.
Fig. 4.

Inhibition of PRAS40 phosphorylation reduces colony forming ability and invasive property of GBC cells. a Colony formation ability of GBC cell lines upon treatment with SGI-1776 (2.5 μM) using DMSO as a control. b Graphical representation of the same. (*p < 0.001). c Invasive property of GBC cell lines upon treatment with SGI-1776 (2.5 μM) using DMSO as a control. d Graphical representation of the same. (p < 0.05; **p < 0.01; ***p < 0.001)
Inhibition of PIM1 modulates mTOR signaling via inhibition of PRAS40 phosphorylation
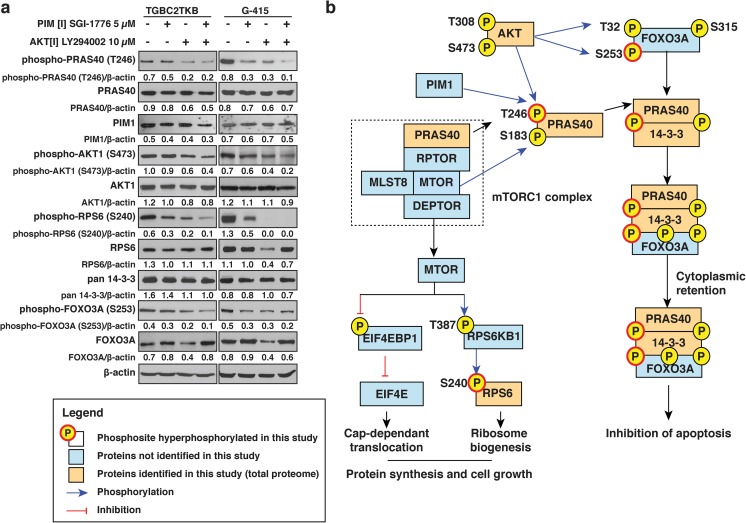
Our results indicate that the inhibition of PIM1 results in decreased phosphorylation of PRAS40 and this in turn may play a role in cell proliferation, invasion and colony forming ability. Phosphorylation of PRAS40 has been shown to relieve its inhibitory control on mTOR signaling (Chong 2016). Studies have reported the activation of mTOR signaling to be associated with tumor progression and poor prognosis in GBC (Cao et al. 2015; Leal et al. 2013). To evaluate the role of PRAS40 in mTOR signaling, we assessed the phosphorylation levels of RPS6, a known mTOR substrate. We observed a decrease in the phosphorylation of RPS6 at S240 in presence of SGI-1776, LY294002 or both in combination (p value <0.05) (Fig. 5a, Supplementary Fig. 4). This indicates that inhibition of PIM1 modulates PRAS40 phosphorylation which in turn results in decreased mTOR signaling.
Fig. 5.
Decrease in PRAS40 phosphorylation leads to a decrease in mTOR signaling and activation of FOXO3A. a GBC cell lines TGBC2TKB and G-415 were treated with PIM1 inhibitor SGI-1776 (5 μM) or PI3K inhibitor LY294002 (10 μM) or both. Western blot analysis of phospho-PRAS40 (T246), total PRAS40, PIM1, phospho-AKT (S473), total AKT, phospho-RPS6 (S240), total RPS6, pan 14–3-3, phospho-FOXO3A (S253) and total FOXO3A. β-actin was used as loading control. b Proposed PRAS40 signaling in GBC
Inhibition of PIM1 leads to activation of FOXO3A in GBC via inhibition of PRAS40 phosphorylation
FOXO3A is a transcriptional factor involved in regulation of cellular processes such as apoptosis. Phosphorylation of FOXO3A at three conserved residues (T32, S253 and S315) promotes its cytoplasmic retention (Brunet et al. 1999). In addition, studies have demonstrated that phosphorylated FOXO3A forms a trimer with 14–3-3 proteins and phosphorylated PRAS40. This also results in cytoplasmic retention of the complex and prevents FOXO3A from transcriptionally activating pro-apoptotic factors (Kim et al. 2011). We evaluated the effect of phospho-PRAS40 (T246) inhibition on the activation of FOXO3A. Western blot analysis revealed a decrease in the expression of phospho-FOXO3A (S253) in presence of either SGI-1776, LY294002 or their combination (p value <0.05) (Fig. 5a, Supplementary Fig. 4). Our results indicate that inhibition of PRAS40 phosphorylation using PIM1 inhibitor leads to loss of phosphorylation of FOXO3A in GBC cell lines.
Discussion
In this study, we employed phosphoproteomic analysis to elucidate the aberrantly activated signaling events associated with tumor progression and aggressiveness in GBC. Quantitative phosphoproteomic analysis of GBC cell lines based on their invasive capabilities led to the identification of 80 proteins that were differentially phosphorylated in invasive GBC cells. Bioinformatics analysis revealed mTOR signaling to be one of the enriched biological pathways in the phosphoproteomic as well as total proteomic data. PRAS40, a regulator of mTOR pathway, was found to be hyperphosphorylated >3-fold at T246 in all 4 invasive GBC cell lines used in this study compared to the non-invasive cell line, TGBC24TKB. Hyperphosphorylation of PRAS40 has been reported in several cancers and has been indicated to play a role in cancer progression and resistance to therapy (Madhunapantula et al. 2007; Kim et al. 2011; Yuan et al. 2015; Lu et al. 2014). PRAS40 is a known substrate of AKT and PIM, which have been shown to phosphorylate PRAS40 at T246 independently. PRAS40 is known to bind to mTORC1 and inhibit mTOR signaling. Phosphorylation of PRAS40 has been demonstrated to relieve its inhibitory control on mTORC1, resulting in the activation of mTOR signaling (Chong 2016). The upstream kinase of PRAS40, PIM1 was not identified in the total proteome data using mass spectrometry. This may be either due to lack of proteotypic peptides for PIM1, incomplete fragmentation of the tryptic peptide or decreased abundance of PIM1 in comparison to the peptides analyzed from other highly abundant proteins. This however does not indicate absence of PIM1 expression in GBC cells and subsequently we found PIM1 to be expressed in GBC cell lines using western blot analysis. PIM1 is a serine threonine kinase and has been reported to play a role in cell survival, proliferation and differentiation (Zhang et al. 2009). It has been implicated in early transformation and tumor progression in several cancers including hepatocellular, prostate and non-small cell lung carcinoma, as well as in hematopoietic malignancies. PIM1 has been found to regulate mTOR activity through phosphorylation of PRAS40 (Zhang et al. 2009). Tissue microarray-based immunohistochemical analyses revealed significant overexpression of phospho-PRAS40 (T246) and a trend towards higher PIM1 expression in gallbladder adenocarcinoma cases compared to its predisposing inflammatory condition, cholecystitis. Supporting these observations, analysis of the signaling pathway activation in a cohort of cholangiocarcinoma tumors from TCGA database predicted that mTOR signaling and AKT pathway were elevated in the majority of cases, and was associated with the higher expression of PIM1 mRNA level in these tumors compared to the non-cancerous controls. Taken together, these findings suggest that phosphorylation of PRAS40 may play a role in orchestrating GBC tumorigenesis.
SGI-1776 is an imidazol [1, 2-b] pyridazine small molecule that inhibits all three PIM kinases (PIM1, PIM2 and PIM3), with higher affinity towards PIM1. Studies have shown that treatment of acute myeloid leukemia and chronic lymphocytic leukemia with SGI-1776 induced apoptosis (Chen et al. 2009, 2011). LY294002 is a highly selective inhibitor for PI3K and has been shown to block PI3K-dependent AKT phosphorylation and kinase activity. We demonstrate that treatment of GBC cells with SGI-1776 and LY294002 resulted in a decreased phosphorylation of PRAS40 at T246. Inhibition of PIM1 also showed a significant decrease in cellular proliferation, colony formation and invasive ability of GBC cells. These findings indicate that targeting PIM1 leads to decreased oncogenic potential of GBC via loss of PRAS40 phosphorylation, suggesting PIM1 as a potential therapeutic target in GBC.
Our phosphoproteomic data as well as western blot analysis revealed increased phosphorylation of the mTOR substrate, RPS6 at S240 in GBC. The role of PI3K/AKT/mTOR signaling in cell survival, activated by hormones and growth factors, has been well established. Activation of mTOR signaling in GBC is well documented (Leal et al. 2013; Cao et al. 2015). Growth factors and hormones activate PIM1 and AKT which, in turn, phosphorylate PRAS40 at T246. Phosphorylated PRAS40 (T246) dissociates from mTORC1 and mTOR, in turn, phosphorylates PRAS40 at S183. Phosphorylation of PRAS40 at T246 and S183 leads to its binding with 14–3-3. mTOR, when activated, is involved in cell survival and growth through activation of its downstream substrates, phospho-RPS6 and EIF4E (Wang et al. 2012). In this study, we show that inhibition of PIM1 leads to decreased phosphorylation of RPS6, indicating a decrease in mTOR signaling.
In our mass spectrometry data, we observed hyperphosphorylation of FOXO3A at S253 in 3 invasive GBC cell lines. Western blot analyses revealed a decrease in phosphorylation of FOXO3A upon inhibition of PIM1, AKT or both in all GBC cells analyzed. FOXO3A belongs to the family of forkhead box O (FOXO) family of transcription factors and is known to regulate targets involved in cell process including cell proliferation, apoptosis, oxidative stress and metabolism (Tzivion et al. 2011). In this study, we show that inhibition of PIM1 leads to a decrease in phosphorylation of FOX3A at S253, indicating its activation and subsequent increase in cell apoptosis.
Taken together, this study along with earlier studies elucidate the role of PIM1-phosphorylated PRAS40 in increased cell survival and growth through the mTOR pathway as well as a decrease in cell apoptosis through the formation of trimer between phospho-PRAS40, 14–3-3 and p-FOXO3A and subsequent inactivation of FOXO3A (Fig. 5b).
In summary, data from this study suggests that PRAS40 phosphorylation via PIM1 may have a role in cell survival through regulation of the mTOR signaling in GBC cells. Although our findings elucidate phospho-PRAS40 as a potential marker in GBC and suggests PIM1 as a potential therapeutic, this study does not rule out the involvement of other factors that may promote GBC cell survival and aggressiveness. Further clinical investigations are warranted to confirm the efficacy of these kinase inhibitors in GBC therapeutics.
Electronic supplementary material
The plots depict overall survival and disease-free status of 36 patients with cholangiocarcinoma as a function of PRAS40 mRNA expression. The plots were visualizing by cBioPortal. (PNG 167 kb)
(a) Scatter plot representing phospho-PRAS40 (T2546) expression versus PIM1 expression in GBC cell lines. (b) Scatter plot representing phospho-PRAS40 (T2546) expression versus AKT expression in GBC cell lines. (c) Scatter plot representing colony forming ability versus phospho-PRAS40 (T246) expression in GBC cell lines. (d) Scatter plot representing invasive ability versus phospho-PRAS40 (T246) expression in GBC cell lines. (PNG 179 kb)
Inhibition of PRAS40 phosphorylation at T246 using inhibitors of its upstream kinases. GBC cell lines TGBC24TKB, TGBC2TKB, OCUG-1, GB-d1 and G-415 were treated with PIM1 inhibitor SGI-1776 (5 μM) or PI3K inhibitor LY294002 (10 μM) or both. Western blot analysis of phospho-PRAS40 (T246), PRAS40, PIM1, phospho-AKT1 (S473) and AKT1 in GBC cell lines. β-actin was used as loading control. (PNG 1091 kb)
Graphical representation of the western blot densitometry analysis for phospho-PRAS40 (T246), total PRAS40, PIM1, phospho-AKT (S473), total AKT, phospho-RPS6 (S240), total RPS6, pan 14–3-3, phospho-FOXO3A (S253) and total FOXO3A in GBC cell lines TGBC2TKB (a) and G-415 (b) treated with PIM1 inhibitor SGI-1776 (5 μM), PI3K inhibitor LY294002 (10 μM) or both. Phospho-PRAS40, phospho-AKT, phospho-RPS6 and phospho-FOXO3A normalized to their total expression levels, PIM1 and 14–3-3 normalized to β-actin (*p < 0.05; **p < 0.01); AU-Relative abundance. (PNG 294 kb)
(PDF 11 kb)
(PDF 932 kb)
(PDF 960 kb)
(PDF 4086 kb)
Acknowledgements
We thank the Department of Biotechnology (DBT), Government of India for research support to the Institute of Bioinformatics. IOB is supported by DBT Program Support on Neuroproteomics and infrastructure for proteomic data analysis (BT/01/COE/08/05). We thank the “Infosys Foundation” for the research support to the Institute of Bioinformatics. This work was supported by the Science and Engineering Research Board, Department of Science and Technology, Government of India grant “miRNAs in chronic tobacco-induced oral cancer (SR/S0/HS-02081/2012)”; NCI’s Clinical Proteomic Tumor Analysis Consortium initiative (U24CA160036) and FAMRI-funded 072017_YCSA. P.K. Tiwari acknowledges research support from the Indian Council of Medical Research (ICMR), MP Council of Science & Technology (MPCST), Bhopal and Department of Science and Technology, Government of India. Harsha Gowda is a Wellcome Trust/DBT India Alliance Early Career Fellow. Juan Carlos Roa acknowledges research support from the National Fund for Scientific and Technological Development (FONDECYT 1170893), and Millennium Institute on immunology and immunotherapy (IMII P09/016-F), Government of Chile. Pamela Leal acknowledges research support from the National Fund for Scientific and Technological Development (FONDECYT 1151008), Government of Chile. Niraj Babu is a recipient of Senior Research Fellowship from the Council for Scientific and Industrial Research (CSIR), Government of India. Remya Raja is a recipient of Research Associateship from Department of Biotechnology, Government of India. Sneha M. Pinto is a recipient of DST INSPIRE Faculty award from Department of Science and Technology, Government of India. We thank Dr. S. K. Shankar of National Institute of Mental Health and Neuro Sciences for providing the use microscope facility.
Abbreviations
- GBC
Gallbladder cancer
- PRAS40
Proline-rich Akt substrate 40 kDa
- TEABC
Triethyl ammonium bicarbonate
- TMT
Tandem mass tag
- IHC
Immunohistochemistry
- TMA
Tissue microarray
- bRPLC
Basic reverse phase liquid chromatography
- PI3K
Phosphatidylinositol 3 kinase
Compliance with ethical standards
Disclosure of interest
The authors report no conflict of interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tejaswini Subbannayya and Pamela Leal-Rojas contributed equally to this work.
Contributor Information
Tejaswini Subbannayya, Email: tejaswini.subbannayya@gmail.com.
Pamela Leal-Rojas, Email: pamela.leal@ufrontera.cl.
Alex Zhavoronkov, Email: alex@insilico.com.
Ivan V. Ozerov, Email: ivan@insilicomedicine.com
Mikhail Korzinkin, Email: mike@insilicomedicine.com.
Niraj Babu, Email: niraj@ibioinformatics.org.
Aneesha Radhakrishnan, Email: aneesha176@gmail.com.
Sandip Chavan, Email: sandip.nc15@gmail.com.
Remya Raja, Email: remya@ibioinformatics.org.
Sneha M. Pinto, Email: sneha@yenepoya.edu.in
Arun H. Patil, Email: arun@ibioinformatics.org
Mustafa A. Barbhuiya, Email: barbhuiyamustafa@gmail.com
Prashant Kumar, Email: prashant@ibioinformatics.org.
Rafael Guerrero-Preston, Email: rafael.guerrerop@gmail.com.
Sanjay Navani, Email: sanjay.hpr@gmail.com.
Pramod K. Tiwari, Email: pk_tiwari@hotmail.com
Rekha Vijay Kumar, Email: rekha_v_kumar@yahoo.co.in.
T. S. Keshava Prasad, Email: keshav@ibioinformatics.org.
Juan Carlos Roa, Email: jcroas@gmail.com.
Akhilesh Pandey, Email: pandey@jhmi.edu.
David Sidransky, Email: dsidrans@jhmi.edu.
Harsha Gowda, Email: harsha@ibioinformatics.org.
Evgeny Izumchenko, Email: eizumch1@jhmi.edu, Email: izumchen@jhmi.edu.
Aditi Chatterjee, Email: aditi@ibioinformatics.org.
References
- Bizama C, Garcia P, Espinoza JA, Weber H, Leal P, Nervi B, Roa JC. Targeting specific molecular pathways holds promise for advanced gallbladder cancer therapy. Cancer Treat Rev. 2015;41:222–234. doi: 10.1016/j.ctrv.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/S0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Cao Y, Liu X, Lu W, Chen Y, Wu X, Li M, Wang XA, Zhang F, Jiang L, Zhang Y, Hu Y, Xiang S, Shu Y, Bao R, Li H, Wu W, Weng H, Yen Y, Liu Y. Fibronectin promotes cell proliferation and invasion through mTOR signaling pathway activation in gallbladder cancer. Cancer Lett. 2015;360:141–150. doi: 10.1016/j.canlet.2015.01.041. [DOI] [PubMed] [Google Scholar]
- Chang X, Ravi R, Pham V, Bedi A, Chatterjee A, Sidransky D. Adenylate kinase 3 sensitizes cells to cigarette smoke condensate vapor induced cisplatin resistance. PLoS One. 2011;6:e20806. doi: 10.1371/journal.pone.0020806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A, Mambo E, Zhang Y, Deweese T, Sidransky D. Targeting of mutant hogg1 in mammalian mitochondria and nucleus: effect on cellular survival upon oxidative stress. BMC Cancer. 2006;6:235. doi: 10.1186/1471-2407-6-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LS, Redkar S, Bearss D, Wierda WG, Gandhi V. Pim kinase inhibitor, SGI-1776, induces apoptosis in chronic lymphocytic leukemia cells. Blood. 2009;114:4150–4157. doi: 10.1182/blood-2009-03-212852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LS, Redkar S, Taverna P, Cortes JE, Gandhi V. Mechanisms of cytotoxicity to Pim kinase inhibitor, SGI-1776, in acute myeloid leukemia. Blood. 2011;118:693–702. doi: 10.1182/blood-2010-12-323022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ. Targeting PRAS40 for multiple diseases. Drug Discov Today. 2016;21:1222–1231. doi: 10.1016/j.drudis.2016.04.005. [DOI] [PubMed] [Google Scholar]
- Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, Lydon NB, Kantarjian H, Capdeville R, Ohno-Jones S, Sawyers CL. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- Goel R, Harsha HC, Pandey A, Prasad TSK. Human protein reference database and human proteinpedia as resources for phosphoproteome analysis. Mol BioSyst. 2012;8:453–463. doi: 10.1039/C1MB05340J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsha HC, Pandey A. Phosphoproteomics in cancer. Mol Oncol. 2010;4:482–495. doi: 10.1016/j.molonc.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsha HC, Jimeno A, Molina H, Mihalas AB, Goggins MG, Hruban RH, Schulick RD, Kamath U, Maitra A, Hidalgo M, Pandey A. Activated epidermal growth factor receptor as a novel target in pancreatic cancer therapy. J Proteome Res. 2008;7:4651–4658. doi: 10.1021/pr800139r. [DOI] [PubMed] [Google Scholar]
- Jordan E, Abou-Alfa GK, Lowery MA. Systemic therapy for biliary cancers. Chin Clin Oncol. 2016;5:65. doi: 10.21037/cco.2016.10.08. [DOI] [PubMed] [Google Scholar]
- Kim W, Youn H, Seong KM, Yang HJ, Yun YJ, Kwon T, Kim YH, Lee JY, Jin YW, Youn B. PIM1-activated PRAS40 regulates radioresistance in non-small cell lung cancer cells through interplay with FOXO3a, 14-3-3 and protein phosphatases. Radiat Res. 2011;176:539–552. doi: 10.1667/RR2609.1. [DOI] [PubMed] [Google Scholar]
- Kim MS, Pinto SM, Getnet D, Nirujogi RS, Manda SS, Chaerkady R, Madugundu AK, Kelkar DS, Isserlin R, Jain S, Thomas JK, Muthusamy B, Leal-Rojas P, Kumar P, Sahasrabuddhe NA, Balakrishnan L, Advani J, George B, Renuse S, Selvan LD, Patil AH, Nanjappa V, Radhakrishnan A, Prasad S, Subbannayya T, Raju R, Kumar M, Sreenivasamurthy SK, Marimuthu A, Sathe GJ, Chavan S, Datta KK, Subbannayya Y, Sahu A, Yelamanchi SD, Jayaram S, Rajagopalan P, Sharma J, Murthy KR, Syed N, Goel R, Khan AA, Ahmad S, Dey G, Mudgal K, Chatterjee A, Huang TC, Zhong J, Wu X, Shaw PG, Freed D, Zahari MS, Mukherjee KK, Shankar S, Mahadevan A, Lam H, Mitchell CJ, Shankar SK, Satishchandra P, Schroeder JT, Sirdeshmukh R, Maitra A, Leach SD, Drake CG, Halushka MK, Prasad TSK, Hruban RH, Kerr CL, Bader GD, Iacobuzio-Donahue CA, Gowda H, Pandey A. A draft map of the human proteome. Nature. 2014;509:575–581. doi: 10.1038/nature13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacina KS, Park GY, Bae SS, Guzzetta AW, Schaefer E, Birnbaum MJ, Roth RA. Identification of a proline-rich Akt substrate as a 14-3-3 binding partner. J Biol Chem. 2003;278:10189–10194. doi: 10.1074/jbc.M210837200. [DOI] [PubMed] [Google Scholar]
- Kris MG, Natale RB, Herbst RS, Lynch TJ, Jr, Prager D, Belani CP, Schiller JH, Kelly K, Spiridonidis H, Sandler A, Albain KS, Cella D, Wolf MK, Averbuch SD, Ochs JJ, Kay AC. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003;290:2149–2158. doi: 10.1001/jama.290.16.2149. [DOI] [PubMed] [Google Scholar]
- Larsen MR, Thingholm TE, Jensen ON, Roepstorff P, Jorgensen TJ. Highly selective enrichment of phosphorylated peptides from peptide mixtures using titanium dioxide microcolumns. Mol Cell Proteomics. 2005;4:873–886. doi: 10.1074/mcp.T500007-MCP200. [DOI] [PubMed] [Google Scholar]
- Lazcano-Ponce EC, Miquel JF, Munoz N, Herrero R, Ferrecio C, Wistuba II, Alonso de Ruiz P, Aristi Urista G, Nervi F. Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin. 2001;51:349–364. doi: 10.3322/canjclin.51.6.349. [DOI] [PubMed] [Google Scholar]
- Leal P, Garcia P, Sandoval A, Buchegger K, Weber H, Tapia O, Roa JC. AKT/mTOR substrate P70S6K is frequently phosphorylated in gallbladder cancer tissue and cell lines. Onco Targets Ther. 2013;6:1373–1384. doi: 10.2147/OTT.S46897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YZ, Deng AM, Li LH, Liu GY, Wu GY. Prognostic role of phospho-PRAS40 (Thr246) expression in gastric cancer. Arch Med Sci. 2014;10:149–153. doi: 10.5114/aoms.2013.36927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- Madhunapantula SV, Sharma A, Robertson GP. PRAS40 deregulates apoptosis in malignant melanoma. Cancer Res. 2007;67:3626–3636. doi: 10.1158/0008-5472.CAN-06-4234. [DOI] [PubMed] [Google Scholar]
- Makarev E, Schubert AD, Kanherkar RR, London N, Teka M, Ozerov I, Lezhnina K, Bedi A, Ravi R, Mehra R, Hoque MO, Sloma I, Gaykalova DA, Csoka AB, Sidransky D, Zhavoronkov A, Izumchenko E. In silico analysis of pathways activation landscape in oral squamous cell carcinoma and oral leukoplakia. Cell Death Discov. 2017;3:17022. doi: 10.1038/cddiscovery.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita S, Onishi H, Nakano K, Nagamatsu I, Imaizumi A, Hattori M, Oda Y, Tanaka M, Katano M. Hedgehog signaling pathway is a potential therapeutic target for gallbladder cancer. Cancer Sci. 2014;105:272–280. doi: 10.1111/cas.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra S, Chaturvedi A, Misra NC. Gallbladder cancer. Curr Treat Options Gastroenterol. 2006;9:95–106. doi: 10.1007/s11938-006-0028-1. [DOI] [PubMed] [Google Scholar]
- Muthusamy B, Thomas JK, Prasad TSK, Pandey A (2013) Access guide to human proteinpedia. Curr Protoc Bioinformatics Chapter 1:Unit 1 21 [DOI] [PMC free article] [PubMed]
- Nagarajha Selvan LD, Kaviyil JE, Nirujogi RS, Muthusamy B, Puttamallesh VN, Subbannayya T, Syed N, Radhakrishnan A, Kelkar DS, Ahmad S, Pinto SM, Kumar P, Madugundu AK, Nair B, Chatterjee A, Pandey A, Ravikumar R, Gowda H, Prasad TSK. Proteogenomic analysis of pathogenic yeast Cryptococcus neoformans using high resolution mass spectrometry. Clin Proteomics. 2014;11:5. doi: 10.1186/1559-0275-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozerov IV, Lezhnina KV, Izumchenko E, Artemov AV, Medintsev S, Vanhaelen Q, Aliper A, Vijg J, Osipov AN, Labat I, West MD, Buzdin A, Cantor CR, Nikolsky Y, Borisov N, Irincheeva I, Khokhlovich E, Sidransky D, Camargo ML, Zhavoronkov A. In silico pathway activation network decomposition analysis (iPANDA) as a method for biomarker development. Nat Commun. 2016;7:13427. doi: 10.1038/ncomms13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathan M, Keerthikumar S, Ang CS, Gangoda L, Quek CY, Williamson NA, Mouradov D, Sieber OM, Simpson RJ, Salim A, Bacic A, Hill AF, Stroud DA, Ryan MT, Agbinya JI, Mariadason JM, Burgess AW, Mathivanan S. FunRich: an open access standalone functional enrichment and interaction network analysis tool. Proteomics. 2015;15:2597–2601. doi: 10.1002/pmic.201400515. [DOI] [PubMed] [Google Scholar]
- Rabindran SK, Discafani CM, Rosfjord EC, Baxter M, Floyd MB, Golas J, Hallett WA, Johnson BD, Nilakantan R, Overbeek E, Reich MF, Shen R, Shi X, Tsou HR, Wang YF, Wissner A. Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res. 2004;64:3958–3965. doi: 10.1158/0008-5472.CAN-03-2868. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan A, Nanjappa V, Raja R, Sathe G, Puttamallesh VN, Jain AP, Pinto SM, Balaji SA, Chavan S, Sahasrabuddhe NA, Mathur PP, Kumar MM, Prasad TSK, Santosh V, Sukumar G, Califano JA, Rangarajan A, Sidransky D, Pandey A, Gowda H, Chatterjee A. A dual specificity kinase, DYRK1A, as a potential therapeutic target for head and neck squamous cell carcinoma. Sci Rep. 2016;6:36132. doi: 10.1038/srep36132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabalin AA, Tjelmeland H, Fan C, Perou CM, Nobel AB. Merging two gene-expression studies via cross-platform normalization. Bioinformatics. 2008;24:1154–1160. doi: 10.1093/bioinformatics/btn083. [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Subbannayya T, Leal-Rojas P, Barbhuiya MA, Raja R, Renuse S, Sathe G, Pinto SM, Syed N, Nanjappa V, Patil AH, Garcia P, Sahasrabuddhe NA, Nair B, Guerrero-Preston R, Navani S, Tiwari PK, Santosh V, Sidransky D, Prasad TSK, Gowda H, Roa JC, Pandey A, Chatterjee A. Macrophage migration inhibitory factor - a therapeutic target in gallbladder cancer. BMC Cancer. 2015;15:843. doi: 10.1186/s12885-015-1855-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbannayya Y, Syed N, Barbhuiya MA, Raja R, Marimuthu A, Sahasrabuddhe N, Pinto SM, Manda SS, Renuse S, Manju HC, Zameer MA, Sharma J, Brait M, Srikumar K, Roa JC, Vijaya Kumar M, Kumar KV, Prasad TSK, Ramaswamy G, Kumar RV, Pandey A, Gowda H, Chatterjee A. Calcium calmodulin dependent kinase kinase 2 - a novel therapeutic target for gastric adenocarcinoma. Cancer Biol Ther. 2015;16:336–345. doi: 10.4161/15384047.2014.972264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taus T, Kocher T, Pichler P, Paschke C, Schmidt A, Henrich C, Mechtler K. Universal and confident phosphorylation site localization using phosphoRS. J Proteome Res. 2011;10:5354–5362. doi: 10.1021/pr200611n. [DOI] [PubMed] [Google Scholar]
- Tzivion G, Dobson M, Ramakrishnan G. FoxO transcription factors; regulation by AKT and 14-3-3 proteins. Biochim Biophys Acta. 2011;1813:1938–1945. doi: 10.1016/j.bbamcr.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Vizcaino JA, Csordas A, del-Toro N, Dianes JA, Griss J, Lavidas I, Mayer G, Perez-Riverol Y, Reisinger F, Ternent T, Xu QW, Wang R, Hermjakob H. 2016 update of the PRIDE database and its related tools. Nucleic Acids Res. 2016;44:D447–D456. doi: 10.1093/nar/gkv1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhang Q, Wen Q, Zheng Y, Lazarovici P, Jiang H, Lin J, Zheng W. Proline-rich Akt substrate of 40kDa (PRAS40): a novel downstream target of PI3k/Akt signaling pathway. Cell Signal. 2012;24:17–24. doi: 10.1016/j.cellsig.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Wu P, Nielsen TE, Clausen MH. FDA-approved small-molecule kinase inhibitors. Trends Pharmacol Sci. 2015;36:422–439. doi: 10.1016/j.tips.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Yuan K, Wu H, Wang Y, Chen H, Jiao M, Fu R. Phospho-PRAS40Thr246 predicts trastuzumab response in patients with HER2-positive metastatic breast cancer. Oncol Lett. 2015;9:785–789. doi: 10.3892/ol.2014.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Beharry ZM, Harris TE, Lilly MB, Smith CD, Mahajan S, Kraft AS. PIM1 protein kinase regulates PRAS40 phosphorylation and mTOR activity in FDCP1 cells. Cancer Biol Ther. 2009;8:846–853. doi: 10.4161/cbt.8.9.8210. [DOI] [PubMed] [Google Scholar]
- Zhang LQ, Zhang XD, Xu J, Wan Y, Qu K, Zhang JY, Wang ZX, Wei JC, Meng FD, Tai MH, Zhou L, Liu C. Potential therapeutic targets for the primary gallbladder carcinoma: estrogen receptors. Asian Pac J Cancer Prev. 2013;14:2185–2190. doi: 10.7314/APJCP.2013.14.4.2185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The plots depict overall survival and disease-free status of 36 patients with cholangiocarcinoma as a function of PRAS40 mRNA expression. The plots were visualizing by cBioPortal. (PNG 167 kb)
(a) Scatter plot representing phospho-PRAS40 (T2546) expression versus PIM1 expression in GBC cell lines. (b) Scatter plot representing phospho-PRAS40 (T2546) expression versus AKT expression in GBC cell lines. (c) Scatter plot representing colony forming ability versus phospho-PRAS40 (T246) expression in GBC cell lines. (d) Scatter plot representing invasive ability versus phospho-PRAS40 (T246) expression in GBC cell lines. (PNG 179 kb)
Inhibition of PRAS40 phosphorylation at T246 using inhibitors of its upstream kinases. GBC cell lines TGBC24TKB, TGBC2TKB, OCUG-1, GB-d1 and G-415 were treated with PIM1 inhibitor SGI-1776 (5 μM) or PI3K inhibitor LY294002 (10 μM) or both. Western blot analysis of phospho-PRAS40 (T246), PRAS40, PIM1, phospho-AKT1 (S473) and AKT1 in GBC cell lines. β-actin was used as loading control. (PNG 1091 kb)
Graphical representation of the western blot densitometry analysis for phospho-PRAS40 (T246), total PRAS40, PIM1, phospho-AKT (S473), total AKT, phospho-RPS6 (S240), total RPS6, pan 14–3-3, phospho-FOXO3A (S253) and total FOXO3A in GBC cell lines TGBC2TKB (a) and G-415 (b) treated with PIM1 inhibitor SGI-1776 (5 μM), PI3K inhibitor LY294002 (10 μM) or both. Phospho-PRAS40, phospho-AKT, phospho-RPS6 and phospho-FOXO3A normalized to their total expression levels, PIM1 and 14–3-3 normalized to β-actin (*p < 0.05; **p < 0.01); AU-Relative abundance. (PNG 294 kb)
(PDF 11 kb)
(PDF 932 kb)
(PDF 960 kb)
(PDF 4086 kb)