Abstract
Menisci are a pair of crescent-shaped fibrocartilages, particularly of which their inner region of meniscus is an avascular tissue. It has characteristics similar to those of articular cartilage, and hence is inferior in healing. We previously reported that low-intensity pulsed ultrasound (LIPUS) treatment stimulates the production of CCN2/CTGF, a protein involved in repairing articular cartilage, and the gene expression of major cartilage matrices such as type II collagen and aggrecan in cultured chondrocytes. Therefore, in this present study, we investigated whether LIPUS has also favorable effect on meniscus cells and tissues. LIPUS applied with a 60 mW/cm2 intensity for 20 min stimulated the gene expression and protein production of CCN2 via ERK and p38 signaling pathways, as well as gene expression of SOX9, aggrecan, and collagen type II in human inner meniscus cells in culture, and slightly stimulated the gene expression of CCN2 and promoted the migration in human outer meniscus cells in culture. LIPUS also induced the expression of Ccn2, Sox9, Col2a1, and Vegf in rat intact meniscus. Furthermore, histological evaluations showed that LIPUS treatment for 1 to 4 weeks promoted healing of rat injured lateral meniscus, as evidenced by better and earlier angiogenesis and extracellular matrix synthesis. The data presented indicate that LIPUS treatment might prevent meniscus from degenerative change and exert a reparative effect on injured meniscus via up-regulation of repairing factors such as CCN2 and that it might thus be useful for treatment of an injured meniscus as a non-invasive therapy.
Keywords: CCN2/CTGF, Low-intensity pulsed ultrasound (LIPUS), Meniscal healing, Meniscus regeneration, Cell migration, ERK, p38
Introduction
Menisci are a pair of crescent-shaped fibrocartilages (Messner and Gao 1998) composed primarily of type I collagen (Tanaka et al. 1999). In the human meniscus, the perimeniscal capillary plexus supplies the outer 10–25% of the meniscus with nutrients and oxygen. On the other hand, inner region is an avascular tissue and hence inferior in healing (Arnoczky and Warren 1982). Outer cells have an oval, fusiform shape and are similar in appearance and behavior to fibroblasts. In contrast, inner cells appear more rounded, are embedded in an extracellular matrix (ECM) comprised largely of type II collagen (Makris et al. 2011), and maintain a stronger chondrogenic phenotype than do the outer cells (Furumatsu et al. 2011). These properties mean that the inner region of the meniscus has characteristics similar to those of articular cartilage.
Menisci have an important role in protecting the knee joint from degenerative change because they provide shock absorption to the joint during walking and assist in overall lubrication of the articular surfaces (Voloshin and Wosk 1983). A meniscal tear is one of the strong risk factors for knee osteoarthritis (OA). Although surgeons make an effort to repair such a tear, a systematic review of the literature demonstrated that the outcome of meniscal repair at greater than 5 years postoperatively showed very similar rates of meniscal failure (22.3% to 24.3%) for all techniques except for all-inside technique (Nepple et al. 2012). Recently, biological augmentative treatment with fibrin clots and platelet-rich plasma (PRP), which include multiple growth factors such as platelet-derived growth factor (PDGF), transforming growth factor-beta (TGF-β), and CCN2/CTGF is performed to improve meniscal healing, particularly in the inner avascular region (Kubota et al. 2004; Griffin et al. 2015; Chahla et al. 2017). However, meniscal repairs with these biological therapies do not always have great outcomes (Kamimura and Kimura 2014; Griffin et al. 2015). Therefore, additional treatment options that improve meniscal healing are required for not only patients undergoing surgical repair but also for those treated with non-operative and non-invasive therapies.
The CCN family of proteins is one of cysteine-rich secreted proteins consisting of 6 members, namely, CCN1/CYR61, CCN2/connective tissue growth factor (CTGF), CCN3/NOV, CCN4-CCN6/WISP1-WISP3 and has a multifunctional role in cellular proliferation, differentiation, ECM synthesis, and tissue regeneration (Takigawa 2013). Among these members, CCN2/CTGF has been the one most extensively investigated. Earlier we demonstrated that CCN2 promotes the proliferation and differentiation of growth-plate chondrocytes and osteoblasts, as well as the proliferation and migration of fibroblasts and vascular endothelial cells (Takigawa 2013). These cellular functions of CCN2 except for cell proliferation are mostly due to its stimulatory effect on gene expression and production of cell type-specific ECM. For example, CCN2 stimulates the gene expression and protein production of aggrecan and collagen type II and type X in growth-plate chondrocytes; whereas it stimulates the gene expression of only aggrecan and collagen type II but not that of collagen type X in articular cartilage cells (Takigawa 2003, 2013, 2018). It also regenerates articular cartilage via increase in gene expression of cartilage-specific components, such as aggrecan and collagen type II, in an osteoarthritis model (Kubota and Takigawa 2011). Tang X et al. recently reported that CCN2 has an important role in joint homeostasis and OA severity by controlling the matrix sequestration and activation of latent TGF-β (Tang et al. 2018). CCN3/NOV has the highest degree of homology to CCN2, but had been believed until now to have functions opposite to those of CCN2 in various biological processes (Perbal 2004; Perbal and Takigawa 2005; Kawaki et al. 2008; Janune et al. 2011, 2017). In fact, CCN3 represses cell proliferation and extracellular matrix production in various types of cells (Kubota and Takigawa 2013). However, we recently found that CCN3 directs the differentiation of epiphyseal chondrocytes toward the articular chondrocyte phenotype (Janune et al. 2011) and protects articular cartilage by promoting an increase in the production of aggrecan and in the gene expression of collagen type II, which are 2 major common markers of various cartilages, and also increases the expression of tenascin-C and lubricin, which are articular cartilage-specific markers (Janune et al. 2017).
We also previously reported that mechanical stretching epigenetically stimulates CCN2 transcription via TGF-β1 release associated with Smad2/3 activation and enhances COL2A1 expression through complex formation between Sry-type high-mobility-group box (SOX) 9 and Smad2/3 in chondrocytic cells (Furumatsu et al. 2013). Furthermore, CCN2 production is detected in human meniscus cells; and CCN2 expression in human meniscus inner cells is significantly induced by mechanical stretching (Furumatsu et al. 2012).
Low-intensity pulsed ultrasound (LIPUS) is an acoustic pressure wave capable of providing localized mechanical stimulus to cells and is commonly used to promote the healing of fractures (Hadjiargyrou et al. 1998). Although the mechanism underlying the repair process is still unclear, LIPUS has been reported to induce chondrocyte proliferation and matrix production (Cheng et al. 2014). Recently, a systematic review and network meta-analysis revealed that LIPUS also has a positive effect on both pain relief and functional improvement in the management of knee OA (Zeng et al. 2014). A past study demonstrated that LIPUS not only reduces the expression of catabolic markers in chondrocytes but also increases chondrocyte migration and proliferation (Uddin et al. 2016). Furthermore, we earlier showed that the expression of chondrocyte differentiation markers and CCN2 production in chondrocytes are increased by LIPUS treatment (Nishida et al. 2017).
From these findings, we hypothesized that LIPUS treatment should promote meniscal healing by inducing cartilage-repairing factors such as CCN2. As far as we know, there is no study that has evaluated the effect of LIPUS on meniscus cells and tissues. Therefore, the purpose of this study was to investigate the effect of LIPUS on meniscus cells and tissues.
Materials and methods
Meniscus cell cultures
Institutional Review Board approval and informed consent were obtained before performing all experimental studies. Human meniscus cells were obtained from human knee joints as described previously (Furumatsu and Ozaki 2017). Macroscopically, intact lateral menisci were obtained at total knee arthroplasty in patients suffering from medial-affected OA. The patients were 64 and 80 years of age. Inner and outer meniscus cells were prepared from inner and outer halves of the meniscus, respectively (Furumatsu et al. 2011). Attached cells (passage 0) were maintained in Dulbecco’s modified Eagle’s medium (DMEM, Wako, Osaka, Japan) containing 10% fetal bovine serum (FBS; HyClone, South Logan, UT) and 1% penicillin/streptomycin (Sigma, St. Louis, MO) at 37 °C with 5% CO2. Meniscus cells were used at the second passage.
Animals and surgery
Male SD rats (Japan SLC, Inc., Shizuoka, Japan) at 7–12 weeks of age were used for these experiments. All animal care and experimentation were conducted in accordance with the institutional guidelines of the Animal Center of Okayama University.
Each 7-week-old rat was anesthetized with isoflurane, after which the same surgery was performed on both knees. The knee placed in full flexion and the knee joint were exposed with a 2-cm straight skin incision. The anterolateral capsule was opened from the patellar joint to the lateral collateral ligament. The surgeon checked the anterior portion of the lateral meniscus (LM) by direct visual confirmation and then cut it at the anterior one-third with a No. 11 scalpel to make a radial tear. After the surgery, the joint surface was washed with sterile saline, and the skin was sutured using 5–0 nylon sutures. The rats were allowed to walk freely in their cages following recovery from the anesthesia.
LIPUS treatment
LIPUS stimulations were applied with an ST-SONIC from ITO Corporation Ltd. (Saitama, Japan). In vitro, meniscus cells were cultured in 35-mm dishes. When the cells reached the appropriate density for each experiment, they were exposed to LIPUS from the bottom of the culture dish for 20 min under the following conditions: 60 mW/cm2, frequency of 3 MHz with a 4.1-cm2 transducer repeated at 100 Hz, as described previously (Nishida et al. 2017). These cells were subsequently maintained in a humidified atmosphere of 5% CO2 in air at 37 °C during the incubation time indicated in each experiment.
For determination of mRNA levels in meniscus tissue in vivo, rat intact right knees were exposed to LIPUS at a frequency of 1.5 MHz and an intensity of 60 mW/cm2 with a 0.8-cm2 transducer repeated at 100 Hz for 20 min under anesthesia with isoflurane. After 4 h, the rats were sacrificed; and their menisci were then separated from bilateral knees. Menisci obtained from left knees were used as controls.
For observation of the in vivo reparative effect of LIPUS on the injured menisci, LIPUS treatment for 20 min per day was performed on only the right knee in each rat under anesthesia with isoflurane. The treatment was given for 1, 2 or 4 weeks from 7 days post-operatively (LIPUS knee) at 60 mW/cm2 and a frequency of 1.5 MHz with a 0.8-cm2 transducer repeated at 100 Hz. The left knee received no treatment (Control knee). Rats were sacrificed at 2, 3, and 5 weeks after the primary surgery (n = 7–8).
Migration scratch assay
Migration assays were performed by use of the scratch assay, as described earlier (Liang et al. 2007). Briefly, meniscus cells (2.5 × 105 cells/dish) were seeded into 35-mm dishes to approximately 90% confluence and allowed to adhere overnight in DMEM containing 10% FBS at 37 °C with 5% CO2. The next day, 90% confluent cultures of cells in the LIPUS group were exposed to LIPUS stimulation under the conditions described above. The cells were submerged in serum-free DMEM with calcein AM (Dojindo, Kumamoto, Japan) for a 30-min incubation. After having been washed with phosphate-buffered saline (PBS), the stained cells were incubated in a serum free DMEM at 37 °C with 5% CO2 for another 2 h in order to remove the extracellular dye. Then, 3 parallel scratches were made on the monolayer of cells by using a 200-μl pipette tip; and the cells were washed with PBS twice and incubated with DMEM containing 10% FBS at 37 °C with 5% CO2. Subsequently, migration was monitored microscopically at time 0 and 24 h. Five fields per dish were randomly examined, and the number of cells that had advanced into the cell-free space were counted at time 24 h. We excluded the fields with highest and lowest number of migrating cells.
Proliferation assay
Meniscus cells (3 × 104 cells/dish) were seeded in 35-mm dishes (on day 0) and allowed to adhere overnight with DMEM containing 10% FBS at 37 °C with 5% CO2. From the next day when the cells had reached 30% confluence, the cultures in the LIPUS group were exposed to LIPUS stimulation at a frequency of 3 MHz and an intensity of 60 mW/cm2 for 20 min at time 24 h or at time 24 and 48 h. Cells in the control group received no treatment. Cell growth was analyzed at time 0, 24 and 48 h with a WST-1 kit (Roche, Basel, Switzerland). The level of orange formazan was quantified by using an ELISA reader at 450 nm, with a reference wavelength at 690 nm.
Western blot analysis
Western blot analysis was carried out essentially as described previously (Aoyama et al. 2012; Kawaki et al. 2017). Briefly, cell lysates were prepared from cultured human meniscus cells with cell lysis buffer and subjected to SDS-polyacrylamide gel electrophoresis (PAGE). Proteins separated by SDS-PAGE were transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad, Hercules, CA) by using a semi-dry blotting apparatus (Bio-Rad). Transferred proteins were detected with anti-CCN2/CTGF antibody (Abcam, Cambridge, UK) and anti-β-actin (Sigma, St. Louis, MO). The amount of CCN2 was determined densitometrically and these amounts were normalized to those of β-actin.
Preparation of total RNA
In vitro, meniscus cells were cultured in 35-mm dishes (3 wells per group) and exposed to LIPUS under the conditions described above. After 20 min of stimulation, mRNA was collected from the cells by using an RNeasy Mini Kit (Qiagen, Hilden, Germany).
In vivo, total RNA was isolated from the menisci of rat knees by using ISOGEN (Nippon Gene, Toyama, Japan) and an RNeasy Mini Kit.
Quantitative real-time PCR
cDNA was synthesized from the isolated total RNA by using a PrimeScript™ RT Reagent Kit (Perfect Real Time; Takara Bio Inc., Shiga, Japan). SYBR® Green Realtime PCR Master Mix (Toyobo Co. Ltd., Osaka, Japan) was used to perform real-time PCR with a StepOnePlus™ Real-Time PCR System (Applied Biosystems, Carlsbad, CA). The nucleotide sequences of the primers are shown in Table 1.
Table 1.
Primer sequences used for real-time PCR
| Gene | Primer sequence | |
|---|---|---|
| Human | ||
| CCN2 | Forward | 5’-GCAGGCTAGAGAAGCAGAGC-3′ |
| Reverse | 5’-ATGTCTTCATGCTGGTGCAG-3′ | |
| CCN3 | Forward | 5’-AGCATGCAGAGTGTGCAGAG-3’ |
| Reverse | 5’-GGTGTGCCACTTACCTGTCC-3’ | |
| SOX9 | Forward | 5’-CAACCAGAATTCCCTTTGGA-3’ |
| Reverse | 5’-TGCTCCATTTAGCCAAGGTT-3’ | |
| ACAN | Forward | 5’-TTCGGGCAGAAGAAGGAC-3’ |
| Reverse | 5’-CGTGAGCTCCGCTTCTGT-3’ | |
| COL1A1 | Forward | 5’-TATGGCGGCCAGGGCTCCGACCCTG-3’ |
| Reverse | 5’-CCAAGGGGGCCACATCGATGATGG G-3’ | |
| COL2A1 | Forward | 5’-GAGGGCAATAGCAGGTTCACGTA-3’ |
| Reverse | 5’-TGGGTGCAATGTCAATGATGG-3’ | |
| GAPDH | Forward | 5’-GCCAAAAGGGTCATCATCTC-3’ |
| Reverse | 5’-GTCTTCTGGGTGGCAGTGAT-3’ | |
| Rat | ||
| Ccn2 | Forward | 5’-CCACCCGAGTTACCAATGAC-3’ |
| Reverse | 5’-GTGCAGCCAGAAAGCTCA-3’ | |
| Ccn3 | Forward | 5’-CTGGACCTCATCAGCATTT-3’ |
| Reverse | 5’-TCAAGGGCCAGCAGTTCA-3’ | |
| Sox9 | Forward | 5’-AGACCAGTACCCGCATCT-3’ |
| Reverse | 5’-CGCTCCGCCTCCTCCAC-3’ | |
| Acan | Forward | 5’-TTGGAGCCGGAGACGACAGA-3’ |
| Reverse | 5’-AGAGGCAGAGGGACTTTCGGT-3’ | |
| Col1a2 | Forward | 5’-CCGTGCTTCTCAGAACATCA-3’ |
| Reverse | 5’-CTTGCCCCATTCATTTGTCT-3’ | |
| Col2a1 | Forward | 5’-TTCCTCCGTCTACTGTCCACTGA-3’ |
| Reverse | 5’-CTACATCATTGGAGCCCTGGAT-3’ | |
| Vegf-a | Forward | 5’-TTCAGAGCGGAGAAAGCATT-3’ |
| Reverse | 5’-GAGGAGGCTCCTTCCTGC-3’ | |
| Gapdh | Forward | 5’-GTCTTCACTACCATGGAGAAGG-3’ |
| Reverse | 5’-TCATGGATGACCTTGGCCAG-3’ | |
Inhibition of MAPK signaling pathways
PD98059 (MEK1 inhibitor) and SB203580 (p38 MAPK inhibitor) were purchased from Merck Millipore (Darmstadt, Germany). After human meniscus cells had reached confluence, the cells were pre-treated with DMSO, PD98059 (50 μM) or SB203580 (10 μM). After 1 h, the cells were treated with LIPUS under the same conditions as described above. Cell lysates were prepared 6 h later, and Western blot analysis was performed as described above.
Gene silencing of CCN2 by siRNA transfection
To knockdown the expression of CCN2, we employed an RNA-mediated interference method. A small interfering RNA (siRNA) mixture containing 3 distinct siRNAs directed against human CCN2 (sc-39329) and non-targeting control siRNA (sc-37007) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Human meniscus inner cells were seeded into 35 mm dish at a density of 1.5 × 105/dish (at 80–90% of confluence) and were transfected with 10 nM of siRNA after 24 h using Lipofectamine® RNAiMAX Regent (Thermo Fisher Scientific, Carlsbad, CA). Twenty-four hours after transfection, LIPUS treatment was performed. Total cellular RNA was harvested 40 min after LIPUS treatment and evaluated for the expression of CCN2, SOX9 and ACAN.
Macroscopic observation
The tibial plateau with menisci was carefully separated from the femoral condyle. Macroscopic pictures were taken with a COOLPIX S9500 (Nikon, Tokyo, Japan).
Histological examination
Rats’ menisci were fixed in 95% ethanol solution with 4% H2O and 1% acetic acid for several days, and then embedded in paraffin wax. The specimens were sectioned in the axial plane at 6 μm and stained with safranin-O. Histological sections were visualized by using an Olympus BX 53 microscope (Olympus, Tokyo, Japan). The repaired meniscus was evaluated by using the quantitative score based on Pauli’s histological score (Ozeki et al. 2015).
Statistical analysis
All experiments were repeated twice or more. The expression of the examined genes and proteins in meniscus cells in response to LIPUS was similar across donors, and representative data from the 2 patients are presented in the figures. Data were expressed as mean ± standard deviation (SD). Mean values were compared by Welch’s t test or one-way analysis of variance, and post hoc comparisons were performed with the Tukey-Kramer test to compare the in vitro expression levels and CCN2 productions between groups. The effect of LIPUS on human meniscus cells’ proliferation and migration was evaluated by using Welch’s t test. The Wilcoxon signed-rank test was used to compare the in vivo expression levels and Pauli’s histological scores between both knees. Significance was set at p < 0.05.
Results
Effect of LIPUS treatment on gene expression of CCN2 and CCN3 and cartilage markers in human meniscus cells
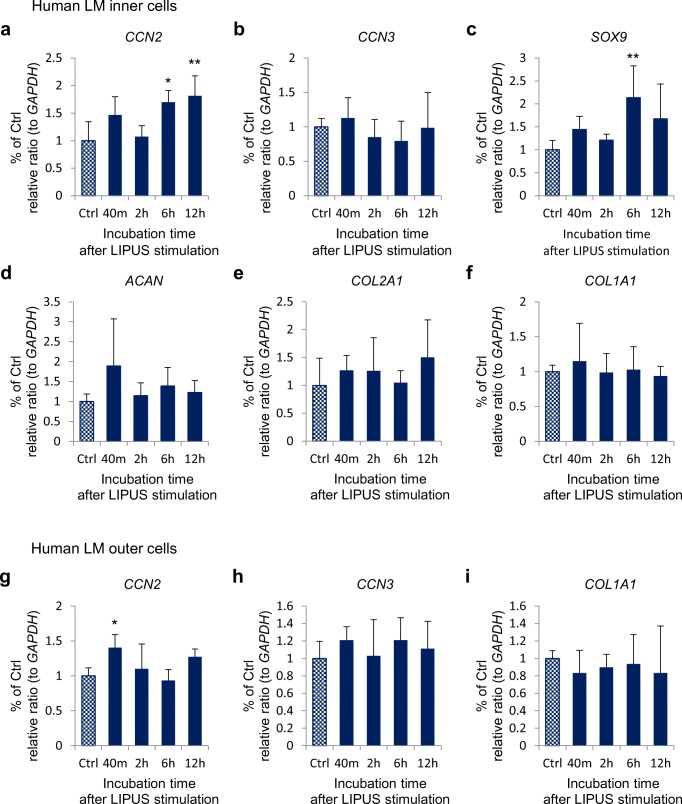
In order to understand the effect of LIPUS on human meniscus cells, we analyzed the time courses of gene expression of CCN2 and CCN3 and cartilage markers by performing quantitative real-time PCR on total RNA isolated from human meniscus cells incubated for 40 min or 2, 6 or 12 h after LIPUS stimulation. In cultured inner meniscus cells, CCN2 expression was induced 40 min after LIPUS stimulation; although there was no significant difference. Furthermore, CCN2 expression was significantly up-regulated 6 and 12 h after LIPUS stimulation to a level approximately 2 fold higher than that of the control, as shown in Fig. 1a. SOX9 expression also significantly increased 6 h after LIPUS stimulation to a level approximately 2 fold higher than its control value (Fig. 1c). The expression level of CCN3, which is involved in the differentiation of epiphyseal chondroblasts toward articular cartilage cells (Janune et al. 2011), did not change after the LIPUS treatment (Fig. 1b). However, expression levels of ACAN and COL2A1, which are major matrix components in cartilage, tended to increase at all time points examined after the LIPUS treatment (Fig. 1d, e). COL1A1 expression was not significantly increased after LIPUS treatment (Fig. 1f).
Fig. 1.
Effect of LIPUS on gene expression of CCNs and cartilage markers in cultured human meniscus cells. Human meniscus cells were treated with LIPUS at a frequency of 3 MHz and an intensity of 60 mW/cm2 for 20 min and then harvested at the times indicated after the LIPUS treatment to extract total RNA. The mRNA levels of (a) CCN2, (b) CCN3, (c) SOX9, (d) ACAN, (e) COL2A1, and (f) COL1A1 in inner cells and (g) CCN2, (h) CCN3, (i) COL1A1 in outer cells were analyzed by use of quantitative RT-PCR. The amounts of these mRNAs were normalized to the amount of GAPDH mRNA. In the all graphs, the ordinate indicates the relative ratio to the Ctrl (ratio = 1.0), and columns and bars represent mean and standard deviation, respectively. The expression of CCN2 in both types of cells (a, g) and SOX9 in inner cells (g) was significantly increased after LIPUS treatment. *p < 0.05 vs. Ctrl. ** p < 0.01 vs. Ctrl (one-way ANOVA, Tukey-Kramer).; n = 6
In cultured outer cells, CCN2 expression showed a significant increase after 40 min from LIPUS stimulation (Fig. 1g). The expression level of CCN3 was slightly elevated at all time points after LIPUS treatment, although the increases were are not statistically significant (Fig. 1h). COL1A1 expression was not significantly increased by LIPUS stimulation (Fig. 1i).
Effect of LIPUS treatment on protein level of CCN2 in human meniscus cells
To confirm whether LIPUS-induced expression of CCN2 gene was followed by CCN2 protein production, we next investigated the level of CCN2 protein in both inner and outer cells by performing Western blot analysis. As shown in Fig. 2, the CCN2 protein level dramatically increased in the inner cells after LIPUS stimulation, whereas the protein level tended to increase in the outer cells.
Fig. 2.
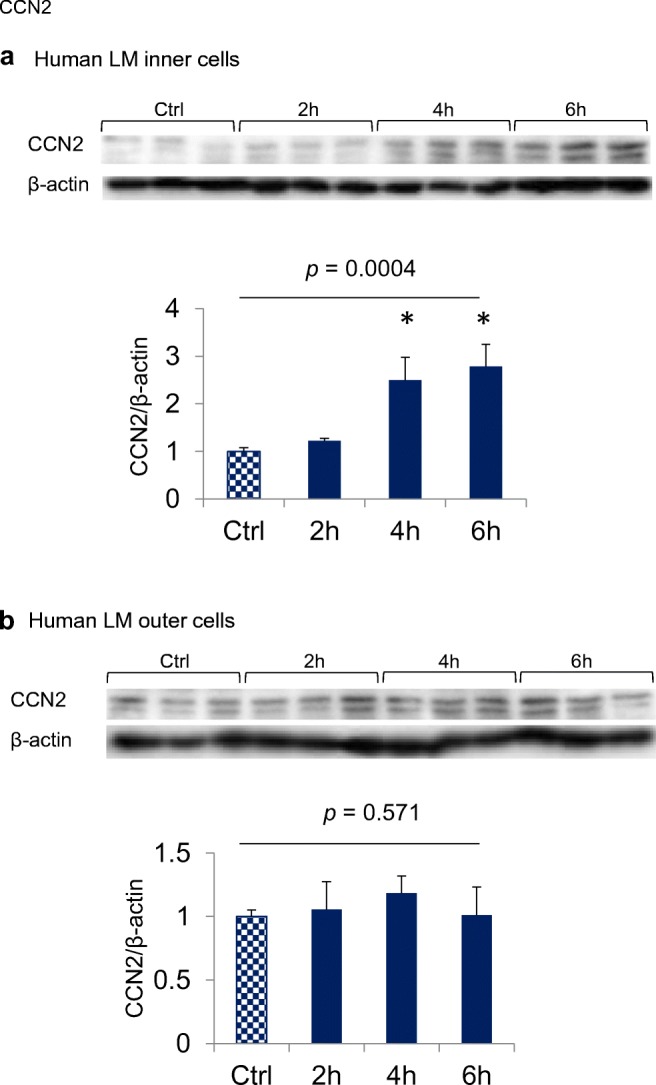
Effect of LIPUS on the level of CCN2 protein. Confluent cultures of inner meniscus cells (a) and outer meniscus cells (b) were treated with LIPUS at a frequency of 3 MHz and an intensity of 60 mW/cm2 for 20 min and harvested at the times indicated. Cell lysates were subjected to Western blotting under the conditions described in “Materials and Methods.” The amount of CCN2 was determined densitometrically and normalized to the amount of β-actin. Relative densitometry values (Ctrl = 1.0) are presented below photographs, and columns and bars represent mean and standard deviation, respectively. CCN2 production in inner cells showed a significant increase at 4 and 6 h following LIPUS treatment, and that in outer cells showed a slight increase after 4 h. * p < 0.01 vs. Ctrl (one-way ANOVA, Tukey-Kramer).; n = 3
Effect of MAPK inhibitors on LIPUS-stimulated CCN2 production in human meniscus cells
A previous report demonstrated that MAPKs pathway in gingival cells is activated via phosphorylated p38 and ERK by LIPUS treatment (Shiraishi et al. 2011). Recently, it was also revealed that LIPUS treatment induced CCN2 production via the activation of MAPKs pathway in cultured chondrocytes (Nishida et al. 2017). Therefore, we investigated whether or not the effect of LIPUS on the production of CCN2 would be affected by PD98059 and SB203580, a specific inhibitor of the MEK1 and the p38, respectively. After human meniscus cells had reached confluence, these cells were treated with LIPUS at a frequency of 3.0 MHz and an intensity of 60 mW/cm2 for 20 min. As shown in Fig. 3, LIPUS-induced CCN2 production was decreased by pre-treatment with PD98059 and SB203580. These results suggest that the production of CCN2 was promoted by LIPUS treatment through ERK and p38 pathways.
Fig. 3.
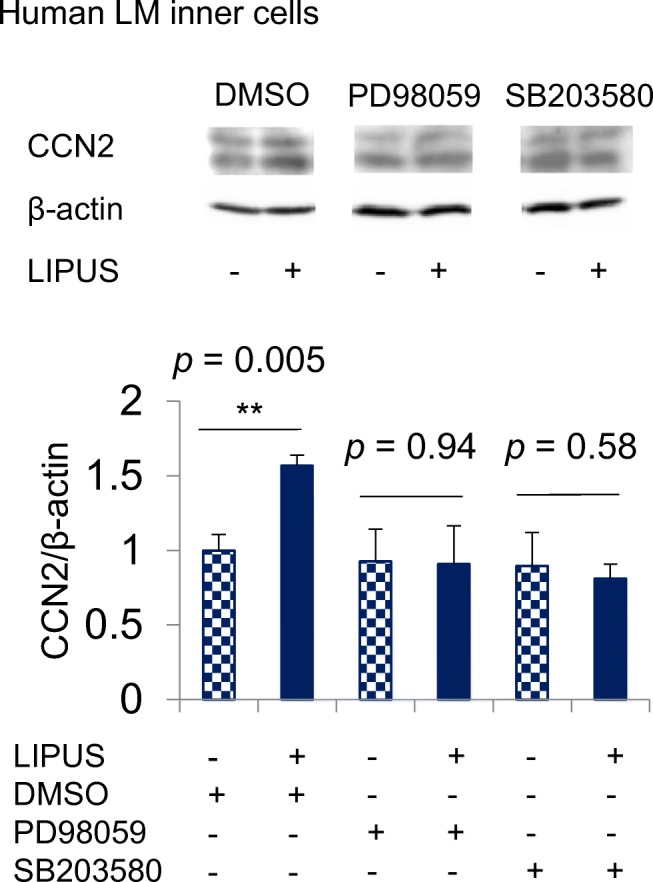
Effect of MEK1 and p38 MAPK inhibitors on LIPUS-induced CCN2 production. After human meniscus inner cells had reached confluence, the cells were pre-treated with PD98059 (MEK1 inhibitor; 50 μM) or SB203580 (p38 MAPK inhibitor; 10 μM). After 1 h, the cells were treated with LIPUS at a frequency of 3.0 MHz and an intensity of 60 mW/cm2 for 20 min. Cell lysates were prepared 6 h later, and Western blot analysis was performed with anti-CCN2 and β-actin antibodies. The amount of CCN2 was determined densitometrically and these amounts were normalized to those of β-actin. Relative densitometry (DMSO without LIPUS = 1.0) from three measurements are presented below photographs. LIPUS treatment significantly induced CCN2-production in pre-treated with DMSO (p = 0.005, Welch’s t test). However, no significant induction was observed in pre-treatment groups with PD98059 and SB203580 (p > 0.05). ** p < 0.01; n = 3
Effects of siRNA-mediated knockdown of CCN2 gene on the induction of chondrogenic marker genes by LIPUS in human meniscus cells
In order to further confirm the involvement of CCN2 in the regulation of chondrogenic marker genes by LIPUS, we applied an siRNA mixture against CCN2 to human meniscus cells. An siRNA with non-targeting scramble sequence was used as a negative control. Quantitative real-time RT-PCR analysis was employed to determine the relative mRNA levels of the chondrogenic marker genes and CCN2 (Fig. 4). Approximately 90% inhibition of CCN2 expression was achieved. Consequently, CCN2 knockdown significantly reduced LIPUS effect on SOX9 and ACAN expressions in meniscus inner cells (Fig. 4).
Fig. 4.

Effect of LIPUS on gene expression of cartilage markers in CCN2 knocked-down meniscus cells. Human meniscus inner cells were seeded into 35 mm dish at a density of 1.5 × 105/dish (at 80–90% of confluence) and were transfected with 10 nM of siRNA against CCN2 or siRNA with non-targeting scramble sequence as a negative control after 24 h. Twenty-four hours after transfection, LIPUS treatment was performed. Total cellular RNA was harvested 40 min after LIPUS treatment and evaluated for the expression of CCN2, SOX9 and ACAN using quantitative real-time RT-PCR analysis. Approximately 90% inhibition of CCN2 expression was achieved. Induced expressions of SOX9 and ACAN by LIPUS treatment were suppressed in CCN2 knocked-down human meniscus inner cells (p > 0.05, Welch’s t test). ** p < 0.01; n = 3
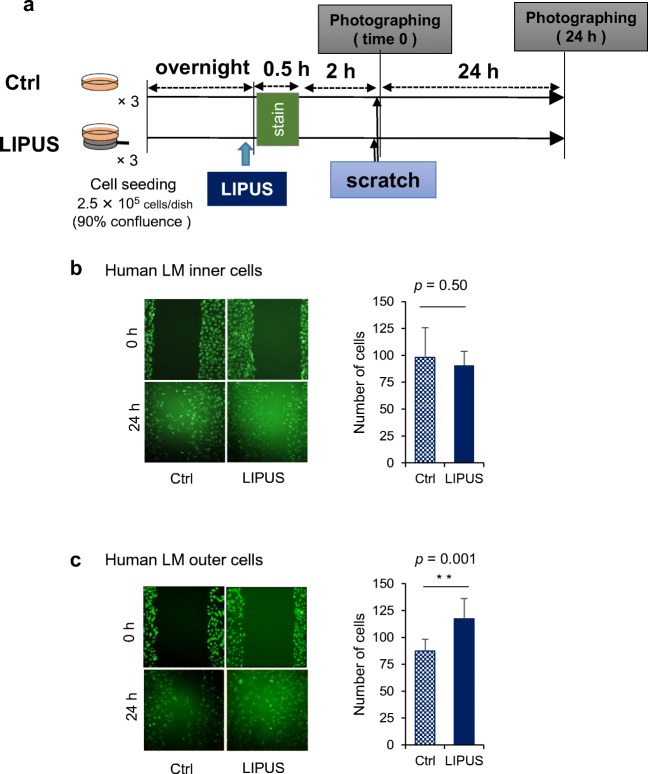
Effect of LIPUS on migration of human meniscus cells
Because CCN2 has been reported to promote the migration of various types of cells such as vascular endothelial cells and fibroblasts (Takigawa 2003; Perbal and Takigawa 2005), we next investigated whether or not LIPUS treatment would stimulate the migration of meniscus cells. In cultured inner cells, there was no significant difference between the control group and the LIPUS group (p = 0.49). However, LIPUS treatment significantly promoted the migration of the outer cells in culture (p = 0.001; Fig. 5).
Fig. 5.
Effect of LIPUS on migration of human meniscus cells. (a) Inner and outer meniscus cells (2.5 × 105 cells/dish) were seeded in 35-mm dishes and allowed to adhere overnight. On the next day when the cells had reached 90% confluence, the cells were stained with calcein AM and submerged in the medium for 30-min, and then 3 parallel scratches were made on the monolayer of cells by using a 200-μl pipette tip. Then, the cells were exposed to LIPUS stimulation at a frequency of 3 MHz and an intensity of 60 mW/cm2 as described in “Materials and Methods”. Migration was monitored microscopically at time 0 and 24 h (left photographs). (b, c) Five sections per dish were randomly selected, and the number of cells that had advanced into the cell-free space was counted at time 24 h (right graphs). In inner cells, there was no significant difference between the Ctrl group and the LIPUS group (p = 0.50, Welch’s t test). In outer cells, however, there was a significant difference between the 2 groups (p = 0.001). ** p < 0.01; n = 9
Effect of LIPUS on proliferation of human meniscus cells
Although it is well known that CCN2 is involved in ECM production, proliferation, and migration of various types of cells, its effect is differs depending on the cell type (Takigawa 2003; Perbal and Takigawa 2005). Therefore, we next investigated whether or not LIPUS would stimulate the proliferation of meniscus cells in culture. As a result, even when the inner cells were exposed to the 20-min LIPUS stimulation once on day 1 or on day 1 and day 2, twice in total, their proliferation rate was not altered as compared with that of the control (Fig. 6).
Fig. 6.
Effect of LIPUS on the proliferation of human meniscus cells. (a) Inner and outer meniscus cells (3 × 104 cells/dish) were seeded in 35-mm dishes and allowed to adhere overnight in DMEM containing 10% FBS at 37 °C with 5% CO2. The next day, when the cells had reached 30% confluence, cells in the LIPUS group were exposed to LIPUS stimulation at a frequency of 3 MHz and an intensity of 60 mW/cm2 for 20 min at time 0 h or at time 0 and 24 h. Cells in the Ctrl group received no treatment. Cell growth was analyzed at time 0, 24, and 48 h with a WST-1 kit. The level of orange formazan was quantified by using an ELISA Reader at 450 nm, with a reference wavelength at 690 nm. (b, c) In the graphs, dots represent the mean. There was no significant difference between the Ctrl group and the LIPUS group (p > 0.05, Welch’s t test).; n = 3
In the case of the outer cells, LIPUS had no effect on their proliferation, either (Fig. 6).
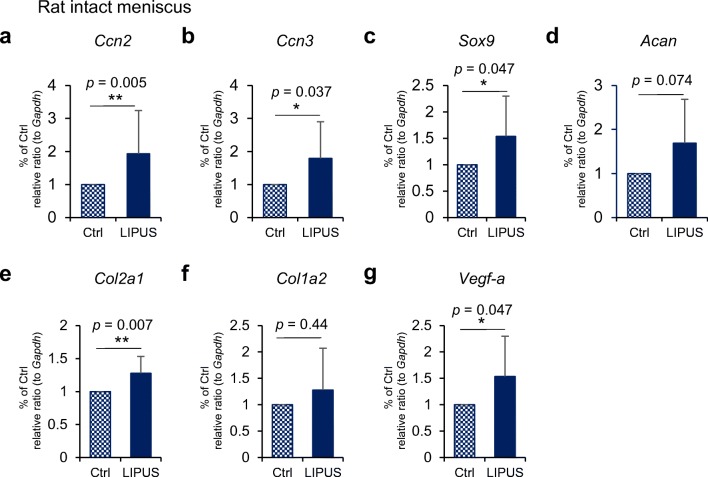
Effect of LIPUS on gene expression of CCN2 and CCN3 and cartilage markers in rats’ intact menisci
Because LIPUS treatment exhibited an anabolic effect on cultured meniscus cells, we next investigated whether a similar anabolic effect could be observed in the in vivo rat knee joints. In the intact menisci, which included both inner and outer cells, LIPUS significantly induced the expression of 2 CCN genes, Ccn2 and Ccn3, and that of Sox9, a marker of chondrogenesis (Fig. 7). LIPUS also increased the expression of one of 2 major cartilage matrix component genes, Col2a1 and tended to increase the other matrix component gene Acan (Fig. 7d, e). On the contrary, there was a non-significant increase in Col1a2 expression in the in vivo meniscus treated by LIPUS (Fig. 7f). Because the meniscus contains blood vessels, we also investigated the effect of LIPUS on the expression of Vegf-a and found a significant increase in response to LIPUS (Fig. 7g).
Fig. 7.
Effect of LIPUS on gene expression of CCNs and cartilage markers in rat intact meniscus. Rat intact right knees were exposed to LIPUS at a frequency of 1.5 MHz and an intensity of 60 mW/cm2 for 20 min under anesthesia with isoflurane. Total RNA was isolated as described in “Materials and Methods.” The mRNA levels of Ccn2 (a), Ccn3 (b), Sox9 (c), Acan (d), Col2a1 (e), Col1a2 (f), and Vegf-a (g) were analyzed by using quantitative RT-PCR. The amounts of these mRNAs were normalized to the amount of Gapdh mRNA. In the all graphs, the ordinate indicates the relative ratio to the Ctrl (ratio = 1.0), and columns and bars represent mean and standard deviation, respectively. Ccn2, Ccn3, Sox9, Col2a1, and Vegf-a expression levels significantly increased after LIPUS treatment. *p < 0.05 vs. Ctrl. ** p < 0.01 vs. Ctrl (Wilcoxon signed-rank test).; n = 10
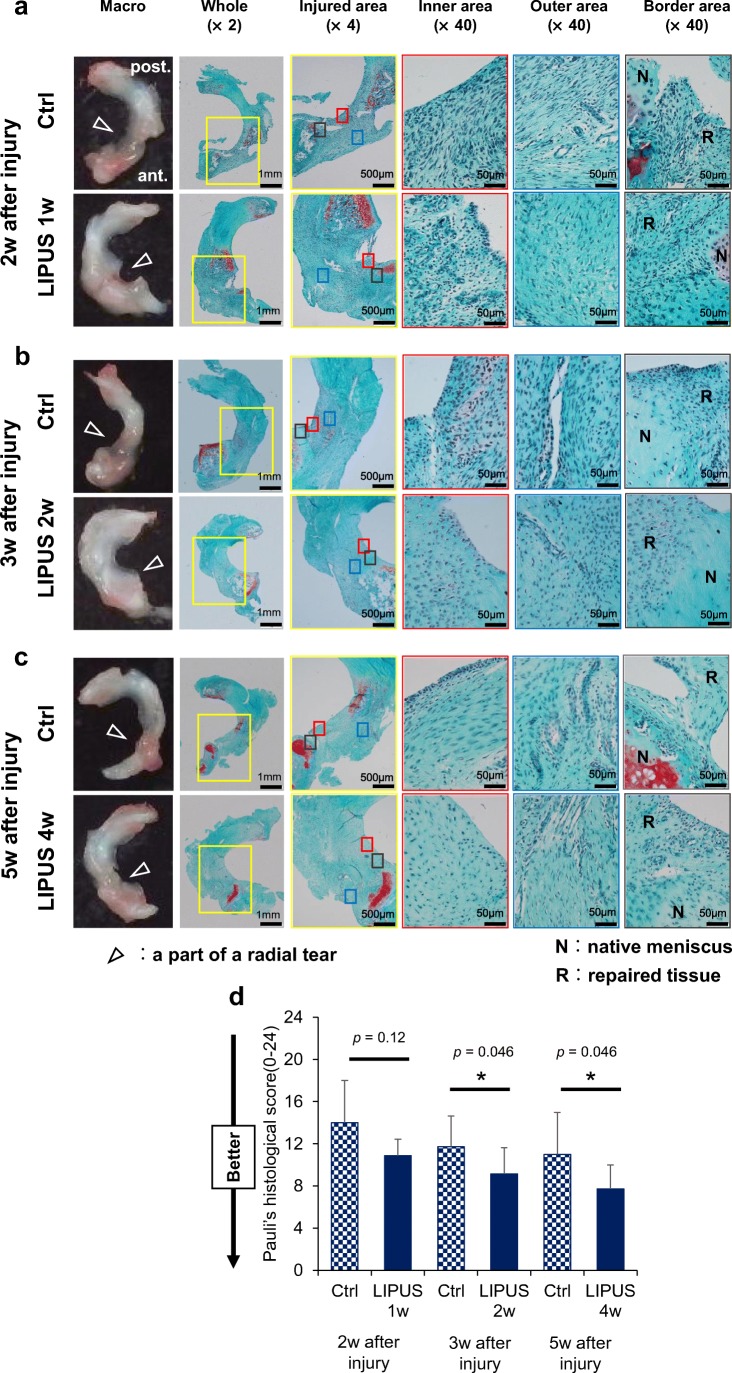
Effect of LIPUS treatment on healing of rat lateral meniscus radial tears
The anabolic effect of LIPUS on cultured meniscus cells and on the meniscus in vivo let us speculate that LIPUS may be useful as a new therapeutics approach for repairing a meniscus injury. To test this possibility, we made surgical tears in the lateral menisci of rat bilateral knee joints and investigated the effect of LIPUS on meniscal healing. Macroscopic examination revealed that the radial tears partially healed in both the LIPUS group and control group after 2, 3, and 5 weeks from surgical injury; although the LIPUS group showed better healing than the control group. Macroscopic connection between both side tissues of tear (closure of tear) was observed in all rats in LIPUS treated group after at all time points after injury; whereas that in the control group after 2, 3, and 5 weeks from injury was 87.5% (7/8), 85.7% (6/7) and 71.4% (5/7), respectively.
Histologically, in the inner area of the LIPUS group, angiogenesis was better at 2 weeks after surgical injury; and extracellular matrix synthesis was much better at 3 and 5 weeks than in that of the control group. The inner edge of the repaired tissue of the LIPUS group at 5 weeks was very smooth. The morphology of the inner cells in the repaired tissue was distinct from that of the meniscal inner chondrocytic cells in the normal meniscus. In the LIPUS group at 5 weeks, however, we could find some round-shaped cells in the inner region. In the control group, the native meniscus and the repaired tissue formed a connection, but the border was still identifiable completely at 2 weeks and partially at 3 and 5 weeks. On the other hand, in the LIPUS group, the border between the native meniscus and the repaired tissue was detectable at 2 and 3 weeks, but it became unclear at 5 weeks. Histological scores of the LIPUS group were significantly better than those of control group at 3 and 5 weeks after the surgical injury (Fig. 8).
Fig. 8.
Effect of LIPUS on healing of rat lateral meniscus radial tears. A surgical procedure was performed on a rat’s bilateral knees to make a radial tear in the lateral meniscus, as described in “Materials and Methods.” Rat right knees were exposed to LIPUS every day starting 1 week later at a frequency of 1.5 MHz and an intensity of 60 mW/cm2 for 20 min/day under anesthesia with isoflurane. Rats were sacrificed at 2, 3, and 5 weeks after the primary surgery. (a, b, c) Macroscopic inspections and histological analysis of lateral menisci at 2, 3, and 5 weeks after the surgical procedure (injury) for the LIPUS group and Ctrl group. Sections were stained with Safranin-O. The left panels in each time period show the macroscopic findings on the whole lateral meniscus. White arrows indicate points of radial tear in the anterior segment of the lateral menisci. The yellow square shows the injured area (×4). The red and blue squares indicate the inner and outer areas, respectively, of the repaired tissue. The gray square shows the border area between the native meniscus and the repaired tissue. Meniscal healings in the LIPUS group were earlier and better than those in the Ctrl group at any time examined. (d) Specimens at 2, 3, and 5 weeks after the injury were quantitatively scored based on Pauli’s histological score for repaired meniscus (Ozeki et al. 2015). The lateral menisci treated with LIPUS for 2 and 4 weeks healed significantly better than those with no treatment. *p < 0.05 vs. Ctrl (Wilcoxon signed-rank test).; n = 7–8
Discussion
In this study, we revealed that LIPUS treatment (60 mW/cm2) promoted CCN2 gene expression in human inner and outer meniscus cells in culture and that up-regulation of the mRNAs by LIPUS in inner cells was followed by the production of CCN2 protein via ERK and p38 signaling pathways (Figs. 1, 2 and 3). The LIPUS treatment also promoted CCN2 gene expression in rat intact meniscus tissues, suggesting increased protein production in vivo (Fig. 7). In clinical practice, LIPUS for the treatment of bone fractures is normally set at a fixed intensity of 30 mW/cm2 with a frequency of 1.5 MHz. However, many laboratory investigations in basic research have used various LIPUS intensities, such as 30, 45 or 60 mW/cm2, and demonstrated the healing effect of LIPUS on bone fractures and chondrocytes. Our latest study showed that the amount of CCN2 protein in human chondrocytic cell line HCS-2/8 was increased in an intensity-dependent manner by LIPUS, reaching a maximum at a frequency of 3 MHz and intensity of 60 mW/cm2 with a 20-min exposure (Nishida et al. 2017). Therefore, we selected this condition presently because meniscus tissues, in particular their inner region, have characteristics similar to those of articular cartilage.
Because LIPUS treatment promoted the gene expression of CCN2 in human chondrocytic cells and mouse primary chondrocytes (Nishida et al. 2017), the difference between the inner and outer cells in terms of the stimulatory effect on CCN2 expression at 6–12 h after LIPUS treatment might be explained by their different characteristics; i.e., the inner meniscus cells have more chondrocytic properties than the outer ones. Therefore we considered that the effect might have been amplified in the inner cells, which are similar to chondrocytes.
In addition to the gene expression of CCN2, that of aggrecan and type II collagen in mouse chondrocytes in culture is enhanced by LIPUS, but the LIPUS-enhanced gene expression of aggrecan and type II collagen is not observed in Ccn2-deficient chondrocytes, indicating that CCN2 expression increased by LIPUS resulted in the increased gene expression of aggrecan and type II collagen in chondrocytes (Nishida et al. 2017). As was shown in Fig. 1d and e, LIPUS also increased the gene expression of aggrecan and type II collagen in cultured inner cells at all times examined after exposure; but these increases were not significant. However, in the in vivo experiments, LIPUS increased the gene expression of aggrecan and collagen type II; but only the increase in that of type II collagen was significant. These subtle stimulatory effects of LIPUS on chondrocyte matrix formation by meniscus cells, which were less than those on chondrocytes, may have been due to the characteristics of inner meniscus cells, which are similar to, but not the same as, those of articular cartilage cells.
LIPUS treatment had no effect on the gene expression of CCN3 in either inner or outer meniscus cells in vitro (Fig. 1), suggesting that CCN3 did not contribute to the trend of a LIPUS-induced increase in the gene expression of aggrecan and type II collagen in the inner meniscus cells. Since CCN3 inhibits the gene expression of aggrecan and collagen type II in growth-plate chondrocytes (Kawaki et al. 2008), but stimulates that in articular chondrocytes (Janune et al. 2017), it is feasible that CCN3 would have no effect on other types of cells such as meniscus cells. However, LIPUS treatment did up-regulate the gene expression of CCN3 in the intact meniscus in vivo (Fig. 7). Considering that LIPUS had no effect on CCN3 in cultured meniscus cells, the LIPUS treatment might have stimulated CCN3 expression in other types of cells in the meniscus. Because there are only a few reports on CCN3 induction by mechanical stress including that by LIPUS, it would be very interesting to investigate what type(s) of cells responds to LIPUS; and so further investigation is needed to clarify this point.
SOX9 is the master chondrogenic transcription factor and is essential for chondrocyte differentiation and cartilage formation (Bi et al. 1999). It is expressed in the processes of mesenchymal condensation and early-to-mid-stage chondrocyte differentiation and is, therefore, an earlier marker of chondrogenesis than aggrecan and type II collagen. As was shown in Fig.1c and Fig. 7c, LIPUS treatment increased SOX9 expression in human inner meniscus cells in culture and in rat meniscus tissues more significantly than the expression of aggrecan and collagen type II, suggesting that LIPUS may more effective on earlier markers of chondrogenesis in inner meniscus cells. In this regard, we previously reported that cyclic tensile strain, another mechanical stress, increases complex formation between phosphorylated Smad2/3 and SOX9 and enhances CCN2 promoter activity (Furumatsu et al. 2013). Also, Oh et al. (Oh et al. 2016) reported that SOX9 directly regulates CCN2 transcription in growth-plate chondrocytes and in nucleus pulposus cells of intervertebral discs. These findings suggest that CCN2 is located downstream of SOX9, and so it is feasible that LIPUS might be more effective on earlier chondrogenesis markers than on aggrecan and type II collagen, at least in meniscus inner cells. However, CCN2 knockdown reduced LIPUS effect on not only ACAN but also SOX9 expressions in meniscus inner cells (Fig. 4). This finding indicates that CCN2 mediates this regulation at least in part. In any case, further investigation on the lineage of meniscus cells is still needed.
Recently, we reported that MAPK signaling pathways play an important role in the CCN2 induction by LIPUS treatment in human cultured chondrocytes (Nishida et al. 2017). Similarly, LIPUS stimulation induced CCN2 production via the activations of ERK and p38 MAPK in human meniscus inner cells (Fig. 3). This finding is consistent with the previous reports with other types of the cells (Shiraishi et al. 2011; Ren et al. 2013; Sato et al. 2014). Therefore, LIPUS stimulation activates MAPK signaling pathways in various cells.
LIPUS treatment had no significant effect on the proliferation of either human inner or outer cells in culture (Fig. 6). However, the treatment did promote the migration of human meniscus outer cells, but had no effect on human meniscus inner cells, in culture (Fig. 5). These findings suggest that the LIPUS treatment promoted chondrogenic differentiation causing cell aggregation and ECM production of inner meniscus cells, while the same treatment stimulated the development of a fibroblast-like phenotype or undifferentiated mesenchymal cell phenotype by the outer meniscus cells, because those cells migrated easily while the mature chondrocytes rather tended to aggregate by producing much aggrecan and collagens. In other words, LIPUS treatment would promote the respective characteristics of both types of meniscus cells. In this regard, it is noteworthy that LIPUS stimulates the migration of both periodontal ligament stem cells (Wang et al. 2018) and undifferentiated chondro-progenitor cells (Jang et al. 2014). It is also noteworthy that LIPUS treatment enhances the gene expression of VEGF, which is an angiogenesis factor (Carmeliet 2003) to promote migration of endothelial cells in rat meniscus tissues, of which their outer region is rich in blood vessels.
The radially torn rat lateral meniscus that had undergone LIPUS treatment for 7, 14 or 28 days tended to heal earlier and better than the one with no treatment (Fig. 8). In the intact rat menisci, LIPUS significantly induced the gene expression of Ccn2, Ccn3, Sox9, Col2a1, and Vegf-a and also increased that of Acan and Col1a2; although the their increase was not significant (Fig. 7). As described earlier, CCN2 is a cartilage-repairing factor and acts as a regenerative factor for various tissues such as articular cartilage (Nishida et al. 2004; Abd El Kader et al. 2014), bone (Kikuchi et al. 2008), and blood vessels (Shimo et al. 1999; Oka et al. 2007). Sox9 is a master transcription factor for directing mesenchymal stem cells (MSCs) toward chondrocytic differentiation (Augello and De Bari 2010). CCN3 is involved in the differentiation of epiphyseal chondroblasts to articular chondrocytes (Janune et al. 2011) and in the maintenance of the articular cartilage phenotype (Janune et al. 2017). Aggrecan and collagen type II are 2 major matrix proteins in cartilage, thus making them 2 major markers of cartilage. Therefore, increases in expression of these molecules may be involved in healing of the inner part of the meniscus (Fig. 9). Collagen type I is a fibroblast marker (Takigawa 2003), and VEGF is an angiogenesis factor (Shibuya 2001). Therefore, up-regulation of these 2 genes may contribute to healing of the outer part of the meniscus (Fig. 9).
Fig. 9.

Summary of LIPUS’s effect on meniscal healing. When LIPUS treatment is performed on an injured meniscus, LIPUS would induce cartilage-repairing factors such as CCN2 and chondrogenesis factors such as SOX9 to promote ECM synthesis via MAPKs pathways in the meniscus inner region, as well as promote the migration of meniscus cells and angiogenesis in the meniscus outer region. LIPUS-induced CCN2 production would also promote SOX9 expression and ECM synthesis in the meniscus inner region. All these phenomena would happen in parallel, to repair the defect of meniscus, resulting in promoted meniscal healing
In the process of wound healing, cells around the wound firstly migrate into the defect. Promoted migration of meniscus cells, especially outer cells, by LIPUS would be important for acceleration of the healing process of the injured meniscus. However, several studies on synovial MSCs revealed that these cells also participate in regeneration of a defective meniscus (Hatsushika et al. 2014; Nakagawa et al. 2015; Ozeki et al. 2015). In the present study, whole rat right knees were exposed to LIPUS, therefore LIPUS might have had an effect on MSCs from synovial and adipose tissues or other cells around the knee joint. In any case, LIPUS treatment had a favorable effect on the injured meniscus, allowing it to heal earlier and better. Further studies are needed to uncover the detailed cellular mechanism of LIPUS action with respect to meniscal healing.
In conclusion, LIPUS stimulated CCN2 production, SOX9 expression, and expression of cartilage matrix genes in the inner meniscus cells via MAPK signaling pathways. On the other hand, it also stimulated CCN2 expression and migration of outer meniscus cells. In addition, LIPUS stimulated angiogenesis, which is important for wound healing, by inducing VEGF. When an injured meniscus is exposed to LIPUS, all these phenomena would happen in parallel, to repair the defective meniscus (Fig. 9). Although this is still just an hypothesis, the data presented in this study indicate that LIPUS treatment exerted a reparative effect on the injured meniscus via up-regulation of CCN2, a regenerating factor, thus suggesting that LIPUS treatment is beneficial not only as a non-invasive intervention, but also as a useful therapy for an injured meniscus.
Acknowledgments
We thank Ms. Yoshiko Miyake for her secretarial assistance. We also thank Masataka Fujii, Yuya Kodama, Tomohito Hino, Yoshiki Okazaki, Shin Masuda and Yuki Okazaki for their clinical supports.
Abbreviations
- CCN
Cysteine-rich 61, Connective tissue growth factor, Nephroblastoma-overexpressed
- CCN2
CCN family member 2, Connective tissue growth factor
- CCN3
CCN family member 3, Nephroblastoma-overexpressed
- DMEM
Dulbecco’s modified Eagle’s medium
- ECM
Extracellular matrix
- FBS
Fetal bovine serum
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- LIPUS
Low-intensity pulsed ultrasound
- OA
Osteoarthritis
- PAGE
Polyacrylamide gel electrophoresis
- PDGF
Platelet-derived growth factor
- PVDF
Polyvinylidene difluoride
- PRP
Platelet-rich plasma
- LM
Lateral meniscus
- siRNA
Small interfering RNA
- SOX9
Sry-type high-mobility-group box 9
- TKA
Total knee arthroplasty
- TGF-β
Transforming growth factor-beta
- VEGF
Vascular endothelial growth factor
Funding
This work was supported in part by grants from the programs Grants-in-Aid for Scientific Research (B) to MT (#JP15H05014), and for Challenging Exploratory Research to MT (#JP17K19757) from Japan Society for the Promotion of Sciences, Japan.
Conflict of interest
NY is a managing director of Ito Co., Ltd.. and MT received research funding from this company. SM’s salary is supported by Teijin Nakashima Medical Co. Ltd. All other authors state that they have no conflicts of interest.
Ethical approval
This article does not contain any studies using human participants.
References
- Abd El Kader T, Kubota S, Nishida T, Hattori T, Aoyama E, Janune D, Hara ES, Ono M, Tabata Y, Kuboki T, Takigawa M. The regenerative effects of CCN2 independent modules on chondrocytes in vitro and osteoarthritis models in vivo. Bone. 2014;59:180–188. doi: 10.1016/j.bone.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Aoyama E, Kubota S, Takigawa M. CCN2/CTGF binds to fibroblast growth factor receptor 2 and modulates its signaling. FEBS Lett. 2012;586:4270–4275. doi: 10.1016/j.febslet.2012.10.038. [DOI] [PubMed] [Google Scholar]
- Arnoczky SP, Warren RF. Microvasculature of the human meniscus. Am J Sports Med. 1982;10:90–95. doi: 10.1177/036354658201000205. [DOI] [PubMed] [Google Scholar]
- Augello A, De Bari C. The regulation of differentiation in mesenchymal stem cells. Hum Gene Ther. 2010;21:1226–1238. doi: 10.1089/hum.2010.173. [DOI] [PubMed] [Google Scholar]
- Bi W, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Sox9 is required for cartilage formation. Nat Genet. 1999;22:85–89. doi: 10.1038/8792. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- Chahla J, Kennedy NI, Geeslin AG, Moatshe G, Cinque ME, DePhillipo NN, LaPrade RF. Meniscal repair with fibrin clot augmentation. Arthrosc Tech. 2017;6:e2065–e2069. doi: 10.1016/j.eats.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K, Xia P, Lin Q, Shen S, Gao M, Ren S, Li X. Effects of low-intensity pulsed ultrasound on integrin-FAK-PI3K/Akt mechanochemical transduction in rabbit osteoarthritis chondrocytes. Ultrasound Med Biol. 2014;40:1609–1618. doi: 10.1016/j.ultrasmedbio.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Furumatsu T, Ozaki T (2017) An analysis of pathological activities of CCN proteins in joint disorders: mechanical stretch-mediated CCN2 expression in cultured meniscus cells. Methods Mol Biol 1489:533–542 [DOI] [PubMed]
- Furumatsu T, Kanazawa T, Yokoyama Y, Abe N, Ozaki T. Inner meniscus cells maintain higher chondrogenic phenotype compared with outer meniscus cells. Connect Tissue Res. 2011;52:459–465. doi: 10.3109/03008207.2011.562061. [DOI] [PubMed] [Google Scholar]
- Furumatsu T, Kanazawa T, Miyake Y, Kubota S, Takigawa M, Ozaki T. Mechanical stretch increases Smad3-dependent CCN2 expression in inner meniscus cells. J Orthop Res. 2012;30:1738–1745. doi: 10.1002/jor.22142. [DOI] [PubMed] [Google Scholar]
- Furumatsu T, Matsumoto E, Kanazawa T, Fujii M, Lu Z, Kajiki R, Ozaki T. Tensile strain increases expression of CCN2 and COL2A1 by activating TGF-beta-Smad2/3 pathway in chondrocytic cells. J Biomech. 2013;46:1508–1515. doi: 10.1016/j.jbiomech.2013.03.028. [DOI] [PubMed] [Google Scholar]
- Griffin JW, Hadeed MM, Werner BC, Diduch DR, Carson EW, Miller MD. Platelet-rich plasma in meniscal repair: does augmentation improve surgical outcomes? Clin Orthop Relat Res. 2015;473:1665–1672. doi: 10.1007/s11999-015-4170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjiargyrou M, McLeod K, Ryaby JP, Rubin C. Enhancement of fracture healing by low intensity ultrasound. Clin Orthop Relat Res. 1998;355S:S216–S229. doi: 10.1097/00003086-199810001-00022. [DOI] [PubMed] [Google Scholar]
- Hatsushika D, Muneta T, Nakamura T, Horie M, Koga H, Nakagawa Y, Tsuji K, Hishikawa S, Kobayashi E, Sekiya I. Repetitive allogeneic intraarticular injections of synovial mesenchymal stem cells promote meniscus regeneration in a porcine massive meniscus defect model. Osteoarthr Cartil. 2014;22:941–950. doi: 10.1016/j.joca.2014.04.028. [DOI] [PubMed] [Google Scholar]
- Jang KW, Ding L, Seol D, Lim TH, Buckwalter JA, Martin JA. Low-intensity pulsed ultrasound promotes chondrogenic progenitor cell migration via focal adhesion kinase pathway. Ultrasound Med Biol. 2014;40:1177–1186. doi: 10.1016/j.ultrasmedbio.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janune D, Kubota S, Nishida T, Kawaki H, Perbal B, Iida S, Takigawa M. Novel effects of CCN3 that may direct the differentiation of chondrocytes. FEBS Lett. 2011;585:3033–3040. doi: 10.1016/j.febslet.2011.08.024. [DOI] [PubMed] [Google Scholar]
- Janune D, Abd El Kader T, Aoyama E, Nishida T, Tabata Y, Kubota S, Takigawa M. Novel role of CCN3 that maintains the differentiated phenotype of articular cartilage. J Bone Miner Metab. 2017;35:582–597. doi: 10.1007/s00774-016-0793-4. [DOI] [PubMed] [Google Scholar]
- Kamimura T, Kimura M. Meniscal repair of degenerative horizontal cleavage tears using fibrin clots: clinical and arthroscopic outcomes in 10 cases. Orthop J Sports Med. 2014;2:2325967114555678. doi: 10.1177/2325967114555678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaki H, Kubota S, Suzuki A, Lazar N, Yamada T, Matsumura T, Ohgawara T, Maeda T, Perbal B, Lyons KM, Takigawa M. Cooperative regulation of chondrocyte differentiation by CCN2 and CCN3 shown by a comprehensive analysis of the CCN family proteins in cartilage. J Bone Miner Res. 2008;23:1751–1764. doi: 10.1359/jbmr.080615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaki H, Kubota S, Takigawa M (2017) Western blotting analysis of CCN proteins in calcified tissues. Methods Mol Biol 1489:43–51 [DOI] [PubMed]
- Kikuchi T, Kubota S, Asaumi K, Kawaki H, Nishida T, Kawata K, Mitani S, Tabata Y, Ozaki T, Takigawa M. Promotion of bone regeneration by CCN2 incorporated into gelatin hydrogel. Tissue Eng A. 2008;14:1089–1098. doi: 10.1089/ten.tea.2007.0167. [DOI] [PubMed] [Google Scholar]
- Kubota S, Takigawa M. The role of CCN2 in cartilage and bone development. J Cell Commun Signal. 2011;5:209–217. doi: 10.1007/s12079-011-0123-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota S, Takigawa M. The CCN family acting throughout the body: recent research developments. Biomol Concepts. 2013;4:477–494. doi: 10.1515/bmc-2013-0018. [DOI] [PubMed] [Google Scholar]
- Kubota S, Kawata K, Yanagita T, Doi H, Kitoh T, Takigawa M. Abundant retention and release of connective tissue growth factor (CTGF/CCN2) by platelets. J Biochem. 2004;136:279–282. doi: 10.1093/jb/mvh126. [DOI] [PubMed] [Google Scholar]
- Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- Makris EA, Hadidi P, Athanasiou KA. The knee meniscus: structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials. 2011;32:7411–7431. doi: 10.1016/j.biomaterials.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner K, Gao J. The menisci of the knee joint. Anatomical and functional characteristics, and a rationale for clinical treatment. J Anat. 1998;193(Pt 2):161–178. doi: 10.1046/j.1469-7580.1998.19320161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y, Muneta T, Kondo S, Mizuno M, Takakuda K, Ichinose S, Tabuchi T, Koga H, Tsuji K, Sekiya I. Synovial mesenchymal stem cells promote healing after meniscal repair in microminipigs. Osteoarthr Cartil. 2015;23:1007–1017. doi: 10.1016/j.joca.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Nepple JJ, Dunn WR, Wright RW. Meniscal repair outcomes at greater than five years: a systematic literature review and meta-analysis. J Bone Joint Surg Am. 2012;94:2222–2227. doi: 10.2106/JBJS.K.01584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida T, Kubota S, Kojima S, Kuboki T, Nakao K, Kushibiki T, Tabata Y, Takigawa M. Regeneration of defects in articular cartilage in rat knee joints by CCN2 (connective tissue growth factor) J Bone Miner Res. 2004;19:1308–1319. doi: 10.1359/JBMR.040322. [DOI] [PubMed] [Google Scholar]
- Nishida T, Kubota S, Aoyama E, Yamanaka N, Lyons KM, Takigawa M. Low-intensity pulsed ultrasound (LIPUS) treatment of cultured chondrocytes stimulates production of CCN family protein 2 (CCN2), a protein involved in the regeneration of articular cartilage: mechanism underlying this stimulation. Osteoarthr Cartil. 2017;25:759–769. doi: 10.1016/j.joca.2016.10.003. [DOI] [PubMed] [Google Scholar]
- Oh CD, Yasuda H, Zhao W, Henry SP, Zhang Z, Xue M, de Crombrugghe B, Chen D. SOX9 directly regulates CTGF/CCN2 transcription in growth plate chondrocytes and in nucleus pulposus cells of intervertebral disc. Sci Rep. 2016;6:29916. doi: 10.1038/srep29916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka M, Kubota S, Kondo S, Eguchi T, Kuroda C, Kawata K, Minagi S, Takigawa M. Gene expression and distribution of connective tissue growth factor (CCN2/CTGF) during secondary ossification center formation. J Histochem Cytochem : Official Journal of The Histochemistry Society. 2007;55:1245–1255. doi: 10.1369/jhc.7A7263.2007. [DOI] [PubMed] [Google Scholar]
- Ozeki N, Muneta T, Matsuta S, Koga H, Nakagawa Y, Mizuno M, Tsuji K, Mabuchi Y, Akazawa C, Kobayashi E, Saito T, Sekiya I. Synovial mesenchymal stem cells promote meniscus regeneration augmented by an autologous Achilles tendon graft in a rat partial meniscus defect model. Stem Cells. 2015;33:1927–1938. doi: 10.1002/stem.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal B (2004) CCN proteins: multifunctional signalling regulators. Lancet 363:62–64 [DOI] [PubMed]
- Perbal B, Takigawa M. 2005. CCN Proteins: A New Family of Cell Growth and Differentiaton Regulator
- Ren L, Yang Z, Song J, Wang Z, Deng F, Li W. Involvement of p38 MAPK pathway in low intensity pulsed ultrasound induced osteogenic differentiation of human periodontal ligament cells. Ultrasonics. 2013;53:686–690. doi: 10.1016/j.ultras.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Sato M, Nagata K, Kuroda S, Horiuchi S, Nakamura T, Karima M, Inubushi T, Tanaka E. Low-intensity pulsed ultrasound activates integrin-mediated mechanotransduction pathway in synovial cells. Ann Biomed Eng. 2014;42:2156–2163. doi: 10.1007/s10439-014-1081-x. [DOI] [PubMed] [Google Scholar]
- Shibuya M. Structure and function of VEGF/VEGF-receptor system involved in angiogenesis. Cell Struct Funct. 2001;26:25–35. doi: 10.1247/csf.26.25. [DOI] [PubMed] [Google Scholar]
- Shimo T, Nakanishi T, Nishida T, Asano M, Kanyama M, Kuboki T, Tamatani T, Tezuka K, Takemura M, Matsumura T, Takigawa M. Connective tissue growth factor induces the proliferation, migration, and tube formation of vascular endothelial cells in vitro, and angiogenesis in vivo. J Biochem. 1999;126:137–145. doi: 10.1093/oxfordjournals.jbchem.a022414. [DOI] [PubMed] [Google Scholar]
- Shiraishi R, Masaki C, Toshinaga A, Okinaga T, Nishihara T, Yamanaka N, Nakamoto T, Hosokawa R. The effects of low-intensity pulsed ultrasound exposure on gingival cells. J Periodontol. 2011;82:1498–1503. doi: 10.1902/jop.2011.100627. [DOI] [PubMed] [Google Scholar]
- Takigawa M. CTGF/Hcs24 as a multifunctional growth factor for fibroblasts, chondrocytes and vascular endothelial cells. Drug News & Perspectives. 2003;16:11–21. doi: 10.1358/dnp.2003.16.1.829302. [DOI] [PubMed] [Google Scholar]
- Takigawa M. CCN2: a master regulator of the genesis of bone and cartilage. J Cell Commun Signal. 2013;7:191–201. doi: 10.1007/s12079-013-0204-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takigawa M. An early history of CCN2/CTGF research: the road to CCN2 via hcs24, ctgf, ecogenin, and regenerin. J Cell Commun Signal. 2018;12:253–264. doi: 10.1007/s12079-017-0414-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Fujii K, Kumagae Y. Comparison of biochemical characteristics of cultured fibrochondrocytes isolated from the inner and outer regions of human meniscus. Knee Surg Sports Traumatol Arthrosc. 1999;7:75–80. doi: 10.1007/s001670050125. [DOI] [PubMed] [Google Scholar]
- Tang X, Muhammad H, McLean C, Miotla-Zarebska J, Fleming J, Didangelos A, Onnerfjord P, Leask A, Saklatvala J, Vincent TL. Connective tissue growth factor contributes to joint homeostasis and osteoarthritis severity by controlling the matrix sequestration and activation of latent TGFbeta. Ann Rheum Dis. 2018;77:1372–1380. doi: 10.1136/annrheumdis-2018-212964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin SM, Richbourgh B, Ding Y, Hettinghouse A, Komatsu DE, Qin YX, Liu CJ. Chondro-protective effects of low intensity pulsed ultrasound. Osteoarthr Cartil. 2016;24:1989–1998. doi: 10.1016/j.joca.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voloshin AS, Wosk J. Shock absorption of meniscectomized and painful knees: a comparative in vivo study. J Biomed Eng. 1983;5:157–161. doi: 10.1016/0141-5425(83)90036-5. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li J, Qiu Y, Hu B, Chen J, Fu T, Zhou P, Song J. Lowintensity pulsed ultrasound promotes periodontal ligament stem cell migration through TWIST1mediated SDF1 expression. Int J Mol Med. 2018;42:322–330. doi: 10.3892/ijmm.2018.3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C, Li H, Yang T, Deng ZH, Yang Y, Zhang Y, Ding X, Lei GH. Effectiveness of continuous and pulsed ultrasound for the management of knee osteoarthritis: a systematic review and network meta-analysis. Osteoarthr Cartil. 2014;22:1090–1099. doi: 10.1016/j.joca.2014.06.028. [DOI] [PubMed] [Google Scholar]