Abstract
Adult-onset xanthogranuloma (AOX) is one of the four uncommon syndromes called adult xanthogranulomatous disease (AXD), which is diagnosed by characteristic histopathology. AXD is rare and heterogeneous group of entities that can affect multiple organ systems. Orbital involvement is included in the xanthogranulomatous disease although less prevalent. This work focuses on the use of external beam radiotherapy in the control of local symptoms of periocular manifestation of AOX as case report and literature review.
Keywords: Radiotherapy, Adult-onset xanthogranuloma , Xanthogranuloma, EBRT, Histiocytic disorder, Erdheim–Chester disease
Introduction
Adult-onset xanthogranuloma (AOX) is one of the four uncommon syndromes called adult xanthogranulomatous disease (AXD) diagnosed by characteristic histopathology. AXD is rare and heterogeneous group of entities with inflammation of multiple organs like bone, heart, lungs, retro-peritoneum or orbit and ocular adnexa. The spectrum of the symptoms varies depending on the localization from hypertension, neurologic manifestations, heart and lung diseases and visual alteration and exophthalmos.
Orbital xanthogranulomatous disease includes four clinical syndromes: adult-onset xanthogranuloma, necrobiotic xanthogranuloma, adult-onset asthma and periocular xanthogranuloma and Erdheim–Chester disease (ECD).
Histopathologically these entities are characterized by the presence of “hallmark cells” especially foamy histiocytes, Touton-type giant cells and varying degrees of fibrosis.
Consequently, in this work is presented an AOX case report with infiltration of unilateral periocular tissues in an adult woman. This work focuses on the use of external beam radiotherapy in the control of local symptoms of periocular manifestation of AOX providing an overview of adult xanthogranulomatous disease and reporting a representative case of treatment with external beam radiotherapy in a patient with adult-onset xanthogranuloma.
Case report
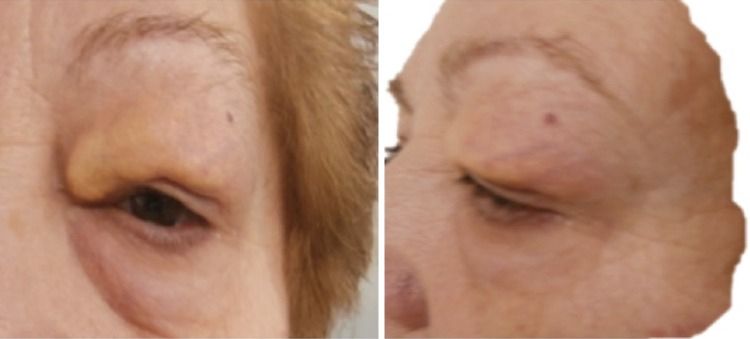
A 64-year-old female in the last 3 years has presented edema, proptosis and upper eyelid induration of left eye. Lacrimation increased and intermittent pruritus was associated. The lesion has had a slow and progressive growth, begins in inner edge with lateral–upper expansion (Fig. 1).
Fig. 1.
Pre-treatment lesion: left eye with edema, proptosis and upper eyelid induration without ocular globe affectation. Reproduced with patient permission
Personal history included hypertension, diabetes, glaucoma and obesity. Oncological record reports left breast carcinoma treated with surgery and radiotherapy with 20-year disease-free survival (DFS).
Ophthalmologist initially assessed the patient; he made visual evoked potentials and electro-oculogram resulting in both within normality.
Subsequently orbital fat biopsy was performed appreciating an infiltrate of histiocytes sparkling, clear cytoplasm with some eosinophils and plasma cells affecting striated muscle fiber and fat tissue. Immunohistochemistry analysis: CD68 (+), S-100 (−), CD1a (−), without atypia and low Ki-67 (proliferative activity <1 %). There is a population of mature interstitial CD3+ lymphocytes and small lymphoid follicles expressing CD20 (+). Histological changes compatibles with adult xanthogranulomatous disease (AXD).
Before the diagnosis of AXD, extension study was performed to rule out involvement in other locations: MRI found 28 × 10 × 18 mm left upper eyelid infiltration without or post-orbital septal extension (Fig. 2). Whole-body bone scan observed no findings compatible with ECD. Systemic involvement was excluded through body CT.
Fig. 2.
a MRI shows a 28 × 10 × 18 mm left upper eyelid infiltration without orbital septal extension. b Simulation CT/MRI fusion: lesion is localized an 18 mm depth
From initial diagnosis, the patient has been operated up to three times with surgical excision and blepharoplasty of the left eyelid injury; recurrence was observed after 6 months post-surgery.
At this point, the patient was referred to radiation oncology department to assess local treatment.
Treatment
The radiotherapy treatment was performed with 6 MeV electron beam (Clinac iX VARIAN), this energy has the therapeutic range at 21 mm and the practical range at 30 mm. The treatment dose was prescribed at 18 mm depth (Fig. 3) using a 3-mm-thick bolus over skin; in addition to modulating the therapeutic depth, the bolus produces a 100 % dose at the entrance. A field size of 5.5 × 2.6 cm (Fig. 4) was set up with a 4-mm-thick customized lead cutout as ocular protector; this lead shield absorbs all the electrons remaining just about 1 % of bremsstrahlung. The patient received a total of 20 Gy in 10 fractions (2 Gy per fraction) daily with five fractions/week.
Fig. 3.

Treatment planning: antero-posterior electron beam was performed using a skin bolus. The color zones correspond to curves treatment. The ocular globe was save according prescribed dose
Fig. 4.

Irradiation field: a field size of 5.5 × 2.6 cm was set up with a 4-mm-thickness customized lead cutout as ocular protector
The treatment was well tolerated by the patient. The dermal acute toxicity was erythema and pruritus grade 1 (CTCAE v4.02) having been resolved with topical treatment. No visual acute alteration post-treatment was observed.
Fifteen days after the treatment, an orbit MRI was performed. Signal and thickness increased in the upper eyelid region is seen on T2 and STIR sequences that corresponds to soft tissue material. Hypo-intense on T1 and evident contrast enhancement that produces its hyper-vascular nature (Fig. 5).
Fig. 5.
Orbit MRI, 15 days after radiotherapy treatment: STIR (a) and T2 axial planes (b), T1 pre (c) and post (d) gadolinium in sagittal planes. Signal and thickness increase in the left upper eyelid region is seen on T2 and STIR sequences (big arrow) that correspond to soft tissue material. Hypo-intense on T1 and evident contrast enhancement (small arrow) that produces its hyper-vascular nature
Last orbit MRI (9 months post-radiotherapy) show reduction of eyelid lesion (Fig. 6).
Fig. 6.
Orbit MRI, 9 months after radiotherapy treatment: STIR (a) and T2 (b) in axial planes, T1 pre (c) and post (d) gadolinium in sagittal planes. Images obtained in the same sequences and locations where size and contrast enhancement reduction of eyelid lesion is seen
At present, the physical examinations show a visible lesion reduction and pain relief. The patient will continue with the follow-up standardized every 6 months.
Discussion
Since the first description of disease in 1930 by the pathologists William Chester and Jacob Erdheim, some 100 cases have been reported in the word literature [1]. Subsequently, in 1987 the histiocytic disorder was classified in many categories according to common characteristics.
Adult xanthogranulomatosis disease is an entity belonging to category 1 of histiocytic disorders and includes many syndromes clustered depending on the systemic involvement, these are: [2, 3]
Adult-onset xanthogranuloma (AOX) described as solitary lesion without systemic findings.
Adult-onset asthma and periocular xanthogranuloma (AAPOX) associated with immune dysfunction like asthma and lymphoproliferative disorders.
Necrobiotic xanthogranuloma (NBX) is similar an AAPOX, however, its main immune dysfunction is as paraproteinemia. Subcutaneous skin lesions with tendency to ulceration.
Erdheim–Chester disease (ECD) adds systemic involvement as pericardial or pleural effusions, retroperitoneal involvement, diabetes insipidus, hepatosplenomegaly, etc. Also associated with bone lesions and more aggressive development.
Eyelid or ocular adnexa skin lesion can be presented in the four syndromes, although there appears to be a predisposition towards different target orbital location. The AOX, AAPOX and NBX mainly have preseptal and anterior orbital involvement while ECD had diffuse intraconal disease [2, 3].
The cellular and clinic characteristics are very close, and its distinction should therefore be made between histology and immunostaining.
By histologic characteristics in relation to type of cellular infiltration may be divided in non-Langerhans and Langerhans. AXD is characterized by non-Langerhans histiocytes with foamy or eosinophilic cytoplasm, polymorphic granulomae and fibrosis, xanthogranulomatosis, proliferating fibroblast and Touton giant cells. Immunohistochemically, presents S-100 (−), CD68 (+), CD1a (−), factor XIIIa (+) while the Langerhans infiltration presents S-100 (+) and Birbeck granules [4–9].
Other causes of histiocytosis are rare but may occur, including: juvenile xanthogranuloma (affects the child mainly), inflammatory idiopathic pseudo-tumor, Rosai–Dorfman syndrome and lymphoma.
In our case, orbital fat biopsy shows an infiltrate of histiocytes sparkling, clear cytoplasm with some eosinophils and plasma cells affecting striated muscle fiber and fat tissue. Immunohistochemistry analysis: CD68 (+), S-100 (−), CD1a (−), without atypia and low Ki-67 (proliferative activity <1 %) that along with the anterior localization of disease, and a lack of systemic affectation we consider the entity as an AOX (adult-onset xanthogranuloma).
AOX is a rare type of tumor within the family of AXD. According to Sivak-Callcott et al. in his publication on BJO (British Journal of Ophthalmology) NBX is the most frequent followed by ECD, and in the literature only exists five cases of AOX over 155 cases reported between AXD. There is no evidence of mortality related to AOX [3].
The treatment of the AXD included various options such as corticosteroid therapy, cyclosporine, interferon, methotrexate, chemotherapy (e.g., cyclophosphamide, doxorubicin, and vinblastine), surgical resection and radiotherapy. Radiation may be presumed to inhibit the xanthogranulomatous proliferation of histiocytes, but radiation responses are variable and often the result is only temporary stabilization [10].
Focusing on the radiation treatment of our specific case (AOX), we have not found a clear and concise scheme about the doses, results and survival rate. However, there are many studies that have used the radiation therapy in ECD cases, the majority of them from retro-orbital location.
Miller et al. [11] in his publication discuss the treatment with radiotherapy in many ECD cases, treating one retro-orbital ECD patient with orbital bone invasion at a dose of 19.8 Gy finding a partial response.
There is no consensus that determines the right dose used in these types of tumors, although many authors describe a 10–35 Gy range for ECD lesions (brain, extra-axial and retrobulbar mainly) [3, 5, 7, 11–15].
Hoffmann et al. [2] describe one case of retrobulbar radiotherapy (20 Gy) and long-term systemic corticosteroid treatment followed, resulting in a partial improvement of the original lesion [2].
In our patient, the recurrent eyelid has been operated upto three times with surgical excision without local control. Therefore, we decided to administer a radiotherapy treatment with electron beam 20 Gy, this is because the superficial skin lesion (18 mm depth) and the lack of ocular invasion. After 12 months of follow-up the size of the tumor evidence a decrease and local control (Figs. 5, 6).
Conclusion
There is at present no standard treatment for AXD. However, there are several therapeutic strategies in the daily practice making possible to treat patients with this pathology.
In our patient, just one left periocular lesion (upper lid), undergoing two failed surgical interventions and progression of the lesion has given raise to a local radiotherapy as alternative treatment. The outcomes show macroscopic improvement and pain relief. The patient will continue with the follow-up standardized every 6 months.
There is no consensus explaining suitable doses and fractionation about radiation of these lesions. However, a similar therapeutic scheme is used in the Langerhans histiocytosis.
In summary, AOX as subtype of adult xanthogranulomatous diseases is rare and pose challenge in daily clinical practice. The therapeutic strategy remains controversial and requires further study.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Chester W. Über lipidgranulomatose. Virchows Archiv. 1930;279:561–602. doi: 10.1007/BF01942684. [DOI] [Google Scholar]
- 2.Hoffman E, Müller-Forell W, Pitz S, Radner H. Erdheim–Chester disease: a case report. Graefe’s Arch Clin Exp Ophthalmol. 2004;242:803–807. doi: 10.1007/s00417-004-0928-5. [DOI] [PubMed] [Google Scholar]
- 3.Sivak-Callcot J, Rootman J, Rasmussen S, et al. Adult xanthogranulomatous disease of the orbit and ocular adnexa: new inmunohistochemical findings and clinical review. Br J Ophthalmol. 2006;90:602–608. doi: 10.1136/bjo.2005.085894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babu RP, Lansen TA, Chadburn A, et al. Erdheim–Chester disease of the central nervous system: report of two cases. J Neurosurg. 1997;86:888–892. doi: 10.3171/jns.1997.86.5.0888. [DOI] [PubMed] [Google Scholar]
- 5.Veyssier-belot C, Cacoub P, Caparros-Lefebvre D, et al. Erdheim–Chester disease. Clinical and radiologic characteristics of 59 cases. Medicine. 2001;75:157–169. doi: 10.1097/00005792-199605000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Martinez R. Erdheim–Chester disease. MR of intraaxial and extraaxial brain stem lesions. AJNR Am J Neuroradiol. 1995;16:1787–1790. [PMC free article] [PubMed] [Google Scholar]
- 7.Masalchi M, Nencini P, Nistri M, et al. Failure of radiation therapy for brain involvement in Erdheim–Chester disease. J. Neurooncol. 2000;59:169–172. doi: 10.1023/A:1019649201324. [DOI] [PubMed] [Google Scholar]
- 8.Shamburek RD, Brewer HB, Caparros-Lefevre D, et al. Erdheim–Chester disease. Clinical and radiologic characteristics of 59 cases. Medicine. 1996;75:157–169. doi: 10.1097/00005792-199605000-00005. [DOI] [PubMed] [Google Scholar]
- 9.mazor RD, Manevich-Mazor M, Shoenfeld Y. Erdheim–Chester disease: a comprehensive review of the literature. Orphanet J Rare Dis. 2013;8:137. doi: 10.1186/1750-1172-8-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katsunori M, Yasushi N, Masahiro H. Radiotherapy for Erdheim–Chester disease. Int J Clin Oncol. 2007;12:238–241. doi: 10.1007/s10147-006-0644-8. [DOI] [PubMed] [Google Scholar]
- 11.Miller R, Villá S, Kamer S, et al. Palliative treatment of Erdheim–Chester disease with radiotherapy: a rare cancer network study. Radiother Oncol. 2006;80:323–326. doi: 10.1016/j.radonc.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 12.Aster JC, Pozdnyakova O, Kutok JL. Hematopathology: a volume in the hight yield pathology series. Amsterdam: Saunders, Elsevier; 2013. pp. 87–88. [Google Scholar]
- 13.Bohlega S, Alwatban J, Tulbah A, et al. Cerebral manifestation of Erdheim–Chester disease: Clinical and neurologic findings. Neurology. 1997;49:1702–17058. doi: 10.1212/WNL.49.6.1702. [DOI] [PubMed] [Google Scholar]
- 14.Kujat C, Martin J, Püschel W. Erdheim–Chester disease. Radiology. 1991;31:297–306. [PubMed] [Google Scholar]
- 15.Wright RA, Hermann RC, Parisis JE. Neurological manifestation of Erdheim–Chester disease. J Neurol Neurosurg Psychiatry. 1999;66:72–75. doi: 10.1136/jnnp.66.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]