Abstract
The recurrence of gastric cancer is rarely associated with cardiac tamponade induced by carcinomatous pericarditis. We encountered a patient in whom cancer recurred as carcinomatous pericarditis 9 years after surgery for advanced gastric cancer. Furthermore, pericardial effusion caused marked subcutaneous edema in her trunk and lower limbs after percutaneous pericardial drainage was applied to treat cardiac tamponade. A 49-year-old woman presented with lower limb edema and exertional dyspnea 9 years after distal gastrectomy for advanced gastric cancer. Chest computed tomography and ultrasonography showed bilateral pleural effusion and pericardial effusion. Pericardial drainage and thoracocentesis were performed, and her symptoms of respiratory distress remitted. Class V adenocarcinoma was detected on cytology from both effusions, and was diagnosed as the recurrence of gastric cancer. After systemic chemotherapy, she was admitted for the aggravation of dyspnea because of recurrent retention of pericardial effusion. Pericardiocentesis was repeated. The pericardial effusion became subcutaneously retained in the trunk below the puncture site over the lower limbs via the drainage route. Edema in the trunk below the abdomen and lower limbs gradually aggravated over time. The skin extended and became sclerotic because of severe edema, liquid leaked from abdominal skin injuries, and the condition became similar to skin lymphorrhea in lymphedema. Neoplastic cardiac tamponade due to gastric cancer has an extremely low incidence and a poor prognosis. We encountered a patient in whom pericardial effusion caused subcutaneous edema in the trunk and lower limbs after percutaneous pericardial drainage was applied to treat carcinomatous pericarditis associated with gastric cancer.
Keywords: Gastric cancer, Metastasis, Neoplastic cardiac tamponade, Carcinomatous pericarditis, Oncologic emergency
Introduction
Cardiac tamponade caused by carcinomatous pericarditis-induced retention of pericardial effusion needs to be treated rapidly as an oncologic emergency [1]. Emergency treatment is necessary to prevent sudden death and relieve symptoms of pericardial effusion retention in neoplastic cardiac tamponade [[2]. The recurrence of gastric cancer is rarely associated with cardiac tamponade induced by carcinomatous pericarditis. We encountered a patient in whom cancer recurrence was associated with carcinomatous pericarditis 9 years after surgery for advanced gastric cancer. Furthermore, pericardial effusion caused marked subcutaneous edema in her trunk and lower limbs after percutaneous pericardial drainage was applied to treat cardiac tamponade. We report the case with a literature review.
Case report
The female patient was 49 years old when carcinomatous pericarditis recurred. She had undergone distal gastrectomy, D2 dissection, and Roux-en-Y reconstruction for advanced gastric cancer at 40 years of age. Histopathologically, the lesion was poorly differentiated adenocarcinoma, T3(SS) N1 M0 Stage IIB, and radical surgery was performed. Adjuvant chemotherapy with S-1 was administered after surgery.
9 years after her surgery, she visited our hospital for lower limb edema and exertional dyspnea. Pleural effusion and retention of pericardial effusion were observed on computed tomography imaging (Fig. 1). Pericardial drainage was performed via an ordinary approach, and 500 mL of pale bloody pericardial effusion was drained. Her blood pressure was 159/117 mmHg and her pulse was 84 beats/min before drainage. These values improved to 106/60 mmHg and 76 beats/min, respectively. Class V adenocarcinoma was detected on cytology. On histocytological examination, pericardial carcinosis appeared to be similar to the primary gastric cancer. Pleural effusion retention was also observed, and thoracocentesis was applied. Cells similar to those in the pericardial effusion were detected on cytology. Her respiratory distress symptoms remitted, and the primary lesion was re-investigated, but no significant findings were observed in the other organs. The disease was judged to be a recurrence of gastric cancer. The following systematic chemotherapy was administered: primary treatment, Tegafur/Gimeracil/Oteracil (S-1) + oxaliplatin; secondary treatment, capecitabine; and tertiary treatment, paclitaxel.
Fig. 1.

A chest computed tomography scan shows bilateral pleural effusion and pericardial effusion before pericardial drainage and thoracocentesis
4 months after initial pericardiocentesis, the patient was admitted for the aggravation of dyspnea because of the recurrent retention of pericardial effusion, and active anticancer treatment was discontinued. Pericardiocentesis was repeated, and 200 mL of pale bloody pericardial effusion was drained. Pericardial effusion became subcutaneously retained in the trunk below the puncture site over the lower limbs via the drainage route. Edema in the trunk below the abdomen and lower limbs gradually aggravated over time (Figs. 2, 3). A puncture was performed after 3 months to treat recurrent retention of pleural effusion. In contrast, the patient’s pericardial effusion did not recur. Flexion of the knee joint gradually became difficult because of marked edema in the trunk over the lower limbs. The skin extended and became sclerotic because of severe edema, liquid leaked from abdominal skin injuries, and the condition became similar to skin lymphorrhea in lymphedema. The skin injury regions were managed using an ostomy pouch. Retention of pleural effusion recurred; however, puncture was not performed because the patient’s general condition was poor and her prognosis was judged to be limited. Her symptoms were relieved by morphine. Home care was continued, and the patient died 9 months after the diagnosis of recurrence. During that period, the retention of pericardial effusion did not recur. Her next of kin provided informed consent for the publication of this case report.
Fig. 2.
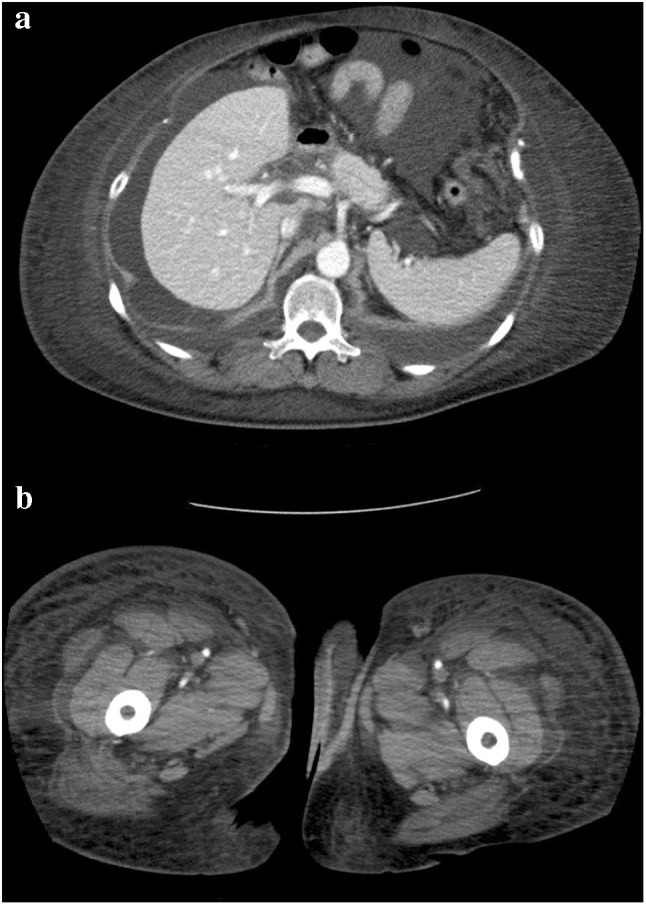
a An abdominal computed tomography scan shows pleural effusion, ascites, and subcutaneous edema in the lower trunk after re-pericardiocentesis. b A computed tomography scan shows subcutaneously edema in the bilateral femurs after re-pericardiocentesis
Fig. 3.
Ultrasonographic examination does not show the venous thromboembolism in the lower limbs; however, high-grade subcutaneous edema can be observed
Discussion
According to a study by Berge and Sievers [3], malignant tumor metastasis to the pericardium was observed in 50% of autopsy cases of malignant tumors with metastasis. Pericardial metastasis does not frequently cause cardiac tamponade. In a recent (2015) case series of carcinomatous pericarditis-induced cardiac tamponade, Takayama et al. reported that neoplastic cardiac tamponade occurred in 113 (0.3%) of 43,735 cancer patients over 22 years; the most frequent cause was lung cancer (59.2%), followed by breast cancer (21.2%), and lymphoma/leukemia (5.3%). Among the 7672 gastric cancer cases, the incidence of neoplastic cardiac tamponade was 0.01% (4 cases), making it a rare condition. The median time from gastric cancer diagnosis to neoplastic cardiac tamponade onset was 37.3 months, and the median survival time after pericardiocentesis was 1.3 months [4].
When neoplastic cardiac tamponade is diagnosed, drainage by pericardiocentesis should be performed as the first procedure to relieve symptoms and prevent sudden death. The guidelines on the diagnosis and management of pericardial diseases published in August 2015 by the European Society of Cardiology (ESC) recommended pericardiocentesis to relieve symptoms and establish the diagnosis of malignant pericardial effusion at evidence level B (based on the results of one randomized or non-randomized clinical study) [5].
Treatment of the primary disease is investigated after pericardial drainage. Based on an analysis of 17 cases of gastric cancer-induced neoplastic cardiac tamponade, Kobayashi et al. suggested that systematic chemotherapy may facilitate long-term survival, although they did not observe any statistically significant differences in survival [6]. For gastric cancer-induced carcinomatous pericarditis, the following systematic chemotherapies have been reported to be useful in previous case reports and case series: S-1, S-1 + cisplatin, oxaliplatin + 5-fluorouracil + leucovorin, S-1 + Irinotecan, and docetaxel [6–11]. Nonetheless, complete cure is difficult [6–11].
To control pericardial effusion, percutaneous drainage, pericardial adhesion by anticancer drug injection, and surgical resection of the pericardium are performed, and the procedure is selected based on the patient’s general condition. In the ESC guidelines, extended pericardial drainage to prevent recurrent retention of carcinomatous pericardial effusion is recommended at evidence level B, consideration of anticancer drug administration to the pericardium is specified at evidence level B, and pericardiotomy is to be considered when pericardiocentesis cannot be performed [5].
In the present case, retention of pericardial effusion recurred. A high rate of recurrence (40–70%) has been reported for the retention of carcinomatous pericarditis-induced pericardial effusion [5]. Percutaneous pericardiocentesis was performed to treat the recurrent retention of pericardial effusion. This procedure caused subcutaneous leakage of pericardial effusion through the pericardial effusion drainage route; however, the retention of drainage from the pericardial effusion did not recur thereafter. Complications of percutaneous pericardiocentesis include cardiac puncture, arrhythmia, myocardial perforation, pneumothorax, internal mammary or coronary artery injury, and infection. The reported incidence of complications has varied markedly from 1.5 to 37.5% [12–15], and has been high in reports that included arrhythmias, such as atrial fibrillation [15]. To our knowledge, there has been no previous report of the complication of subcutaneous edema after the drainage of pericardial effusion.
The placement of a continuous drainage tube in the pericardium might have prevented subcutaneous edema; however, dyspnea resulting from retention of oncologic pericardial effusion did not occur, and home care was possible during the terminal phase.
For various cancer types, advancements in active anticancer therapy are expected to improve prognosis and survival. Appropriate drainage and systemic chemotherapy may improve the prognosis, even in patients with carcinomatous pericarditis and cardiac tamponade. In this case, it was possible to continue palliative care without the recurrence of cardiac tamponade because of the subcutaneous leakage of pericardial effusion.
Conclusion
We encountered a patient in whom pericardial effusion caused subcutaneous edema in the trunk and lower limbs after percutaneous pericardial drainage was applied to treat carcinomatous pericarditis associated with gastric cancer.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Vaitkus PT, Herrmann HC, LeWinter MM. Treatment of malignant pericardial effusion. JAMA. 1994;272:59–64. doi: 10.1001/jama.1994.03520010071035. [DOI] [PubMed] [Google Scholar]
- 2.Buck M, Ingle JN, Guiliani ER, et al. Pericardial effusion in women with breat cancer. Cancer. 1987;60:263–269. doi: 10.1002/1097-0142(19870715)60:2<263::AID-CNCR2820600225>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 3.Berge T, Sievers J. Myocardial metastases. A pathological electrocardiographic study. Br Heart J. 1968;30:383–390. doi: 10.1136/hrt.30.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takayama T, Okura Y, Okada Y, et al. Characteristics of neoplastic cardiac tamponade and prognosis after pericardiocentesis: a single-center study of 113 consecutive cancer patients. Int J Clin Oncol. 2015;20:872–877. doi: 10.1007/s10147-015-0794-7. [DOI] [PubMed] [Google Scholar]
- 5.Adler Y, Charron P, Imazio M, et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases. Eur Heart J. 2015;36:2921–2964. doi: 10.1093/eurheartj/ehv318. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi M, Okabayashi T, Okamoto K, et al. Clinicopathological study of cardiac tamponade due to pericardial metastasis originating from gastric cancer. World J Gastroenterol. 2005;11:6899–6904. doi: 10.3748/wjg.v11.i44.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varvarigos N, Kamaradou H, Kourti A, et al. Cardiac tamponade as the first manifestation of gastric cancer and remission after chemotherapy. Dig Dis Sci. 2001;46:2333–2335. doi: 10.1023/A:1012382610671. [DOI] [PubMed] [Google Scholar]
- 8.Kusaba H, Fujihara M, Nagashima R, et al. Systemic chemotherapy of TS-1 and cisplatin for gastric signet-ring cell carcinoma presenting as cardiac tamponade. Med Oncol. 2008;25:241–244. doi: 10.1007/s12032-007-9010-6. [DOI] [PubMed] [Google Scholar]
- 9.Zhang BL, Xu RL, Zheng X, Qin YW. A case with cardiac tamponade as the first sign of primary gastric signet-ring cell carcinoma treated with combination therapy. Med Sci Mon Int Med J Exp Clin Res. 2010;16:CS41-44. [PubMed] [Google Scholar]
- 10.Kuga Y, Okanobu H, Kitamura S, et al. A case of advanced gastric cancer with carcinomatous pericarditis effectively treated by S-1/CDDP combined therapy (in Japanese with English abstract) Jpn J Cancer Chemother. 2013;40:87–89. [PubMed] [Google Scholar]
- 11.Arisoy A, Memic K, Karavelioglu Y, Sen F. Cardiac tamponade as the first clinical sign of gastric adenocarcinoma: a rare condition. Turk Kardiyol Der Ars. 2014;42:377–379. doi: 10.5543/tkda.2014.24892. [DOI] [PubMed] [Google Scholar]
- 12.Tsang TS, Seward JB, Barnes ME, et al. Outcomes of primary and secondary treatment of pericardial effusion in patients with malignancy. Mayo Clin Proc. 2000;75:248–253. doi: 10.1016/S0025-6196(11)65028-3. [DOI] [PubMed] [Google Scholar]
- 13.Saltzman AJ, Paz YE, Rene AG, et al. Comparison of surgical pericardial drainage with percutaneous catheter drainage for pericardial effusion. J Invasive Cardiol. 2012;24:590–593. [PMC free article] [PubMed] [Google Scholar]
- 14.Ho MY, Wang JL, Lin YS, et al. Pericardiocentesis adverse event risk factors: a nationwide population-based cohort study. Cardiology. 2015;130:37–45. doi: 10.1159/000368796. [DOI] [PubMed] [Google Scholar]
- 15.Labbe C, Tremblay L, Lacasse Y. Pericardiocentesis versus pericardiotomy for malignant pericardial effusion: a retrospective comparison. Curr Oncol. 2015;22:412–416. doi: 10.3747/co.22.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]



