Abstract
Salivary duct carcinoma (SDC) has a poor prognosis owing to the high incidence of distant metastasis. Approximately 40 % of SDCs are positive for human epidermal growth factor receptor 2 (HER2), suggesting that anti-HER2 therapy for cases with distant metastasis may be effective. However, the pathological response of SDC metastases to anti-HER2 therapy has not been reported. A 44-year-old man was diagnosed with HER2-positive SDC of the submandibular gland with multiple distant metastases. After six cycles of trastuzumab/docetaxel therapy, the patient achieved clinical partial response for the primary tumor and complete response for the neck and distant metastases. Subsequently, the patient underwent submandibular gland resection and neck dissection. On histopathological examination, very few viable cancer cells were identified in the resected primary tumor. Unfortunately, the patient died of multiple distant metastases 12 months after initial treatment. Trastuzumab/docetaxel therapy can elicit a clinical and pathological response in HER2-positive SDC.
Keywords: Salivary duct carcinoma, HER2, Trastuzumab, Docetaxel, Metastases
Introduction
Salivary duct carcinoma (SDC) is a rare malignant tumor accounting for 9 % of all malignant salivary tumors; it has been defined as “an aggressive adenocarcinoma that resembles high-grade breast ductal carcinoma” [1]. SDC is classified as a high-grade malignancy and has a poor prognosis, with a mortality rate of more than 60 % [2]; the effects of chemotherapy for this disease have been limited [3]. Human epidermal growth factor receptor 2 (HER2) is overexpressed in 20–40 % of SDCs, a significantly higher rate than that expressed in other malignant salivary tumors [4–7]. Recently, the efficacy of the HER2 inhibitor, trastuzumab, in treating SDC has been reported [8, 9]. Here, we report a case of HER2-positive SDC in the submandibular gland and the pathological response of that SDC to trastuzumab/docetaxel therapy.
Case report
Clinical course
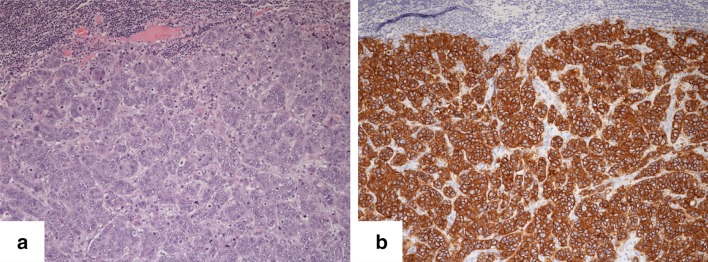
A 44-year-old man who had been diagnosed with submandibular adenocarcinoma at another hospital visited our institution. He had noticed a hard mass in his right submandibular gland 8 months earlier, and had experienced lumbago since 2 months prior to his diagnosis. He had no complicating disease. His Eastern Cooperative Oncology Group (ECOG) performance status (PS) was 0, and he exhibited no organ function or blood evaluation abnormalities. Positron emission tomography–computed tomography (PET–CT) revealed high fludeoxyglucose (FDG) uptake in the neck, lungs, liver, and bone (Fig. 1). On reviewing a primary submandibular gland tumor specimen collected from the patient at another hospital, we observed an eosinophilic granular matrix with a growth pattern characteristic of dysplastic epithelium, as well as large nuclei and clear nucleoli (Fig. 2a). Immunohistochemistry [IHC; PATHWAY anti-HER2/neu (4B5), Roche Diagnostics, Penzberg, Germany) indicated positive staining for HER2 protein (Fig. 2b), and fluorescence in situ hybridization (FISH; FISH HER2 PharmDx, Dako, Glostrup, Denmark) revealed that the HER2/centromere probe of chromosome 17 (CEP17) ratio was 2.6, indicating HER2/neu gene amplification. The IHC staining score was 3+, according to the guideline of ASCO/CAP [10]. Based on these findings, we diagnosed the patient with submandibular HER2 positive salivary duct carcinoma stage T3N2bM1.
Fig. 1.
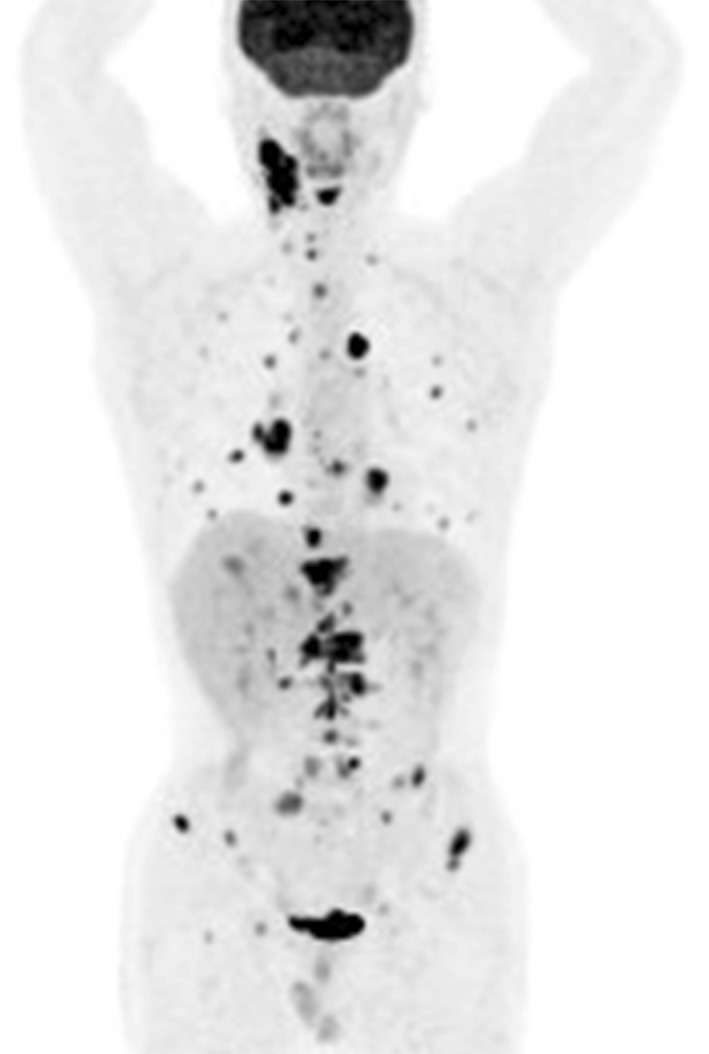
A whole body positron emission tomography/computed tomography scan before treatment. High fludeoxyglucose uptake was observed in the neck, lungs, liver, and bone
Fig. 2.
Histological and immunohistochemical examinations before treatment. a Eosinophilic granular matrix and a dysplastic epithelium growth pattern with large nuclei and clear nucleoli were identified. b Cancer cells were positive for overexpression of the HER2 protein
The patient received six cycles of trastuzumab (8 mg/kg loading dose, followed by 6 mg/kg triweekly) and docetaxel (70 mg/m2, triweekly) therapy in addition to zoledronic acid. This regimen was selected according to the regimen for metastatic HER2 positive breast cancer that had been approved by our institutional review board. Adverse events included grade 4 leukopenia and neutropenia, grade 1 anemia, hypocalcemia, edema, and appetite loss.
Five months after initiation of trastuzumab/docetaxel therapy, PET–CT revealed FDG uptake only in the submandibular gland and not in the areas of distant lesions (Fig. 3). Thus, the patient exhibited a partial response in the submandibular gland and a complete response in the neck and distant metastases. Subsequently, use of trastuzumab/docetaxel therapy was discontinued in accordance with the patient’s wishes. We therefore performed a submandibular gland resection and neck dissection for loco-regional control. Figure 4 illustrates the histopathology of the primary tumor and neck lymph node metastases after trastuzumab/docetaxel therapy. Very few viable cancer cells were identified in the primary tumor (Fig. 4a, b). HER2 protein expression was identified only in viable cancer cells, whereas it was absent in other lesions (Fig. 4c, d). No cancer cells were identified in the cervical lymph nodes.
Fig. 3.

A whole body positron emission tomography/computed tomography scan after six cycles of trastuzumab/docetaxel therapy. Fludeoxyglucose uptake was undetectable except in the right submandibular gland
Fig. 4.
Histological and immunohistochemical examinations of the resected tumor. a, b Very few viable cancer cells were identified in the primary tumor. c, d On immunohistochemistry, the HER2 protein was identified only in viable cancer cells, and was absent from other lesions
The patient received postoperative concurrent chemoradiotherapy comprising weekly docetaxel administration (10 mg/m2); however, brain metastases developed and chemoradiotherapy was discontinued. The patient died of multiple brain, lung, and liver metastases 12 months after the initial treatment, following a progression-free survival interval of 6.3 months.
Discussion
This is the first case report to show a pathological response of HER2-positive SDC to trastuzumab/docetaxel therapy. A high incidence of HER2 or androgen receptor positivity has been reported in SDCs [1, 4–6, 11]. HER2 positivity is a strong prognostic factor for breast carcinoma [12], and the efficacy of trastuzumab, a monoclonal HER2 antibody, for HER2-positive breast carcinoma has been established [13]. Approximately 20–40 % of SDC cases are HER2-positive as determined on IHC and FISH [4–7]. Similar to breast carcinoma, HER2-positivity has been reported to be a prognostic factor for SDC [7, 11], but recent reports have contradicted this claim [4–6], demonstrating that trastuzumab mono-therapy for HER2-positive SDC is not effective [14]. Combined therapy regimens consisting of trastuzumab and cytotoxic agents such as docetaxel, paclitaxel, or carboplatin are more effective compared to cytotoxic agent monotherapy [8, 9].
The current patient exhibited distant metastases at the initial visit, but achieved complete response in the neck and distant metastases by using trastuzumab/docetaxel therapy. Among patients with aggressive breast cancer subtypes, a pathological complete response achieved by preoperative chemotherapy is predictive of a better prognosis. In addition, a histological evaluation after preoperative chemotherapy is recommended to confirm the treatment effect and predict prognosis [15]. Therefore, a pathological examination of SDC after chemotherapy might be considered during treatment. However, the patient developed brain metastases during postoperative chemoradiotherapy for the loco-regional site, and died. One report suggests that anti-HER2 therapy might increase the lifespan of patients with recurrent or metastatic disease [9]. As residual HER2-positive cells were identified in the resected tissue of the patient described in this case, continuation of anti-HER2 therapy may have resulted in the prolongation of his life.
Several case series have reported the efficacy of anti-HER2 therapy [7, 8]; however, studies with a large sample size and randomized controlled studies are lacking. In addition, previous studies of anti-HER2 therapy included cases with 1–3+ HER2 positivity on IHC or those that were not analyzed using FISH and some reports have indicated that the concordance rate of HER2-positivity on IHC and FISH for SDC was lower than that for breast cancer [7, 16]. For these reasons, more research is necessary to establish the utility of anti-HER2 therapy for SDC. Further multi-institutional studies to evaluate the efficacy of anti-HER2 therapy are needed.
Conflict of interests
All authors state that they have no potential conflicts of interest to disclose.
Statements of human rights
For this type of study, formal consent is not required.
Informed consent
Informed consent was obtained from the patient included in this report. This study was supported in part by JSPS Grants-in-Aid for Scientific Research (C) to Y. Tada (No. 15K10823).
References
- 1.Brandwein-Gensler MS, Skalova A, Nagao T. Salivary duct carcinoma. In: Barnes L, Eveson JW, Reichart P, Sidranksky D, editors. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. pp. 236–237. [Google Scholar]
- 2.Barnes L, Rao U, Krause J, Contis L, Schwartz A, Scalamogna P. Salivary duct carcinoma. Part I. A clinicopathologic evaluation and DNA image analysis of 13 cases with review of the literature. Oral Surg Oral Med Oral Pathol. 1994;78:64–73. doi: 10.1016/0030-4220(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 3.Laurie SA, Licitra L. Systemic therapy in the palliative management of advanced salivary gland cancers. J Clin Oncol. 2006;24:2673–2678. doi: 10.1200/JCO.2005.05.3025. [DOI] [PubMed] [Google Scholar]
- 4.Williams MD, Roberts D, Blumenschein GR, Jr, et al. Differential expression of hormonal and growth factor receptors in salivary duct carcinomas: biologic significance and potential role in therapeutic stratification of patients. Am J Surg Pathol. 2007;31:1645–1652. doi: 10.1097/PAS.0b013e3180caa099. [DOI] [PubMed] [Google Scholar]
- 5.Otsuka K, Imanishi Y, Habu N, et al. Survival analysis and immunohistochemical study of HER-2 and AR (androgen receptor) expression in salivary duct carcinoma. Nihon Jibiinkoka Gakkai Kaiho. 2013;116:1024–1032. doi: 10.3950/jibiinkoka.116.1024. [DOI] [PubMed] [Google Scholar]
- 6.Masubuchi T, Tada Y, Maruya S, et al. Clinicopathological significance of androgen receptor, HER2, Ki-67 and EGFR expressions in salivary duct carcinoma. Int J Clin Oncol. 2015;20:35–44. doi: 10.1007/s10147-014-0674-6. [DOI] [PubMed] [Google Scholar]
- 7.Skálová A, Stárek I, Vanecek T, et al. Expression of HER-2/neu gene and protein in salivary duct carcinomas of parotid gland as revealed by fluorescence in situ hybridization and immunohistochemistry. Histopathology. 2003;42:348–356. doi: 10.1046/j.1365-2559.2003.01600.x. [DOI] [PubMed] [Google Scholar]
- 8.Nabili V, Tan JW, Bhuta S, Sercarz JA, Head CS. Salivary duct carcinoma: a clinical and histologic review with implications for trastuzumab therapy. Head Neck. 2007;29:907–912. doi: 10.1002/hed.20614. [DOI] [PubMed] [Google Scholar]
- 9.Limaye SA, Posner MR, Krane JF, et al. Trastuzumab for the treatment of salivary duct carcinoma. Oncologist. 2013;18:294–300. doi: 10.1634/theoncologist.2012-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wollf AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 11.Etges A, Pinto DS, Jr, Kowalski LP, Soares FA, Araújo VC. Salivary duct carcinoma: immunohistochemical profile of an aggressive salivary gland tumour. J Clin Pathol. 2003;56:914–918. doi: 10.1136/jcp.56.12.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 13.Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol. 2005;23:4265–4274. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 14.Haddad R, Colevas AD, Krane JF, et al. Herceptin in patients with advanced or metastatic salivary gland carcinomas. A phase II study. Oral Oncol. 2003;39:724–727. doi: 10.1016/S1368-8375(03)00097-6. [DOI] [PubMed] [Google Scholar]
- 15.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 16.Cornolti G, Ungari M, Morassi ML, et al. Amplification and overexpression of HER2/neu gene and HER2/neu protein in salivary duct carcinoma of the parotid gland. Arch Otolaryngol Head Neck Surg. 2007;133:1031–1036. doi: 10.1001/archotol.133.10.1031. [DOI] [PubMed] [Google Scholar]